Abstract
Background and Aims
Soil waterlogging often causes oxygen deficiency in the root systems of plants and severely inhibits plant growth. Formation of aerenchyma – interconnected spaces that facilitate the movement of gases between and within the aerial and submerged parts of plants – is an adaptive trait for coping with waterlogged conditions. Soybean (Glycine max) forms porous secondary tissues known as aerenchymatous phellem (AP), which are derived from the outermost cell layer of phellogen. To understand what factors other than waterlogging are involved in phellogen and AP formation, we examined how their formation in soybean seedlings was affected by darkness, CO2 deficiency and blockage of phloem transport.
Methods
Aerenchymatous phellem and phellogen formation were expressed as area ratios in cross-sections of hypocotyl. CO2 was depleted by use of calcium oxide and sodium hydroxide. Phloem transport was blocked by heat-girdling of hypocotyls. Sucrose levels were measured by spectrophotometry.
Key Results
Under light conditions, waterlogging induced the accumulation of high concentrations of sucrose in hypocotyls, followed by phellogen and AP formation in hypocotyls. Phellogen formation and AP formation were inhibited by darkness, CO2 deficiency and blockage of phloem transport. Phellogen formation and AP formation were also inhibited by excision of shoots above the epicotyl, but they recovered following application of sucrose (but not glucose or fructose application) to the cut surface.
Conclusions
The results demonstrate that sucrose derived from leaves is essential for AP and phellogen formation in soybean hypocotyls under waterlogged soil conditions. Maintenance of a high sucrose concentration is thus essential for the development of phellogen and AP and the differentiation of phellogen to AP.
Keywords: Aerenchymatous phellem, phellogen, phloem transport, soybean, sucrose, waterlogging
INTRODUCTION
Excess water stress caused by soil waterlogging or submergence often leads to oxygen deficiency in plants and severely suppresses their growth, particularly in non-wetland plants. Plants have evolved several adaptive traits for coping with waterlogged or submerged conditions (Bailey-Serres and Voesenek, 2008; Voesenek and Bailey-Serres, 2015), one of which is the formation of aerenchyma. Aerenchyma are interconnected gas spaces that provide a low-resistance pathway for gas movement between shoots and roots, and thus are an essential feature for internal aeration in plants under excess water conditions (Colmer and Voesenek, 2009; Takahashi et al., 2014b).
Aerenchyma can be largely classified as primary aerenchyma, which forms in primary tissues (e.g. cortex), and secondary aerenchyma, which forms in secondary tissues (e.g. phellem). Phellem is produced by phellogen cells (cork cambium), which are meristematic cell layers. The phellogen cells undergo successive divisions, producing phellem cells in a radially outward direction. Under waterlogging, phellem cells expand (Scott and Wager, 1888; Arber, 1920; Fraser, 1931; Jackson and Armstrong, 1999; Shimamura et al., 2003) to form a white, spongy, highly porous tissue called aerenchymatous phellem (AP) (Yamauchi et al., 2013; Takahashi et al., 2014b). In response to waterlogging stress, some legume plants, including soybean, form phellogen and AP in the stem, hypocotyl, adventitious roots, taproots and nodules (Yamauchi et al., 2013; Takahashi et al., 2014b).
In soybean, waterlogging conditions induce the formation of phellogen and AP (Shimamura et al., 2003; Thomas et al., 2005), possibly triggered by a reduction in abscisic acid concentrations (Shimamura et al., 2014). The surfaces of hypocotyls and roots (i.e. epidermis and cortex) split during the formation of phellogen and AP cells, resulting in the formation of lenticels, pores that allow gas exchange between the atmosphere and internal tissues (Shimamura et al., 2010). The gas-filled spaces of AP enable internal aeration between the aerial and submerged parts of plants (Stevens et al., 1997; Shimamura et al., 2003, 2010; Teakle et al., 2011; Verboven et al., 2012).
One of the factors involved in waterlogging tolerance in legumes appears to be sucrose, as waterlogging-tolerant plants were found to utilize sucrose better than waterlogging-intolerant plants (Striker and Colmer, 2016). Sucrose is required for cell division and extension in growing tissues and organs (Eveland and Jackson, 2012). Sucrose is produced by photosynthesis in source tissues (e.g. leaves), then loaded into the phloem, and subsequently transported to and deposited in the sink tissues (e.g. growing tissues or storage tissues) (Turgeon and Wolf, 2009; De Schepper et al., 2013; Ruan, 2014). Phloem is a vascular tissue that allows the translocation of photoassimilates (e.g. sucrose) from source to sink tissues (Turgeon and Wolf, 2009; De Schepper et al., 2013; Ruan, 2014). Sucrose is converted into several hexoses that are used in various metabolic pathways (e.g. energy production, starch synthesis, cellulose synthesis) in plants (Ruan, 2014), and thus spatial and temporal patterns of accumulation of sucrose are crucial to plant growth and tissue organization. For example, in Arabidopsis, sucrose promotes lateral root formation (MacGregor et al., 2008) and adventitious root initiation under dark conditions (Takahashi et al., 2003). Sucrose depletion causes arrest of cell division in meristematic cells and root growth (Francis and Halford, 2006). In seedlings at early growth stages, photosynthetically produced sucrose is translocated to the roots and is important for root development (Kircher and Schopfer, 2012). Sucrose is also involved in the regulation of apical dominance (Mason et al., 2014; Barbier et al., 2015). Limiting the amount of sucrose transport to axillary buds represses their growth and exogenous sucrose application initiates axial bud growth (Mason et al., 2014). Such results indicate that the transport of source tissue-derived sugars (such as sucrose) via phloem plays an important role in the growth of newly developed plant tissues and organs.
Although waterlogging clearly induces phellogen and AP formation, it is unclear what other factors may be involved. Here, we report that light exposure and photosynthesis-produced sucrose are required for phellogen and AP formation in soybean hypocotyls under waterlogging conditions.
MATERIALS AND METHODS
Plant materials and growth conditions
We grew soybean seedlings following Shimamura et al. (2010) with minor modifications. Soybean (Glycine max L. ‘Enrei’) seeds used in this study were collected from field-grown plants. Plastic pots (400 mL) were filled with 200 mL of silica sand (18–26 mesh). In each pot, a 2-cm-deep hole was dug, into which a single seed was sown and covered with a small amount of silica sand. Soybean plants were germinated and grown at 25 °C under 14-h light/10-h dark conditions for approximately 10 d [light conditions: 180–200 µmol m−2 s−1]. Deionized (DI) water was added to the pots daily beginning 2 d after sowing. Soybean seedlings were ready for use in the experiments when the unifoliate leaves were fully expanded (~10 d old), with seedlings of this age being used for all experiments. The soil was waterlogged by raising DI water levels to 3 cm above the soil surface for several days under treatments of 24-h light, 14-h light/10-h dark, 7-h light/17-h dark and 24-h dark conditions. Observations of phellogen and AP formation were conducted by examining cross-sections of seedling hypocotyls taken at 1 cm above the soil surface (i.e. 2 cm below the water surface) at 0, 1, 3, 5, and 7 d after the onset of waterlogging for subsequent time-course analyses.
CO2 removal, hypocotyl heat-girdling and sugar supplementation
To further investigate the effects of photoassimilate supply on phellogen and AP formation, we examined waterlogged plants subjected to three additional treatments: CO2 removal, hypocotyl heat girdling and sugar application.
To remove CO2, seedlings under waterlogged soil conditions were placed in a tightly closed container (internal dimensions: φ234 mm × 298 mm), along with 10 g calcium oxide and 10 g sodium hydroxide, for 5 d. Concentrations of CO2 fell within a few hours after the containers were closed (Supplementary Data Fig. S1).
To inhibit phloem transport, we used a modification of the heat-girdling method (Malone and Alarcon, 1995). A razor blade with a dull side and sharp side was held with tweezers and heated with an alcohol lamp. Cells all the way around the hypocotyl surface, parenchyma and phloem 1.5 cm above the soil surface were killed by gently touching them with the dull side of the heated razor for 1–2 s.
For the sugar (sucrose, glucose, fructose and mannitol) supplementation experiment, epicotyls were cut 2 cm above the cotyledons once unifoliate leaves were fully expanded. The cut end of the epicotyl was inserted into the tip of a 1.0-mL syringe that was cut at the 0.4-mL mark on the syringe scale, and the junction was sealed with silicone rubber (Supplementary Data Fig. S2). In total, 300 µL of various sugar solutions (0, 0.1, 0.25, 0.5 and 1 m) were then loaded into the syringe, from which they were gravity-fed into the cut surface of the epicotyl. Seedlings were subsequently grown under waterlogged soil conditions for 7 d. The sugar solution was replaced every day.
Anatomical observations
Transverse sections of seedling hypocotyls were taken at 1 cm above the soil surface following Shimamura et al. (2010) with minor modifications. Sections of 100–140 µm thickness were prepared using a Plant Microtome MTH-1 (Nippon Medical and Chemical Instruments Co., Ltd, Osaka, Japan) and stained with 0.05 % (w/v) toluidine blue O for 30 min. Each section was photographed using a light microscope (DM5000 B; Leica Microsystems, Wetzlar, Germany) and a CCD camera (DFC310 FX; Leica Microsystems). The areas of phellogen, AP and stele (a vascular cylinder) were measured using Image J software (v.1.46r; National Institutes of Health, Bethesda, MD, USA). The respective areas of phellogen, AP and stele depicted in Figs 2, 4, 8 and 9 are shown in Table S1. Areas were expressed as ratios of the area of the stele.
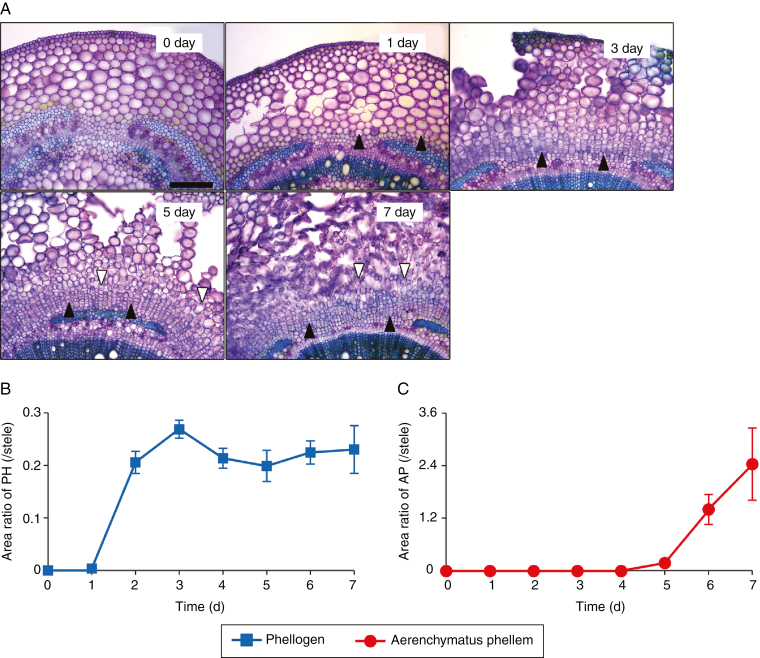
Fig. 2.
Time-course of phellogen and aerenchymatous phellem (AP) formation in response to waterlogging stress. Soybean seedlings were grown under drained soil conditions until unifoliate leaves were fully expanded. Phellogen and AP formation in cross-sections taken from hypocotyls at 1 cm above the soil surface were observed at 0, 1, 3, 5 and 7 d after waterlogging (A). Black and white arrowheads indicate phellogen and elongated cells in the AP, respectively. Scale bar = 250 µm. Phellogen and AP areas were measured daily for 7 d, and the area ratios of phellogen (PH) and AP to stele (i.e. vascular cylinder) were calculated (B, C). Values are means ± SD (n = 3).
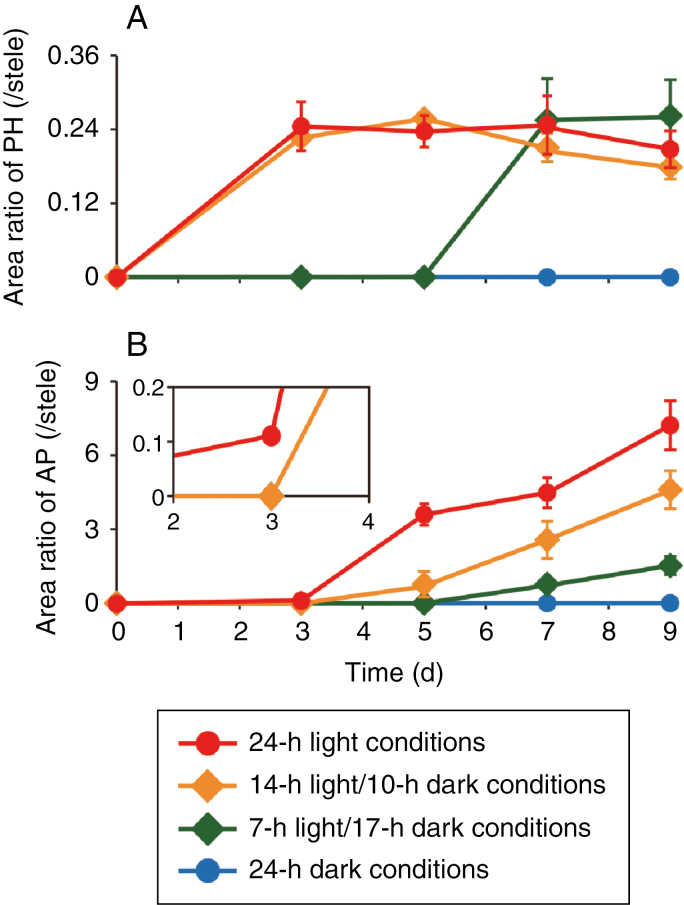
Fig. 4.
Phellogen and aerenchymatous phellem (AP) formation under different photocycles. Soybean seedlings were grown under drained soil conditions. When unifoliate leaves were fully expanded, the soil was waterlogged and seedlings were grown for 9 d under 24-h light, 14-h light/10-h dark, 7-h light/17-h dark, and 24-h dark conditions. Cross-sections of hypocotyls were taken at 1 cm above the soil surface from seedlings grown under each condition. Area ratios of phellogen [PH; (A)] and AP (B) to stele were calculated. Values are means ± SD (n = 3).
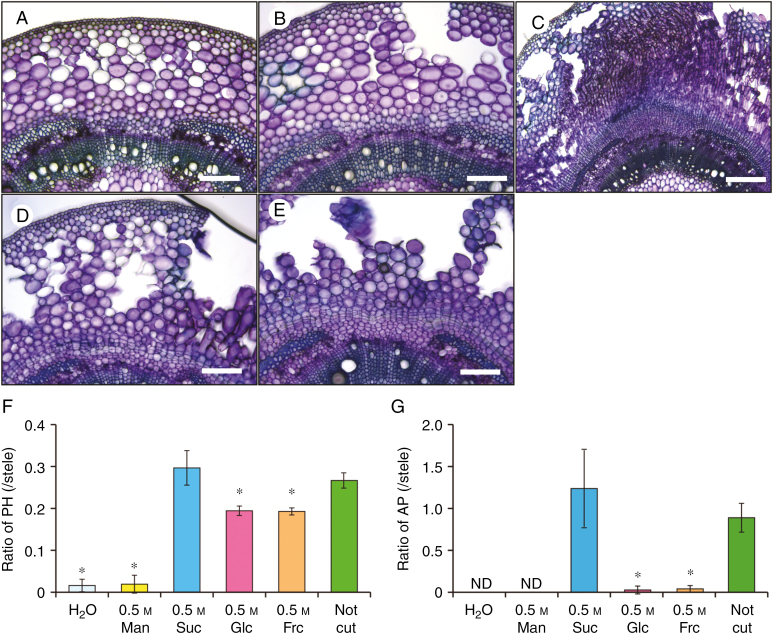
Fig. 8.
Effect of sugar administration to the cut epicotyl surfaces on phellogen and aerenchymatous phellem (AP) formation in the hypocotyls. Soybean seedlings were grown under drained soil conditions. When unifoliate leaves were fully expanded, an incision was made in the epicotyl of each seedling at 2 cm above the cotyledon. The cut surfaces were treated with water (H2O), 0.5 m mannitol (Man), sucrose (Suc), glucose (Glc) or fructose (Fru). Seedlings without epicotyl cutting (Not cut) served as controls. Seedlings were then grown under waterlogged conditions for 7 d. Cross-sections were taken at 1 cm above the soil surface from seedlings grown under each condition [H2O (A), Man (B), Suc (C), Glc (D)]. Scale bars = 500 µm (C), or 200 µm (A, B, D, E). Area ratios of phellogen [PH; (F)] and AP (G) to stele were calculated. Values are means ± SD (n = 3). ND, not determined. Asterisks indicate a significant difference in comparison with seedlings without epicotyl cutting (P < 0.05, two samples, t-test).
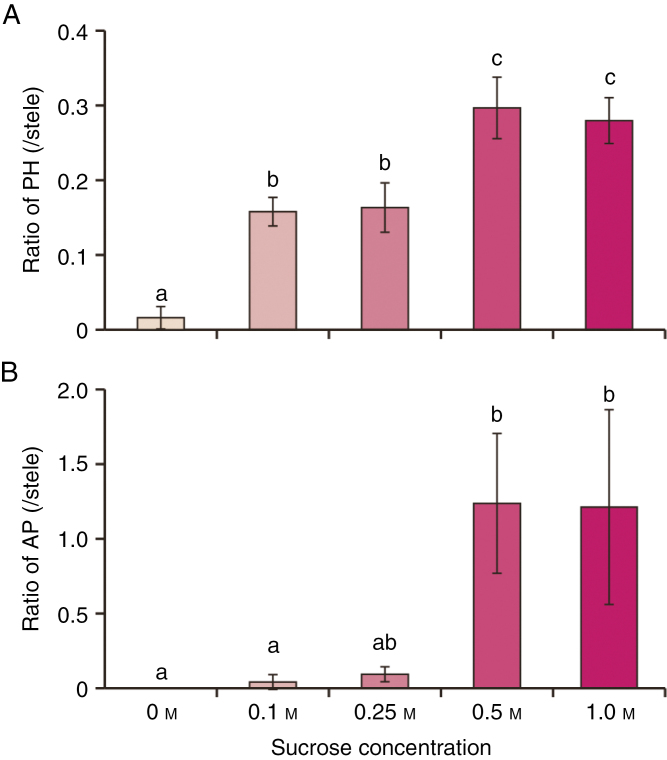
Fig. 9.
Effect of sucrose concentration on phellogen and aerenchymatous phellem (AP) formation in seedlings pinched at epicotyls. Soybean seedlings were grown under drained soil conditions. When unifoliate leaves were fully expanded, an incision was made in the epicotyl of each seedling at 2 cm above the cotyledon. The cut surfaces were treated with different concentrations of sucrose, following which seedlings were grown under waterlogged conditions for 7 d. Cross-sections were taken at 1 cm above the soil surface from seedlings under each condition. Area ratios of phellogen [PH; (A)] and AP (B) to stele were calculated. Values are means ± SD (n = 3). The area ratio of phellogen showed equal variances and normal distribution. The area ratio of AP showed unequal variance and a normal distribution. Different letters indicate significant differences among all conditions (P < 0.05, one-way ANOVA followed by Tukey’s HSD post-hoc test in phellogen or Kruskal–Wallis analysis in AP for multiple comparisons).
Extraction and determination of metabolites
To measure the sugar concentrations in tissues, hypocotyls were harvested from 0–1 cm above the soil surface at the beginning of the light cycle, weighed, plunged into liquid nitrogen, freeze-dried (Freezone 1; Labconco Corp., Kansas City, MO, USA) and weighed again. Soluble sugars were extracted from the freeze-dried hypocotyls (Takahashi et al., 2014a). Sucrose concentrations were determined using TC Sucrose/D-Glucose kits (Roche Diagnostics, Mannheim, Germany) and expressed as mmoles divided by the volume of water (L) in tissues, with water content calculated by subtracting the dry weights from the fresh weights.
Statistical analysis
Data are presented as the mean ± standard deviation. Statistical analysis was performed using IBM SPSS Statistics 19.0 (IBM Corp., Armonk, NY, USA). Multiple comparisons were performed using one-way ANOVA followed by Tukey’s HSD post-hoc test or Kruskal–Wallis analysis at a confidence level of 95 %.
RESULTS
Phellogen and AP formation under waterlogged soil conditions
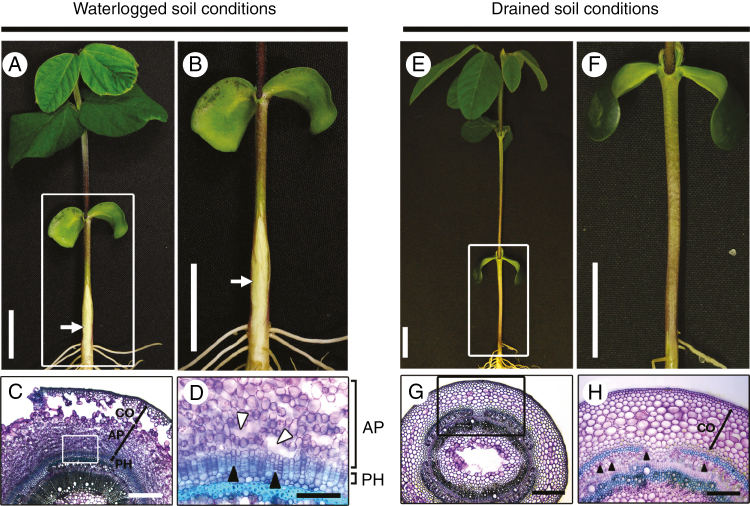
Secondary aerenchyma formed in submerged soybean hypocotyls under waterlogged soil conditions (arrows in Fig. 1A, B), but not under drained soil conditions (Fig. 1E, F). This spongy material consisted of two different tissues, AP and phellogen. Under waterlogged soil conditions, AP formed a porous tissue inside the primary cortex, whereas phellogen formed a several cell layers inside of the AP (Fig. 1C, D). Phellogen cells were closely packed, whereas large air spaces remained between the AP cells (Fig. 1D). These tissues were not observed under drained soil conditions (Fig. 1G, H) in agreement with a previous report (Shimamura et al., 2003). Several phellogen cells were initially observed 1 d after waterlogging treatment, and subsequently increased to form several cell layers (Fig. 2A). After about 2 d, waterlogging caused the surface of the hypocotyl to split and form lenticels. Cell proliferation during phellogen and AP formation squeezed the epidermal and cortical cells, causing them to rupture (Fig. 2A). The area ratio of phellogen remained more or less constant after 3 d (Fig. 2B). Formation of AP was initially observed 5 d after waterlogging treatment, and then gradually increased (Fig. 2A, C).
Fig. 1.
Phellogen and aerenchymatous phellem (AP) formation in soybean hypocotyls under waterlogged soil conditions. After unifoliate leaves were fully expanded, seedlings were grown for 7 d under waterlogged soil conditions (A) or drained soil conditions (E). (B) and (F) are higher magnification images of the squared areas shown in (A) and (E), respectively. White arrows indicate AP. Hypocotyl cross-sections were taken 1 cm above the soil surface from seedlings under waterlogged soil (C) and drained soil (G) conditions. (D) and (H) are higher magnification images of the squared areas shown in (C) and (G), respectively. Phellogen (PH) and aerenchymatous phellem (AP) are formed inside cortical cells (CO) under waterlogged soil conditions (C), but not under drained soil conditions (H). White arrowheads in (D) indicate intercellular gas spaces created by cell elongation in the AP; black arrowheads in (D) indicate phellogen (PH) cells. Black arrowheads in (H) indicate phloem cells. Scale bars = 2 cm (A, B, E, F), 500 µm (C, G), 250 µm (H), or 125 µm (D).
Requirement of photosynthesis for phellogen and AP formation
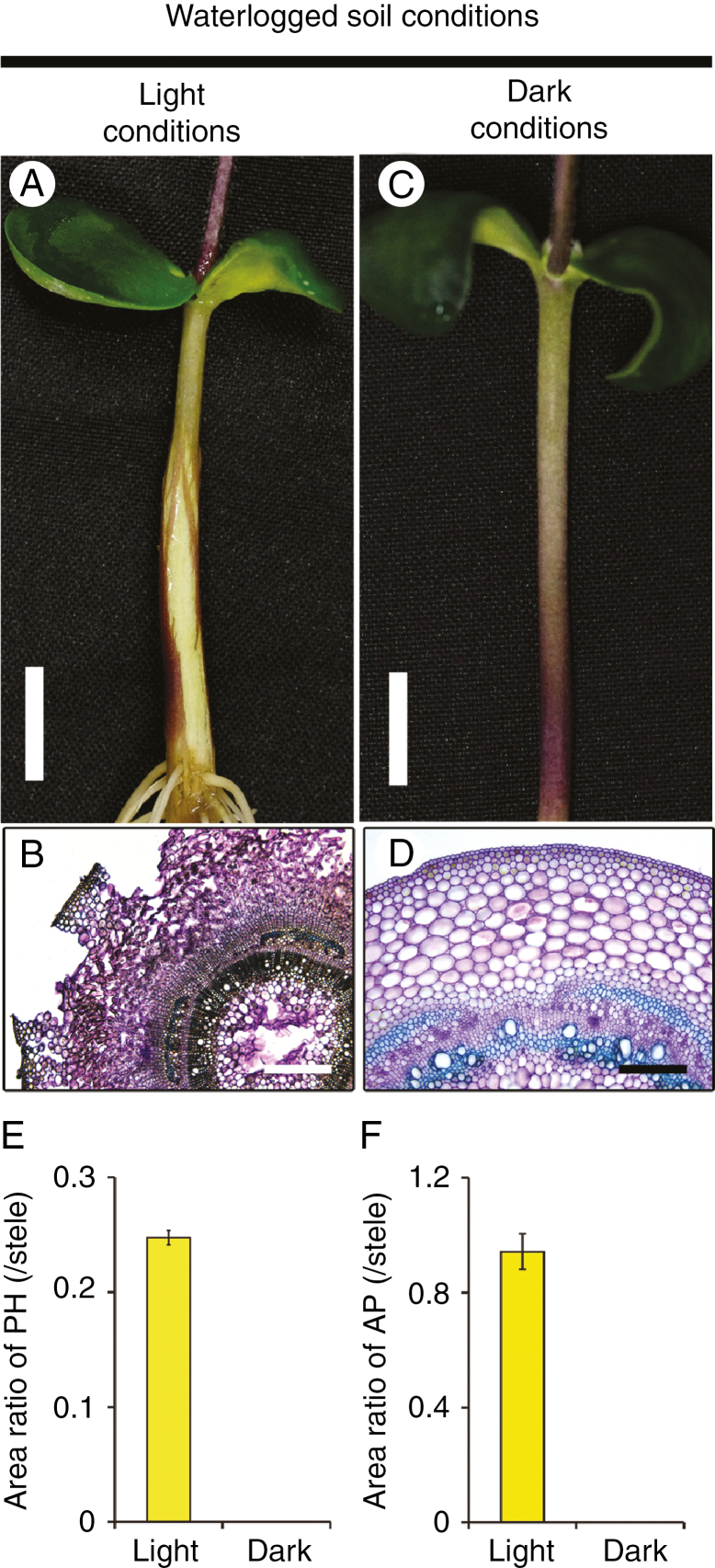
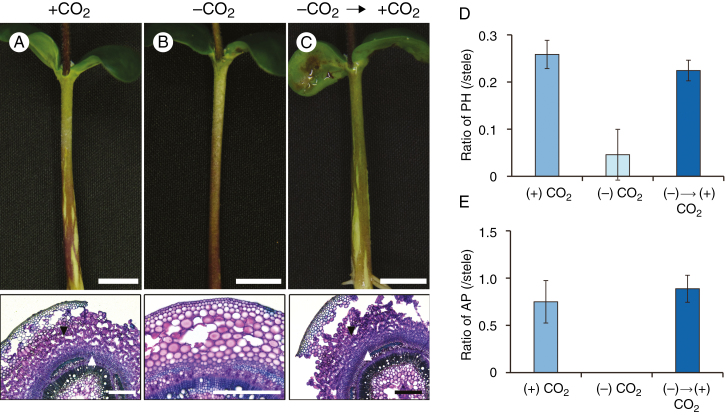
Interestingly, phellogen and AP did not form under soil waterlogging conditions when the seedlings were grown in darkness (Fig. 3) and the amounts decreased with decreasing day length (Fig. 4A, B, respectively). For AP, increasing the day length increased not only the amount of AP but also the speed with which it increased (Fig. 4B). That is, the amount of time it took to detect AP formation decreased with increasing day length, apparently due to the greater amount of photoassimilates available. As another indication of the importance of light, phellogen formation was slightly inhibited and AP formation was severely inhibited when all leaves apart from the cotyledons were covered with aluminium foil under waterlogged and light conditions (Supplementary Data Fig. S3). To determine whether these results were due to a lack of photosynthesis, we suppressed photosynthesis by removing CO2. Under waterlogged conditions without CO2, phellogen formation and AP formation were severely or completely inhibited (Fig. 5B) compared to the control (Fig. 5A). However, formation of both phellogen and AP recovered when CO2 was restored (Fig. 5C). These recoveries are shown quantitatively in Fig. 5D and E, respectively. These results show that the dark-induced loss of phellogen and AP formation in soybean hypocotyls was due to a lack of photosynthesis.
Fig. 3.
Inhibition of phellogen and aerenchymatous phellem (AP) formation under darkness. Soybean seedlings were grown under drained soil conditions. When unifoliate leaves were fully expanded, soil was waterlogged and seedlings were grown for 7 d under light (A) or dark conditions (C). Cross-sections of hypocotyls were taken at 1 cm above the soil surface from waterlogged seedlings under light (B) and dark (D) conditions. Scale bars = 1 cm (A, C), 500 µm (B) or 250 µm (D). Area ratios of phellogen (PH) and AP to stele were calculated (E and F, respectively). Values are means ± SD (n = 3).
Fig. 5.
Phellogen (PH) and aerenchymatous phellem (AP) formation under CO2-deprived, waterlogged conditions. After unifoliate leaves were fully expanded, seedlings were grown with or without CO2 for 5 d (A, B). After 5 d, CO2-deprived seedlings were transferred to CO2 conditions (C). Cross-sections of hypocotyls were taken at 1 cm above the soil surface (2 cm below the water surface). Scale bars: upper panels, 1 cm; lower panels, 500 μm. (D, E) Area ratios of PH and AP to stele. Values are means ± SD (n = 3).
Involvement of phloem-mediated transport of leaf-derived substance(s) in phellogen and AP formation
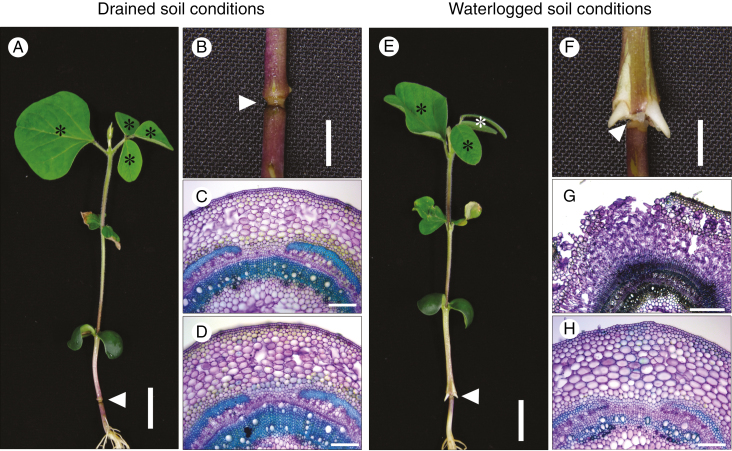
Waterlogging-induced phellogen and AP formation was prevented when all leaves apart from the cotyledons were removed from seedlings (Supplementary Data Fig. S4), even under long-day (14-h light/10-h dark) conditions. In contrast, phellogen and AP formed when the cotyledons, but not other leaves, were removed (Fig. S4). Thus, photoassimilates transported from the leaves via the phloem, and not substances translocated from the cotyledons, seem to play a role in phellogen and AP formation. However, it is possible that locally accumulated substances are also used for phellogen and AP formation. To examine this possibility, we disrupted the phloem stream in the hypocotyls of seedlings via heat-girdling, and then grew the heat-girdled seedlings under drained soil or waterlogged soil conditions for 7 d. Heat-girdling did not interfere with xylem transport, as evidenced by the emergence of non-wilted leaves of heat-girdled seedlings grown under drained soil conditions (indicated by the asterisks in Fig. 6A). Under drained soil conditions, neither phellogen nor AP formed in hypocotyls either above or below the heat-girdled point (Fig. 6B–D). In contrast, phellogen and AP formed in hypocotyls above the heat-girdled point (Fig. 6F, G), but not below it (Fig. 6F, H), under waterlogged soil conditions, and adventitious roots emerged from the hypocotyls above the heat-girdled point (Fig. 6F). These results indicated that photoassimilates transported via the phloem are involved in phellogen and AP formation.
Fig. 6.
Effect of hypocotyl heat-girdling on phellogen and aerenchymatous phellem (AP) formation. Soybean seedlings were grown under drained soil conditions until unifoliate leaves were fully expanded. Heat-girdling of the hypocotyls was performed 1.5 cm above the soil surface, following which seedlings were grown under either drained soil (A) or waterlogged (E) conditions for 7 d. (B) and (F) are higher magnification images of the heat-girdled positions shown in (A) and (E), respectively. White arrowheads indicate the position of heat-girdling. Cross-sections were taken above (C, G) and below (D, H) the point of heat-girdling. Scale bars = 2 cm (A, E), 0.5 cm (B, F), 500 µm (G) or 200 µm (C, D, H).
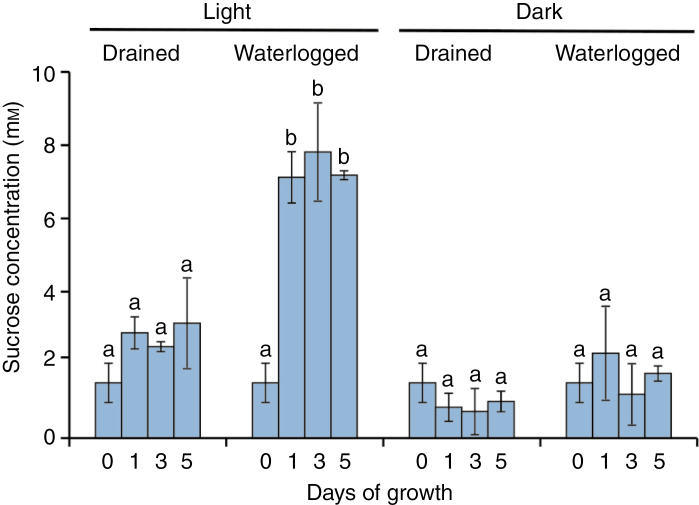
Sucrose accumulates in the hypocotyl under light and waterlogged soil conditions
Sucrose is a major component of the photoassimilated material transported from source organs (e.g. leaves) to sink organs via the phloem. Over a period of 5 d, the accumulation of sucrose in hypocotyls was significantly greater under waterlogged light conditions than under other conditions (Fig. 7), suggesting that sucrose is the photoassimilate that is needed for phellogen and AP formation.
Fig. 7.
Sucrose concentrations in the hypocotyls during phellogen and aerenchymatous phellem (AP) formation. Soybean seedlings were grown under drained soil conditions until unifoliate leaves were fully expanded. Seedlings were grown for 1, 3 and 5 d under drained soil and 14-h light/10-h dark conditions, waterlogged soil and 14-h light/10-h dark conditions, drained soil and 24-h dark conditions, and waterlogged soil and 24-h dark conditions. Sections of submerged hypocotyl were collected to determine sucrose concentrations, which were expressed as tissue-water concentrations. Values are means ± SD (n = 3). All data showed equal variances and normal distribution. Different letters indicate significant differences among the conditions (P < 0.05, one-way ANOVA followed by Tukey’s HSD post-hoc test for multiple comparisons).
Effect of exogenous sucrose on phellogen and AP formation
To examine whether sucrose induces phellogen and AP formation in soybean hypocotyls, we cut seedlings at the epicotyl, removed the upper parts, and applied 0.5 m sucrose solution to the cut surface. These seedlings were then exposed to waterlogged soil conditions for 7 d. Other seedlings received similar treatment with alternative sugars (0.5 m mannitol, glucose or fructose), and a water-only treatment served as a control (Fig. 8). Neither phellogen nor AP formation occurred in the seedlings subjected to H2O or mannitol treatment (Fig. 8A, B). In contrast, sucrose treatment recovered both phellogen and AP formation (Fig. 8C) to the same levels as that occurring in uncut seedlings (Fig. 8F, G). Phellogen formation was observed in both glucose- (Fig. 8D) and fructose-treated (Fig. 8E) seedlings, although in these instances phellogen formation was only partial (Fig. 8F). However, neither glucose nor fructose induced AP formation (Fig. 8G). Furthermore, application of 0.1 m or 0.25 m sucrose partially induced phellogen formation and slightly induced AP formation, whereas application of higher concentrations (0.5 or 1 m) of exogenous sucrose induced vigorous phellogen and AP formation.
DISCUSSION
Photosynthesis in leaves and phloem-mediated sucrose transport are essential for phellogen and AP formation in soybean hypocotyls under waterlogged soil conditions
The formation of phellogen and AP were induced in soybean hypocotyls in response to soil waterlogging under light conditions (Figs 1 and 2), as was reported previously (Shimamura et al., 2003; Thomas et al., 2005). However, they did not form at all under dark conditions (Fig. 3). Furthermore, their formation was inhibited by suppressing photosynthesis (Supplementary Data Fig. S3, Fig. 5). Moreover, leaves (although not cotyledons) were necessary for phellogen and AP formation (Fig. S4). These results indicate that photosynthesis in leaves is important for waterlogging-induced phellogen and AP formation.
Phellogen and AP formation was prevented by cutting at the epicotyl, but could be recovered by application of sucrose, indicating that sucrose (a major component of leaf photoassimilates) plays an important role in phellogen and AP formation in the hypocotyl of waterlogged plants. Although sucrose may be newly produced from starch stored in the hypocotyl, phellogen and AP formed above but not below the point of heat-girdling under waterlogged soil conditions (Fig. 6E–H). Because the soybean cultivar ‘Asoaogari’ also forms phellogen and AP under waterlogged soil conditions (Shimamura et al., 2003), we examined the effect of heat-girdling in this plant and found that it did have the same effect on phellogen and AP formation (Supplementary Data Fig. S5). This suggests that long-distance transport of sucrose from leaves to the hypocotyl via the phloem makes a substantial contribution to phellogen and AP formation. Not only primary (i.e. root and shoot apices) but also secondary (e.g. vascular cambium) meristems can assume the role of sink tissues (De Schepper et al., 2013; Tognetti et al., 2013). Thus, phellogen and AP represent sink tissues that are newly formed in response to waterlogging stress. Moreover, the longer the light period to which seedlings were exposed, the more rapid and efficient was the formation of phellogen and AP under waterlogged soil conditions (Fig. 4). As sucrose is produced for both export and storage in leaves during the day (Gordon et al., 1980), the amount of sucrose exported would be expected to increase in tandem with extension of the photoperiod for phellogen and AP formation. This idea is supported by the fact that longer periods of light exposure – and thus larger amounts of sucrose produced – promoted further efficient development of phellogen and AP (Fig. 4).
Sucrose concentrations in the phloem normally range between 0.18 and 0.6 m (Taiz and Zeigler, 1991; Nadwodnik and Lohaus, 2008; Merchant et al., 2010; Jensen et al., 2013; Patrick, 2013). The present results (Fig. 9) indicate that higher concentrations of sucrose in phloem sap (≥0.5 m) are a crucial factor for efficient phellogen and AP formation. Phellogen formation and AP formation were restored to the uncut level by a high concentration of sucrose, but glucose, fructose or low concentration of sucrose only partially restored phellogen formation and had negligible effect on AP formation (Figs 8 and 9). Thus, phellogen formation and AP formation appear to have a specific requirement for sucrose. Why glucose and fructose were less effective than sucrose is unclear, but it may be because they must be converted to sucrose before being loaded into the phloem sap (Giaquinta, 1977). Otherwise, there might be sugar preference in phellogen development.
Lower concentrations of sucrose (0.1 and 0.25 m) partially recovered phellogen formation but had little effect on AP formation (Fig. 9). Because AP is derived from phellogen, this suggests that AP cannot develop from phellogen unless the phellogen is fully developed.
Sucrose accumulation in the hypocotyl is required for phellogen and AP formation
The concentrations of sucrose in the hypocotyls of seedlings grown under waterlogged soil and light conditions increased markedly after just 1 d – a response that was suppressed when waterlogged seedlings were transferred to dark conditions (Fig. 7). As neither phellogen nor AP formation was observed in darkness even under waterlogged soil conditions (Figs 3 and 4), elevated sucrose concentrations, as observed in the hypocotyls of waterlogged seedlings, may be an essential condition for phellogen and AP formation. These results indicate that sucrose is predominantly allocated to the hypocotyls below the water level for phellogen and AP formation.
Sucrose plays an important role in organ and tissue development in plants. Indeed, disaccharides, including sucrose, have been shown to induce vascular differentiation in callus tissue in Phaseolus vulgaris (Jeffs and Northcote, 1967), and sucrose is known to regulate both lateral root formation in Arabidopsis thaliana (MacGregor et al., 2008) and tuber formation in potato plants in vitro (Xu et al., 1998). In the present study, phellogen formation was initiated 1 d after waterlogging, and further developed at 3 d and thereafter (Fig. 2A, B; Shimamura et al., 2003), and high sucrose concentrations were maintained during these processes (Fig. 7). These observations imply that in addition to waterlogging stress, higher concentrations of sucrose in the hypocotyl trigger phellogen development. Endogenous sucrose in the hypocotyls was maintained at higher concentrations until AP formation was initially observed (Figs 2 and 7). Moreover, application of sucrose to the cut epicotyl restored AP formation (Fig. 8C, G), suggesting that sucrose accumulation in the hypocotyl may also be a prerequisite for AP development.
Why and how does sucrose accumulate to high concentrations in soybean hypocotyls under waterlogged soil and light conditions? Photosynthetic activity was shown to be inhibited in alfalfa (Castonguay et al., 1993) and wheat (Huang et al., 1994; Malik et al., 2001; Zheng et al., 2009) grown in waterlogged soils, but sucrose concentrations in the phloem sap of Eucalyptus globulus grown under similar conditions remained stable (Merchant et al., 2010). On the basis of these observations, we assumed that sucrose concentration would gradually decrease under waterlogged soil conditions, whereas it actually increased (Fig. 7). In the roots of plant species intolerant to waterlogging, sucrose concentrations generally increase under flooded conditions, as a consequence of the limitation of sucrose usage (Castonguay et al., 1993; Herzog et al., 2016; Striker and Colmer, 2016). Likewise, sucrose accumulation may rise in the hypocotyls of waterlogged soybean as a consequence of lower sucrose consumption in the waterlogged parts of the plant. On the other hand, spatial and temporal sugar accumulation is important for the development of meristematic tissues (Francis and Halford, 2006). Sugars may be translocated to the hypocotyl in response to waterlogging, with subsequent induction of phellogen and AP formation. Although it remains unclear whether sucrose concentration is a trigger for the initiation of phellogen, maintaining high levels of sucrose in the hypocotyl is necessary for the development of phellogen and AP and the differentiation from phellogen to AP under waterlogged soil conditions (Fig. 9). Recently, Mason et al., (2014) reported that apical dominance (i.e. inhibition of axillary bud outgrowth) is maintained by limitation of sugar accumulation in axillary buds, and that increasing the supply of sugars to the axillary buds is a prerequisite for the release of bud outgrowth from suppression. Thus, sucrose is one of the limiting factors for the development of phellogen and AP. Further studies are needed to determine whether it acts as an energy source or, as often occurs, as a signalling molecule (Eveland and Jackson, 2012; Tognetti et al., 2013; Lastdrager et al., 2014).
In conclusion, we hypothesize that sucrose synthesized in leaves by photosynthesis and transported elsewhere via the phloem regulates phellogen and AP formation in the soybean hypocotyl under waterlogged soil and light conditions. We conclude that the maintenance of a high sucrose concentration is essential for the development of phellogen and AP and the differentiation from phellogen to AP, although it remains to be determined whether high concentrations of sucrose trigger the initiation of phellogen.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Fig. S1: Change of CO2 concentration in a tightly closed container. Fig. S2: Method of sugar treatment of soybean seedlings cut 2 cm above the cotyledon. Fig. S3: Effect of leaf wrapping using aluminium foil on phellogen and aerenchymatous phellem formation. Fig. S4: Effect of leaf and cotyledon removal on phellogen and aerenchymatous phellem formation. Fig. S5: Effect of hypocotyl heat-girdling and sucrose treatment on phellogen and aerenchymatous phellem formation in the soybean cultivar ‘Asoaogari’. Table S1: Areal extent of phellogen, aerenchymatous phellem and stele.
ACKNOWLEDGEMENTS
We are grateful to Timothy D. Colmer, Al Imran Malik and Takaki Yamauchi for stimulating discussions. This work was supported by JSPS KAKENHI, Grant Numbers 15H06272 and 16K07552. The authors have no conflict of interest to declare. H.T., Q.X. and S.S. planned and designed the research. H.T., Q.X., S.S. and A.Y. performed the research; H.T., Q.X. and S.S. analysed the data; and H.T. wrote the manuscript. All authors reviewed and commented on the manuscript.
LITERATURE CITED
- Arber A. 1920. Water plants: a study of aquatic angiosperms. Cambridge: Cambridge University Press. [Google Scholar]
- Bailey-Serres J, Voesenek LACJ. 2008. Flooding stress: acclimations and genetic diversity. Annual Review of Plant Biology 59: 313–339. [DOI] [PubMed] [Google Scholar]
- Barbier FF, Lunn JE, Beveridge CA. 2015. Ready, steady, go! A sugar hit starts the race to shoot branching. Current Opinion in Plant Biology 25: 39–45. [DOI] [PubMed] [Google Scholar]
- Castonguay Y, Nadeau P, Simard RR. 1993. Effect of flooding on carbohydrate and ABA levels in roots and shoots of alfalfa. Plant, Cell and Environment 16: 695–702. [Google Scholar]
- Colmer TD, Voesenek LACJ. 2009. Flooding tolerance: suites of plant traits in variable environments. Functional Plant Biology 36: 665–681. [DOI] [PubMed] [Google Scholar]
- De Schepper V, De Swaef T, Bauweraerts I, Steppe K. 2013. Phloem transport: a review of mechanisms and controls. Journal of Experimental Botany 64: 4839–4850. [DOI] [PubMed] [Google Scholar]
- Eveland AL, Jackson DP. 2012. Sugars, signaling, and plant development. Journal of Experimental Botany 63: 3367–3377. [DOI] [PubMed] [Google Scholar]
- Francis D, Halford NG. 2006. Nutrient sensing in plant meristems. Plant Molecular Biology 60: 981–993. [DOI] [PubMed] [Google Scholar]
- Fraser L. 1931. The reaction of Viminaria denudata to increased water content of the soil. Proceedings of the Linnean Society of New South Wales 56: 391–406. [Google Scholar]
- Giaquinta R. 1977. Phloem loading of sucrose. pH dependence and selectivity. Plant Physiology 59: 750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AJ, Ryle GJA, Powell CE, Mitchell D. 1980. Export, mobilization, and respiration of assimilates in uniculm barley during light and darkness. Journal of Experimental Botany 31: 461–473. [Google Scholar]
- Herzog M, Striker GG, Colmer TD, Pedersen O. 2016. Mechanisms of waterlogging tolerance in wheat – a review of root and shoot physiology. Plant, Cell and Environment 39: 1068–1086. [DOI] [PubMed] [Google Scholar]
- Huang B, Johnson JW, Nesmith S, Bridges DC. 1994. Growth, physiological and anatomical responses of two wheat genotypes to waterlogging and nutrient supply. Journal of Experimental Botany 45: 193–202. [Google Scholar]
- Jackson MB, Armstrong W. 1999. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biology 1: 274–287. [Google Scholar]
- Jeffs RA, Northcote DH. 1967. The influence of indol-3yl acetic acid and sugar on the pattern of induced differentiation in plant tissue culture. Journal of Cell Science 2: 77–88. [DOI] [PubMed] [Google Scholar]
- Jensen KH, Savage JA, Holbrook NM. 2013. Optimal concentration for sugar transport in plants. Journal of the Royal Society Interface 10: 20130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher S, Schopfer P. 2012. Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 109: 11217–11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastdrager J, Hanson J, Smeekens S. 2014. Sugar signals and the control of plant growth and development. Journal of Experimental Botany 65: 799–807. [DOI] [PubMed] [Google Scholar]
- MacGregor DR, Deak KI, Ingram PA, Malamy JE. 2008. Root system architecture in Arabidopsis grown in culture is regulated by sucrose uptake in the aerial tissues. The Plant Cell 20: 2643–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik AI, Colmer TD, Lambers H, Schortemeyer M. 2001. Changes in physiological and morphological traits of roots and shoots of wheat in response to different depths of waterlogging. Australian Journal of Plant Physiology 28: 1121–1131. [Google Scholar]
- Malone M, Alarcon JJ. 1995. Only xylem-borne factors can account for systemic wound signalling in the tomato plant. Planta 196: 740–746. [Google Scholar]
- Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA. 2014. Sugar demand, not auxin, is the initial regulator of apical dominance. Proceedings of the National Academy of Sciences of the United States of America 111: 6092–6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant A, Peuke AD, Keitel C, Macfarlane C, Warren CR, Adams MA. 2010. Phloem sap and leaf δ13C, carbohydrates, and amino acid concentrations in Eucalyptus globulus change systematically according to flooding and water deficit treatment. Journal of Experimental Botany 61: 1785–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadwodnik J, Lohaus G. 2008. Subcellular concentrations of sugar alcohols and sugars in relation to phloem translocation in Plantago major, Plantago maritima, Prunus persica, and Apium graveolens. Planta 227: 1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick JW. 2013. Does Don Fisher’s high-pressure manifold model account for phloem transport and resource partitioning?Frontiers in Plant Science 4: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YL. 2014. Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annual Review of Plant Biology 65: 33–67. [DOI] [PubMed] [Google Scholar]
- Scott DH, Wager H. 1888. On the floating-roots of Sesbania aculeata, Pers. Annals of Botany 1: 307–314. [Google Scholar]
- Shimamura S, Mochizuki T, Nada Y, Fukuyama M. 2003. Formation and function of secondary aerenchyma in hypocotyl, roots and nodules of soybean (Glycine max) under flooded conditions. Plant and Soil 251: 351–359. [Google Scholar]
- Shimamura S, Yamamoto R, Nakamura T, Shimada S, Komatsu S. 2010. Stem hypertrophic lenticels and secondary aerenchyma enable oxygen transport to roots of soybean in flooded soil. Annals of Botany 106: 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura S, Yoshioka T, Yamamoto R, Hiraga S, Nakamura T, Shimada S, Komatsu S. 2014. Role of abscisic acid in flood-induced secondary aerenchyma formation in soybean (Glycine max) hypocotyls. Plant Production Science 17: 131–137. [Google Scholar]
- Stevens KJ, Peterson RL, Stephenson GR. 1997. Morphological and anatomical responses of Lythrum salicaria L. (purple loosestrife) to an imposed water gradient. International Journal of Plant Sciences 158: 172–183. [Google Scholar]
- Striker GG, Colmer TD. 2016. Flooding tolerance of forage legumes. Journal of Experimental Botany 68: 1851–1872. [DOI] [PubMed] [Google Scholar]
- Taiz L, Zeigler E. 1991. Plant physiology. Redwood City, CA: The Benjamin Cummings Publishing Company, Inc. [Google Scholar]
- Takahashi F, Sato-Nara K, Kobayashi K, Suzuki M, Suzuki H. 2003. Sugar-induced adventitious roots in Arabidopsis seedling. Journal of Plant Research 116: 83–91. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Greenway H, Matsumura H, Tsutsumi N, Nakazono M. 2014a Rice alcohol dehydrogenase 1 promotes survival and has a major impact on carbohydrate metabolism in the embryo and endosperm when seeds are germinated in partially oxygenated water. Annals of Botany 113: 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Yamauchi T, Colmer TD, Nakazono M. 2014b Aerenchyma formation in plants. In: van Dongen JT, Licausi F, eds. Low oxygen stress in plants. Heidelberg: Springer, 247–265. [Google Scholar]
- Teakle NL, Armstrong J, Barrett-Lennard EG, Colmer TD. 2011. Aerenchymatous phellem in hypocotyl and roots enables O2 transport in Melilotus siculus. New Phytologist 190: 340–350. [DOI] [PubMed] [Google Scholar]
- Thomas AL, Guerreiro SMC, Sodek L. 2005. Aerenchyma formation and recovery from hypoxia of the flooded root system of nodulated soybean. Annals of Botany 96: 1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetti JA, Pontis HG, Martínez-Noël GMA. 2013. Sucrose signaling in plants: a world yet to be explored. Plant Signaling & Behavior 8: e23316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R, Wolf S. 2009. Phloem transport: cellular pathways and molecular trafficking. Annual Review of Plant Biology 60: 207–221. [DOI] [PubMed] [Google Scholar]
- Verboven P, Pedersen O, Herremans E et al. . 2012. Root aeration via aerenchymatous phellem: three-dimensional micro-imaging and radial O2 profiles in Melilotus siculus. New Phytologist 193: 420–431. [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Bailey-Serres J. 2015. Flood adaptive traits and processes: an overview. New Phytologist 206: 57–73. [DOI] [PubMed] [Google Scholar]
- Xu X, van Lammeren AAM, Vermeer E, Vreugdenhil D. 1998. The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiology 117: 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Shimamura S, Nakazono M, Mochizuki T. 2013. Aerenchyma formation in crop species: a review. Field Crops Research 152: 8–16. [Google Scholar]
- Zheng C, Jiang D, Liu F, Dai T, Jing Q, Cao W. 2009. Effects of salt and waterlogging stresses and their combination on leaf photosynthesis, chloroplast ATP synthesis, and antioxidant capacity in wheat. Plant Science 176: 575–582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.