Abstract
A recent study shows that spermidine has beneficial effects on health and lifespan in mice, and that these effects are the result of improved cardiovascular function. Similar effects of spermidine on humans are supported by epidemiological studies.
Aging is a major risk factor for chronic diseases, including cardiovascular disease (CVD). In developed countries, the leading causes of death are ischemic heart disease and stroke, and their incidence increases exponentially with age. Therapeutic approaches developed in the past three decades for CVD have been focused on interventions that target particular risk factors, such as high cholesterol levels and high blood pressure, rather than targeting the underlying mechanisms for the development of CVD. As the heart ages, there is a progressive decline in cardiac structure and function that is characterized by hypertrophy, increases in collagen, decreases in diastolic filling rates and impaired left ventricular function. The underlying molecular mechanisms of cardiac aging involve altered macromolecular composition, mitochondrial dysfunction, increased oxidative stress, loss of proteostasis and declines in autophagy—a mechanism of cellular repair—among others. In this issue of Nature Medicine, Eisenberg et al.1 report that they delivered the polyamine spermidine as a dietary supplement to laboratory animals and achieved striking results, including a decrease in cardiac hypertrophy and a delay in the onset of diastolic dysfunction with aging. Furthermore, life-long supplementation of mice with spermidine led to substantial increases in median lifespan, which was also observed when implementation occurred late in life (Fig. 1).
Figure 1.
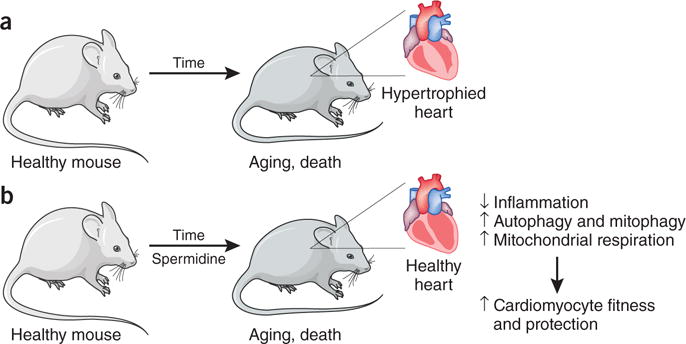
The effects of spermidine on aging and cardiovascular health. (a) Aging increases the risk of cardiovascular disease, which manifests as increased hypertrophy and decreased diastolic function. (b) Eisenberg et al.1 show that dietary supplementation of mice with spermidine decreases the risk of CVD and death upon aging via an increase in autophagy, which results in increased cardiomyocyte fitness.
Spermidine is a polyamine that is present in our diet and is essential for the proper function of many metabolic processes. Polyamines are involved in nearly every cellular function, including the activation of autophagy, DNA stability, transcription, translation and apoptosis, among others2. In their study, Eisenberg et al.1 expand their previous observations in yeast, flies and worms, in which they showed that lifespan and health span can be extended through the induction of autophagy by spermidine3. Spermidine also delayed both age-associated memory loss and motor impairment in flies. Furthermore, it has been shown that autophagy is required for the prevention of cardiomyopathy associated with age4. As of now, calorie restriction is the most robust non-genetic intervention known to extend lifespan in invertebrates and mammals, which it does by improving mitochondrial respiratory efficiency and delaying age-associated diseases. Different natural compounds considered to be calorie-restriction mimetics, such as resveratrol, rapamycin, acarbose and metformin, can also increase health span in mammals5.
Here Eisenberg et al.1 show that dietary supplementation of spermidine given to wild-type mice leads to an extension of lifespan that is associated with a decrease in cardiac hypertrophy and a delay of age-induced diastolic dysfunction. The authors also elegantly demonstrate that spermidine confers protection of the elasticity and mechanosensitivity function of cardiomyocytes, at least in part, through structural preservation of the cytoskeleton and the myofibrils, attenuation of inflammation and preservation of mitochondrial respiration, all of which are essential for the maintenance of cardiac health during aging6. The authors further show that the treatment of old male and female mice with spermidine late in life is effective at extending their lifespans. To understand the mechanism by which spermidine improves diastolic function and thus delays cardiac aging, they studied hypertension-induced cardiac hypertrophy and remodeling and heart-failure progression in the salt-sensitive rat strain (Dahl) fed a high-salt diet (a model for human hypertension-induced congestive heart failure), and further demonstrated the cardioprotective functions of spermidine in this model.
Importantly, through pharmacological means and the use of transgenic mice, the authors were able to show that dietary supplementation of spermidine leads to an upregulation of autophagy—indicated by the presence of enhanced autophagosomes and autolysosomes (and, importantly, enhanced autophagic flux)—and a substantial increase of mitophagy in cardiomyocytes of young and old mice treated with spermidine. The cardioprotective effects of spermidine were dependent on Atg5, a critical regulator of autophagy. They also found in the Dahl rat evidence for a role of autophagy in the cardioprotective effects of spermidine in these rats.
Resveratrol and spermidine increase autophagy through a similar mechanism7, in parallel with a reduction in inflammation and oxidative stress that improves cardiovascular protection8. Because calorie restriction and its mimetics (for example, resveratrol) confer substantial health benefits to aging animals, it could be suggested that spermidine might be another calorie-restriction mimetic4. Notably, using data from a prospective population-based cohort, these authors1 also show that humans given high levels of dietary spermidine had lower protein markers of cardiac failure, a finding that is in agreement with the benefits observed in animal models.
A key question for the future is whether the effects of spermidine on CVD and longevity are translatable to humans. Joining a growing list of compounds that have been shown to extend lifespan or health span in rodents (for example, metformin, resveratrol, rapamycin and acarbose), spermidine is now a leading candidate for treating the underlying mechanisms of age-related CVD in humans9. Unlike other compounds, there are no side effects known for spermidine. In fact, spermidine is present in many foods in our diet, including beans, fermented cheeses, meats, fruits, vegetables and mushrooms. There is evidence that the levels of spermidine in humans decrease with age, and that a diet rich in spermidine is able to increase blood and tissue spermidine levels10. Thus, it could be relatively easy for most people to get the benefits of spermidine through dietary modifications or by supplementation, and thus validate its potential as a new therapeutic approach for cardioprotection against aging, particularly in those with diastolic heart failure. Eisenberg et al.1 have clearly demonstrated the beneficial effects of spermidine in cardiac protection during aging, including humans, by preserving mitochondrial function and turnover and sustained autophagy in cardiomyocytes. These properties of spermidine open up new avenues for the treatment of diastolic dysfunction and protection against age-associated cardiac failure.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Rafael de Cabo, National Institute on Aging, US National Institutes of Health, Baltimore, Maryland, USA.
Pácido Navas, Centro Andaluz de Biología del Desarrollo, CABD-CSIC, CIBERER, Instituto Carlos III, Universidad Pablo de Olavide, Sevilla, Spain.
References
- 1.Eisenberg T, et al. Nat Med. 2016;22:1428–1438. doi: 10.1038/nm.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pegg AE. J Biol Chem. 2016;291:14904–14912. doi: 10.1074/jbc.R116.731661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenberg T, et al. Nat. Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 4.Taneike M, et al. Autophagy. 2010;6:600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 5.de Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN, Madeo F. Cell. 2014;157:1515–1526. doi: 10.1016/j.cell.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López-Otín C, Galluzzi L, Freije JM, Madeo F, Kroemer G. Cell. 2016;166:802–821. doi: 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Morselli E, et al. J Cell Biol. 2011;192:615–629. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz M, Degens H, Vanhees L, Austin C, Azzawi M. Exp Gerontol. 2016;85:41–47. doi: 10.1016/j.exger.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Minois N. Gerontology. 2014;60:319–326. doi: 10.1159/000356748. [DOI] [PubMed] [Google Scholar]
- 10.Soda K, et al. J Nutr Sci Vitaminol (Tokyo) 2009;55:361–366. doi: 10.3177/jnsv.55.361. [DOI] [PubMed] [Google Scholar]


