Abstract
microRNAs are small non-coding RNA molecules involved in the regulation of gene expression and play critical roles in human malignancies. Next generation sequencing analysis of the MCF-7 breast cancer cell line overexpressing miR-335-5p and miR-335-3p demonstrated that the miRNA duplex repressed genes involved in the ERα signaling pathway, and enhanced resistance of MCF-7 cells to the growth inhibitory effects of tamoxifen. These data suggest that despite its conventional role in tumor suppression, the miR-335 transcript can also play an oncogenic role in promoting agonistic estrogen signaling in a cancerous setting.
Keywords: estrogen receptor, isoform, microRNA, miRNA-335, breast cancer, endocrine resistance
Introduction
Breast cancer is a multifaceted disease that comprises tumor subgroups with considerable differences in biology, clinical behavior, and treatment, making it one of the most common causes of death in US women [1]. Classifications of breast cancer including luminal A, luminal B, triple negative/basal-like, and V-Erb-B2 Erythroblastic Leukemia Viral Oncogene Homolog 2 (HER2) amplified are based on molecular characteristics of estrogen receptor (ERα), progesterone receptor (PGR), and HER2/neu [2], with each displaying diverse clinical outcomes and responses to therapeutics. Among the subtypes, ERα+ hormone responsive breast cancers rely on estrogen stimulation to maintain tumorigenesis [3], account for approximately 70% of all newly diagnosed cases, and comprise the luminal A and luminal B cohorts. Although the majority of patients with ERα+ breast cancer benefit from endocrine therapies targeting ERα (tamoxifen, fulvestrant/faslodex) or obstruction of estrogen biosynthesis (aromatase inhibitors or AIs) [3–5], these therapeutics are ineffective in approximately 40–50% of patients where resistance pathways remain unknown [3–6]. Endocrine resistance, attained through loss of either ERα expression or function, occurs through multiple mechanisms including methylation, promoter inhibition, and most recently microRNAs (miRNAs) regulation.
miRNAs are small non-coding RNA molecules involved in the regulation of gene expression at the level of translation. Conventional miRNA biogenesis pathways consist of one strand of a miRNA duplex preferentially selected for entry into RNA-induced silencing complex (RISC) for gene silencing, while the other strand is degraded [7, 8]. There are many miRNAs which have been demonstrated to regulate ERα through miRNA binding to the 3′UTR of ERα mRNA and inducing downstream gene silencing [9–12]. Previous studies demonstrate both conventionally believed tumor suppressive and oncogenic miRNAs can target ERα, elucidating variable effects on the breast cancer phenotype and clinical prognosis. For example, miR-221/222 induces resistance to endocrine therapies and metastasis, and miR-206 induces an anti-proliferative effect, despite elevated levels of miR-206 in ERα− breast cancer cell lines [12–14]. Further exploration of miRNA pathways is essential for understanding their physiological role and their implications associated with the regulation of breast cancer. Here we describe the effects of miR-335, a conventional tumor suppressive miRNA which targets ERα and alters the endocrine response in ERα+ breast cancer cell lines.
Materials and Methods
Cells and reagents
MCF-7 human breast cancer cell line was purchased from American Type Culture Collection (Manassas, VA). The MCF-7-TR and MCF-7-FR cell lines were generated as previously described [15]. Cells lines were cultured as previously described [16]. Liquid nitrogen stocks were made upon receipt and maintained until the start of study. ERE–luciferase and/or qPCR for ERα and PGR were used to confirm MCF-7 sustained estrogen responsiveness. Morphology and doubling times were also recorded regularly to ensure maintenance of phenotype for all cell lines. Cells were used for no more than 6 months in culture. Cells were maintained in 10% FBS DMEM as previously described [17]. MCF-7 parental cells were thawed at passage 65 and were not used past passage 80. MCF-7-miR-335 cell line was used at passage 9 to passage 18. ICI 182,780 was purchased from Tocris Bioscience, 4hydroxy (4OH) tamoxifen was purchased from (Calbiochem, Damstadt, Germany), and 1 nM 17β-estradiol (E2) was purchased from Sigma (Sigma-Aldrich, St. Louis, MO).
Transfection of MCF-7 Cell Line
Parental MCF-7 cell line (passage 65) was stably transfected with pLEMIR-RFP-vector or pLEMIR-RFP-pre-miR-335 (Open Biosystems) with Lipofectamine 2000 per manufacturer’s protocol (Invitrogen, Grand Isles, NY). Parental MCF-7 cells were grown in 100mm dishes. Plasmid (5μg) was added to 100μl serum free opti-MEM followed by 15ul Lipofectamine. After 30-minute incubation, opti-MEM containing plasmid was added. The following day pLEMIR transfected cells were treated with 300ng/ml puromycin. Cells were grown in 10% DMEM and treated with 300ng/ml puromycin (pLEMIR) every two days for 2 weeks. Colonies were pooled and verification of miR-335 overexpression was confirmed using qPCR. Stable pools of transfected cells were maintained in 10% DMEM as described above and were not used beyond passage 18.
Crystal Violet Assay
MCF-7-vector and MCF-7-miR-335 cells were grown in 5% phenol free DMEM for 24 hours and then plated in 24 well plates (7,500 cells per well) for 24 hours prior to pre-treatment with 100nM ICI 182,780 or 100nM 4OH tamoxifen followed by a one-time treatment with 1nM E2 or DMSO. After 72 hours, cells were washed once with PBS and fixed and stained using 0.1% crystal violet (in 20% methanol) for 10 minutes. Cells were washed with water and lysed with 1% SDS. Absorbance at wavelength 570 was determined using a Gene5 plate reader. To account for variance in plating and cell adhesion between cell lines, each cell line was normalized to its respective DMSO treated group designated as 100.
RNA Extraction and Quantitative Real Time RT-PCR
MCF-7-pLEMIR-vector and MCF-7-pLEMIR-MCF7-335 cells were harvested for total RNA extraction using Qiagen RNeasy (Qiagen). Quantity and quality of the RNA was determined by absorbance at 260 and 280 nm using the NanoDrop ND-1000. Total RNA (1ug) was reverse-transcribed using the iScript kit (BioRad Laboratories, Hercules, CA) and qPCR was performed using SYBR-green and 300ng cDNA (Bio-Rad Laboratories, Hercules, CA). β-Actin, PGR, ERα, and ERα-36 genes were amplified n=3. ERα forward GGCATGGTGGAGATCTTCGA, ERα reverse CCTCTCCCTGCAGATTCATCA, ERα-36 forward CAAGTGGTTTCCTCGTGTCTAAAGC, ERα-36 reverse TGTTGAGTGTTGGTTCCAGG, PGR forward TACCCGCCCTATCTCAACTACC, PGR reverse TGCTTCATCCCCACAGATTAAACA, β-actin forward TGAGCGCGGCTACAGCTT, β-actin reverse CCTTAATGTCACACACGATT. Data was analyzed by comparing relative target gene expression to β-actin. Relative gene expression was analyzed using 2-ΔΔCt method. qPCR for miRNA was as follows, total RNA was extracted using the Qiagen miRNeasy kit (Qiagen, Valencia, CA) as per manufacturers protocol and small RNA fraction was not selected for. 1.5 ug of total RNA was reverse transcribed using the Qiagen miScript II kit and qPCR was performed using miScript SYBR green and primers for pre-mir-335, miR-335-5p, miR-335-3p and U6 purchased from Qiagen. Normalization was to U6.
RNA-Sequencing Analysis
RNA-sequencing was performed as in our previously described methods for read preparation, repeat masking, and read mapping. [31]. Edge R software was used to determine differential gene expression [version 2.6.0] where the raw gene counts where input [33]. The estimatecommonDisp and estimateTagwiseDisp methods were used to estimate dispersion [33,34] and a prior.n value = 10 was used for running estimateTagwiseDisp. Pathway analysis was performed using the curated data base provided by NCI-Nature Pathway Interaction Database (analysis performed 2014) [35].
Data Sources
The Cancer Genome Atlas (TCGA) research network breast cancer gene expression data (RNA-seq deep sequencing data) was viewed through the University California Santa Cruz (UCSC) Cancer Genomics Browser [18–20]. The breast invasive carcinoma TCGA data set (total of n=1032 tumor samples) was used and analyzed for gene expression aligned through the IlluminaHiSeq system. Gene signatures were based on receptor status (ERα, PGR, and HER2) and molecular subtype (Luminal A, Luminal B, HER2-enriched, and basal-like).
Western Blot
MCF-7-vector and MCF-7-miR-335 cells were grown in 10% FBS DMEM. Cells were washed with phosphate-buffered saline (PBS) and lysed with M-Per lysis buffer supplemented with 1% protease inhibitor and 1% phosphatase inhibitors (I/II) (Invitrogen). Supernatant containing protein extracts was obtained through centrifugation at 12,000 rpm (5415, Eppendorf, Westbury, NY, USA) for 10 min at 4 °C. Protein extracted per sample was determined by absorbance at 260 and 280 nm. Proteins were heat denatured and loaded on Bis-Tris-nuPAGE gel (Invitrogen). Protein transfer to nitrocellulose through iBlot and iBlot transfer stacks was per the manufacturer’s protocol (Invitrogen). Nonspecific binding was blocked by incubation in 3% milk (in 1% Tris buffered saline-Tween (TBS-T)) for 1 h. Overnight incubation of membrane with primary antibody for ERα (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:400 at 4 °C followed by 3 × 15 min washes in 1% TBS-T. Membranes were incubated for 1 h in secondary antibody 1:10,000 dilution (LiCor Bioscience, Lincoln, NE, USA) followed by 3 × 10 min washes in 1% TBS-T. Band density was determined by LiCor gel imager. Normalization was to Rho GDI-α (Santa Cruz Biotechnology).
Results
miR-335-3p and miR-335-5p are Ubiquitously Expressed Across Breast Cancer Subtypes
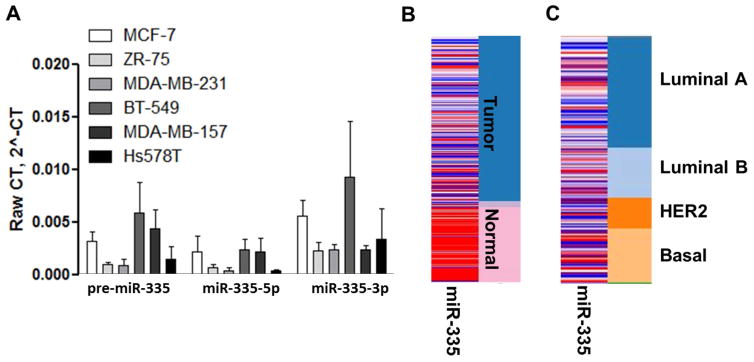
miRNAs can directly target ERα and induce an endocrine resistant advanced phenotype. miR-335-5p, while conventionally described as a tumor suppressor, represses ERα in breast cancer cells and is induced by estrogen stimulation, suggesting it may be involved in altered estrogen signaling [21]. To gain greater insight into the effects of the miR-335 transcript on estrogen signaling we performed qRT-PCR to determine basal expression levels of pre-miR-335, miR-335-5p, and miR-335-3p across a series of breast cancer cell lines (ERα+ and ER−). miR-335-3p was included in our analysis due to recent evidence suggesting a functional role for both strands of the miRNA duplex [22]. qRT-PCR results demonstrated that both miR-335-5p and miR-335-3p were robustly expressed across all breast cancer cell lines regardless of receptor status, with expression at physiologically relevant levels (Figure 1A). There was no correlation with pre-miR-335, miR-335-5p or miR-335-3p to a specific breast cancer subtype. To determine if the observed enhanced expression of miR-335 in cell lines translated to a similar expression in clinical breast tumor samples, The Cancer Genome Atlas (TCGA) was used to determine expression levels of miR-335 across subsets of breast tumor samples. miR-335 was observed to have overall higher expression levels in normal tissue compared to that of tumor tissue (Figure 1B), consistent with previous studies identifying miR-335 as a tumor suppressor. However, in evaluation of clinical tumor samples, miR-335 was observed to be expressed in all tumor subtypes with no indication of a subtype specific expression mechanism (Figure 1C).
Figure 1. miR-335-5p and miR-335-3p are Ubiquitously Expressed Across Breast Cancer Subtypes.
(A) A cohort of ER+ (MCF-7, ZR-75) and ER- (MDA-MB-231, BT-549, MDA-MB-157, Hs578t) breast cancer cell lines were tested by qRT-PCR to determine basal miR-335-5p and miR-335-3p expression. U6 was used for internal normalization and results represent raw gene expression. Error bars represent SEM. (B-C) miRNA deep sequencing of TCGA breast cancer samples was analyzed for miR-335 expression in correlation with (B) tumor tissue versus normal tissue and (C) tumor subtype. Red = high miRNA expression levels and blue = low miRNA expression. Orange = positive expression and blue = negative expression for tumor subtypes (Luminal A, Luminal B, HER2, Basal).
miR-335-3p and miR-335-5p Dual Target mRNAs
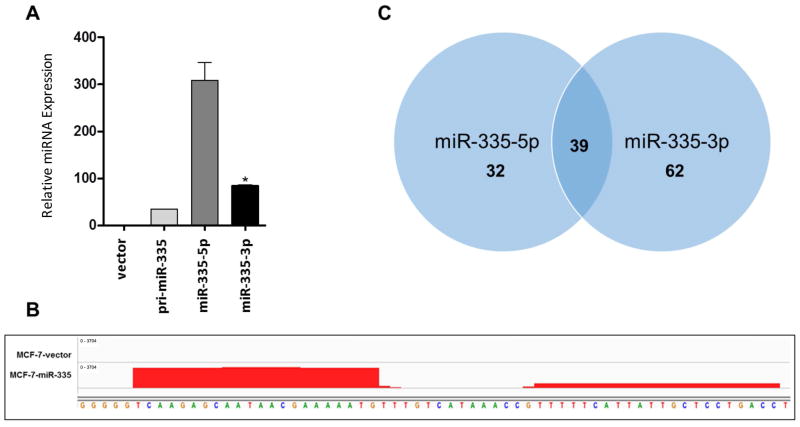
Since both strands of the miR-335 duplex were observed to be expressed across breast cancer cell lines without apparent correlation with clinical tumor subtypes, we next determined the effects of forced expression of the miR-335 transcript on an ERα+ breast cancer cell line. The parental MCF-7 cell line was stably transfected with a pre-miR-335 expression vector, and qPCR was used to confirm overexpression of both miR-335-5p and miR-335-3p in the MCF-7-miR-335 cell line (Figure 2A). Next generation sequencing was performed on the MCF-7-miR-335 cell line and vector transfected cells to determine the overall changes in miR-335 induced gene expression. As viewed in Integrative Genomics Viewer (IGV), miR-335-5p and miR-335-3p overexpression was observed in the MCF-7-miR-335 cell line but not in the -vector cell line (Figure 2B). Analysis of the miR-335-5p and miR-335-3p transcript in IGV demonstrated that the miR-335 transcript was processed without alterations on either the 3′ or 5′ ends. Our results suggest that conventional miR-335 duplex strands, miR-335-5p and miR-335-3p, are expressed and that normal miRNA/mRNA targeting should occur.
Figure 2. Effects of Forced Expression and Deep Sequencing Analysis of miR-335.
(A) qPCR for expression was performed for –vector vs. pre-mir-335, miR-335-5p and miR-335-3p cell lines. (B) Representation of gene expression for miR-335 expression in –vector vs. –miR-335 cell line viewed in IGV (Interactive Genomics Viewer, https://www.broadinstitute.org/igv/). (C) Venn diagram of all down regulated predicted targets for miR-335-3p and miR-335-5p. * Significantly different p < 0.05
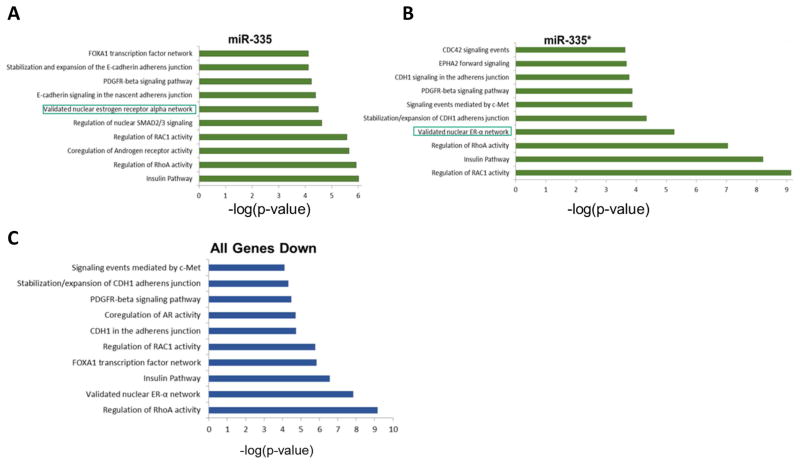
Given that miR-335-5p and miR-335-3p were both overexpressed in our MCF-7-miR-335 cell line and contained intact 3-p and 5-p miRNAs, we next determined if the function of one miRNA strand was more prominent than the other. To evaluate this, we profiled all significantly down-regulated genes from our deep sequencing analysis for seed sites to either miR-335-5p or miR-335-3p independently. Seed sites were determined through our in house program, Seedfinder [23]. Both miRNA strands had similar numbers of overall genes which contained a 3′UTR seed site and similar percentages of gene alteration. A total of 2799 genes contained a seed site for both miR-335-5p and miR-335-3p, and of these genes only 38 were observed to be significantly repressed in our RNA sequencing (FDR < 0.05) (Supplemental Table S1). Furthermore, 32 and 62 genes were observed to be significantly repressed that contained only seed sites for miR-335-5p or miR-335-3p respectively (Figure 2C). Since both miR-335-5p and miR-335-3p exhibited similar numbers of repressed genes, we next performed pathway analysis for significantly down regulated genes that contained a seed site for either miR-335-5p or miR-335-3p. Pathway analysis of genes repressed by miR-335-5p and miR-335-3p suggested that there was an overlap in target genes as well as pathways (Figure 3A and 3B). Furthermore, pathway analysis demonstrated that based on repressed gene sets, both miR-335-5p and miR-335-3p had the potential to alter CDH1 stability, RAC1 activity, PDGFR signaling, and ERα signaling networks. To determine the global effect of the miR-335 construct on the MCF-7 breast cancer phenotype, we next determined the overall top down-regulated pathways as observed in our RNA sequencing, irrespective of genes possessing a miR-335-5p or miR-335-3p seed site (Figure 3C). Our results indicated that the ERα signaling pathway was one of the top down-regulated pathways.
Figure 3. Pathway Analysis of Genes Repressed by miR-335-5p and miR-335-3p.
(A) Pathways associated with repression by miR-335-5p. (B) Pathways associated with repression by miR-335-3p. (C) Down-regulated pathways, irrespective of genes possessing a miR-335-5p or miR-335-3p seed site.
miR-335-3p and miR-335-5p Regulate ERα mRNA Expression
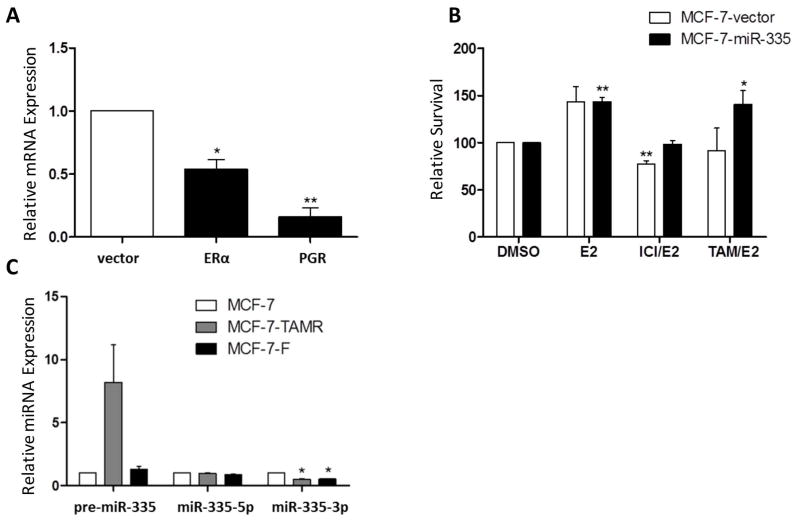
To further investigate the ERα signaling network, we used qPCR analysis to evaluate hormone receptor expression of both ERα and PGR in our MCF-7-miR-335 cell line. Results demonstrated that both ERα and PGR were significantly decreased in the MCF-7-miR-335 cell line compared to the -vector (Figure 4A), in accordance with previous studies [21]. To investigate the effects of the loss of ERα on cellular response to endocrine therapies, the MCF-7-miR-335 cell line was pre-treated with tamoxifen (4OH) or ICI 182,780 (ICI) for 30 minutes prior to stimulation with E2 and analyzed for cell survival using the crystal violet assay. Compared to anti-estrogen-sensitive MCF7 cells, tamoxifen pre-treatment significantly increased MCF-7-miR-335 cell proliferation compared to the control MCF-7-vector cell line, ICI 182,780 had no effect (Figure 4B). The loss of inhibition of tamoxifen in the E2/tamoxifen treated group has been observed in previous studies [24]. These data suggest that altered estrogen signaling due to miR-335 resulted in induced agonistic-activity of tamoxifen. To determine if the miR-335 transcript may be facilitating endocrine resistance we next profiled the expression of pre-miR-335, miR-335-5p and miR-335-3p in both MCF-7 tamoxifen resistant and ICI 182,780 resistant cell lines, MCF-7-TR and MCF-7-FR respectively. Evaluation of the miR-335 transcript expression revealed an increase in pre-miR-335 expression in MCF-7-TR cells compared to the parental MCF-7 cell line, in addition to a significant repression of miR-335-3p in both endocrine resistant cell lines. There was no observed change in miR-335-5p expression in either endocrine resistant cell lines compared to the parental MCF-7 cells (Figure 4C). Since there was no correlation with enhanced miR-335 expression and endocrine resistant cell lines, we next determined a mechanism for the loss of response to endocrine therapy in our MCF-7-miR-335 cell line. While ERα contained seed sites to both miR-335-5p and miR-335-3p, it was not observed to be significantly repressed in our next generation sequencing (Supplemental Table S1). However, ERα was significantly repressed by qPCR and has been previously reported to be a direct target of miR-335.
Figure 4. miR-335 is a Regulator of ERα Signaling.
(A) qPCR for ERα and PGR expression in the MCF-7-miR-335 cell line vs. –vector. (B) Crystal Violet assay for cellular survival in the MCF-7-miR-335 cell line vs. -vector following the pretreatment treatment of E2 (1 nM) for 30 minutes followed by treatment with the endocrine therapies ICI 182,780 (100 nM) and 4-OH tamoxifen (100 nM). Normalization is to vehicle treated cell line designated as 100. (C) qPCR for pre-mir-335, miR-335-5p, and miR-335-3p in the MCF-7-parental, 4-OH tamoxifen (TAMR) resistant and ICI 182,780 (F) resistant cell lines. Normalization was to MCF-7-parental and U6. Error bars represent SEM. * p < 0.05, ** p < 0.01.
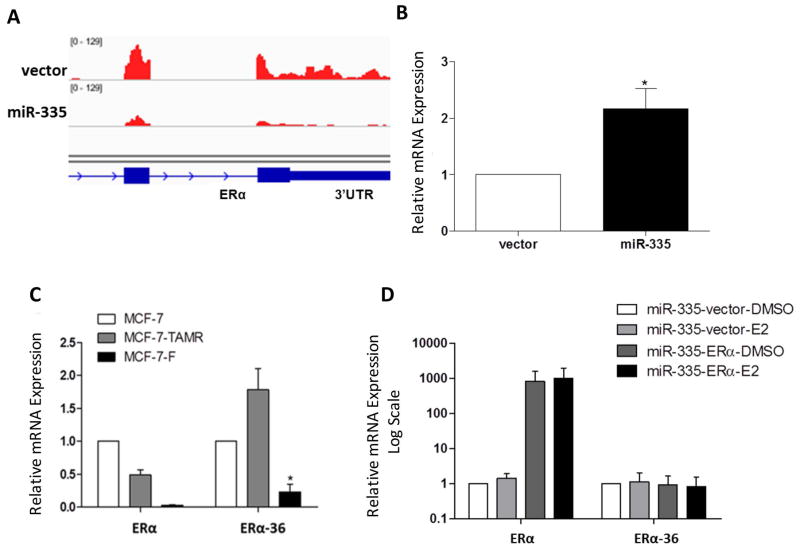
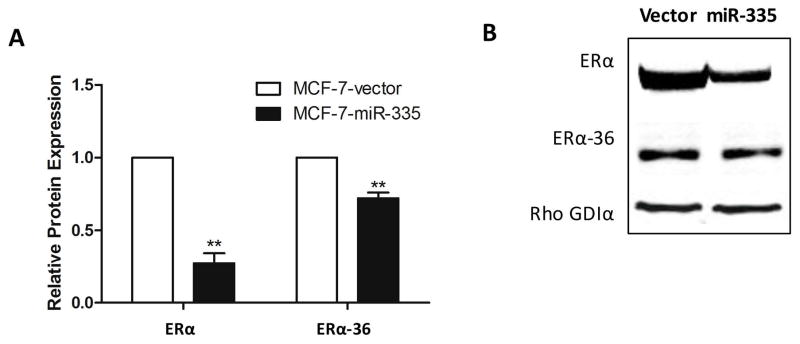
To further evaluate these discrepancies and to fully understand the ERα/miR-335 interplay, we analyzed the 3′UTR of ERα for miR-335-5p and miR-335-3p seed sites using the UCSC Genome Browser and our next generation sequencing data. Although our results demonstrated ERα repression in the MCF-7-miR-335 cell line compared to the -vector, there were differences observed in the expression levels of ERα across the exons with severely repressed expression towards the 3′UTR (Figure 5A). To further investigate, we analyzed truncated isoforms of ERα which do not contain a 3′UTR as conventional ERα isoforms, and thus are not targeted by the same miRNAs. ERα-36 is an isoform which does not possess a 3′UTR with miR-335-5p or miR-335-3p seed sites. Additionally, the ERα-36 variant is known to activate rapid estrogen signaling pathways through MAPK and PI3K/AKT, inducing endocrine resistance [4]. qPCR demonstrated that ERα-36 expression levels were significantly increased in the MCF-7-miR-335 cell line compared to the control vector (Figure 5B) and unlike other ERα variants, ERα-36 has a severely truncated 3′UTR that does not contain a miR-335 seed site (Supplemental Table S2). Furthermore, MCF-7-TR and MCF-7-FR cell lines did not display significantly enhanced expression of ERα-36, which correlated to the lack of miR-335 change in these cell lines (Figure 5C). Full length ERα is known to represses ERα-36 expression, so we next determined if loss of full length ERα expression induced the observed enhanced expression of ERα-36 [25]. We used a truncated ERα without 3′UTR to negate effects of miR-335/ERα targeting. Truncated ERα was transiently transfected in the MCF-7-miR-335 cell line. Following treatment of cells with E2 for 24 hours, there was no observed change in ERα-36 expression in MCF-7-miR-335 cells transiently expressing ERα basally or following E2 stimulation, compared to MCF-7-miR-335-vector transfected cells (Figure 5D). Western blot analysis for ERα and ERα-36 protein expression demonstrated decreased expression of both proteins in the MCF-7-miR-335 cell line versus vector (Figure 6).
Figure 5. miR-335 Alters ER Isoform Expression and Endocrine Resistance.
(A) 3′UTR of ERα for miR-335-5p and miR-335-3p seed sites in –vector vs. –miR-335 cell line viewed in IGV (Interactive Genomics Viewer) (B) qPCR for ERα-36 expression in MCF-7-miR-335 vs. -vector (C) qPCR for ERα and ERα-36 expression in MCF-7-parental, 4-OH tamoxifen (TAMR) resistant and ICI 182,780 (F) resistant cell lines. (D) qPCR for ERα and ERα-36 in MCF-7-miR-335 cells transiently transfected with ERα containing a truncated 3′UTR and +/− E2 for 24 hours. Error bars represent SEM. * p < 0.05.
Figure 6. miR-335 Decreases ERα and ERα-36 Expression.
(A-B) Western blot analysis for ERα and ERα-36 protein expression in the MCF-7-miR-335 cell line versus vector. Normalization was to Rho GDIα. Error bars represent SEM. ** p <0.01. All n = 4 biological replicates.
Given that we observed no enhancement of ERα-36 protein expression in the MCF-7-miR-335 cell line, we next evaluated activity in MAPKs and growth factor mediated genes involved in endocrine resistance pathways. As shown in Table 1, we analyzed MAPK and growth factor mediated genes in our MCF-7-miR-335 sequencing data and observed an increase in select MAPK mediated pathways including p38, p42/44, ERK1/2, and JNK. These data suggest repression of ERα by miR-335 is not the mechanism driving ERα-36 expression and that growth factor mediated resistance may be involved.
Table 1.
Genes Altered by Tamoxifen Treatment and Associated MAPK Cascade
Discussion
Numerous miRNAs have been involved in acquired resistance to endocrine therapies, providing a novel platform for gene regulation that has yet to be been fully explored. Despite a strong correlation between expression of ERα and a favorable response to endocrine therapy, 40–50% of patients with ERα+ breast cancer develop resistance or exhibit de novo resistance [35], and patients with luminal B and ERα+/PGR− breast cancer exhibit a poor response to tamoxifen. The underlying mechanism appears to be deregulation in estrogen receptor signaling pathways [2, 5] due to crosstalk of growth factor signaling pathways such as PI3K/AKT/mTOR and epidermal growth factor receptor (EGFR) crosstalk with ERα signaling to enhance pro-proliferative ERα regulated gene expression and suppression of PGR gene expression [2]. We explored miRNA regulatory pathways interfering with ERα expression and demonstrated a miRNA induced mechanism for endocrine resistance. The forced expression of miR-335 repressed ERα expression in addition to PGR, the classically regulated ERα gene. Furthermore, we demonstrated that not all isoforms of ERα were equally repressed by miR-335, with preferential repression of ERα over the truncated ERα-36 variant observed in the MCF-7-miR-335 cell line. The preferential repression of ERα was associated with increased survival in the MCF-7-miR-335 cell line following co-treatment of E2 and tamoxifen. Additionally, MCF-7 cells which had acquired tamoxifen or ICI resistance did not demonstrate enhanced miR-335 transcript expression or ERα-36 expression. These differences may represent fundamental alterations in signaling between acquired endocrine resistance and de novo resistance. miR-335 is regulated by estrogen stimulation, which suggests a feedback mechanism for the miR-335/ERα axis and a potential mechanism for endocrine resistance. Future studies may be warranted in the investigation of miRNAs such as miR-335 and de novo resistance.
Although we did not observe increased protein expression of ERα-36 in the MCF-7-miR-335 cell line, we suspect increased MAPK expression may be an another mechanism leading to endocrine resistance. Overexpression and activation of growth factor HER2 has been shown to regulate ERα-36 expression through increased AP1 activation [36]. Additionally, activation of growth factor receptors, such as EGFR and IGF-1R, has been shown to drive proliferation and survival through activation of MAPK signaling pathways in endocrine resistant breast cancers [37]. The role of miRNAs associated with endocrine resistance has been explored through the differential expression of miRNAs in tamoxifen-resistant cells [12–14, 38, 39]. Enhanced understanding of resistances to current therapies would elucidate novel methods for intervention. miRNAs that inhibit ERα such as miR-221/222 are strongly associated with resistance to tamoxifen [13, 14] and fulvestrant in basal-like breast cancer [40]. Differential miRNA expression between endocrine-resistant and -sensitive breast cancer cells has also been demonstrated [41]. In another tamoxifen-resistant cell model, miR-375 was identified as one of the top repressed miRNAs, and re-expression of miR-375 resulted in reversal of the tamoxifen-resistant phenotype [39]. miR-342 was also repressed in tamoxifen-resistant cell lines and believed to target genes associated with cell death and cell cycle [38]. While miR-335 has conventionally been described as tumor suppressive, our data demonstrated agonistic estrogen signaling and enhanced proliferation following combined treatment with E2 and tamoxifen. Additionally, TCGA sequencing data demonstrated that despite high expression levels in normal tissue versus breast tumor tissue, miR-335 expression was not correlated with molecular subtype. This was further confirmed in vitro through our analysis of breast cancer cell lines with miR-335-5p, miR-335-3p, and pre-miR-335 expressed uniformly at robust levels across all subtypes. Our results suggest that miRNAs can have both tumor suppressive and oncogenic functions depending on the cellular system. While miR-335 has been conventionally characterized as tumor suppressive in normal/non-diseased tissue, it can promote cancer drug resistance in estrogen responsive tumors. Furthermore, our research demonstrates that miRNA function is dictated in a cell type specific manner and dependent on the cellular transcriptome.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health- CA125806, and CA174785 (BM Collins), The Office of Naval Research N00014-16-1-1136 (ME Burow) and National Center for Research Resources P20RR020152 and The Department of Defense Breast Cancer Research Program BC085426 (BM Collins-Burow) and NCI-U54 CA113001-07 Epigenetic Changes in Cancer Genomes (The Integrative Cancer Biology Program (ICBP): Centers for Cancer Systems Biology (CCSB) (KP Nephew). Supported in part by U54 GM104940 (EC Martin) from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ERα
estrogen receptor
- PGR
progesterone receptor
- HER2/neu
V-Erb-B2 Erythroblastic Leukemia Viral Oncogene Homolog 2
- miRNA
microRNA
- RISC
RNA-induced silencing complex
- EGFR
epidermal growth factor receptor
References
- 1.Group, U.S.C.S.W. United States Cancer Statistics: 1999–2012 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2015. Available from: http://www.cdc.gov/uscs. [Google Scholar]
- 2.Cui X, et al. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005;23(30):7721–35. doi: 10.1200/JCO.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer. 2002;2(2):101–12. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 4.Lin SL, et al. ER-alpha36, a variant of ER-alpha, promotes tamoxifen agonist action in endometrial cancer cells via the MAPK/ERK and PI3K/Akt pathways. PLoS One. 2010;5(2):e9013. doi: 10.1371/journal.pone.0009013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348(24):2431–42. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- 6.Li CI, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol. 2003;21(1):28–34. doi: 10.1200/JCO.2003.03.088. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz DS, et al. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115(2):199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 8.Winter J, et al. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11(3):228–34. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 9.de Souza Rocha Simonini P, et al. Epigenetically deregulated microRNA-375 is involved in a positive feedback loop with estrogen receptor alpha in breast cancer cells. Cancer Res. 2010;70(22):9175–84. doi: 10.1158/0008-5472.CAN-10-1318. [DOI] [PubMed] [Google Scholar]
- 10.Li X, et al. MicroRNA-27a Indirectly Regulates Estrogen Receptor {alpha} Expression and Hormone Responsiveness in MCF-7 Breast Cancer Cells. Endocrinology. 2010;151(6):2462–73. doi: 10.1210/en.2009-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spizzo R, et al. miR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-alpha in human breast cancer cells. Cell Death Differ. 2010;17(2):246–54. doi: 10.1038/cdd.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams BD, Furneaux H, White BA. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-alpha (ERalpha) and represses ERalpha messenger RNA and protein expression in breast cancer cell lines. Mol Endocrinol. 2007;21(5):1132–47. doi: 10.1210/me.2007-0022. [DOI] [PubMed] [Google Scholar]
- 13.Miller TE, et al. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J Biol Chem. 2008;283(44):29897–903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao JJ, et al. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J Biol Chem. 2008;283(45):31079–86. doi: 10.1074/jbc.M806041200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Fan M, et al. Diverse gene expression and DNA methylation profiles correlate with differential adaptation of breast cancer cells to the antiestrogens tamoxifen and fulvestrant. Cancer Res. 2006;66(24):11954–66. doi: 10.1158/0008-5472.CAN-06-1666. [DOI] [PubMed] [Google Scholar]
- 16.Struckhoff AP, et al. Novel ceramide analogs as potential chemotherapeutic agents in breast cancer. J Pharmacol Exp Ther. 2004;309(2):523–32. doi: 10.1124/jpet.103.062760. [DOI] [PubMed] [Google Scholar]
- 17.Salvo VA, et al. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin Cancer Res. 2006;12(23):7159–64. doi: 10.1158/1078-0432.CCR-06-1426. [DOI] [PubMed] [Google Scholar]
- 18.Goldman M, et al. The UCSC Cancer Genomics Browser: update 2013. Nucleic Acids Res. 2013;41(Database issue):D949–54. doi: 10.1093/nar/gks1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanborn JZ, et al. The UCSC Cancer Genomics Browser: update 2011. Nucleic Acids Res. 2011;39(Database issue):D951–9. doi: 10.1093/nar/gkq1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaske CJ, et al. Inference of patient-specific pathway activities from multi-dimensional cancer genomics data using PARADIGM. Bioinformatics. 2010;26(12):i237–45. doi: 10.1093/bioinformatics/btq182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyn H, et al. MicroRNA miR-335 is crucial for the BRCA1 regulatory cascade in breast cancer development. Int J Cancer. 2011;129(12):2797–806. doi: 10.1002/ijc.25962. [DOI] [PubMed] [Google Scholar]
- 22.Rhodes LV, et al. Dual regulation by microRNA-200b-3p and microRNA-200b-5p in the inhibition of epithelial-to-mesenchymal transition in triple-negative breast cancer. Oncotarget. 2015;6(18):16638–52. doi: 10.18632/oncotarget.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin EC, et al. Preferential star strand biogenesis of pre-miR-24-2 targets PKC-alpha and suppresses cell survival in MCF-7 breast cancer cells. Mol Carcinog. 2014;53(1):38–48. doi: 10.1002/mc.21946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin I, Arteaga CL. Expression of active Akt protects against tamoxifen-induced apoptosis in MCF-7 Cells. IUBMB Life. 2006;58(11):664–9. doi: 10.1080/15216540601001681. [DOI] [PubMed] [Google Scholar]
- 25.Zou Y, et al. Estrogen receptor-alpha (ER-alpha) suppresses expression of its variant ER-alpha 36. FEBS Lett. 2009;583(8):1368–74. doi: 10.1016/j.febslet.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moro T, et al. Inhibition of Cdk6 expression through p38 MAP kinase is involved in differentiation of mouse prechondrocyte ATDC5. J Cell Physiol. 2005;204(3):927–33. doi: 10.1002/jcp.20350. [DOI] [PubMed] [Google Scholar]
- 27.Gusterson R, et al. The transcriptional co-activators CBP and p300 are activated via phenylephrine through the p42/p44 MAPK cascade. J Biol Chem. 2002;277(4):2517–24. doi: 10.1074/jbc.M104626200. [DOI] [PubMed] [Google Scholar]
- 28.Svensson S, et al. ERK phosphorylation is linked to VEGFR2 expression and Ets-2 phosphorylation in breast cancer and is associated with tamoxifen treatment resistance and small tumours with good prognosis. Oncogene. 2005;24(27):4370–9. doi: 10.1038/sj.onc.1208626. [DOI] [PubMed] [Google Scholar]
- 29.Jang JW, Boxer RB, Chodosh LA. Isoform-specific ras activation and oncogene dependence during MYC- and Wnt-induced mammary tumorigenesis. Mol Cell Biol. 2006;26(21):8109–21. doi: 10.1128/MCB.00404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salmeron A, et al. Activation of MEK-1 and SEK-1 by Tpl-2 proto-oncoprotein, a novel MAP kinase kinase kinase. EMBO J. 1996;15(4):817–26. [PMC free article] [PubMed] [Google Scholar]
- 31.Tsatsanis C, Patriotis C, Tsichlis PN. Tpl-2 induces IL-2 expression in T-cell lines by triggering multiple signaling pathways that activate NFAT and NF-kappaB. Oncogene. 1998;17(20):2609–18. doi: 10.1038/sj.onc.1202460. [DOI] [PubMed] [Google Scholar]
- 32.Becker E, et al. Nck-interacting Ste20 kinase couples Eph receptors to c-Jun N-terminal kinase and integrin activation. Mol Cell Biol. 2000;20(5):1537–45. doi: 10.1128/mcb.20.5.1537-1545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pombo CM, et al. Activation of the SAPK pathway by the human STE20 homologue germinal centre kinase. Nature. 1995;377(6551):750–4. doi: 10.1038/377750a0. [DOI] [PubMed] [Google Scholar]
- 34.Datta NS, et al. Distinct roles for mitogen-activated protein kinase phosphatase-1 (MKP-1) and ERK-MAPK in PTH1R signaling during osteoblast proliferation and differentiation. Cell Signal. 2010;22(3):457–66. doi: 10.1016/j.cellsig.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Romancer M, et al. Cracking the estrogen receptor’s posttranslational code in breast tumors. Endocr Rev. 2011;32(5):597–622. doi: 10.1210/er.2010-0016. [DOI] [PubMed] [Google Scholar]
- 36.Kang L, et al. A positive cross-regulation of HER2 and ER-alpha36 controls ALDH1 positive breast cancer cells. J Steroid Biochem Mol Biol. 2011;127(3–5):262–8. doi: 10.1016/j.jsbmb.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholson RI, et al. Growth factor signalling in endocrine and anti-growth factor resistant breast cancer. Rev Endocr Metab Disord. 2007;8(3):241–53. doi: 10.1007/s11154-007-9033-5. [DOI] [PubMed] [Google Scholar]
- 38.Cittelly DM, et al. Downregulation of miR-342 is associated with tamoxifen resistant breast tumors. Mol Cancer. 2010;9:317. doi: 10.1186/1476-4598-9-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward A, et al. Re-expression of microRNA-375 reverses both tamoxifen resistance and accompanying EMT-like properties in breast cancer. Oncogene. 2013;32(9):1173–82. doi: 10.1038/onc.2012.128. [DOI] [PubMed] [Google Scholar]
- 40.Rao X, et al. MicroRNA-221/222 confers breast cancer fulvestrant resistance by regulating multiple signaling pathways. Oncogene. 2011;30(9):1082–97. doi: 10.1038/onc.2010.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J, et al. Endocrine resistance in breast cancer: Current status and a perspective on the roles of miRNAs (Review) Oncol Lett. 2013;6(2):295–305. doi: 10.3892/ol.2013.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.