Abstract
Background and Aims
Boraginales are often characterized by a dense cover of stiff, mineralized trichomes, which may act as a first line of defence against herbivores. Recent studies have demonstrated that the widely reported silica and calcium carbonate in plant trichomes may be replaced by calcium phosphate. The present study investigates mineralization patterns in 42 species from nine families of the order Boraginales to investigate detailed patterns of mineralization and the possible presence of a phylogenetic signal in different mineralization patterns.
Methods
The distribution of biominerals was analysed by scanning electron microscopy (SEM) including cryo-SEM and energy-dispersive X-ray analyses with element mapping. The observed distribution of biominerals was plotted onto a published phylogeny of the Boraginales. Three colours were selected to represent the principal elements: Si (red), Ca (green) and P (blue).
Key Results
Calcium carbonate was present in the mineralized trichomes of all 42 species investigated, silica in 30 and calcium phosphate in 25; multiple mineralization with calcium carbonate and silica or calcium phosphate was found in all species, and 13 of the species were mineralized with all three biominerals. Trichome tips featured the most regular pattern – nearly all were exclusively mineralized with either silica or calcium phosphate. Biomineralization of the trichome shafts and bases was found to be more variable between species. However, the trichome bases were also frequently mineralized with calcium phosphate or silica, indicating that not only the tip is under functional constraints requiring specific patterns of chemical heterogeneity. The complete absence of either silica or phosphate may be an additional feature with systematic relevance.
Conclusions
This study demonstrates that complex, site-specific and differential biomineralization is widespread across the order Boraginales. Calcium phosphate, only recently first reported as a structural plant biomineral, is common and appears to be functionally analogous to silica. A comparison with the phylogeny of Boraginales additionally reveals striking phylogenetic patterns. Most families show characteristic patterns of biomineralization, such as the virtual absence of calcium phosphate in Cordiaceae and Boraginaceae, the triple biomineralization of Heliotropiaceae and Ehretiaceae, or the absence of silica in Namaceae and Codonaceae. The complex chemical and phylogenetic patterns indicate that trichome evolution and functionalities are anything but simple and follow complex functional and phylogenetic constraints.
Keywords: Biomineralization, Boraginales, calcium phosphate, silica, calcium carbonate, trichomes, SEM, EDX
INTRODUCTION
Boraginales is a monophyletic plant group with subcosmopolitan distribution. It comprises approx. 2700 species in 125 genera and eleven families (Luebert et al., 2016). The order Boraginales falls into two major clades, Boraginales I resolved as [Codonaceae, [Wellstediaceae, [Boraginaceae]]], and Boraginales II resolved as [Hydrophyllaceae, [Namaceae, [Heliotropiaceae, [Coldeniaceae, [Cordiaceae, [Hoplestigmataceae, [Ehretiaceae, Lennoaceae]]]]]]] (Weigend et al., 2014; Luebert et al., 2016). Leaves and stems of most Boraginales plants have long been known to be clothed in – often mineralized – trichomes (Jonová, 1926; Selvi and Bigazzi, 2001; Retief and van Wyk, 2005; Weigend and Hilger, 2010; Aleemuddin et al., 2011; Mehrabian et al., 2014; Weigend et al., 2014, 2016). This is also reflected in the old Latin name for the group ‘Asperifoliae’ and the modern German counterpart ‘Rauhblattgewächse’ (= ‘rough leaf plants’). These mineralized trichomes show a range of structural features, including a large diversity of density, shape and size, often dependent on their specific role and plant habitat (Diane et al., 2003). Mineralized trichomes are thought to play a crucial role in physical plant defence, e.g. via restricting herbivore movement in general and their access to vegetative and reproductive organs in particular (Reynolds and Rodriguez, 1981; Reynolds et al., 1986, 1989; He et al., 2012; Szyndler et al., 2013). Additional, non-defence functions of trichomes comprise a protection against water loss, high temperature and UV-B radiation (Bickford, 2016). Mineral deposits in the sharp trichome tips render them hard and enhance their mechanical stability (Johnson, 1975), but their relevance for other functionalities has not been studied. The formation of biogenic minerals in the outer cell walls of trichomes is a widespread phenomenon found across many plant groups, but their mineral abundance and distribution in different types of trichomes varies widely between taxa (Thurston and Lersten, 1969; Ensikat et al., 2017; Mustafa et al., 2017). Calcium carbonate and silica have been widely reported as biominerals in the trichomes of a wide range of plant families in general, and Boraginales in particular (Hilger et al., 1993; Selvi and Bigazzi, 2001). In general, however, the detailed patterns of biomineralization remain poorly studied (He et al., 2014). Nevertheless, our series of recent studies has demonstrated the occurrence of multiple biomineralization (calcium phosphate, calcium carbonate, silica) in stinging trichomes belonging to several angiosperm plant families such as Caricaceae, Loasaceae, Urticaceae and Namaceae (Ensikat et al., 2016, 2017, Mustafa et al., 2017). Using scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) analyses, the present study was undertaken to investigate the distribution of different biominerals across morphologically distinct types of trichomes in the order Boraginales. Preliminary data indicated that biomineralization in Boraginales is complex and variable and this triggered a more detailed study of biomineralization across the order Boraginales. The study addresses the following questions: (1) Which biominerals are present in Boraginales trichomes and what is their distribution within trichomes? (2) Can differential functionalities be inferred from the distribution patterns of individual biominerals? (3) Are there phylogenetic patterns in the distribution of different biominerals?
MATERIALS AND METHODS
Sample preparation and microscopy
Fresh plant material was cultivated at the Botanical Garden of the University of Bonn, Germany, whereas herbarium specimens were collected from the herbaria of the Nees-Institute for Biodiversity of Plants (BONN); Royal Botanical Garden, Kew, UK; and Institut für Biologie – Systematische Botanik und Pflanzengeographie, Freie Universität Berlin/Botanische Gärten der Universität Berlin-Dahlem (BSB/B). At the Botanical Garden of the University of Bonn the plants were grown under three different temperature regimes depending on their regions of origin: a temperate glasshouse with a minimum temperature of 13 °C and an average temperature of 15–17 °C, a conservatory with minimum temperature of 10 °C, and in the open during the summer months, respectively outdoor all year (most species). As a potting soil we used a peat-based mixture of 70 % Einheitserde ED73 and 30 % sand. Einheitserde ED73 comprises 70 % peat and 30 % clay dust with a pH of 5.8 and pre-fertilized with an N: P: K ratio of 14: 16: 18 plus a slow-release fertilizer with an N: P: K ratio of 20: 10: 15. Plants were usually watered every other day. A total of 42 species representing nine families of the Boraginales were studied. Details of the species examined and their voucher information are given in Table 1. For EDX analysis and element mapping, fresh leaf samples were fixed in formaldehyde solution (70 % ethanol + 4 % formaldehyde) for 24 h, dehydrated, critical-point dried (CPD 020, Balzers Union, Liechtenstein), and sputter-coated with silver or palladium (SCD 040 Sputter-Coater; Balzers), using standard procedures described elsewhere (Ensikat et al., 2016).
Table 1.
Examined taxa from nine plant families and their voucher information
| Order | Family | Taxon | Region of origin | Herbarium |
|---|---|---|---|---|
| Boraginales | Codonaceae | Codon royenii D. Royen | Namibia | T. Joßberger 1247 |
| Boraginales | Codonaceae | Codon schenckii Schinz | Namibia | T. Joßberger 653 |
| Boraginales | Wellstediaceae | Wellstedia dinteri Pilg. | No locality data | Dinter 4845 |
| Boraginales | Boraginaceae | Borago officinalis L. | France | T. Joßberger 1720 |
| Boraginales | Boraginaceae | Borago pygmaea (DC.) Chater & Greuter | Italy | T. Joßberger 1721 |
| Boraginales | Boraginaceae | Echium italicum S. G. Gmel. subsp. biebersteinii (Lacaita) Greuter & Burdet | Georgia | T. Joßberger 759 |
| Boraginales | Boraginaceae | Echium vulgare L. | Germany | T. Joßberger 1037 |
| Boraginales | Boraginaceae | Cerinthe major L. subsp. oranensis (Batt.) Selvi & L. Cecchi | Germany | F. Selvi & M. Bigazzi 04.46 |
| Boraginales | Boraginaceae | Myosotis sylvatica Hoffm. | Hungry | T. Joßberger 1039 |
| Boraginales | Boraginaceae | Ogastemma pusillum (Coss. & Durieu ex Bonnet & Barratte) Brummitt | Israel | T. Joßberger 1609 |
| Boraginales | Boraginaceae | Onosma alborosea Fisch. & C. A. Mey. | Turkey | M. Ackermann 578 |
| Boraginales | Boraginaceae | Podonosma orientalis (L.) Feinbrun | Israel | T. Joßberger 1620 |
| Boraginales | Boraginaceae | Symphytum caucasicum M. Bieb. | Germany | T. Joßberger 1487 |
| Boraginales | Boraginaceae | Symphytum officinale L. | Germany | T. Joßberger 1723 |
| Boraginales | Boraginaceae | Trachystemon orientalis (L.) G. Don | Georgia | T. Joßberger 1438 |
| Boraginales | Boraginaceae | Trichodesma africanum (L.) Sm. | Israel | T. Joßberger 953 |
| Boraginales | Hydrophyllaceae | Hydrophyllum canadense L. | No locality data | T. Joßberger 1584 |
| Boraginales | Hydrophyllaceae | Hydrophyllum virginianum L. | Austria | T. Joßberger 1820 |
| Boraginales | Hydrophyllaceae | Hydrophyllum tenuipes A. Heller | USA | T. Joßberger 1556 |
| Boraginales | Hydrophyllaceae | Nemophila menziesii Hook. & Arn. | USA | T. Joßberger 1889 |
| Boraginales | Hydrophyllaceae | Phacelia brachyantha Benth. | Chile | F. Luebert 3182 |
| Boraginales | Hydrophyllaceae | Phacelia malvifolia Cham. | USA | T. Joßberger 1502 |
| Boraginales | Hydrophyllaceae | Phacelia secunda J. F. Gmel. | Chile | T. Joßberger 1722 |
| Boraginales | Hydrophyllaceae | Draperia systyla Torr. | USA | T. Joßberger 1752 |
| Boraginales | Hydrophyllaceae | Eriodictyon crassifolium Benth. | USA | T. Joßberger 1611 |
| Boraginales | Namaceae | Nama rothrockii A. Gray | USA | T. Joßberger 38768 |
| Boraginales | Namaceae | Wigandia caracasana Kunth | Peru | T. Joßberger 1619 |
| Boraginales | Namaceae | Wigandia ecuadorensis Cornejo | Ecuador | T. Joßberger 899 |
| Boraginales | Namaceae | Wigandia urens Ruiz & Pav.) Kunth | Peru | T. Joßberger 1717 |
| Boraginales | Heliotropiaceae | Heliotropium corymbosum Ruiz & Pav. | Peru | T. Joßberger 820 |
| Boraginales | Heliotropiaceae | Heliotropium europaeum L. | Turkmenistan, Turkestan | T. Joßberger 762 |
| Boraginales | Heliotropiaceae | Heliotropium rufipilum I. M. Johnst. | Ecuador | T. Joßberger 696 |
| Boraginales | Heliotropiaceae | Tournefortia johnstonii Standl. | No locality data | Det. James S. Miller, 2000 |
| Boraginales | Heliotropiaceae | Tournefortia cuspidata Kunth | No locality data | Jose Gonzalez 787, Det. James S. Miller |
| Boraginales | Ehretiaceae | Ehretia dicksonii Hance | Germany | T. Joßberger 1220 |
| Boraginales | Ehretiaceae | Ehretia microphylla Lam. | No locality data | T. Joßberger 1715 |
| Boraginales | Ehretiaceae | Tiquilia cf. dichotoma (Ruiz & Pav.) Pers. | Peru | M. Weigend, H. Förther and N. Dostert 688 |
| Boraginales | Ehretiaceae | Tiquilia cf. elongata (Rusby) A. T. Richardson | Peru | M. Weigend & F. Cáceres-H. 9081 (MW_06) |
| Boraginales | Coldeniaceae | Coldenia procumbens L. | Burkina Faso | T. Joßberger 1305 |
| Boraginales | Cordiaceae | Cordia monoica Roxb. | Kenya | T. Joßberger 1874 |
| Boraginales | Cordiaceae | Varronia bahamensis (Ubs.) Millsp. | Bahamas | M. A. Hamilton 64831000 |
| Boraginales | Cordiaceae | Varronia rupicola (Ubs.) Britton | Puerto Rico | M. A. Hamilton 2014-384 |
Topographic imaging and EDX analyses were performed using a Cambridge S200 Stereoscan and an LEO 1450 scanning electron microscope (Cambridge, UK), which include secondary electron (SE) and backscattered electron (BSE) detectors and an EDX analysis system (Oxford Instruments, Oxford, UK). Material contrast images were acquired from frozen, hydrated samples at approx. −100 °C using a cryo stage (Ensikat and Weigend, 2013). EDX was used for detection and mapping of the elements silicon (Si), phosphorus (P) and calcium (Ca). It has been previously demonstrated for trichome walls (Ensikat et al., 2017; Mustafa et al., 2017) that P is generally associated with Ca in the form of calcium phosphate, whereas Ca without P corresponds to a calcium carbonate-based composite, associated with organic compounds such as cellulose. This calcium carbonate composite is here simply referred to as calcium carbonate. All obtained images were recorded with a digital image acquisition system (‘DISS 5’; Point Electronic, Halle, Germany). Image processing was carried out with standard image processing software (Paint Shop Pro X8, Corel GmbH, München, Germany). Material contrast colour images were generated by combining SE and BSE images, reflecting an increasing content of mineral elements (with higher atomic numbers than the main organic elements carbon and oxygen) in yellow to red hues, depending on the BSE contrast. Element colour images were generated by combining SE images with one or two corresponding element mapping images, either Ca, P or Si distribution mappings. Three colours (red, green and blue) were chosen to demonstrate the distribution of the elements Si, Ca and P. The observed distribution of biominerals was plotted onto the phylogeny of the Boraginales given by Luebert et al. (2016).
RESULTS
Overview of trichome mineralization
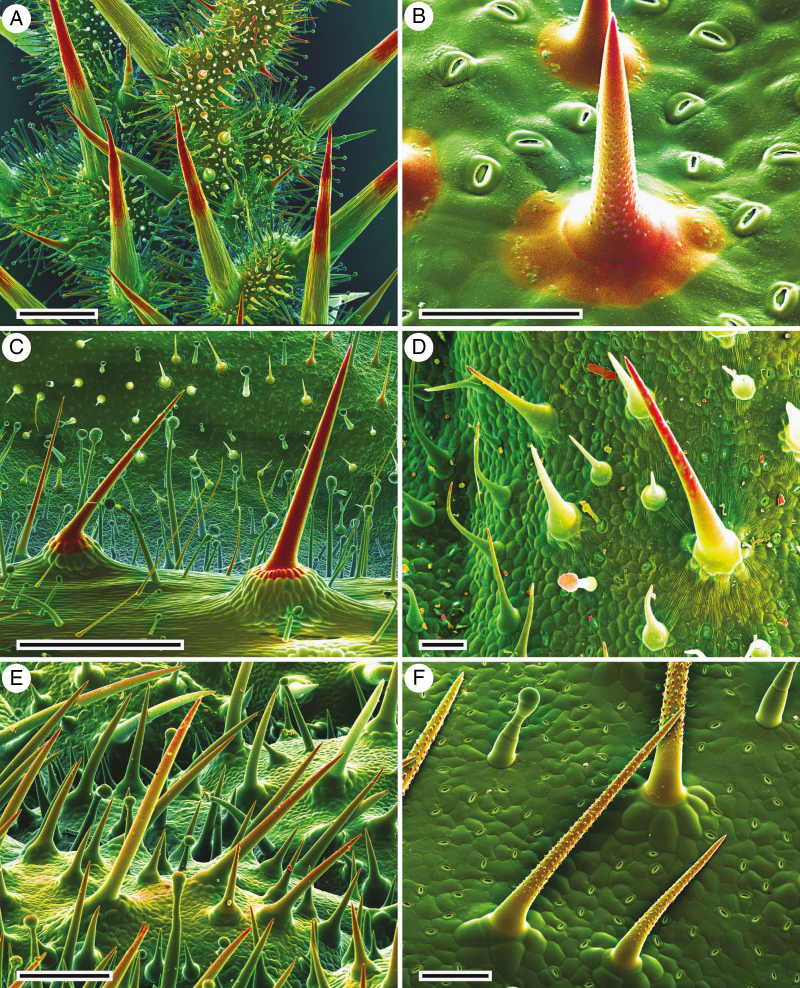
Leaf surfaces of all the investigated taxa are covered by morphologically different types of mineralized trichomes in variable densities and shapes. Figure 1 illustrates typical trichome morphologies and the extent of the mineral deposits in their outer walls. The dual-detector cryo-SEM images show mineralized parts in yellow-to-red colours, reflecting an increasing content of mineral elements such as Ca, P or Si. Many taxa from different families of the Boraginales have small non-mineralized glandular trichomes with a rounded apical cell and usually larger, eglandular, unicellular, mineralized trichomes with sharp tips (Fig. 1A–F). The mineralized trichomes generally consist of a single trichome cell, usually basally surrounded by a ring of foot cells. In most of the species examined the pluricellular bases are non-mineralized, but in some species they are found to be partially mineralized [e.g. Onosma alborosea and Phacelia malvifoliva, Fig. 1B, C)], sometimes extensively so. The foot cells may form a pedestal carrying the large trichome cell, with Codon royenii (Fig. 1A) representing an extreme case with the unicellular mineralized trichome sitting atop a massive, pluricellular ‘spine’, which is distally heavily mineralized. Several species (e.g. Codon royenii, Phacelia malvifolia, Fig. 1A, C) carry clearly distinguishable trichomes of different sizes: large ones sitting atop a pluricellular pedestal and much smaller ones covering the epidermis without a pedestal and occurring at very high densities. The mineralized trichomes universally have a sharp tip; the trichome surface is sometimes smooth (Codon royenii, Phacelia malvifolia, Nama rothrockii; Fig. 1A, C, D), sometimes grooved (Onosma hispida, Heliotropium corymbosum; Fig. 1B, E) or densely verrucose (Coldenia procumbens; Fig. 1F).
Fig. 1.
Cryo-SEM micrographs of typical trichomes on the leaves of different Boraginales plant families. (A) Codon royenii (Codonaceae); large trichomes consist of a mineralized single apical cell located on a high, pluricellular, distally mineralized pedestal. In between are numerous small, partially mineralized trichomes and non-mineralized glandular trichomes. (B) Onosma hispida (Boraginaceae); stiff unicellular trichomes with a pointed tip, inserted on a ring of indistinct foot cells; mineralization (yellow to red color) extends onto the foot cells. (C) Phacelia malvifolia (Hydrophyllaceae); unicellular trichomes with multicellular base and needle-shaped unicellular, mineralized trichomes inserting on one row of mineralized foot cells, and non-mineralized glandular trichomes are also abundantly represented. (D) Nama rothrockii (Namaceae); sharp-tipped unicellular trichomes with a smooth surface. (E) Heliotropium corymbosum (Heliotropiaceae); unicellular hispid trichomes with granular surface pattern. (F) Coldenia procumbens (Coldeniaceae); spinulose, mineralized trichomes and non-mineralized glandular trichomes. The combined topographical and compositional contrast images show regions with higher mineral concentrations in yellow to red, whereas green indicates the absence of mineral elements. Scale bars: A, C = 1000 µm; B, D, F = 100 µm; E = 300 µm.
Systematic distribution of patterns of biomineralization
Our data clearly document the widespread occurrence of Ca, P and Si, indicating the presence of three different biominerals in the plant trichomes: Ca in the absence of P indicates various inorganic or organic calcium carbonate compounds; plant trichomes usually contain calcium carbonate-based composites with organic material such as cellulose. Here we refer to this phenomenon as biomineralization with calcium carbonate. The simultaneous detection of Ca and P indicates the presence of calcium phosphate – P is always found in combination with Ca in trichome walls and appears to be present in the form of a calcium phosphate-based composite with varying parts of organic matter and calcium carbonate. Si occurs as biomineral silica (SiO2).
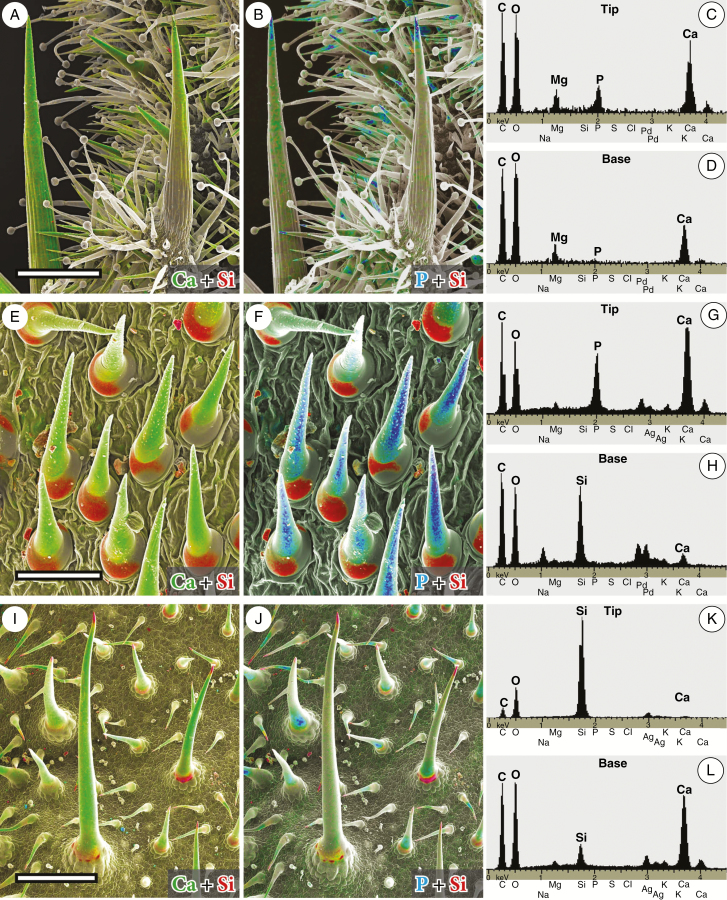
The combined topography and element-mapping images in Fig. 2 show either Ca or P in green colours (Ca: yellow-to-green, or P: green-to-blue, depending on increasing concentration) and Si in red. The colorized combined element distribution images of representative species of three different plant families show remarkable differences in the mineral components, particularly in the tips and in the bases of the trichomes. Ca (green in Fig. 2A, E, I) over the entire shafts of trichomes was found in the majority of the taxa investigated. In contrast, the tips and bases of the trichomes show characteristic mineralization patterns, which can be assigned to three different basic patterns of biomineralization. The first pattern represents calcified trichomes with calcium phosphate tips [e.g. Codon royenii (Fig. 2A, B)] and no silica. The second pattern represents calcified trichomes with calcium phosphate in the trichome tips, whereas silica is dominant in the bases [e.g. Tiquilia cf. elongata (Fig. 2E, F)]. In some cases silica and calcium phosphate are found side-by-side at the trichome bases. The third pattern represents calcified trichomes with silica at high concentrations in their tips and in varying amounts at their bases, sometimes accompanied by calcium phosphate [e.g. Borago officinalis (Fig. 2I, J)].
Fig. 2.
Combined topographic and element-mapping micrographs of critical-point dried leaves of the representative species Codon royenii (Codonaceae, A–D), Tiquilia cf. dichotoma (Ehretiaceae, E–H) and Borago officinalis (Boraginaceae, I–L) showing the various mineralization patterns. Colours identify the distribution of the elements Ca (green) and Si (red) in A, E and I, respectively, and P (blue) and Si (red) in B, F and J, respectively. The EDX spot spectra from the tips and bases of the trichomes illustrate the presence of the mineral elements Si, P and Ca. The large trichomes of Codon contain Ca in the main cell and some adjacent cells of the pedestal, whereas P (calcium phosphate) is restricted to the tip. Si is absent. The trichomes of Tiquilia contain Ca and P in high concentrations in their tips and shafts. The bases contain mostly Si, but also Ca and P in an adjacent area. Borago has a very high concentration of Si in the tips; the bases contain Si and P; the shafts only Ca. The peaks of Ag or Pd in the spectra are due to sputter coating with these metals to avoid charging during SEM analyses. Scale bars: A, B = 500 µm; E, F =100 µm; I, F = 500 µm.
Magnesium (Mg) was frequently found at low and variable concentrations in the trichomes of several plant species of Boraginales (Fig. 2C, D, G, H, L). It occurred together with Ca in the entire trichomes, but the concentration ratio of Mg to Ca was highly variable, arguing against a fixed mineral formation.
Thus, the trichome tips were found to show relatively uniform mineralization patterns across all species examined, containing either silica or calcium phosphate in high concentration. In many cases the occurrence of calcium phosphate or silica in the tips was restricted to a very small region at the apex of the trichomes, but in several species they extended over wider parts of the shafts (e.g. Fig. 2F). In contrast to the trichome tips, the shafts showed rather less characteristic patterns. Calcium carbonate was mostly dominant, but silica and calcium phosphate were also found in varying concentrations. The biomineral patterns of the trichome bases generally differed from those of the shafts, but the variability was much higher than in the trichome tips. In particular, basal regions of young, developing trichomes or partially mineralized trichomes appeared non-mineralized, because mineralization usually starts at the apex and thus the bases are the last part of the trichome to become fully mineralized (Mustafa et al., 2017). EDX spectra of tips and bases of the selected examples illustrate the high mineral concentrations particularly in the tips. A comparison of the height ratios of the P and Ca peaks with those of pure apatite-like calcium phosphate shows that many trichome tips contain (more or less pure) calcium phosphate, whereas lower P/Ca ratios indicate mixed phases containing both calcium phosphate and carbonate. We hesitate to present quantitative data, because the concentrations of the light elements carbon and oxygen cannot be determined with any accuracy with the technique used here: surface topography, detector position, surface coatings such as cutin and metal coating strongly influence the carbon and oxygen measurements.
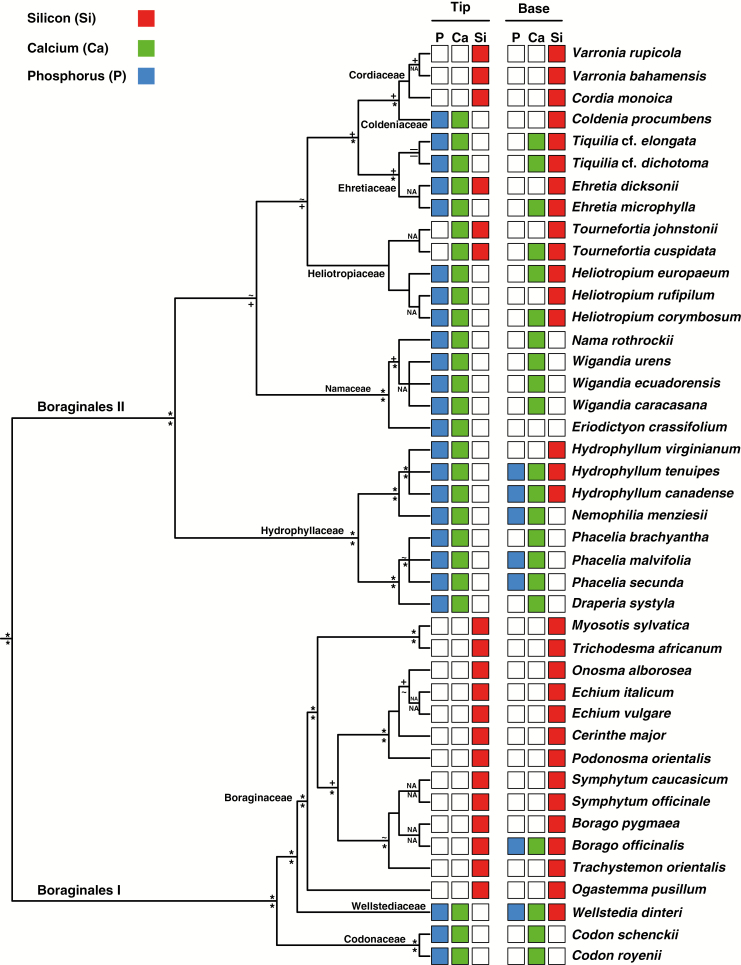
In Fig. 3 the biomineralization data are mapped onto a phylogeny of Boraginales, differentiating between the occurrence of the three biominerals in the tips and basal regions of the species examined so far. It is clear that, in particular, the mineral contents of the trichome tips correlate with the phylogenetic position of the species. In general, the families (with all their species studied so far) can be assigned to one of the mineralization patterns defined above.
Fig. 3.
Phylogeny of the Boraginales with specific trichome zones plotted onto the terminal branches. The selected colours demonstrate the principal distribution of the elements Si (red), Ca (green) and P (blue). The backbone topology is based on Weigend et al. (2014). The topologies of some of the individual families are based on the following publications: Boraginaceae, Chacón et al. (2016); Ehretiaceae, Gottschling et al. (2014); Hydrophyllaceae, Walden et al. (2014); Cordiaceae, Hamilton (2016). Support values are taken from Weigend et al. (2014) and the above cited sources. Symbols above the lines indicate Bayesian posterior probability, while those below the lines indicate maximum-likelihood bootstrap support. Clades with an asterisk (*) are very highly supported groups (Bayesian posterior probability = 1.0, likelihood bootstrap value 100). Clades marked with a (+) are moderately supported (Bayesian posterior probability 0.99–0.9, maximum-likelihood bootstrap values 99–75) in Weigend et al. (2014). Clades with a tilde (~) are weakly supported (Bayesian posterior probability 0.89–0.80, maximum-likelihood bootstrap values 74–50). Clades marked with a minus sign (–) have no support (Bayesian posterior probability <0.80, maximum-likelihood bootstrap values <50), whereas non-assigned support values are shown as ‘NA’.
Based on Fig. 3, trichomes of Pattern 1 (calcium phosphate tips, no silica) occur in 12 out of 42 species. Trichomes with calcium phosphate tips in general (Patterns 1 and 2; trichome bases with or without silica) are reported from seven plant families, namely Codonaceae, Coldeniaceae, Ehretiaceae, Heliotropiaceae, Hydrophyllaceae, Namaceae and Wellstediaceae. In 16 species from two families, Boraginaceae and Cordiaceae, the trichomes tips were strongly mineralized with silica (Pattern 3). In our limited sampling of Heliotropiaceae we found species with silica tips (Tournefortia) and others with calcium phosphate tips (Heliotropium), but our sampling of these large genera appears too small to draw any conclusion at the genus level. Silica, sometimes co-occurring with calcium phosphate, was found in the trichome bases of a wider range of species, including representatives of Boraginaceae, Coldeniaceae, Cordiaceae, Ehretiaceae, Heliotropiaceae and some Hydrophyllaceae. Biominerals in the shafts showed considerable variability within plant families; the main component calcium carbonate was often accompanied by silica or calcium phosphate in varying concentrations. Overall, the data presented indicate a considerable phylogenetic signal in the detailed patterns of biomineralization. Only two of the 42 species had trichomes with aberrant mineral patterns for their respective phylogenetic group: Ehretia microphylla has short and stiff trichomes with pointed tips mineralized with silica and calcium phosphate; Eriodictyon crassifolium has long, thin, partially mineralized trichomes with calcium phosphate in the tips while the basal regions are not mineralized. Overall, both Boraginales I and Boraginales II appear to have clades which are mineralized predominantly or exclusively with calcium carbonate and phosphate, whereas the more derived families in both clades appear to be increasingly mineralized with silica.
Detailed localization of the biominerals
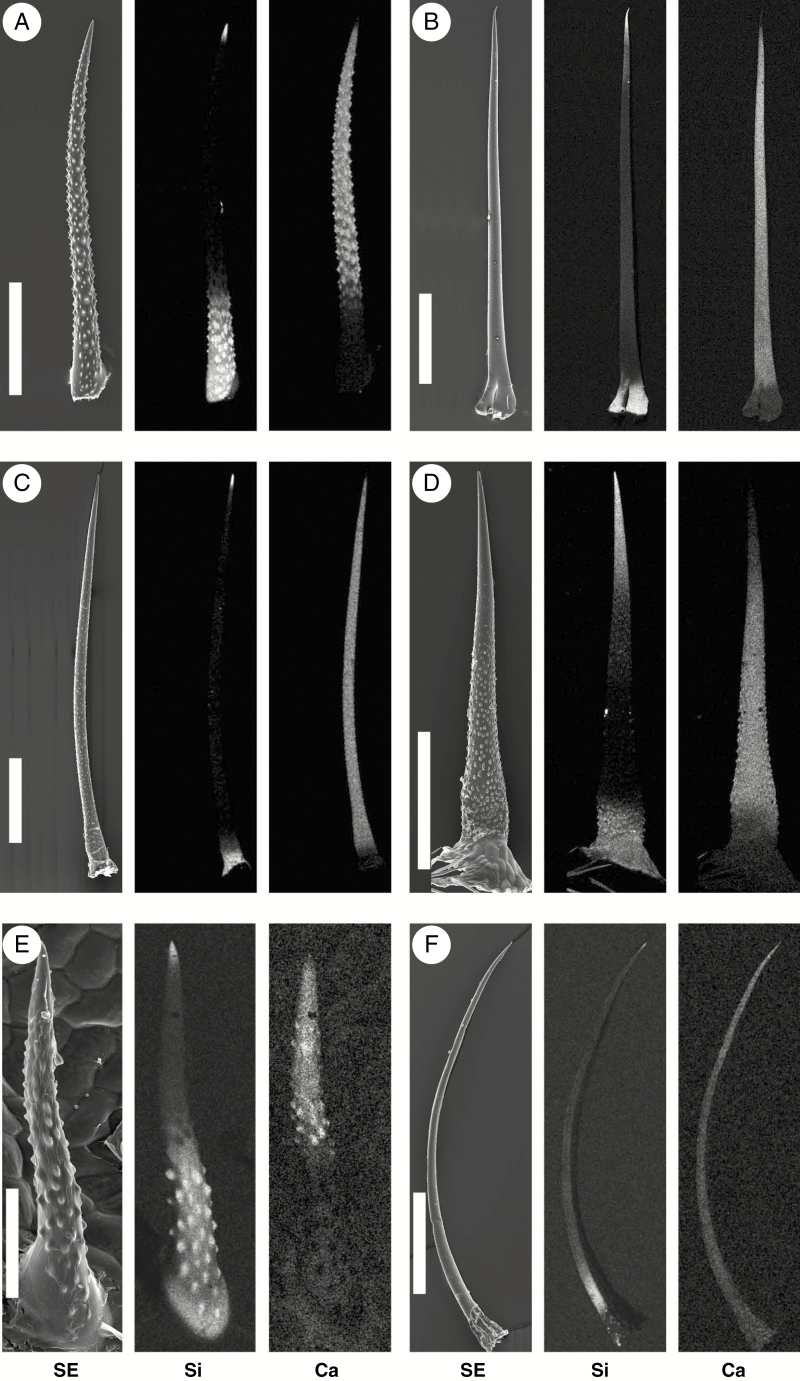
Figure 4 and Supplementary Data Figs S1–3 show some detailed patterns of biomineralization. The colorized images of one or a few representative species of each plant family illustrate the localization of calcium phosphate and silica, represented by the elements P and Si, in isolated single trichomes. Figure 4A–C shows three examples of ‘Pattern 1’ with calcium phosphate (green), but no silica (red). In Codon schenckii and particularly Nama rothrockii, calcium phosphate is limited to the apex (Fig. S1). Separate greyscale mapping images are used here, as they are more suitable for illustrating co-localized elements present at low concentrations. In Phacelia malvifolia, calcium phosphate was found along the entire trichome, with elevated concentrations at the base and the apex. The examples of ‘Pattern 2’ (Figs 4D–G and S2) show calcium phosphate in high concentrations in the tips. Three species have silica in the base [Ehretia dicksonii, Heliotropium corymbosum, Coldenia procumbens (Fig. 4E–G)]. One species, Hydrophyllum canadense (Fig. 4D), contains both silica and calcium phosphate in its base, but in two clearly delimited regions. The presence of three biominerals at different locations can also be demonstrated for Wellstedia dinteri (Fig. S3). Calcium is present in all parts of the trichomes (not colorized) except in locations with high Si concentrations.
Fig. 4.
SEM images showing element distribution in the trichomes of representative species of Codonaceae, Hydrophyllaceae, Namaceae, Heliotropiaceae, Ehretiaceae and Coldeniaceae. (A–C) Occurrence of calcium phosphate but no silica. (A) Codon schenckii; high concentrations of calcium phosphate are restricted to the tip regions. (B) Phacelia malvifolia; both tip and base regions are mineralized with high concentrations of calcium phosphate, with lower P concentrations in the shaft. (C) Nama rothrockii; calcium phosphate is restricted to a very short apical region of the trichome tip. (D–G) Calcium phosphate tips and silica in the bases. (D) Hydrophyllum canadense; (E) Heliotropium corymbosum; (F) Ehretia dicksonii; (G) Coldenia procumbens. Combined topographic and EDX element-mapping images show the distribution of P in green and Si in red. Calcium is present in the entire trichomes but is not colorized. Scale bars: A, C–G = 100 µm; B = 250 µm.
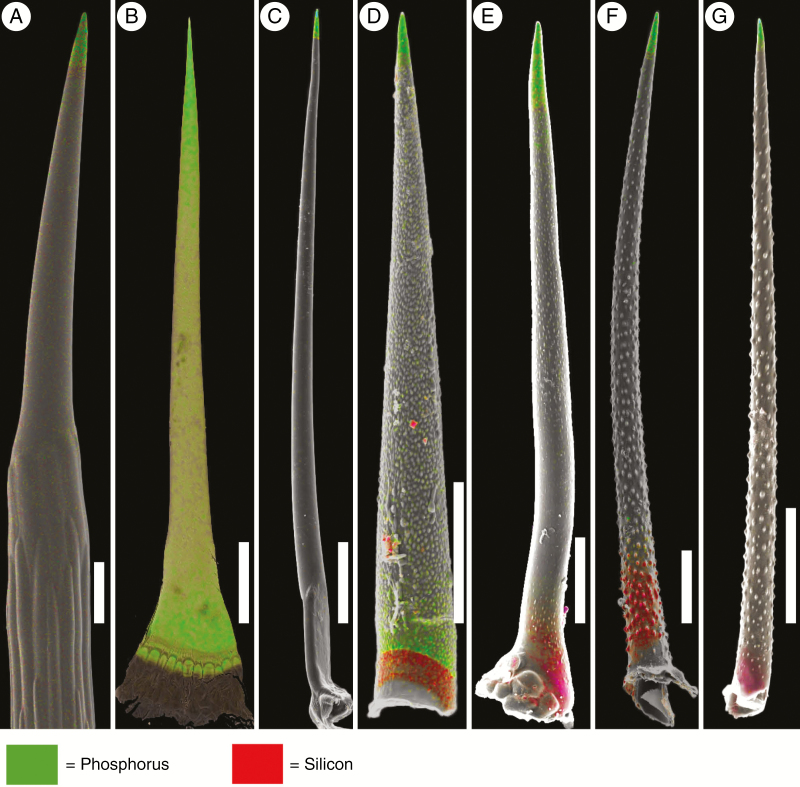
Figure 5 shows details of the element distribution in ‘Pattern 3’ (trichomes with silica tips) in separate greyscale mapping images for Si and Ca. The extent of the silicified regions varies strongly. Small silica deposits in the apex and at the base were found in Echium italicum and Tournefortia cuspidata. In Trichodesma africanum and Varronia bahamensis, the silicified regions are more extensive, whereas the trichomes of Trachystemon orientalis are completely silicified. Trichomes of Borago officinalis show high concentrations of Si in both the base and the apex and lower Si concentrations in the shaft. Ca is found in all parts except regions with high Si concentrations. Sometimes, P occurs at low concentrations, such as in Trichodesma africanum and in Borago officinalis.
Fig. 5.
Topographic (secondary electron, SE) and element-mapping images of trichomes of selected Boraginaceae, Cordiaceae and Heliotropiaceae species with silicified tips. (A) Ogastemma pusillum; (B) Borago officinalis; (C) Echium italicum; (D) Trichodesma africanum; (E) Varronia bahamensis; (F) Tournefortia cuspidata. The distribution of elements appears to be highly structured: The tips and bases of all trichomes are exclusively silicified, but the extent of the silica deposits varies strongly. Ca is present in all parts except the regions with high Si concentration. P was generally absent or present at trace concentrations only and is therefore not shown here. Scale bars: A, E = 200 µm; B–D, F = 500 µm.
DISCUSSION
The present study expands the range of structural biominerals in Boraginales from calcium carbonate and silica to calcium phosphate, recently documented for the first time as a structural plant biomineral in Loasaceae (Cornales: Ensikat et al., 2016). Calcium carbonate is present in the mineralized trichomes of all 42 species investigated, silica in 30 and calcium phosphate in 25; 13 species are mineralized with all three biominerals. Biomineralization is thus varied and complex across the plant order, similar to what has been reported from the family Loasaceae (Ensikat et al., 2017).
Within the individual trichomes, there are clear patterns of biomineral distribution. The tips generally contain a hard biomineral at high concentration, either silica or calcium phosphate. The trichome shafts usually contain calcium carbonate, often with varying portions of silica or calcium phosphate. The bases of fully developed trichomes often contain silica or calcium phosphate at specific locations, together with calcium carbonate. The highly specific localization of silica and calcium phosphate deposits, found in most trichomes, indicates a strict cellular and genetic control of their deposition and, probably, a crucial functional component. The areas generally mineralized with calcium phosphate and silica are those where particular hardness is required. This is true for the apex of the trichomes, which is considered to mechanically damage herbivore skin and which is mineralized with at least one of the two minerals in all 42 species studied. Similarly, the base of the trichome may need to be particularly stiff to ensure that the trichome is not easily broken off and has the required rigidity to perform its defensive role. This portion of the trichome is mineralized with either calcium phosphate and/or silica in 33 of the 42 species studied. Universally, wherever a particularly hard or stiff biomineral is required, either calcium phosphate or silica appear to be employed rather interchangeably across Boraginales. The nine species without calcium phosphate or silica in their base comprise five (genera Codon and Wigandia) with massive, pluricellular pedestals, which evidently require no re-enforcement by biominerals to provide the requisite rigidity. The mineralized trichome bases are usually surrounded by a ring of foot cells. The remaining taxa are species of Nama, Draperia, Eriodictyon and Phacelia with very dense and relatively soft pubescence, probably with a primary role in drought protection rather than herbivore defence. This indicates that biomineralization in the base is reduced in taxa in which organic compounds take over their role due to larger size of the base (pedestal) and/or more massive cell walls, where mechanical rigidity is not a central aspect of trichome functions, because the defence function is secondary.
The detailed patterns of biomineral distribution show some degree of phylogenetic sorting. The early branching families in both major clades of Boraginales appear to be primarily (Namaceae, Hydrophyllaceae) or exclusively (Codonaceae, Wellstediaceae) mineralized with calcium carbonate and phosphate. Conversely, the more derived groups (Broaginales I: Boraginaceae; Boraginales II: Coldeniaceae, Ehretiaceae, Cordiaceae and Heliotropiaceae) show either triple mineralization or silica largely replacing calcium phosphate. This is, to the best of our knowledge, the first time that a clear phylogenetic signal can be documented for patterns of multiple biomineralization. In most of the species investigated the foot cells were non-mineralized, but in some species they were found to be heavily mineralized with silica, i.e. Wellstedia dinteri (Fig. S3) and Onosma hispida. This agrees with a study by Selvi and Bigazzi (2001), demonstrating massive silica deposits in the trichome foot cells of Anchusa formosa and Trachystemon orientalis, indicating that this may be a widespread pattern at least in the corresponding subgroup Boraginaceae subfam. Boraginoideae (Chacón et al., 2016). Selvi and Bigazzi (2001) also mention the presence of phosphorus and sulphur in the tubercles of some species of Nonea, but without elaborating on the exact distribution. Calcium sulphate (gypsum) has been reported as a biomineral in plants, such as in the form of crystals in different plant tissues (Acacia robeorum: He et al., 2012; Caryophyllaceae, Cistaceae and Fabaceae: Palacio et al., 2014). A recent study on leaves collected from 23 gypsophiles and related non-endemic taxa growing on non-gypsum soils demonstrated the presence of gypsum in the leaves of several Chihuahuan Desert gypsum endemics, including the gypsophiles Nama carnosa and Tiquilia hispidissima (Muller et al., 2017) as representatives of the Boraginales. However, no conclusive evidence for structural biomineralization with gypsum has been brought forward, i.e. the incrustation of trichomes or glochidia with calcium phosphate as here demonstrated for calcium phosphate, calcium carbonate and silica. Future studies could investigate the possible presence of gypsum as a structural biomineral in gypsum-endemic species of Boraginaceae such as Cryptantha gypsophila (Reveal and Broome, 2006) and Moltkia gypsacea (Rabizadeh et al., 2017). In general, it seems that biomineralization in Boraginales is more diversified than previously assumed. The complex distribution of biominerals within individual plants and the phylogenetic patterns demonstrated here invite studies on the genetic basis of trichome evolution and mineralization, but also on the differential functionalities of mineralized trichomes across the taxa investigated here.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: EDX mapping of selected trichomes. Figure S2: EDX mapping images of selected trichomes containing calcium phosphate in the tips and silica in the bases; separate greyscale images of the trichomes are shown in Fig. 4D–G. Figure S3: Element distribution in the trichome foot cells of Wellstedia dinteri.
ACKNOWLEDGEMENTS
We are grateful to F. Luebert (Nees Institut für Biodiversität der Pflanzen, BONN), M. Hamilton (Research Leader, UKOTs; Royal Botanical Garden, Kew, UK) and T. Henning (Botanischer Garten und Botanisches Museum Berlin-Dahlem) for organizing the plant specimens. We thank J. Jeiter for helping with collecting plant material, coding the phylogenetic tree and useful suggestions in writing the manuscript. We would like to express our sincere gratitude to T. Joßberger for vouchering the collections and preparing the herbarium specimens.
LITERATURE CITED
- Aleemuddin MA, Karthikeyan M, Rajasekar S. 2011. Coldenia procumbens Linn. – A phytopharmacological review. International Journal of Pharmaceutical Sciences Review and Research 11: 133. [Google Scholar]
- Bickford CP. 2016. Ecophysiology of leaf trichomes. Functional Plant Biology 43: 807–814. [DOI] [PubMed] [Google Scholar]
- Chacón J, Luebert F, Hilger HH et al. . 2016. The borage family (Boraginaceae s. str.): a revised infrafamilial classification based on new phylogenetic evidence, with emphasis on the placement of some enigmatic genera. Taxon 65: 523–546. [Google Scholar]
- Diane N, Jacob C, Hilger HH. 2003. Leaf anatomy and foliar trichomes in Heliotropiaceae and their systematic relevance. Flora 198: 468–485. [Google Scholar]
- Ensikat HJ, Weigend M. 2013. Cryo-scanning electron microscopy of plant samples without metal coating, utilizing bulk conductivity. Microscopy and Analysis 27: 7–10. [Google Scholar]
- Ensikat HJ, Geisler T, Weigend M. 2016. A first report of hydroxylated apatite as structural biomineral in Loasaceae – plants’ teeth against herbivores. Scientific Reports 6: 26073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensikat HJ, Mustafa A, Weigend M. 2017. Complex patterns of multiple biomineralization in single-celled plant trichomes of the Loasaceae. American Journal of Botany 104: 195–206. [DOI] [PubMed] [Google Scholar]
- Gottschling M, Luebert F, Hilger HH, Miller JS. 2014. Molecular delimitations in the Ehretiaceae (Boraginales). Molecular Phylogenetics and Evolution 72: 1–6. [DOI] [PubMed] [Google Scholar]
- Hamilton MA. 2016. Boraginaceae Varronia rupicola (Urb.) Brtton: biogeography, systematic placement and conservation genetics of a threatened species endemic to the Caribbean. PhD thesis, Birkbeck, University of London, UK. [Google Scholar]
- He H, Bleby TM, Veneklaas EJ, Lambers H, Kuo J. 2012. Morphologies and elemental compositions of calcium crystals in phyllodes and branchlets of Acacia robeorum (Leguminosae: Mimosoideae). Annals of Botany 109: 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Veneklaas EJ, Kuo J, Lambers H. 2014. Physiological and ecological significance of biomineralization in plants. Trends in Plant Science 19: 166–174. [DOI] [PubMed] [Google Scholar]
- Hilger HH, Hoppe JR, Hofmann M. 1993. Energiedispersive Röntgenmikroanalyse (EDX) von Boraginaceae subfam. Boraginoideae - Klausenoberflächen. Sind Silicium- und Calcium- Einlagerungen in die Fruchtwand systematisch verwertbare Merkmale?Flora 188: 387–398. [Google Scholar]
- Johnson HB. 1975. Plant pubescence: an ecological perspective. The Botanical Review 41: 233–258. [Google Scholar]
- Jonová M. 1926. Anatomie a morfologie trichomu u Borraginacei s ohledem na systematiku této celedi (L’anatomie et al morphologie des trichomes des Borraginées à l’égard du système de cette famille) [Anatomie und Morphologie der Trichome bei den Borraginaceen mit Rücksicht auf die Systematik dieser Familie]. Vestnik Král. Ceské Spolecn. Nauk, Trida Mathematicko-Prirodovedecka 2: 1–66. [Google Scholar]
- Luebert F, Cecchi L, Frohlich MW et al. . 2016. Familial classification of the Boraginales. Taxon 65: 502–522. [Google Scholar]
- Mehrabian AR, Sheidai M, Mozaffarian V. 2014. Micromorphology of leaf trichomes in Onosma (Boraginaceae) and their systematic relevance in Iran. Phytologia Balcanica 20: 41–56. [Google Scholar]
- Muller CT, Moore MJ, Feder Z, Tiley H, Drenovsky RE. 2017. Phylogenetic patterns of foliar mineral nutrient accumulation among gypsophiles and their relatives in the Chihuahuan Desert. American Journal of Botany 104: 1442–1450. [DOI] [PubMed] [Google Scholar]
- Mustafa A, Ensikat HJ, Weigend M. 2017. Ontogeny and the process of biomineralization in the trichomes of Loasaceae. American Journal of Botany 104: 367–378. [DOI] [PubMed] [Google Scholar]
- Palacio S, Aitkenhead M, Escudero A, Montserrat-Martí G, Maestro M, Robertson AJ. 2014. Gypsophile chemistry unveiled: Fourier transform infrared (FTIR) spectroscopy provides new insight into plant adaptations to gypsum soils. Plos One 9: e107285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabizadeh F, Zare-Maivan H, Kazempour S. 2017. Endemic gypsophytes composition delimitated by soil properties and altitude from calciphytes and halophytes in the south‐central Alborz Ranges. Nordic Journal of Botany, in press. doi:10.1111/njb.01568 [Google Scholar]
- Retief E, van Wyk AE. 2005. Boraginaceae: Codonoideae, a new subfamily based on Codon. Bothalia 35: 78–80. [Google Scholar]
- Reveal JL, Broome CR. 2006. Cryptantha gypsophila (Boraginaceae: Boraginoideae), a new species from western Colorado. Brittonia 58: 178–181. [Google Scholar]
- Reynolds GW, Rodriguez E. 1981. Prenylated hydroquinones: contact allergens from trichomes of Phacelia minor and P. parryi. Phytochemistry 20: 1365–1366. [Google Scholar]
- Reynolds GW, Epstein WL, Rodriguez E. 1986. Unusual contact allergens from plants in the family Hydrophyllaceae. Contact Dermatitis 14: 39–44. [DOI] [PubMed] [Google Scholar]
- Reynolds GW, Gafner F, Rodriguez E. 1989. Contact allergens of an urban shrub Wigandia caracasana. Contact Dermatitis 21: 65–68. [DOI] [PubMed] [Google Scholar]
- Selvi F, Bigazzi M. 2001. Leaf surface and anatomy in Boraginaceae tribe Boragineae with respect to ecology and taxonomy. Flora 196: 269–285. [Google Scholar]
- Szyndler MW, Haynes KF, Potter MF, Corn RM, Loudon C. 2013. Entrapment of bed bugs by leaf trichomes inspires microfabrication of biomimetic surfaces. Journal of the Royal Society Interface 10: 20130174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston EL, Lersten NR. 1969. The morphology and toxicology of plant stinging hairs. The Botanical Review 35: 393–412. [Google Scholar]
- Walden GK, Garrison LM, Spicer GS, Cipriano FW, Patterson R. 2014. Phylogenies and chromosome evolution of Phacelia (Boraginaceae: Hydrophylloideae) inferred from nuclear ribosomal and chloroplast sequence data. Madroño 61: 16–47. [Google Scholar]
- Weigend M, Hilger HH. 2010. Codonaceae – a newly required family name in Boraginales. Phytotaxa 10: 26–30. [Google Scholar]
- Weigend M, Luebert F, Gottschling M, Couvreur TL, Hilger HH, Miller JS. 2014. From capsules to nutlets – phylogenetic relationships in the Boraginales. Cladistics 30: 508–518. [DOI] [PubMed] [Google Scholar]
- Weigend M, Selvi F, Thomas DC, Hilger HH. 2016. Boraginaceae. In: Kubitzki K, ed. The families and genera of vascular plants, vol. 14 Heidelberg: Springer, 41–102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.