Abstract
Background and Aims
Although there has been much experimental work on leaf colour change associated with selection generated by abiotic environmental factors and antagonists, the role of leaf colour change in pollinator attraction has been largely ignored. We tested whether whitening of the apical leaves subtending the inflorescences of Saururus chinensis during flowering enhances pollinator attraction, and whether re-greening of the white leaves after flowering increases carbon assimilation and promotes seed development.
Methods
White leaves were removed or covered, and the effects of these manipulations on pollinator visitation and subsequent reproductive success were assessed. The net photosynthetic rates of leaves of different colour were measured and their photosynthetic contributions to seed development were evaluated.
Key Results
Saururus chinensis is able to self-pollinate autonomously, but depends largely on flies for pollination. White leaves had different reflectance spectra from green leaves, and white leaves attracted significantly more pollinators and led to significantly higher fruit and seed set. Although leaf whitening resulted in a reduction in photosynthetic capacity, it translated into only a small decrease in seed mass. When leaves had turned back from white to green after flowering their photosynthetic capacity was similar to that of ‘normal’ green leaves and promoted seed development.
Conclusions
The reversible leaf colour change in S. chinensis appears to be adaptive because it enhances pollination success during flowering, with a small photosynthetic cost, while re-greening of these leaves after flowering helps to meet the carbon requirements for seed development.
Keywords: Carbon assimilation, leaf colour change, net photosynthetic rate, pollinator attraction, seed development
INTRODUCTION
The organs of many higher plants exhibit remarkable colour changes during development; these include both reproductive (i.e. flowers and fruits) and vegetative (i.e. leaves and stems) parts (Lev-Yadun et al., 2004). Such ontogenetic colour changes have been interpreted as adaptations to several different selective forces (Haberlandt, 1914; Kursar and Coley, 1992, 2003; Weiss, 1995; Feild et al., 2001; Ida and Kudo, 2003; Herrera, 2005; Karageorgou and Manetas, 2006; Karageorgou et al., 2008; Pélabon et al., 2015). Compared with the considerable attention that has been paid to colour changes of reproductive organs, the adaptive significance of colour change in leaves has been largely overlooked (Lev-Yadun et al., 2002; but see Kursar and Coley, 1992, 2003).
In most cases, leaf colour change occurs in young, developing leaves or in old, senescing leaves, mainly because of the accumulation of anthocyanins (together with other pigments), the breakdown of chlorophyll (Lev-Yadun et al., 2002), and/or the reversible transformation of plastids (Ikeda, 1979). The prevailing view among plant physiologists is that leaf colour change is associated with resistance to photoinhibition because of the immature photosynthetic machinery in young leaves and the risk of reactive oxygen production in senescing leaves (Miranda et al., 1981; Matile, 2000). An alternative, more ecologically oriented view links leaf colour change with (1) reduced insect herbivory by signalling to insects that the leaves are well defended or are a poor nutrient resource, such as red young leaves or variegated adult leaves (Kursar and Coley, 1992, 2003; Numata et al., 2004; Lev-Yadun and Gould, 2007; Archetti et al., 2009; Chen and Huang, 2013); (2) increased leaf temperature in cold regions (Taulavuori et al., 2011); or (3) enhanced seed dispersal by providing visual guides to animals (Stiles, 1982). Thus far, no single general role of leaf colour change in plant fitness has been accepted. In addition, it is worth noting that, in some plant species, some leaves change colour during flowering, suggesting that they may take part in plant reproduction, e.g. attracting pollinators by advertising flowers through high-contrast colourful leaves, such as showy extrafloral structures (e.g. bracts), helping to attract pollinators by enhancing visual displays (Armbruster, 1997; Herrera, 1997; Borges et al., 2003; Armbruster et al., 2005; Sun et al., 2008; Song et al., 2013). Furthermore, such visual signals in extrafloral organs may be especially important in plant species lacking perianths or with inconspicuous corollas (Keasar et al., 2009). To date, however, very few studies have demonstrated the role of colour changes in leaves in pollinator attraction and reproductive fitness.
Although plants may benefit reproductively from leaf colour change, such colour changes usually involve trade-offs in terms of reduced photosynthesis and cost of pigment synthesis. In the process of leaves changing from green to red, white or other colours, the breakdown of chlorophyll and synthesis of anthocyanins and/or other pigments that absorb visible light but have no direct role in photosynthesis may impose biosynthetic and photosynthetic costs (Burger and Edwards, 1996; Karageorgou and Manetas, 2006; Karageorgou et al., 2008). For example, compared with green leaves, in Coleus blumei quantum yield was reduced by 52 % in red leaves because of the absorption of photosynthetically active light by anthocyanin, resulting in a significant reduction in net carbon gain (Burger and Edwards, 1996). Thus, colour change in leaves due to physiological or other causes may negatively affect plant fitness in terms of fruit and seed production. In this context, leaf colour change can be favoured by natural selection only when (1) the photosynthetic cost can be paid off by a fitness benefit associated with the colour change, and/or (2) the post-pollination re-greening of leaves allows plants to minimize photosynthetic losses and meet the increased carbon requirements of developing fruits and seeds. Consequently, a complete understanding of the adaptive significance of various types of leaf colour changes in plants requires the consideration of both their benefits and costs.
In Saururus chinensis (Saururaceae), two or three apical leaves subtending the inflorescences show ontogenetic colour change from green before flowering to white during flowering, and then reversion back to green during fruit development (Fig. 1A–F). We investigated the possible functions of this green-to-white-to-green colour change of apical leaves during flowering and fruit development. We hypothesized that the white colour of the leaves during flowering increases pollinator attraction by enhancing the visual display (flowers are inconspicuous, being small and lacking a perianth). In addition, we hypothesized that the re-greening of leaves after flowering could enhance photosynthetic capacity to the level of normal green leaves and thereby increase the carbon supply for developing seeds. To determine whether the colour change from green to white increased pollination success, we compared pollinator visitation rates, and the subsequent fruit and seed set, in plants with and without white leaves. In order to determine the possible costs of leaf whitening and the function of leaf re-greening, we measured the net photosynthetic rates of leaves of different colour and examined the effects of leaf removal at different developmental stages on seed development.
Fig. 1.
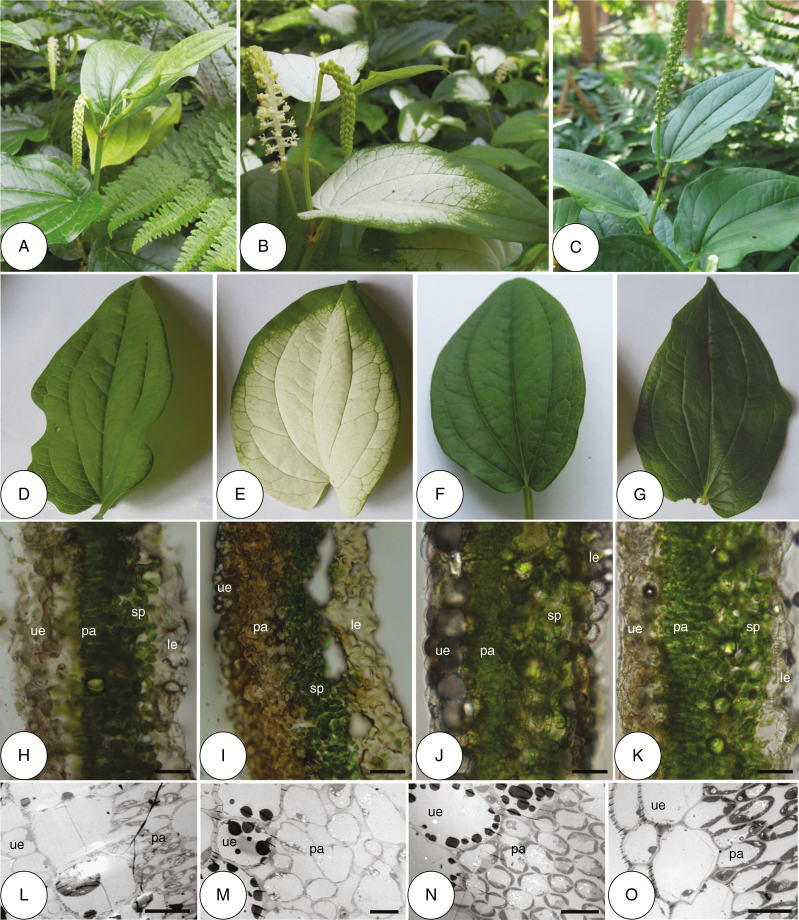
Leaves of Saururus chinensis at different developmental stages. (A) A plant at pre-anthesis stage. (B) A plant at anthesis stage. (C) A plant at fruit development stage. (D, H, L) A green leaf at pre-anthesis stage and its cross-section. (E, I, M) A white leaf at anthesis stage and its cross-section. (F, J, N) A re-greened leaf and its cross-section. (G, K, O) A normal green leaf and its cross-section. Scale bars: (H–K) = 100 μm; (L–O) = 10 μm. le = lower epidermis, pa = palisade parenchyma, sp = spongy parenchyma, ue = upper epidermis.
MATERIALS AND METHODS
Study species and sites
Saururus chinensis is a perennial medicinal herb in the Saururaceae; it has alternate leaves, grows up to 1 m tall, and is found mainly in moist sites in southern China from sea level to 1700 m a.s.l. (Chen et al., 1982). Reproduction is exclusively by seeds. The plant flowers between late May and late July. Each plant produces two or three lateral inflorescences from the axils of terminal leaves, each subtending an inflorescence; each inflorescence comprises a single spike with 80–160 tiny flowers that mature in an acropetal succession (Liang et al., 1996). The flowers are 3.08 ± 0.06 mm (n = 30) wide, have no perianth and produce no nectar. At the beginning of anthesis, the apical two or three leaves subtending the inflorescences change colour from green to white (Fig. 1A, B, D, E). After ~15 d (depending on the duration of anthesis), when all flowers have been pollinated, the white leaves turn back to green (Fig. 1C, F). The fruits mature between late August and late September (B. Song, pers. obs.).
This study was conducted at two sites: Jinfoshan Mountain (29°12′06″ N, 107°22′41″ E, 766 m a.s.l.), in Chongqing Municipality, Southwest China; and Kunming Botanical Garden (25°08′42″N, 102°44′31″E, 1788 m a.s.l.), in Kunming City, Yunnan Province, Southwest China.
Pollinator observation and pollination experiments
Pollinators visiting the flowers of S. chinensis were observed from 08:00 to 20:00 h for 5 d at both sites in 2016. During one of the five nights of observation at both sites, no visiting pollinators were observed. The visiting insects were collected and sent to the Xishuangbanna Tropical Botanical Garden of the Chinese Academy of Sciences for identification. In addition, the behaviour of visitors on flowers was observed. During the observation period, the weather was clear and without strong winds.
To determine whether wind pollination plays a role in S. chinensis reproduction, the method of Yuan et al. (2008) was used. In the Jinfoshan Mountain population, five flowering plants were selected and microscope slides covered with petroleum jelly were placed around these plants at 0.5-m intervals, 30–50 cm above the ground, to a distance of 5 m away. The slides were collected after 2 d and were checked under a light microscope for wind-dispersed S. chinensis pollen. To determine whether pollination of S. chinensis depends on pollinators, two pollination treatments were applied to the Jinfoshan Mountain population: (1) natural pollination: 30 inflorescences were randomly marked and these inflorescences were not manipulated; and (2) autonomous self-pollination: 30 inflorescences were selected randomly and these inflorescences were bagged with nylon mesh bags throughout their anthesis to exclude pollinating insects. When fruits were ripe, all infructescences were collected and taken to the laboratory to determine fruit set and seed set (measured, here and throughout, as seed number per fruit).
Leaf morphology and anatomy
Between 25 May and 15 August 2016, leaf development in S. chinensis was monitored by examining 50 plants (25 for flowering plants and the rest for non-flowering plants) selected randomly at both Kunming Botanical Garden and Jinfoshan Mountain. For the apical two or three leaves subtending the inflorescences that change colour during anthesis and post-anthesis on each plant, three colour types were distinguished: (1) young green leaves: leaves that were formed, but had not yet turned white (pre-anthesis); (2) white leaves: leaves that had changed from green to white (anthesis); and (3) re-greened leaves: leaves that had changed from white back to green (post-anthesis). In addition, we defined leaves without colour changes as (4) normal green leaves.
The four colour types of leaves described above were fixed on-site in formalin–acetic acid–alcohol (FAA) then examined and photographed using a scanning electron microscope (S-3000N, Hitachi High-Technologies, Japan) at Kunming Medical University. In addition, the four types of leaves were also collected and were processed for frozen sectioning with a Leica CM3050 cryostat (Leica Instruments, Germany) following the method of Vogel-Mikuš et al. (2008). The frozen section thickness was set at 20 μm and sections were photographed using an Olympus microscope (Olympus, Japan).
Colour measurement and pollinator visitation
One green and one white leaf were randomly collected from each of 25 plants in the Kunming Botanical Garden, and their reflectance spectra between 300 and 700 nm at 5-nm intervals were measured relative to magnesium sulphate (highly ultraviolet-reflecting white standard), at an angle of 45°, using a spectroradiometer (USB Ocean Optics 2000+) equipped with a Xenon Pulse X2 lamp light source, following Song et al. (2015). These leaves were kept fresh and measured within 30 min.
To determine whether the presence of white leaves enhances visitation by pollinating insects, 15 flowering plants at the Jinfoshan Mountain site were selected randomly each day between 15 June and 19 June 2016, and separated into three treatment groups: (1) plants kept intact; (2) plants with all white leaves removed; and (3) plants with all white leaves covered by recently detached green leaves and held in place with transparent tape. Inflorescences were clipped as necessary in order to ensure that there were exactly two on each experimental plant. We recorded pollinator visits following a schedule in which we observed each plant for 30 min, then moved to the next plant for another 30 min, etc., until all five plants in each group had been monitored. Observations were carried out at 9:00, 12:00, 15:00 and 18:00 h by three observers simultaneously. The plants used for this experiment were relatively isolated from each other, ruling out the possibility of neighbouring plants adding to and confounding the attraction effect. All censuses were carried out in warm and clear weather, and each plant was considered to be a replicate.
To determine whether white leaves enhance fruit and seed set through attracting pollinators, 75 plants about to flower were selected randomly from the Jinfoshan Mountain population and the aforementioned three experimental treatments were applied before young green leaves began to turn white. Inflorescences were clipped as necessary in order to ensure that there were two on each plant, as described above. The selected plants were randomly assigned to the three treatments. When fruits were ripe, all infructescences were collected and taken to the laboratory to determine fruit and seed set. Each plant was treated as a replicate.
Photosynthetic capacity, fecundity and progeny quality
In order to investigate whether leaves of different colour types (described above) differ in their photosynthetic capacity, we measured their net photosynthetic rates in the Kunming Botanical Garden. For this, a portable photosynthesis measurement system with a fluorescence chamber head (LI-6400-40, Li-Cor, Lincoln, NE, USA) was used (with CO2 concentration at ~400 μmol mol−1 and quantum flux at ~1200 μmol m−2 s−1, representing sunny conditions). Preliminary light response curves showed the selected light level to be saturating but not inhibitory (data not shown). Measurements were made on two leaves of each colour type per plant randomly selected on five different plants, and means were calculated for each plant before analysis.
To measure the effects of leaf whitening and re-greening on reproductive success in terms of seed development, plants about to flower were selected randomly from the Jinfoshan Mountain population and used for the experimental treatments described below. Inflorescences were clipped as necessary in order to ensure that there were two on each plant; supplementary hand-pollination was performed before or after leaf treatment depending on treatment type. The selected plants were exposed to the following five treatments: (1) natural control: plants were left undisturbed after hand pollination; (2) young green leaves were removed just before they turned white, thus preventing them contributing to photosynthesis during flowering and fruiting; (3) young green leaves were trimmed by ~1 mm along their edge just before they turned white as a control for leaf damage whilst mostly maintaining their size; (4) white leaves were removed just before re-greening, thus preventing them contributing to photosynthesis during fruiting; and (5) half of each of the white leaves was removed just before re-greening (the cut was made along the central vein, retaining half the leaf from its petiole attachment to the leaf tip). When the fruits were ripe, all infructescences were collected and taken to the laboratory to determine fruit set, seed set and mass of ten randomly selected seeds from each plant. Plants were treated as replicates, and each treatment included 25 plants.
It was difficult to measure directly the effects of leaf whitening and re-greening on carbon balance, so the effects were estimated indirectly. Since the reduced net photosynthetic rates of white leaves compared with young green leaves was close to the net photosynthetic rates of white leaves (see the Results section), we used the decreased fecundity in plants from treatment (2) compared with treatment (4) to estimate the photosynthetic cost associated with the colour change from green to white. Given that the net photosynthetic rate of white leaves was nearly half that of the re-greened leaves (see Results section), we use the differences in fecundity from plants between treatment (4) and treatment (5) to estimate the increased photosynthetic contribution of the leaves after re-greening to carbon gain after the end of the white-leaf phase.
Data analysis
Data obtained from spectral measurements were used to calculate the brightness of white and green leaves. Four types of photoreceptors were involved in the colour vision of flies, R7p, R7y, R8p and R8y (Troje, 1993). The brightness of an object was calculated by simply summing up the photon catch of these receptors. One-way ANOVA was used to test the effects of pollination treatment on fruit and seed set, and the effects of leaf treatment on pollinator visitation, fruit set, seed set and seed mass. Multiple comparisons of means were performed using Tukey’s test at the 0.05 significance level. A Mann–Whitney U-test was used to compare the length and width of leaves between white and re-greened leaves, and the brightness between white and green leaves. All analyses were performed using SPSS version 18.0. Measurements are reported as means ± 1 s.e.
RESULTS
Pollinator observation and pollination experiments
During our field observation, flowers of S. chinensis were visited by seven insect species (Table 1). Pollen-feeding syrphid flies (Syrphidae), including Eristalis sp., Eupeodes luniger and Episyrphus balteatus, and the tachinid fly Leiophora sp. (Tachinidae) were the major visitors to the flowers at both sites. Of 998 and 1527 visits observed at Kunming Botanical Garden and Jinfoshan Mountain, 96.1 % and 98.8 %, respectively, were by flies. The flies tended to move from one flower to the next within an inflorescence before moving to another inflorescence. Together, these observations suggest that flies are the most effective pollinators of S. chinensis.
Table 1.
Insect visitors observed on flowers of Saururus chinensis at Kunming Botanical Garden and Jinfoshan Mountain
| Taxon | Kunming Botanical Garden | Jinfoshan Mountain | ||
|---|---|---|---|---|
| Total number of visits | Percentage of total visits | Total number of visits | Percentage of total visits | |
| Diptera | ||||
| Eristalis sp. | 898 | 90.0 | 1362 | 89.2 |
| Eupeodes luniger | 42 | 4.2 | 97 | 6.4 |
| Leiophora sp. | 19 | 1.9 | 18 | 1.2 |
| Episyrphus balteatus | 31 | 2.0 | ||
| Hymenoptera | ||||
| Anthophora sp. | 22 | 2.2 | 19 | 1.2 |
| Coleoptera | ||||
| Oenopia sp. | 8 | 0.8 | ||
| Lepidoptera | ||||
| Spindasis syama | 9 | 0.9 | ||
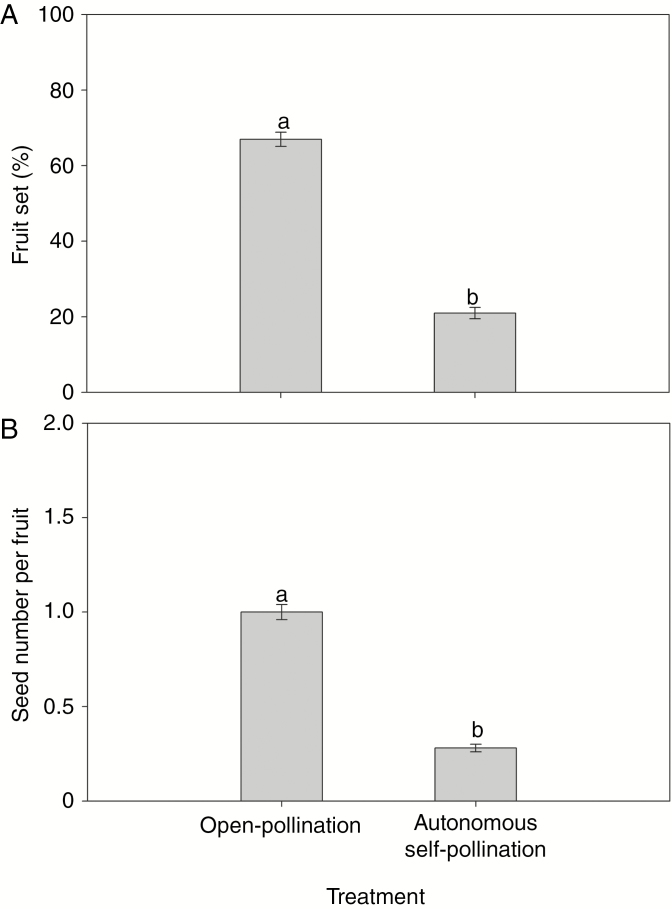
No pollen grains were captured on the microscope slides (14 cm2) coated with petroleum jelly, suggesting that wind pollination is unlikely to play a role in the pollination of S. chinensis. Flowers self-pollinated autonomously, but fruit set of bagged flowers was much lower than in open-pollinated flowers (22.0 ± 1.50 versus 67.0 ± 1.87 %, respectively; F1,58 = 368.16, P < 0.001; Fig. 2A). Similarly, seed number per fruit in autonomously self-pollinated flowers was significantly lower than that in open-pollinated flowers (0.28 ± 0.02 versus 1.01 ± 0.04, respectively; F1,58 = 248.08, P < 0.001; Fig. 2B). These results suggest that pollination of S. chinensis depends mainly on visiting insects.
Fig. 2.
Fruit set (A) and seed number per fruit (B) of Saururus chinensis flowers that were open-pollinated and autonomously self-pollinated (bagged). Different letters denote significant differences at P < 0.05.
Leaf morphology and anatomy
At both sites, leaves subtending the inflorescences always showed colour change from green to white before the first flower opened, and then reverted back to green after all flowers had been pollinated; leaves on non-flowering plants showed no such colour change. Leaf size increased significantly as colour changed from white to green (Z = 2.167, n = 50, P < 0.05; Z = 2.44, n = 50, P < 0.05 for length and width, respectively, Mann–Whitney U-test; data from the two sites were pooled since no significant difference was found between the two sites (data not shown)). The leaves were 7.48 ± 0.12 cm long and 4.74 ± 0.06 cm wide when the colour was white, and 7.83 ± 0.13 cm long and 4.94 ± 0.06 cm wide after re-greening.
The parenchyma cells of young green leaves contained many chloroplasts (Fig. 1H, L). When the leaves became white (during flowering), the palisade parenchyma cells contained few chloroplasts (Fig. 1I, M) because of the transformation of chloroplasts into leucoplasts. However, when the white leaves turned back to green after flowering, the palisade parenchyma cells were again full of chloroplasts, as in normal green leaves (Fig. 1J, K, N, O), because the leucoplasts had transformed into chloroplasts again.
Colour measurement and pollinator visitation
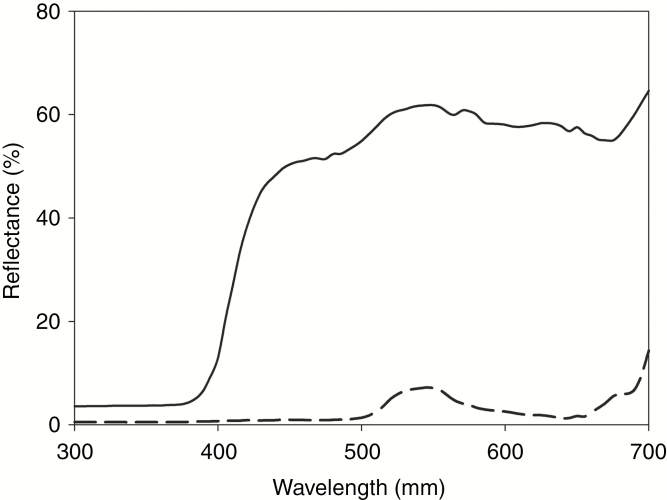
The spectral measurements revealed that the reflectance of white leaves is significantly different from that of green leaves in the visible range, but not in the ultraviolet range (Fig. 3). Based on the sum of the excitation values of the four fly photoreceptors, the white leaves were significantly brighter than green leaves (Z = 6.06, n = 25, P < 0.001, Mann–Whitney U-test).
Fig. 3.
Spectral reflectance of white leaves (solid line) and green leaves (dashed line) of Saururus chinensis.
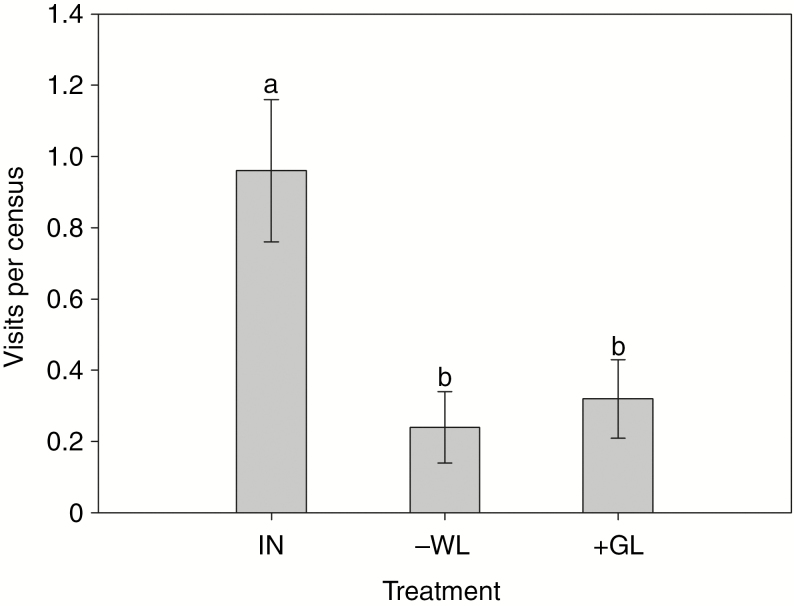
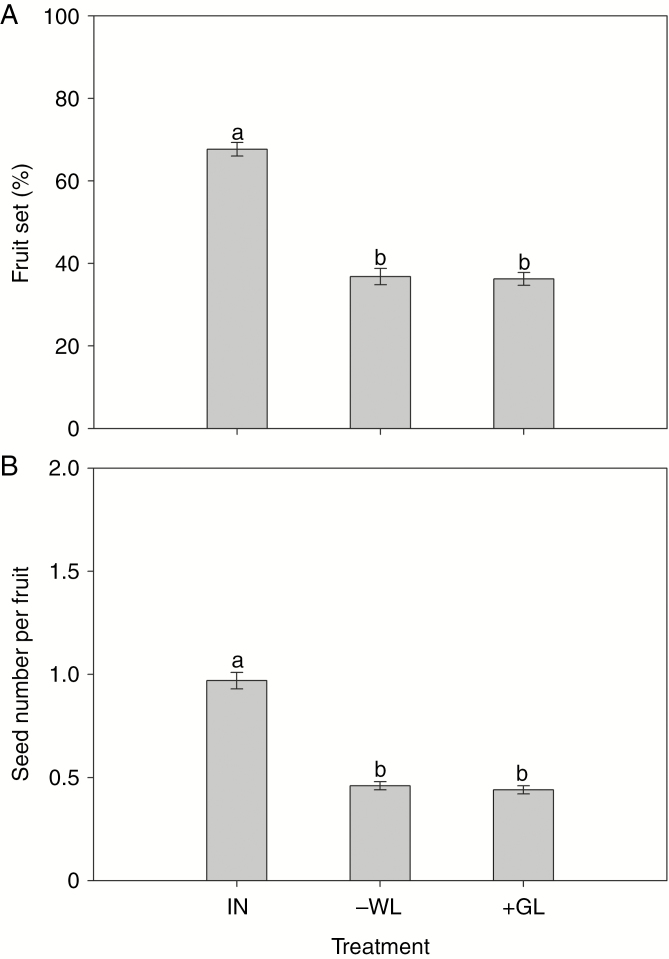
Fly visitation was significantly affected by leaf treatment (F2,72 = 7.20, P < 0.01; Fig. 4). The number of fly visits to plants from which white leaves were removed (0.24 ± 0.10 visits per 30 min) and plants with white leaves covered by green leaves (0.32 ± 0.11 per 30 min) were not different from each other, but both were significantly lower than numbers of visits to intact plants (0.96 ± 0.20 per 30 min). Similarly, fruit and seed set were also affected significantly by leaf treatment (fruit set, F2,72 = 108.38, P < 0.001; seed set, F2,72 = 111.39, P < 0.001; Fig. 5). Fruit set and seed number per fruit for plants with white leaves removed (fruit set, 36.8 ± 1.97 %; seed number per fruit, 0.46 ± 0.02) and plants with white leaves covered by green leaves (fruit set, 36.3 ± 1.55 %; seed number per fruit 0.44 ± 0.02) were not different from each other but both were significantly lower than for intact plants (fruit set 67.7 ± 1.64 %; seed number per fruit, 0.97 ± 0.04). Fruit and seed set increased by ~87 and 120 %, respectively, as a result of leaf colour change from green to white during flowering.
Fig. 4.
Number of fly visits per census (30 min) to plants subjected to three experimental treatments. Different letters denote significant differences at P < 0.05. IN, intact; -WL, all white leaves on a plant were removed; +GL, all white leaves on a plant were covered by green leaves.
Fig. 5.
Fruit set (A) and seed set (B) of Saururus chinensis flowers subjected to three experimental treatments. Different letters denote significant differences at P < 0.05. IN, intact; -WL, leaves were removed just before whitening; +GL, leaves were covered by green leaves just before whitening.
Photosynthetic capacity, fecundity and progeny quality
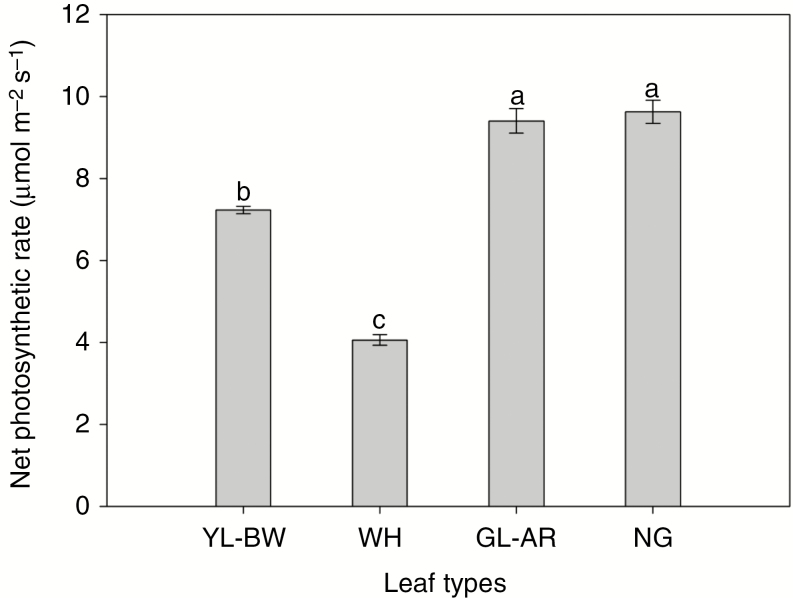
The net photosynthetic rate differed significantly between leaves of different colours (F3,36 = 201.58, P < 0.001; Fig. 6). The net photosynthetic rate of white leaves was significantly lower than that of young green leaves (young green leaves, 7.23 ± 0.09 μmol m−2 s−1; white leaves, 4.06 ± 0.13 μmol m−2 s−1; reduced by ~44 %). However, when white leaves turned back to green these rates were significantly increased (~132 %), and did not differ from the normal green leaves (re-greened leaves, 9.40 ± 0.30 μmol m−2 s−1; normal green leaves, 9.62 ± 0.28 μmol m−2 s−1).
Fig. 6.
Net photosynthetic rates of leaves of different colour. Different letters denote significant differences at P < 0.05. YL-BW, young green leaves before whitening; WH, white leaves; GL-AR, green leaves after re-greening; NG, normal green leaves.
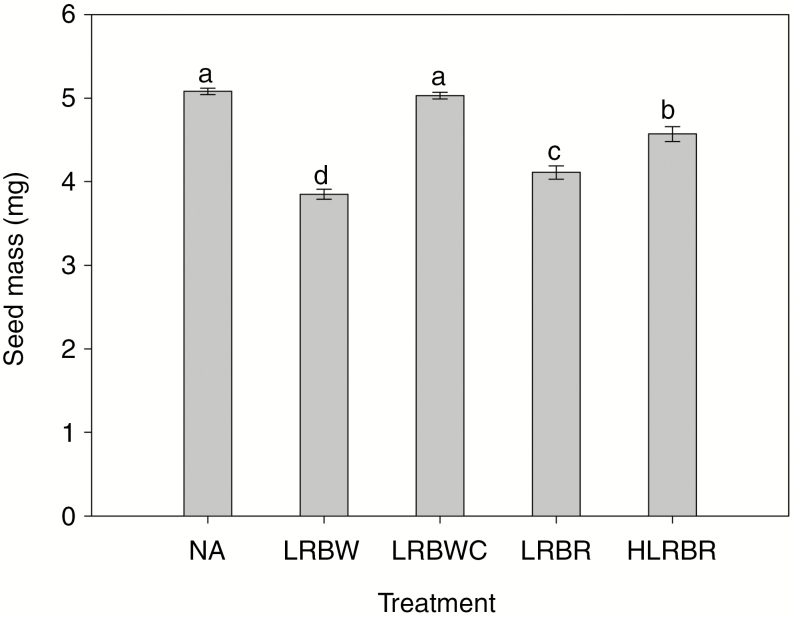
Leaf treatments (removing leaves at different developmental stages) had no significant effects on fruit and seed set when flowers were hand-pollinated (fruit set, F4,120 = 0.39, P = 0.82; seed set, F4,120 = 0.29, P = 0.88). On average, 81 % of flowers produced fruits and each flower produced 1.61 seeds. For seed mass, however, significant differences were found between leaf treatments (F4,120 = 66.62, P < 0.001; Fig. 7). Mean seed mass in plants with young green leaves removed just before changing to white (3.86 ± 0.06 mg) was 0.25 mg less than that from plants with white leaves removed just before re-greening (4.11 ± 0.08 mg). This suggests that white leaves contribute to the photosynthetic budget, albeit at a lower rate than would green leaves. This result also suggests that reduced photosynthetic capacity due to leaf colour change from green to white eventually translated into an associated cost in terms of decreased seed mass (~6 %), given that the reduced net photosynthetic rates of white leaves compared with young green leaves was close to the net photosynthetic rate of white leaves. Compared with the control plants (5.08 ± 0.04 mg), plants with white leaves removed just before re-greening produced seeds that were 0.97 mg (~20 %) lighter. Seed mass in the plants with half of each of the white leaves removed just before re-greening (4.57 ± 0.09 mg) was greater by 0.46 mg (~11 %) than in plants with white leaves completely removed just before re-greening, but was significantly lower than in the control plants. The mass of seeds from the trimmed control treatment was not different from that in the control treatment, indicating that leaf damage alone did not affect carbon provisioning.
Fig. 7.
Seed mass of Saururus chinensis flowers when the two or three apical leaves that will change colour were subjected to different experimental treatments. Flowers used for the experimental treatment were hand-pollinated. Different letters denote significant differences at P < 0.05. NA, natural; LRBW, leaves were removed just before whitening; LRBWC, leaves were trimmed ~1 mm from their edge just before whitening; LRBR, white leaves were removed just before re-greening; HLRBR, half of each of the white leaves was removed just before re-greening.
DISCUSSION
Here, we examined experimentally the possible functions of leaf colour change during flowering and fruiting in S. chinensis. Unlike previous studies on leaf colour change, which mainly focused on protection against abiotic factors such as ultraviolet radiation, low temperature or oxygen toxicity, or against insect herbivory or fungal attacks (Miranda et al., 1981; Matile, 2000; Lev-Yadun et al., 2002; Numata et al., 2004; Archetti et al., 2009; Taulavuori et al., 2011), our study indicates that the colour change in leaves of S. chinensis is functionally important because whitening of young green leaves enhances pollinator visitation through an enhanced visual display during flowering. In addition, re-greening of white leaves after anthesis appears to be advantageous because it promotes the development of ripening seeds through increased photosynthetic capacity during fruiting. Compared with the pollination benefit, the photosynthetic cost as a result of the temporary whitening of the leaves during flowering, in terms of seed development, is modest. Consequently, our results suggest that reversible leaf colour change in S. chinensis is an adaptive feature favoured by natural selection.
Leaf colour change from green to white during flowering
The mean pollinator visitation rate was 67 % lower in plants with their white leaves removed than in the controls, indicating that the whitening of leaves subtending the inflorescences in S. chinensis is important for pollinator attraction. This is in agreement with previous reports on the relationship between visual display by plants and pollinator attraction (Herrera, 1997; Borges et al., 2003; Armbruster et al., 2005; Sun et al., 2008; Keasar et al., 2009; Song et al., 2013, 2015). These findings can be explained as follows. First, white leaves of S. chinensis contrast strongly with green leaves in all spectral ranges except the ultraviolet. This, together with the increased brightness, should make the white leaves detectable to the pollinating insects over long distances, since many fly species have been found to have the ability to discriminate colours based on brightness (Vargas et al., 1991; Cornelius et al., 1999), as is the case for the ‘detection effect’ by the bracts of Davidia involucrata (Nyssaceae) and Mussaenda frondosa (Rubiaceae) (Borges et al., 2003; Sun et al., 2008). Furthermore, several studies have shown that this detection effect is more prominent for plants occurring at low densities (Herrera, 1997; Borges et al., 2003; Keasar et al., 2009). In the field, S. chinensis plants commonly grow several metres apart (B. Song, pers. obs.), making it difficult for insects to find the flowering plants. Second, a high synchrony between flower maturation and presentation of white leaves suggested that the white colour may provide information on plant quality and direct pollinating insects to rewarding flowers, similar to the role played by floral colour change in some other plant species (Ida and Kudo, 2003; Sun et al., 2005). What is important is that increased pollinator visitation did result in greatly increased pollination success (fruit and seed set were increased by 87 and 120 %, respectively). Consequently, our results suggest that leaf colour change from green to white during flowering greatly increased the reproductive fitness of S. chinensis through enhancing pollinator visitation and subsequent pollination success. However, we cannot rule out the possibility that floral scents may play a role in pollinator attraction. Further study should be conducted to test the relative roles of visual and olfactory signals provided by white leaves and floral scent in pollinator attraction of S. chinensis. It is possible that leaf whitening might protect against various abiotic factors (e.g. ultraviolet irradiation, water shortage; Lev-Yadun et al., 2002). However, we largely rule out such a possibility, as leaves on non-flowering plants did not change their colour during the growth season, albeit growing under environmental conditions similar to those of plants with white leaves subtending the inflorescences, at least in the two study sites.
In previous studies on the interactions between plants and their pollinators, enhancement of visual display mainly takes the form of larger flowers, aggregating flowers into inflorescences, retaining of old flowers or developing conspicuous secondary structures associated with flowers, such as the showy bracts of many Araceae, Bromeliaceae, Euphorbiaceae, Nyctaginaceae and Rubiaceae (Heywood, 1978; Harder and Barrett, 1996; Herrera, 1997; Blarer et al., 2002; Suzuki and Ohashi, 2014; Gagliardi et al., 2016). In contrast to these findings, where visual displays are located in flowers themselves or in secondary structures associated with flowers, our study demonstrates that pollinator attraction has exerted a selective pressure on vegetative organs, namely leaves. Because of the sharp reduction in chloroplasts in the palisade mesophyll, the colour change from green to white to attract pollinators undoubtedly comes at a photosynthetic cost, with photosynthetic rate decreased by at least 44 % compared with the young green leaves. However, from a reproductive viewpoint, the effect of temporary decreased photosynthesis on reproductive fitness (e.g. fruit set, seed set and seed mass) as a result of the presence of the white leaves requires direct validation. The subtle decrease in seed mass (~6 %) in plants with young green leaves removed just before they turned white compared with plants with white leaves removed just before re-greening, in combination with the absence of obvious variations in fruit and seed set, suggested that the cost of reduced photosynthesis was modest compared with the enhanced pollination benefit from the presence of the white leaves. A weakness of this study was that we examined only indirectly the photosynthetic cost of changing colour in terms of reduction in seed development. However, the reduced value in net photosynthetic rate due to the colour change from green to white was lower than that of white leaves. Thus, the real photosynthetic cost of white leaves in terms of reduction in seed mass should not be more than the contribution of white leaves that we estimated (6 %). In addition, our results also suggest that in the measurement of reproductive effort, just assessing the reproductive parts may underestimate the reproductive cost if the investment in associated vegetative parts is not taken into account (Bazzaz and Carlson, 1979; Obeso, 2002). It is also possible that the presence of white leaves during flowering might increase floral herbivory, and thus reduce plant reproductive fitness, as traits that are attractive to pollinators may also attract florivores (Armbruster, 1997; Pérez-Barrales et al., 2013). However, we did not find any evidence of floral predation (B. Song et al., unpubl. res.), indicating that leaf whitening in S. chinensis was unlikely to result in a florivory cost, at least at the two sites studied.
Leaf colour change from white to green during fruiting
Leaf re-greening after flowering was associated with a significant increase in photosynthetic capacity, to a level comparable with normal green leaves, suggesting that the re-greened leaves may help to meet the carbon requirements of the developing seeds in S. chinensis. As has been mentioned previously, however, this proposition also needs to be verified directly, because even if the assimilates produced by re-greened leaves increased, this was not necessarily translated into a significant enhancement of fruit or seed development: assimilates could have remained in the leaves or been transferred to storage organs other than nearby seeds as a result of architectural constraints (Watson and Casper, 1984; Nakano et al., 1997). In the treatment where we removed half of each white leaf just before re-greening, there was no significant effect on fruit and seed set, but seed mass was significantly greater (~11 %) than in plants with white leaves removed completely just before re-greening. We largely eliminated the possibility that the variation in seed mass resulted from the damage inflicted because there was no difference in seed mass between the trimmed control treatment and the control treatment. These results indicate that the re-greened leaves of S. chinensis contributed substantial assimilates to the development of seeds, similar to the way that re-greening in persistent reproductive organs, such as bracts and sepals, contributes to reproductive output in other species. For example, the involucral bracts in Dalechampia scandens permit carbon assimilation that contributes to the carbon demand of developing seeds by changing colour from white to green after flowering (Pélabon et al., 2015). In S. chinensis, white leaves accounted for up to 38 % of the total number of leaves on an individual plant. Thus, it is not surprising that re-greening of white leaves substantially contributes to the carbon needs of developing seeds in S. chinensis, in contrast to the limited overall contribution of bract photosynthesis for seed development in D. scandens (Pélabon et al., 2015). Thus, re-greening of the white leaves after flowering increased the local production of photosynthates and decreased the earlier costs imposed by leaf colour change from green to white to enhance pollination success during flowering.
In conclusion, the colour change from green to white of the apical two or three leaves in S. chinensis during flowering enhances the visual display and increases pollination success by increasing pollinator attraction, with only limited photosynthetic cost in terms of seed development. Furthermore, these leaves present a photosynthetic capacity similar to that of normal green leaves after turning back to full green during fruiting, and they help to meet the carbon requirements of the developing seeds, thereby also compensating for the photosynthetic cost resulting from leaf whitening during flowering. Thus, our results suggest that the reversible leaf colour change in S. chinensis is an adaptive strategy resulting from selection for enhanced pollination success during flowering combined with providing a carbon supply for seed development. Although leaf colour change occurs in many plants, this study is the first to document experimentally that selection from pollinators is likely to play an important role in leaf colour change, and thereby it contributes to our understanding of how the interaction between plant and pollinator can allow leaves to evolve a new function in flowering plants.
ACKNOWLEDGEMENTS
We sincerely thank Guowei Zheng, Yang Niu, Fei Li, JinJin Hu and Mei Jia for experimental assistance, and Prof. Darong Yang for insect identification. We also thank Prof. Simcha Lev-Yadun for providing very constructive comments on the manuscript. The authors declare no conflict of interests. This work was supported by the National Key Research and Development Program of China (2017YFC0505200), the Major Program of the National Natural Science Foundation of China (31590820, 31590823), the National Natural Science Foundation of China (31570228, 31770249), the Young Academic and Technical Leader Raising Foundation of Yunnan Province (2017HB062), the Youth Innovation Promotion Association CAS (2017437) and the CAS ‘Light of West China’ Program to B.S.
LITERATURE CITED
- Archetti M, Doring TF, Hagen SB et al. 2009. Unravelling the evolution of autumn colours: an interdisciplinary approach. Trends in Ecology and Evolution 24: 166–173. [DOI] [PubMed] [Google Scholar]
- Armbruster WS. 1997. Exaptations link evolution of plant-herbivore and plant-pollinator interactions: a phylogenetic inquiry. Ecology 78: 1661–1672. [Google Scholar]
- Armbruster WS, Antonsen L, Pélabon C. 2005. Phenotypic selection on Dalechampia blossoms: honest signaling affects pollination success. Ecology 86: 3323–3333. [Google Scholar]
- Bazzaz FA, Carlson RW. 1979. Photosynthetic contribution of flowers and seeds to reproductive effort of an annual colonizer. New Phytologist 82: 223–232. [Google Scholar]
- Blarer A, Keasar T, Shmida A. 2002. Possible mechanisms for the formation of flower size preferences by foraging bumblebees. Ethology 108: 341–351. [Google Scholar]
- Borges RM, Gowda V, Zacharias M. 2003. Butterfly pollination and high-contrast visual signals in a low-density distylous plant. Oecologia 136: 571–573. [DOI] [PubMed] [Google Scholar]
- Burger J, Edwards GE. 1996. Photosynthetic efficiency, and photodamage by UV and visible radiation, in red versus green leaf Coleus varieties. Plant and Cell Physiology 37: 395–399. [Google Scholar]
- Chen YQ, Chen DZ, Wu GF et al. 1982. Flora of China. Beijing: Science Press. [Google Scholar]
- Chen YZ, Huang SQ. 2013. Red young leaves have less mechanical defence than green young leaves. Oikos 122: 1035–1041. [Google Scholar]
- Cornelius ML, Duan JJ, Messing RH. 1999. Visual stimuli and the response of female oriental fruit flies (Diptera: Tephritidae) to fruit-mimicking traps. Journal of Economic Entomology 92: 121–129. [Google Scholar]
- Feild TS, Lee DW, Holbrook NM. 2001. Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiology 127: 566–574. [PMC free article] [PubMed] [Google Scholar]
- Gagliardi KB, Cordeiro I, Demarco D. 2016. Protection and attraction: bracts and secretory structures in reduced inflorescences of Malpighiales. Flora 220: 52–62. [Google Scholar]
- Haberlandt G. 1914. Physiological plant anatomy. London: Macmillan. [Google Scholar]
- Harder LD, Barrett SCH. 1996. Pollen dispersal and mating patterns in animal-pollinated plants. In: Lloyd DG, Barrett SCH, eds. Floral biology: studies on floral evolution in animal-pollinated plants. New York: Chapman & Hall, 140–190. [Google Scholar]
- Herrera CM. 2005. Post-floral perianth functionality: contribution of persistent sepals to seed development in Helleborus foetidus (Ranunculaceae). American Journal of Botany 92: 1486–1491. [DOI] [PubMed] [Google Scholar]
- Herrera J. 1997. The role of colored accessory bracts in the reproductive biology of Lavandula stoechas. Ecology 78: 494–504. [Google Scholar]
- Heywood VH. 1978. Flowering plants of the world. Oxford: Oxford University Press. [Google Scholar]
- Ida T, Kudo G. 2003. Floral colour change in Weigela middendorffiana (Caprifoliaceae): reduction of geitonogamous pollination by bumble bees. American Journal of Botany 90: 1751–1757. [DOI] [PubMed] [Google Scholar]
- Ikeda T. 1979. Electron microscopic evidence for the reversible transformation of Euonymus plastids. Botanical Magazine Tokyo 92: 23–30. [Google Scholar]
- Karageorgou P, Manetas Y. 2006. The importance of being red when young: anthocyanins and the protection of young leaves of Quercus coccifera from insect herbivory and excess light. Tree Physiology 2: 613–621. [DOI] [PubMed] [Google Scholar]
- Karageorgou P, Buschmann C, Manetas Y. 2008. Red leaf color as a warning signal against insect herbivory: honest or mimetic?Flora 203: 648–652. [Google Scholar]
- Keasar T, Sadeh A, Gerchman Y, Shmida A. 2009. The signaling function of an extra-floral display: what selects for signal development?Oikos 118: 1752–1759. [Google Scholar]
- Kursar TA, Coley PD. 1992. Delayed greening in tropical leaves – an antiherbivore defense?Biotropica 24: 256–262. [Google Scholar]
- Kursar TA, Coley PD. 2003. Convergence in defense syndromes of young leaves in tropical rainforests. Biochemical Systematics and Ecology 31: 929–949. [Google Scholar]
- Lev-Yadun S, Gould KS. 2007. What do red and yellow autumn leaves signal?Botanical Review 73: 279–289. [Google Scholar]
- Lev-Yadun S, Inbar M, Izhaki I, Ne’eman G. 2002. Colour patterns in vegetative parts of plants deserve more research attention. Trends in Plant Science 7: 59–60. [DOI] [PubMed] [Google Scholar]
- Lev-Yadun S, Dafni A, Flaishman MA et al. 2004. Plant colouration undermines herbivorous insect camouflage. BioEssays 26: 1126–1130. [DOI] [PubMed] [Google Scholar]
- Liang HX, Pan KY, Chen ZD. 1996. Floral organogenesis in Saururus chinensis (Saururaceae). Acta Phytotaxonomica Sinica 34: 565–568. [Google Scholar]
- Matile P. 2000. Biochemistry of Indian summer: physiology of autumn leaf coloration. Experimental Gerontology 35: 145–158. [DOI] [PubMed] [Google Scholar]
- Miranda V, Baker NR, Long SP. 1981. Limitations of photosynthesis in different regions of the Zea mays leaf. New Phytologist 89: 179–190. [Google Scholar]
- Nakano R, Yonemori K, Sugiura A. 1997. Photosynthesis by calyx lobes has no contribution to early fruit development in persimmon. Acta Horticulturae 436: 346–353. [Google Scholar]
- Numata S, Kachi N, Okuda T, Manokaran N. 2004. Delayed greening, leaf expansion, and damage to sympatric Shorea species in a lowland rain forest. Journal of Plant Research 117: 19–25. [DOI] [PubMed] [Google Scholar]
- Obeso JR. 2002. The costs of reproduction in plants. New Phytologist 155: 321–348. [DOI] [PubMed] [Google Scholar]
- Pélabon C, Hennet L, Strimbeck R, Johnson H, Armbruster WS. 2015. Blossom colour change after pollination provides carbon for developing seeds. Functional Ecology 29: 1137–1143. [Google Scholar]
- Pérez-Barrales R, Bolstad GH, Pélabon C, Hansen TF, Armbruster WS. 2013. Pollinators and seed predators generate conflicting selection on Dalechampia blossoms. Oikos 122: 1411–1428. [Google Scholar]
- Song B, Zhang ZQ, Stöcklin J et al. 2013. Multifunctional bracts enhance plant fitness during flowering and seed development in Rheum nobile (Polygonaceae), a giant herb endemic to the high Himalayas. Oecologia 172: 359–370. [DOI] [PubMed] [Google Scholar]
- Song B, Niu Y, Stöcklin J et al. 2015. Pollinator attraction in Cornus capitata (Cornaceae): the relative role of visual and olfactory cues. Journal of Plant Ecology 8: 173–181. [Google Scholar]
- Stiles EW. 1982. Fruit flags: two hypotheses. American Naturalist 120: 500–509. [Google Scholar]
- Sun JF, Gong YB, Renner SS, Huang SQ. 2008. Multifunctional bracts in the dove tree Davidia involucrata (Nyssaceae: Cornales): rain protection and pollinator attraction. American Naturalist 171: 119–124. [DOI] [PubMed] [Google Scholar]
- Sun SG, Liao K, Xia J, Guo YH. 2005. Floral colour changes in Pedicularis monbeigiana (Orobanchaceae). Plant Systematics and Evolution 255: 77–85. [Google Scholar]
- Suzuki MF, Ohashi K. 2014. How does a floral colour-changing species differ from its non-colour-changing congener? – a comparison of trait combinations and their effects on pollination. Functional Ecology 28: 549–560. [Google Scholar]
- Taulavuori K, Pihlajaniemi H, Huttunen S, Taulavuori E. 2011. Reddish spring colouring of deciduous leaves: a sign of ecotype?Trees 25: 231–236. [Google Scholar]
- Troje N. 1993. Spectral categories in the learning behaviour of blowflies. Zeitschrift für Naturforschung C 48: 96–104. [Google Scholar]
- Vargas R, Stark JD, Prokopy RJ, Green TA. 1991. Response of oriental fruit fly (Diptera: Tephritidae) and associated parasitoids (Hymenoptera: Braconidae) to different-color spheres. Journal of Economic Entomology 84: 1503–1507. [Google Scholar]
- Vogel-Mikuš K, Simčič J, Pelicon P et al. 2008. Comparison of essential and non-essential element distribution in leaves of the Cd/Zn hyperaccumulator Thlaspi praecox as revealed by micro-PIXE. Plant Cell & Environment 31: 1484–1496. [DOI] [PubMed] [Google Scholar]
- Watson MA, Casper BB. 1984. Morphogenetic constraints on patterns of carbon distribution in plants. Annual Review of Ecology and Systematics 15: 233–258. [Google Scholar]
- Weiss MR. 1995. Floral color change: a widespread functional convergence. American Journal of Botany 82: 167–185. [Google Scholar]
- Yuan LC, Luo YB, Thien LB et al. 2008. Pollination of Kadsura longipedunculata (Schisandraceae), a monoecious basal angiosperm, by female, pollen-eating Megommata sp. (Cecidomyiidae: Diptera) in China. Biological Journal of the Linnean Society 93: 523–536. [Google Scholar]