Abstract
Background and Aims
The onset of xylogenesis plays an important role in tree growth and carbon sequestration, and it is thus a key variable in modelling the responses of forest ecosystems to climate change. Temperature regulates the resumption of cambial activity, but little is known about the effect of water availability on the onset of xylogenesis in cold but semi-arid regions.
Methods
The onset of xylogenesis during 2009–2014 was monitored by weekly microcoring Juniperus przewalskii trees at upper and lower treelines on the north-eastern Tibetan Plateau. A logistic regression was used to calculate the probability of xylogenic activity at a given temperature and a two-dimensional reverse Gaussian model to fit the differences between the observed and estimated days of xylogenesis onset at given temperatures and precipitation within a certain time window.
Key Results
The thermal thresholds at the beginning of the growing season were highly variable, suggesting that temperature was not the only factor initiating xylem growth under cold and dry climatic conditions. The onset of xylogenesis was well predicted for climatic thresholds characterized by a cumulative precipitation of 17.0 ± 5.6 mm and an average minimum temperature of 1.5 ± 1.4 °C for a period of 12 d.
Conclusions
Xylogenesis in semi-arid regions with dry winters and springs can start when both critical temperature and precipitation thresholds are reached. Such findings contribute to our knowledge of the environmental drivers of growth resumption that previously had been investigated largely in cold regions without water shortages during early growing seasons. Models of the onset of xylogenesis should include water availability to improve predictions of xylem phenology in dry areas. A mismatch between the thresholds of temperature and moisture for the onset of xylogenesis may increase forest vulnerability in semi-arid areas under forecasted warmer and drier conditions.
Keywords: Tree growth, temperature, precipitation, critical thresholds, xylogenesis, Juniperus przewalskii, xylem formation, drought, two-dimensional reverse Gaussian model, semi-arid area, Tibetan Plateau
INTRODUCTION
Interest in xylem phenology (xylogenesis) and its sensitivity to climate change is growing because wood is a major sink of carbon in terrestrial ecosystems (Chaffey, 2002; Cuny et al., 2015; Pérez-de-Lis et al., 2017). Temperature is increasingly recognized as the primary driver of growth reactivation in cold climates (Rossi et al., 2007, 2008). Both observations and controlled experiments have demonstrated that cambial activity is limited by low air temperatures in cold climates (Oribe et al., 2001; Gricar et al., 2006; Rossi et al., 2008; Seo et al., 2008; Gruber et al., 2010; Begum et al., 2013; Li et al., 2013). In addition, the onset of xylem production is delayed at higher latitudes and altitudes, confirming the role of temperature for xylogenesis (Moser et al., 2010; Oladi et al., 2010; Huang et al., 2011). In particular, Rossi et al. (2008) reported a critical daily minimum temperature for xylogenesis in conifers of 4–5 °C in cold climates. Shen et al. (2015), however, highlighted the impact of precipitation on the starting date of vegetation phenology (canopy greening) in cold and arid or semi-arid regions, indicating that cold and drought stress both affected the onset of growth. Ren et al. (2015) found a delay in the initiation of xylogenesis in Qilian junipers (Juniperus przewalskii Kom.) under extremely dry spring conditions in a cold and dry climate, which suggested a potential influence of water availability on the start of xylogenesis, i.e. on the onset of cambial reactivation after the cold dormant season (winter in the Northern Hemisphere).
The effect of precipitation on the growth dynamics of forest ecosystems needs to be quantified to better understand the adaptation of plants to a changing climate, which may be characterized by warmer and drier conditions (Allen et al., 2015). In addition, climatic thresholds for the resumption of xylem phenology may provide keys to better understand the mechanisms of forest resilience (e.g. post-drought recovery) as the climate changes. Water acts on several important growth processes in plants. The expansion of xylem cells is turgor-driven, and depends on the uptake of cellular water and on solute accumulation. Drought stress affects the loss of turgor of differentiating cells (Kozlowski and Pallardy, 2002), so shifts in the onset of xylogenesis might be affected by variation in moisture, especially in the arid and semi-arid regions of the world. The available literature, however, is limited to studies conducted in regions characterized by rains prior to the onset of xylogenesis (from winter to spring), such as the Mediterranean basin (Camarero et al., 2010, 2015; Vieira et al., 2013), or by abundant water released during snowmelt, such as alpine valleys (Gruber et al., 2010; Eilmann et al., 2011; Swidrak et al., 2011). Soil moisture could be a less important limiting factor for the resumption of xylem formation at these sites than in arid or semi-arid areas. We investigated how cold and dry conditions could drive the onset of xylogenesis by determining the relative influence of these two climatic stressors.
We selected a forested area on the north-eastern Tibetan Plateau to test the effect of soil moisture on the onset of xylogenesis. The dry climate of this area is characterized by scarce winter precipitation, a very thin snowpack and the dependence of moisture availability for vegetation activity on the first rains of spring (Dai, 1990). The climate is described as cold and dry, with a mean annual temperature of 3.1 °C and a mean annual precipitation of approx. 200 mm. Winter is extremely dry, and rain mainly falls from May to September (Dai, 1990). The Qilian juniper forests in this area are stressed by both drought and cold (Zheng et al., 2008). A recent study reported that spring drought could delay the onset of xylogenesis in Qilian juniper despite optimal thermal conditions (Ren et al., 2015). In addition, warmer spring conditions on the plateau are increasing the vulnerability of forests to dry spells, as indicated by a marked decrease in growth and an increase in the frequency of missing tree rings (Liang et al., 2014, 2016). These findings suggested a potential interaction between precipitation and temperature for the onset of xylogenesis under cold and dry conditions.
The objective of this study was to use Qilian juniper as a model species to investigate the onset of xylogenesis at the upper and lower altitudinal boundaries of its distribution during six growing seasons (2009–2014) and to identify the thresholds of temperature and precipitation controlling the onset of xylogenesis. We hypothesized that the onset of xylogenesis in Qilian juniper was constrained more by water deficit than by low temperatures.
MATERIALS AND METHODS
Study site, field sampling and sample preparation
The study was carried out in an undisturbed Qilian juniper forest near Dulan County on the north-eastern Tibetan Plateau (36°00′N, 98°11′E). Two sites, at 3850 and 4210 m a.s.l. with slopes of 15°, were selected at the lower and upper treeline. Five trees were randomly selected at each site. Diameter at breast height was 54 ± 7 and 53 ± 4 cm at the lower and upper treeline sites, respectively, and the average height was 8 m. Microcores were extracted weekly from 2009 to 2014 from the stems at a height of 1.0–1.3 m using a Trephor microborer (Rossi et al., 2006) and stored in a formalin–ethanol–acetic acid (FAA) solution. The microcores were prepared to obtain transverse sections (9–12 µm in thickness) using a Leica RM 2245 rotary microtome (Leica Microsystems, Wetzlar, Germany), and the sections were stained using a mixture of safranin, Astra Blue and ethanol and then permanently fixed. See Ren et al. (2015) for more details on sampling strategy and slide preparation.
Identification of the onset of xylogenesis
The xylem sections were observed under a microscope at a magnification of 100× with visible and polarized light to distinguish the differentiating xylem cells. We concentrated on the radial-enlargement phase, which indicates the beginning of xylem growth (Antonova and Stasova, 1993). Tracheids in the radial-enlarging phase contained a protoplast enclosed in thin primary cell walls, and their radial diameters were at least twice that of a cambial cell (Rossi et al., 2006). The tracheids had light-blue walls under normal light during this phase but were not visible under polarized light due to the lack of a secondary wall. Xylogenesis was considered to have begun for each tree when at least one radial file of enlarging cells was observed in spring.
Meteorological data
Meteorological data were recorded at each site from October 2012 by automatic stations (HOBO; ONSET, Pocasset, MA, USA). Air temperature and precipitation were measured every 30 min and stored in data loggers. Minimum, mean and maximum daily temperatures and daily precipitation were calculated for subsequent analyses. Data for January 2009 to September 2012 were estimated using the measurements collected from a meteorological station in Dulan (36°18′N, 98°06′E; 3190 m a.s.l.), 32 km from the study sites. The consistency of the estimates was based on the high correlations (r > 0.92) between the climatic data (temperature and precipitation) at the two sites with those at the Dulan station (Supplementary Data Fig. S1).
Statistical analyses to predict climatic thresholds of xylogenesis
Based on previous research (Ren et al., 2015), air temperature and precipitation were selected as potential climatic drivers of the beginning of xylogenesis.
Logistic regression [LOGISTIC procedure in SAS 9.4 (SAS Institute Inc., Cary, NC, USA)] was used to calculate the probability of xylogenic activity at a given temperature; responses were coded as non-active (value zero) or active (value 1). Temperature thresholds were calculated when the probability of xylogenesis being active was 0.5 (see Rossi et al., 2007, 2008, for more details on the calculation of temperature thresholds and model verification). The model was fitted with the minimum, mean and maximum temperatures for each tree, site and year. None of 180 models (2 sites × 6 years × 5 trees × 3 temperature series) applied was excluded because of a lack of fit (in all cases R2 > 0.90). Thermal thresholds were then compared between years using an ANOVA.
Two-dimensional reverse Gaussian models were used to calculate the difference between the observed day of onset of xylogenesis and the estimated day according to a given temperature and precipitation within a certain time window. The Gaussian model generates a funnel-surface plot, with a circular-to-elliptical cross-section with the general form:
where Zxy is the mean absolute difference between the observed day of onset of xylogenesis (Supplementary Data Table S1) and the estimated day with a given average temperature x and cumulative precipitation y within the time window t across trees, sites and years, Z0 is the distance from the edge of the surface to the plane z = 0, A is the height of the trough, x0 and y0 are the coordinates defining the position of the centre of the surface, W1 and W2 are the spreads of the surface on the x- and y-axes, respectively, and θ is the clockwise rotation angle of the surface (Supplementary Data Fig. S2). The model was fitted with the corresponding temperature (minimum, mean and maximum air temperatures) and precipitation data from all sites and all years within different time windows. The coefficient of determination (R2) of the model was provided to evaluate the goodness of fits. The culmination of R2 was considered to correspond to the optimal time window t. The critical average temperature (x) and cumulative precipitation (y) were calculated when Zxy was near 0 at the optimal time window t. Standardized residuals were calculated for model verification. Model validation was performed by comparing the observations (onset of xylogenesis) with the predicted values calculated using data for precipitation and temperature as predictors. Standardized regression coefficients were calculated by dividing a parameter estimate by the ratio of the sample standard deviation of the dependent variable to the sample standard deviation of the regressor (Bring, 1994). Standardized regression coefficients allow us to estimate the specific contribution of the independent variables (i.e. temperature and precipitation) on the dependent variable (onset of xylogenesis).
RESULTS
Spring meteorological conditions
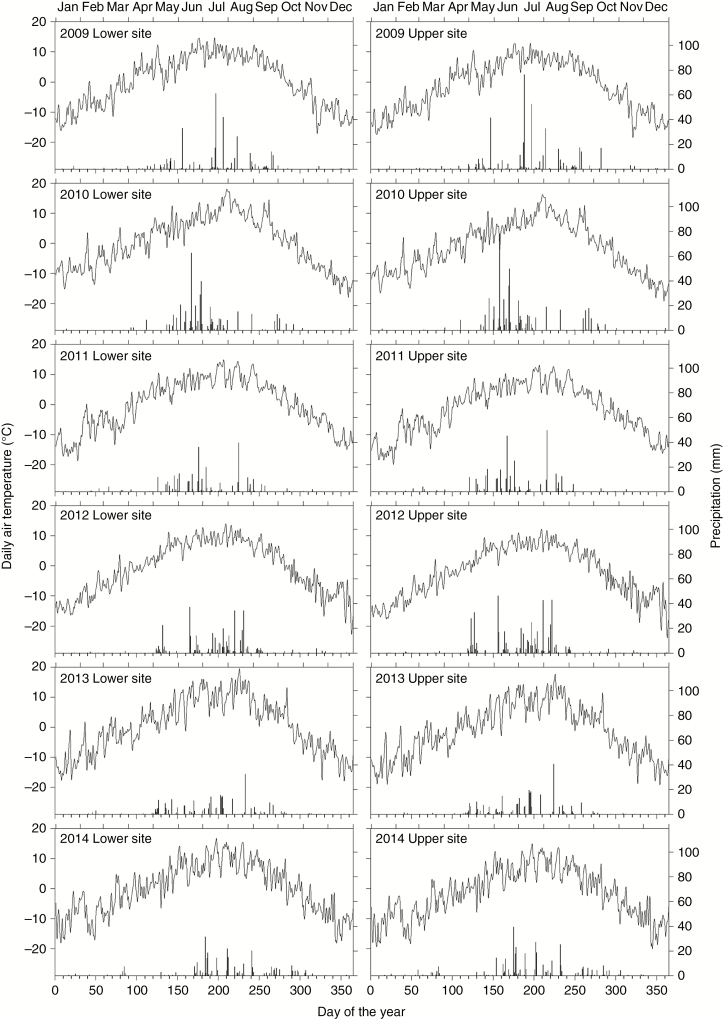
During the study period, annual mean temperature at the upper and lower treeline sites was −1.49 and 0.04 °C, respectively. The years 2012 and 2013 were the coldest (−2.16 and −0.65 °C at upper and lower treelines) and warmest (−1.14 and 0.39 °C), respectively. Annual precipitation at the upper and lower treeline sites was 433.1 and 364.0 mm, respectively. The wettest (537.2 and 443.3 mm) and driest (320.2 and 308.3 mm) years occurred in 2010 and 2013. Daily mean temperatures in March at the upper and lower treeline sites were −4.7 and −3.2 °C, respectively, reaching 1.8 and 3.4 °C in May. Monthly precipitation, on average, increased tenfold, from 5–6 mm in March to 50–60 mm in May (Fig. 1).
Fig. 1.
Daily air temperature (lines) and precipitation (bars) during 2009–2014 at the lower and upper study treeline sites.
Spring (March to May) conditions varied among years (Fig. 1). The warmest spring during the study was in 2009, with daily mean temperatures reaching 1.4 and −0.2 °C at the lower and upper treeline sites, respectively. The coldest and driest springs were in 2014, with mean temperatures of −0.2 and −1.8 °C and total precipitation of 25.8 and 31.0 mm at the lower and upper treeline sites, respectively. Monthly precipitation in March 2014 ranged between 17.4 and 21.8 mm, which represented the highest amount of spring rain during the study period.
Threshold temperatures
The average threshold temperature with a probability of 0.5 for active xylogenesis was calculated for each year and site (Table 1). Thermal thresholds at the lower treeline varied within large ranges, 0–5, 4–9 and 10–14 °C for the daily minimum, mean and maximum temperatures, respectively. Thresholds were significantly higher in 2010 than in other years and were lowest in 2014 (P < 0.001). The thermal thresholds were lower at the upper treeline, but also with large ranges, 0–5, 3–8 and 8–12 °C for the daily minimum, mean and maximum temperatures, respectively. The thresholds at the upper treeline also differed significantly between years (P < 0.001). Thresholds were significantly higher in 2010 than in the other years and were lower in 2014 for the daily minimum and mean temperatures and in 2012 and 2014 for the maximum temperature.
Table 1.
Threshold minimum, mean and maximum temperatures corresponding to the 95 % probability of active xylogenesis in Juniperus przewalskii estimated during 2009–2014 at the lower and upper study treeline sites
| Site | Temperature (°C) | Year | F | P | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | ||||
| Lower treeline | Minimum | 2.5 ± 0.4a | 5.0 ± 0.7b | 2.4 ± 0.7a | 1.8 ± 0.4a | 2.0 ± 0.9a | 0.0 ± 0.7c | 29.93 | <0.001 |
| Mean | 7.2 ± 0.4a | 9.3 ± 0.8b | 7.5 ± 0.7a | 6.0 ± 0.4a | 6.3 ± 0.9a | 4.5 ± 0.7c | 26.33 | <0.001 | |
| Maximum | 12.0 ± 0.5a | 14.2 ± 0.9b | 12.0 ± 1.0a | 10.0 ± 0.4c | 11.3 ± 0.9a,c | 9.8 ± 0.8c | 21.80 | <0.001 | |
| Upper treeline | Minimum | 1.9 ± 0.2a,b | 4.5 ± 0.3c | 2.4 ± 0.4a | 1.0 ± 0.6b | 1.2 ± 0.9b | -0.5 ± 0.4d | 53.83 | <0.001 |
| Mean | 5.9 ± 0.2a,b | 8.0 ± 0.3c | 6.4 ± 0.3a | 4.5 ± 0.6d | 5.0 ± 0.9b,d | 3.4 ± 0.4e | 52.06 | <0.001 | |
| Maximum | 10.1 ± 0.2a,b | 12.2 ± 0.3c | 10.8 ± 0.5a | 7.9 ± 0.6d | 9.4 ± 0.9b | 8.1 ± 0.4d | 51.31 | <0.001 | |
Results from an ANOVA are reported as F and P statistics. Different letters within a row indicate significant differences at P < 0.05.
Two-dimensional Gaussian models
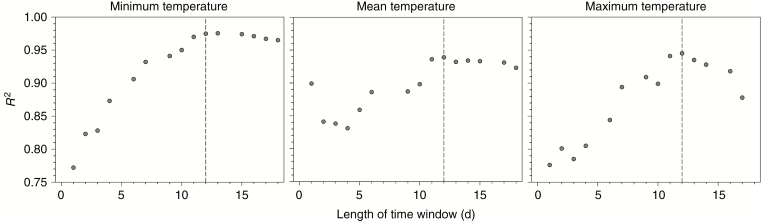
R 2 of the Gaussian models varied with the length of the time window (Fig. 2). R2 increased for longer time windows, culminating with a time window of 12 d when R2 reached 0.97, 0.93 and 0.94 for the minimum, mean and maximum temperatures, respectively. R2 decreased slightly (minimum and mean temperature) or substantially (maximum temperature) for time windows longer than 12 d.
Fig. 2.
Coefficient of determination (R2) for the two-dimensional Gaussian models within the time window from 1 to 18 d. Dotted lines indicate the time windows (in days) corresponding to maximum R2.
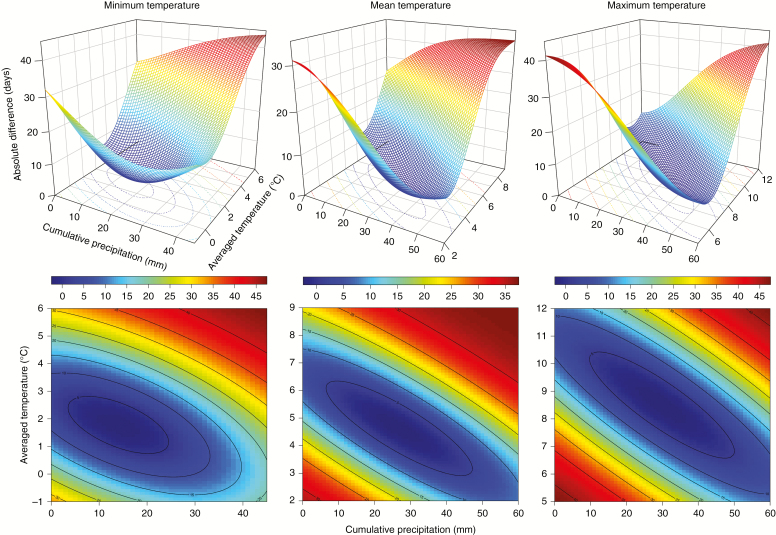
The minimal Zxy was 2.21 d for a time window of 12 d. The critical cumulative precipitation was 17.0 ± 5.6 mm and the average minimum temperature was 1.5 ± 1.4 °C when Zxy was <2.5 d (Fig. 3). The spreads of this trough on the x- and y-axes were 48.0 mm and 2.04 °C, respectively, with an anticlockwise rotation of 4.73°. In the model with the average mean temperature, the minimal Zxy was 1.85 d. The critical precipitation and temperature were 26.9 ± 3.9 mm and 4.6 ± 1.8 °C, respectively, when Zxy was <2 d. The spreads of this trough on the x- and y-axes were 44.1 mm and 1.94 °C, respectively, with an anticlockwise rotation of 8.22°. The minimal Zxy was 1.90 d in the model with the average maximum temperature. The critical precipitation and temperature were 29.9 ± 3.0 mm and 8.5 ± 1.8 °C, respectively, when Zxy was <2 d. The spreads of this trough on the x- and y-axes were 51.1 mm and 2.39 °C, respectively, with an anticlockwise rotation of 9.52°. Most of the standardized residuals of these three models converged from −2 to 2 (Supplementary Data Fig. S3). The standardized regression coefficients revealed similar contributions of the independent variables for explaining the onset of xylogenesis, with temperature and precipitation accounting for 47 % and 53 % of the variability in the date of xylogenesis onset, respectively.
Fig. 3.
Surface plots and the corresponding level sets showing the two-dimensional Gaussian distribution of the absolute difference between the observed day of onset of xylogenesis and the estimated day with a given average temperature and cumulative precipitation in the time window of 12 d. Note that the axes have different scales.
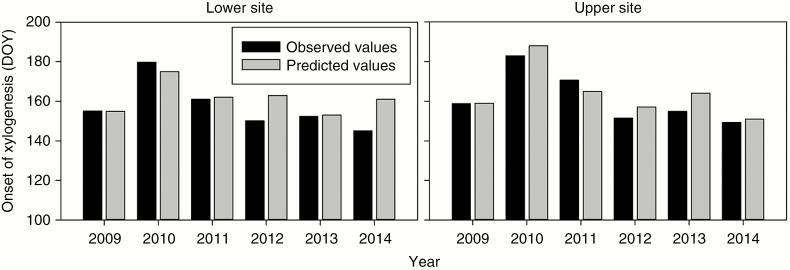
The absolute differences between the observed and predicted dates of onset of xylogenesis using average minimum temperature and cumulative precipitation in a time window of 12 d were smaller than the sampling interval by averages of 5.9 and 4.6 d at the lower and upper treeline sites, respectively (Fig. 4). The largest divergence between observations and predictions was 16 d in 2014 at the lower treeline.
Fig. 4.
Observed and predicted days of onset of xylogenesis (DOY, day of the year) in Juniperus przewalskii during 2009–2014 at the lower and upper study treeline sites. Predictions were obtained using the threshold average minimum temperature and cumulative precipitation calculated by the models in the time window of 12 d.
DISCUSSION
We challenged the general opinion that temperature was the only driver of growth reactivation at high elevations by analysing the onset of xylogenesis of Qilian juniper subjected to cold and dry climatic conditions on the north-eastern Tibetan Plateau. Published threshold temperatures for the onset of xylogenesis in trees for boreal forest or within a dry inner Alpine valley range from 2 to 3 °C (Rossi et al., 2007, 2008; Swidrak et al., 2011; Boulouf Lugo et al., 2012), whereas on the south-eastern Tibetan Plateau the critical minimum temperature occurred at 0.7 ± 0.4 °C (Li et al., 2017). The large range (5 °C) in the thermal thresholds for the onset of xylogenesis in Qilian juniper provides additional evidence that temperature was not the only factor initiating xylem growth under cold and dry climatic conditions (Table 1). More reliable predictions were attained when both thermal and precipitation thresholds for the onset of xylogenesis were included in the fitted models (Fig. 4). The interaction between temperature and precipitation satisfactorily explained the day of onset of xylogenesis in 2010, which was delayed by approx. 3 weeks compared with 2009 and 2011, despite the warm conditions during that spring (Ren et al., 2015). This finding suggests that spring precipitation is also an important factor in the resumption of xylem formation in Qilian juniper.
Water availability is an important determinant of xylem formation. Before the start of xylem phenology, trees must compensate for the water lost during winter and spring to recover an adequate water balance, because turgor is an important requisite for xylem cell growth (Sevanto et al., 2006). Rehydration time in spring can exceed 6 weeks, and stems are fully rehydrated 1 month before the onset of radial growth (Turcotte et al., 2009). Both cell division and expansion in the xylem are sensitive to changes in water potential (Abe and Nakai, 1999; Savidge, 2001). The water potential in the cambium regulates mitosis and influences cell extension and the deposition of wall polymers (Abe and Nakai, 1999; Cosgrove, 2005; Arend and Fromm, 2007). Springs were rainy or water was abundantly supplied by snowmelt in the cold regions of previous studies, so the initiation of xylem growth was not limited by rehydration, and trees responded more to temperature than to precipitation (Turcotte et al., 2009). Warmer springs in such areas can substantially advance xylem phenology (Rossi et al., 2011). The response to seasonal changes, including both initiation and cessation of growth, can also be controlled by photoperiod in many trees of the boreal and temperate regions (Körner and Basler, 2010; Maurya and Bhalerao, 2017). Winter and spring similarly are often wet in cold and drought-prone regions such as continental Mediterranean forests, and moisture is not considered the only factor in the resumption of xylem formation (Camarero et al., 2010). With scarce snow, winter is extremely dry in our study area; consequently, water availability is low before growth reactivation. Moreover, drought stress would be higher under drier and warmer conditions, which would thus slow the onset of xylogenesis, as observed in spring 2010. Soil moisture occasionally can be increased by snowfall, such as the snowfall in 2014, as also occurs in boreal forests (Vaganov et al., 1999). The amount of water available during the snowmelt in our study increased soil moisture and possibly advanced the onset of xylogenesis, probably explaining the difference of 16 d between observations and predictions in 2014 at the lower treeline. This research found that the onset of xylogenesis in Qilian juniper should meet the prerequisite for both critical temperature and precipitation.
This study is the first statistical attempt to demonstrate that the onset of xylogenesis is driven by an interaction between thermal and precipitation thresholds. The selected time window of 12 d agrees with the period required for tracheid expansion and differentiation (Vaganov et al., 2006; Cuny et al., 2015). Temperature is a well-recognized factor controlling the onset of xylem formation, but our findings provide new insights into the climatic forcing of growth. The critical temperatures and precipitation provide keys for modelling the response of forest ecosystems subjected to cold and dry constraints in response to climate change and could help our understanding of the regime shifts in these ecosystems (Scheffer et al., 2001; Zhu et al., 2014). In addition, temperature and precipitation were more efficient for modelling the onset of xylogenesis than the Standardized Precipitation Evapotranspiration Index (SPEI) (Ren et al., 2015). The data for daily temperature and precipitation are absolute values obtained by direct measurement, while the SPEI drought index is quantified as monthly values and is estimated based on climatic data (Vicente-Serrano et al., 2010). Our findings also support the constraint of growth by drought stress in high-elevation forests or near the alpine treeline, as indicated by previous studies (Liang et al., 2014; Piper et al., 2016).
On the other hand, our predictions differed from observations when the winter prior to tree growth was extremely cold or dry. For example, the mean temperature from March to April in 2012 at the lower treeline was very low (−2.2 °C), allowing for long-term storage of water as snow. Snowmelt combined with increasing temperature in spring can yield sufficient soil moisture for the onset of xylogenesis. A similar situation happened in 2014 at the lower treeline. However, at the upper treeline, a deeper snowpack in spring may delay the onset of xylogenesis by reflecting radiation and reducing the heat received by plants. In 2013, less snow (1.4 mm in precipitation) in March at the upper treeline was linked to an earlier onset of xylogenesis.
Tree growth in semi-arid areas is generally limited by drought stress resulting from lower precipitation and high evapotranspiration caused by high temperatures in the early growing season (Allen et al., 2015). A similar constraint has been reported for the Tibetan Plateau and other Asian mountain areas (Shao et al., 2005; Liang et al., 2006, 2016; Liu et al., 2006; Gou et al., 2014; Pederson et al., 2014; Yang et al., 2014; Zhang et al., 2015). Warming-induced drought stress has been decreasing generalized tree growth and increasing mortality in semi-arid areas across Asia (Dulamsuren et al., 2010; Liu et al., 2013; Allen et al., 2015; Liang et al., 2016). In particular, the failure to produce stem wood in a particular year (missing rings) is a response to dry and warm spring conditions, and an increasing frequency of missing tree rings is also evident in response to the warming in recent decades (Liang et al., 2014, 2016). Moreover, the frequency of missing rings has been strongly linked to tree mortality (Liang et al., 2016). We hypothesize that a failure to reach critical water availability for growth reactivation or a delay in cambial resumption in response to increasing drought stress could be primary factors in the failure to form a complete ring and portend lower growth and forest dieback. A mismatch between critical temperatures and amounts of moisture for the onset of xylogenesis under the drought conditions of global climate change and the acceleration of dryland expansion (Peñuelas et al., 2007; Allen et al., 2015; Huang et al., 2015) will reduce forest resilience and risk regime shifts in vulnerable semi-arid forests. Reyer et al. (2015) proposed the assessment of forest resilience and potential tipping points at various levels, from leaf to biosphere, and our study has stressed that climatic thresholds for the onset of xylogenesis might be key indicators of forest resilience and tipping points under changing climates.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: Mean ± s.d. of onset of xylogenesis (DOY, day of the year) during 2009–2014 at the lower and upper treeline sites. Figure S1: Correlations between the daily minimum, mean and maximum temperatures recorded at the Dulan meteorological station and the corresponding temperatures recorded during 2012–2014 at the lower and upper study treeline sites. Figure S2: Sample surface plot and the corresponding level sets of a two-dimensional Gaussian model. Figure S3: The distribution of standardized residuals in the time window of 12 d as a function of minimum, mean and maximum temperatures.
ACKNOWLEDGEMENTS
We thank Macairangjia and Chengye He for the weekly microcore sampling. This work was supported by the International Partnership Program of the Chinese Academy of Sciences [131C11KYSB20160061] and the National Natural Science Foundation of China [41525001 and 41171161]. J.P.’s research was supported by the European Research Council Synergy grant [ERC-2013-SyG 610028-IMBALANCE-P].
LITERATURE CITED
- Abe H, Nakai T. 1999. Effect of the water status within a tree on tracheid morphogenesis in Cryptomeria japonica D. Don. Trees 14: 124–129. [Google Scholar]
- Allen CD, Breshears DD, McDowell NG. 2015. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6: art129. [Google Scholar]
- Antonova G, Stasova V. 1993. Effects of environmental factors on wood formation in Scots pine stems. Trees 7: 214–219. [Google Scholar]
- Arend M, Fromm J. 2007. Seasonal change in the drought response of wood cell development in poplar. Tree Physiology 27: 985–992. [DOI] [PubMed] [Google Scholar]
- Begum S, Nakaba S, Yamagishi Y, Oribe Y, Funada R. 2013. Regulation of cambial activity in relation to environmental conditions: understanding the role of temperature in wood formation of trees. Physiologia Plantarum 147: 46–54. [DOI] [PubMed] [Google Scholar]
- Boulouf Lugo J, Deslauriers A, Rossi S. 2012. Duration of xylogenesis in black spruce lengthened between 1950 and 2010. Annals of Botany 110: 1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bring J. 1994. How to standardize regression coefficients. The American Statistician 48: 209–213. [Google Scholar]
- Camarero JJ, Olano JM, Parras A. 2010. Plastic bimodal xylogenesis in conifers from continental Mediterranean climates. New Phytologist 185: 471–480. [DOI] [PubMed] [Google Scholar]
- Camarero JJ, Gazol A, Sangüesa-Barreda G, Oliva J, Vicente-Serrano SM. 2015. To die or not to die: early warnings of tree dieback in response to a severe drought. Journal of Ecology 103: 44–57. [Google Scholar]
- Chaffey N. 2002. Why is there so little research into the cell biology of the secondary vascular system of trees?New Phytologist 153: 213–223. [Google Scholar]
- Cosgrove DJ. 2005. Growth of the plant cell wall. Nature Reviews Molecular Cell Biology 6: 850–861. [DOI] [PubMed] [Google Scholar]
- Cuny HE, Rathgeber CB, Frank D et al. . 2015. Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nature Plants 1: 1–6. [DOI] [PubMed] [Google Scholar]
- Dai J. 1990. The climate of the Tibetan Plateau.Beijing: China Meteorological Press; (in Chinese). [Google Scholar]
- Dulamsuren C, Hauck M, Leuschner C. 2010. Recent drought stress leads to growth reductions in Larix sibirica in the western Khentey. Global Change Biology 16: 3024–3035. [Google Scholar]
- Eilmann B, Zweifel R, Buchmann N, Graf Pannatier E, Rigling A. 2011. Drought alters timing, quantity, and quality of wood formation in Scots pine. Journal of Experimental Botany 62: 2763–2771. [DOI] [PubMed] [Google Scholar]
- Gou X, Deng Y, Gao L et al. . 2014. Millennium tree-ring reconstruction of drought variability in the eastern Qilian Mountains, northwest China. Climate Dynamics 45: 1761–1770. [Google Scholar]
- Gricar J, Zupancic M, Cufar K, Koch G, Schmitt U, Oven P. 2006. Effect of local heating and cooling on cambial activity and cell differentiation in the stem of Norway spruce (Picea abies). Annals of Botany 97: 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber A, Strobl S, Veit B, Oberhuber W. 2010. Impact of drought on the temporal dynamics of wood formation in Pinus sylvestris. Tree Physiology 30: 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JG, Bergeron Y, Zhai L, Denneler B. 2011. Variation in intra-annual radial growth (xylem formation) of Picea mariana (Pinaceae) along a latitudinal gradient in western Quebec, Canada. American Journal of Botany 98: 792–800. [DOI] [PubMed] [Google Scholar]
- Huang J, Yu H, Guan X, Wang G, Guo R. 2015. Accelerated dryland expansion under climate change. Nature Climate Change 6: 166–171. [Google Scholar]
- Körner C, Basler D. 2010. Phenology under global warming. Science 327: 1461–1462. [DOI] [PubMed] [Google Scholar]
- Kozlowski TT, Pallardy SG. 2002. Acclimation and adaptive responses of woody plants to environmental stresses. The Botanical Review 68: 270–334. [Google Scholar]
- Li X, Liang E, Gricar J, Prislan P, Rossi S, Cufar K. 2013. Age dependence of xylogenesis and its climatic sensitivity in Smith fir on the south-eastern Tibetan Plateau. Tree Physiology 33: 48–56. [DOI] [PubMed] [Google Scholar]
- Li X, Liang E, Gricar J, Rossi S, Cufar K, Ellison AM. 2017. Critical minimum temperature limits xylogenesis and maintains treelines on the southeastern Tibetan Plateau. Science Bulletin 62: 804–812. [DOI] [PubMed] [Google Scholar]
- Liang E, Liu X, Yuan Y et al. . 2006. The 1920s drought recorded by tree rings and historical documents in the semi-arid and arid areas of Northern China. Climatic Change 79: 403–432. [Google Scholar]
- Liang E, Dawadi B, Pederson N, Eckstein D. 2014. Is the growth of birch at the upper timberline in the Himalayas limited by moisture or by temperature?Ecology 95: 2453–2465. [Google Scholar]
- Liang E, Leuschner C, Dulamsuren C, Wagner B, Hauck M. 2016. Global warming-related tree growth decline and mortality on the north-eastern Tibetan plateau. Climatic Change 134: 163–176. [Google Scholar]
- Liu H, Park Williams A, Allen CD et al. . 2013. Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Global Change Biology 19: 2500–2510. [DOI] [PubMed] [Google Scholar]
- Liu Y, An Z, Ma H et al. . 2006. Precipitation variation in the northeastern Tibetan Plateau recorded by the tree rings since 850 AD and its relevance to the Northern Hemisphere temperature. Science in China Series D 49: 408–420. [Google Scholar]
- Maurya JP, Bhalerao RP. 2017. Photoperiod- and temperature-mediated control of growth cessation and dormancy in trees: a molecular perspective. Annals of Botany 120: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser L, Fonti P, Büntgen U et al. . 2010. Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiology 30: 225–233. [DOI] [PubMed] [Google Scholar]
- Oladi R, Pourtahmasi K, Eckstein D, Bräuning A. 2010. Seasonal dynamics of wood formation in Oriental beech (Fagus orientalis Lipsky) along an altitudinal gradient in the Hyrcanian forest, Iran. Trees 25: 425–433. [Google Scholar]
- Oribe Y, Funada R, Shibagaki M, Kubo T. 2001. Cambial reactivation in locally heated stems of the evergreen conifer Abies sachalinensis (Schmidt) masters. Planta 212: 684–691. [DOI] [PubMed] [Google Scholar]
- Pérez-de-Lis G, Olano JM, Rozas V et al. . 2017. Environmental conditions and vascular cambium regulate carbon allocation to xylem growth in deciduous oaks. Functional Ecology 31: 592–603. [Google Scholar]
- Peñuelas J, Ogaya R, Boada MS, Jump A. 2007. Migration, invasion and decline: changes in recruitment and forest structure in a warming-linked shift of European beech forest in Catalonia (NE Spain). Ecography 30: 829–837. [Google Scholar]
- Pederson N, Hessl AE, Baatarbileg N, Anchukaitis KJ, Di Cosmo N. 2014. Pluvials, droughts, the Mongol Empire, and modern Mongolia. Proceedings of the National Academy of Sciences USA 111: 4375–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper FI, Viñegla B, Linares JC et al. . 2016. Mediterranean and temperate treelines are controlled by different environmental drivers. Journal of Ecology 104: 691–702. [Google Scholar]
- Ren P, Rossi S, Gričar J, Liang E, Čufar K. 2015. Is precipitation a trigger for the onset of xylogenesis in Juniperus przewalskii on the north-eastern Tibetan Plateau?Annals of Botany 115: 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyer CPO, Brouwers N, Rammig A et al. . 2015. Forest resilience and tipping points at different spatio-temporal scales: approaches and challenges. Journal of Ecology 103: 5–15. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T. 2006. Assessment of cambial activity and xylogenesis by microsampling tree species: an example at the alpine timberline. IAWA Journal 27: 383–394. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T, Carraro V. 2007. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 152: 1–12. [DOI] [PubMed] [Google Scholar]
- Rossi S, Deslauriers A, Griçar J et al. . 2008. Critical temperatures for xylogenesis in conifers of cold climates. Global Ecology and Biogeography 17: 696–707. [Google Scholar]
- Rossi S, Morin H, Deslauriers A, Plourde P-Y. 2011. Predicting xylem phenology in black spruce under climate warming. Global Change Biology 17: 614–625. [Google Scholar]
- Savidge R. 2001. Intrinsic regulation of cambial growth. Journal of Plant Growth Regulation 20: 52–77. [Google Scholar]
- Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. 2001. Catastrophic shifts in ecosystems. Nature 413: 591–596. [DOI] [PubMed] [Google Scholar]
- Seo JW, Eckstein D, Jalkanen R, Rickebusch S, Schmitt U. 2008. Estimating the onset of cambial activity in Scots pine in northern Finland by means of the heat-sum approach. Tree Physiology 28: 105–112. [DOI] [PubMed] [Google Scholar]
- Sevanto S, Suni T, Pumpanen J et al. . 2006. Wintertime photosynthesis and water uptake in a boreal forest. Tree Physiology 26: 749–757. [DOI] [PubMed] [Google Scholar]
- Shao X, Huang L, Liu H, Liang E, Fang X, Wang L. 2005. Reconstruction of precipitation variation from tree rings in recent 1000 years in Delingha, Qinghai. Science in China Series D 48: 939–949. [Google Scholar]
- Shen M, Piao S, Cong N, Zhang G, Jassens IA. 2015. Precipitation impacts on vegetation spring phenology on the Tibetan Plateau. Global Change Biology 21: 3647–3656. [DOI] [PubMed] [Google Scholar]
- Swidrak I, Gruber A, Kofler W, Oberhuber W. 2011. Effects of environmental conditions on onset of xylem growth in Pinus sylvestris under drought. Tree Physiology 31: 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte A, Morin H, Krause C, Deslauriers A, Thibeault-Martel M. 2009. The timing of spring rehydration and its relation with the onset of wood formation in black spruce. Agricultural and Forest Meteorology 149: 1403–1409. [Google Scholar]
- Vaganov EA, Hughes MK, Kirdyanov AV, Schweingruber FH, Silkin PP. 1999. Influence of snowfall and melt timing on tree growth in subarctic Eurasia. Nature 400: 149–151. [Google Scholar]
- Vaganov EA, Hughes MK, Shashkin AV. 2006. Growth dynamics of conifer tree rings: images of past and future environments.Berlin: Springer-Verlag. [Google Scholar]
- Vicente-Serrano SM, Begueria S, Lopez-Moreno JI. 2010. A multiscalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index. Journal of Climate 23: 1696–1718. [Google Scholar]
- Vieira J, Rossi S, Campelo F, Freitas H, Nabais C. 2013. Xylogenesis of Pinus pinaster under a Mediterranean climate. Annals of Forest Science 71: 71–80. [Google Scholar]
- Yang B, Qin C, Wang J et al. . 2014. A 3,500-year tree-ring record of annual precipitation on the northeastern Tibetan Plateau. Proceedings of the National Academy of Sciences USA 111: 2903–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QB, Evans MN, Lyu L. 2015. Moisture dipole over the Tibetan Plateau during the past five and a half centuries. Nature Communications 6: 8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Liang E, Zhu H, Shao X. 2008. Response of radial growth of Qilian juniper to climatic change under different habitats. Journal of Beijing Forestry University 30: 7–12. [Google Scholar]
- Zhu K, Woodall CW, Ghosh S, Gelfand AE, Clark JS. 2014. Dual impacts of climate change: forest migration and turnover through life history. Global Change Biology 20: 251–264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.