Abstract
The sensory neurons innervating the urinary bladder and distal colon project to similar regions of the central nervous system and often are affected simultaneously by various diseases and disorders, including spinal cord injury. Anatomical and physiological commonalities between the two organs involve the participation of shared spinal-derived pathways, enabling mechanisms of communication between the bladder and colon. Prior electrophysiological data from our lab suggests that the bladder also may receive sensory innervation from a non-spinal source through the vagus nerve, which innervates the distal colon as well. The present study therefore aimed to determine if anatomical evidence exists for vagal innervation of the male rat urinary bladder and assess whether those vagal afferents also innervate the colon. Additionally, the relative contribution to bladder and colon sensory innervation of spinal and vagal sources was determined. Using lipophilic tracers, neurons that innervated the bladder and colon in both the nodose ganglia (NG) and L6/S1 and L1/L2 dorsal root ganglia (DRG) were quantified. Some single vagal and spinal neurons provided dual innervation to both organs. The proportions of NG afferents labeled from the bladder did not differ from spinal afferents labeled from the bladder when considering the collective population of total neurons from either group. Our results demonstrate evidence for vagal innervation of the bladder and colon and suggest that dichotomizing vagal afferents may provide a neural mechanism for cross-talk between the organs.
Keywords: vagus, pelvic, visceral, convergence, RRID: AB_1502299, RRID: AB_2307446
Introduction
Neural innervation of the lower urinary tract to/from the spinal cord includes sensory and somato-motor components as well as a lumbar sympathetic and sacral parasympathetic supply. Furthermore, the micturition reflex requires descending input from central sources located in an area of the pons referred to now as the pelvic organ stimulating center (POSC,) since it also coordinates other pelvic organ eliminative functions (Holstege et al., 1979; Holstege and Kuypers, 1982; Holstege et al., 1986; Blok et al., 1997; Huynh et al., 2013; Beckel and Holstege, 2014). In most species, voiding is initiated when information regarding bladder fullness, conveyed by A-δ fibers, projects to the pelvic organ spinal relay center (POSRC) (Beckel and Holstege, 2011a, 2011b) located near the dorsal horn of the sacral spinal cord, before ascending to the periaqueductal gray (PAG) (VanderHorst et al., 1996; Holstege, 2005; Klop et al., 2005), a main relay center in the midbrain. From there, receiving cues from the brain indicating voiding is appropriate (Blok et al., 1997a; Blok et al. 1998), the PAG activates the POSC to initiate micturition (Beckel and Holstege, 2011b). In addition to spinal and supraspinal derived sources of neural innervation, numerous animal studies have shown that many pelvic viscera are supplied also by a non-spinal vagal component (Burden et al., 1983; Gattone et al., 1986; Jancso and Maggi, 1987; Ortega-Villalobos et al., 1990; Altschuler et al., 1993; Hubscher and Berkley, 1995; Komisaruk et al., 1997; Collins et al., 1999; Ersoz and Akyuz, 2004; Hubscher et al., 2004; Komisaruk et al., 2004). Dual sources of innervation by non-spinal routes that convey sensory information to the brain from visceral organs are likely of high functional significance under pathological conditions including chronic spinal cord injury.
Prior neuroanatomical tracing experiments in normal adult rats have shown that the abdominal branches of the vagus nerve innervate all areas of colon, but not the rectum (Altschuler et al., 1993) and comparative studies in rats receiving a complete spinal cord transection at T8 confirm vagal innervation of the colon (Vizzard et al., 2000). Note that the majority of vagal afferent fibers outnumber efferent fibers by a ratio of 10:1 (Grundy D, 2002). The degree of vagal contribution to the bladder, however, has not previously been determined. Furthermore, examining the possible connection between the bladder and colon through the vagus nerve is important since cross-talk between pelvic organ systems is part of normal visceral functioning and therefore, physiologically important for maintaining homeostasis. For example, urinary bladder micturition and defecation occur alternately through mutually-inhibitory reflexes. Distention of the urinary bladder leads to contractions of the external anal sphincter, preventing defecation and allowing for micturition (Basinski et al., 2003). The opposite is true in the process of allowing for defecation while inhibiting micturition. If both organs are distended, micturition occurs prior to defecation since it is thought that voiding should happen in a timelier manner due to the fact that prolonged urinary retention could irritate the urothelial lining (De Wachter et al., 2007). The vagus nerve also displays important regulatory cross-talk as seen with the baroreceptor reflex, demonstrating its role in maintaining stable blood pressure and heart rate (Dampney, 1994; Spyer, 1994). Injury or trauma leading to disruption with the coordination of any of a number of vagal- or spinal-mediated processes can cause the pathological organ to affect the functionality of the non-injured one through a number of mechanisms: cross-excitatory or dorsal root/axonal reflexes, intraganglionic and interaxonal interactions, axon collateral activation, as well as central sensitization (Malykhina et al., 2004; Pezzone et al., 2005; Kaddumi and Hubscher, 2006; Malykhina et al., 2006; Ustinova et al., 2006; Liang et al., 2007; Malykhina, 2007; Ustinova et al., 2007; Brumovsky and Gebhart, 2010; Pan et al., 2010; Ustinova et al., 2010). Influential effects from one organ to another are evident through convergence of inputs at the primary afferent level as well as at second order neurons and higher integrative centers.
Based on our earlier electrophysiological research on responsiveness of medullary reticular formation neurons to stimulation of abdominal branches of the vagus nerve, we found evidence for non-specific afferent induced plasticity with chemical irritation of the bladder, suggesting that vagal afferents may innervate the lower urinary tract (Kaddumi and Hubscher, 2006; Kaddumi and Hubscher, 2007). We therefore sought to determine, through anatomical tracing, if evidence of a vagal neural connection to the bladder exists and to assess the degree of convergence (i.e. dichotomizing afferents - reflective of the direction of information flow) with the known vagal supply of the distal colon. We hypothesized that there are distinct subsets of NG neurons innervating the bladder or colon, as well as a subset innervating both visceral structures. For comparison, we simultaneously processed and assessed within the same group of animals spinal ganglia (L1/L2 and L6/S1 dorsal root ganglia (DRG)) that are known to innervate both the bladder and colon in the rat. We hypothesized that a greater degree of bladder, colon, and convergent labeling are present in the L6/S1 DRG compared to the NG.
Materials and Methods
Animals
All experimental procedures were carried out according to NIH guidelines and protocols reviewed and approved by the Institutional Animal Use and Care Committee at the University of Louisville School of Medicine. All adult male Wistar rats (n=12, Harlan Sprague Dawley, Inc, Indianapolis, IN), approximately 250 grams in weight, were individually housed in an animal room with a 12-hour light and dark cycle. They had ad libitum access to water and food (Laboratory Rodent Diet).
Injection of retrograde tracers into the urinary bladder and distal colon
One group of adult male Wistar rats (n=8), anesthetized under ketamine (80mg/kg of body weight) and xylazine (10mg/kg), received a ventral/caudal midline peritoneal incision to expose the urinary bladder and distal colon. The bladder was emptied and the abdominal viscera were gently shifted to the side for exposure of the distal colon. Using a protocol previously published (Rau et al., 2007), the fluorescent tracer FAST DiO™ oil (3,3’-dilinoleyloxacarbocyanine perchlorate, 5mg dye dissolved in 0.1ml methanol, Molecular Probes Inc., Eugene, OR) was injected with a dye-dedicated 33-gauge needle coupled to a Hamilton microsyringe (Fisher Scientific, Pittsburgh, PA) into the distal colon wall, followed by a separate dye-dedicated 33-gauge microsyringe injection of FAST DiI™ oil (1,1’-dilinoleyl-3,3,3’,3’,-tetramethylindo-carbocyanine perchlorate, 5mg dye dissolved in 0.1ml methanol, Molecular Probes, Inc., Eugene, OR) into the bladder wall. Note that the abbreviations DiI and DiO are used throughout the text/figures/legends to refer to FAST DiI™ oil and FAST DiO™ oil, individually and respectively, and the term carbocyanine to refer to them collectively. For the distal colon, injections were made 1-3 centimeters rostral to the anus (10μl volume per animal divided into 10 injections of 1 μl each). For the bladder, bilateral injections were made to the trigone and dome (10μl volume per animal divided into 10 injections of 1ul each). Injections were made into the distal colon prior to bladder injections as to avoid organ to organ contact and potential tracer contamination. For tracer comparisons, a set of additional rats (n=2, 4 ganglia) received bladder injections of either tetramethlyrhodamine (TMR) (anionic, lysine-fixable, 3000MW, 2.0% concentration in 0.9% saline, Molecular Probes, Inc., Eugene, OR) or Cholera Toxin Subunit B (CTB)– 594 (0.5% injection, Molecular Probes, Inc., Eugene, OR) and distal colon injections of either Fluorescein Dextran (FD) (anionic, lysine-fixable, 3000MW, 2% concentration in 0.9% saline, Molecular Probes, Inc., Eugene, OR) or CTB–488 (0.5% injection, Molecular Probes, Inc., Eugene, OR). A final subset of rats (n=2, 4 ganglia) also received colon-only injections of DiI, utilizing the same protocol above.
Animal body temperature was maintained at 37-40°C during surgery via a warm water recirculator (Gaymar, Kent Scientific, Winston-Salem, NC). After each injection, the needle was removed slowly; any leakage was controlled by cotton-tipped applicators, and the site was rapidly sealed with n-butyl cyanoacrylate monomer glue (Henkel Consumer Adhesives, Avon, OH). After injections were completed, the intestines were rehydrated with 5% Dextrose Lactated Ringers, the abdominal musculature was sutured closed (Ethicon 4-0 non-absorbable surgical suture), the skin closed with Michel clips (Fine Science Tools, Foster City, CA), and a topical antibiotic (Bacitracin, Actavis Mid Atlantic LLC, Lincolnton, NC) applied. Following surgery, animals were placed on a heating pad and core temperature monitored. They were given subcutaneous injections of ketoprofen (Ketofen, 2.5mg/kg, Fort Dodge Animal Health, Fort Dodge, IA) for analgesia twice a day for 2 days, 0.5ml of dual penicillin (PenJect ®, The Butler Company, Columbus, OH) single dose peri-operatively as a general prophylactic and 5mg/kg gentamicin (GentaFuse®, Butler Schein, Dublin, OH) once per day for 5 days to prevent infections. All animals were monitored daily to inspect the surgical incision and identify any changes in an animal's general condition.
Perfusion and Tissue Collection
Ten days after tracer injection, animals were deeply anesthetized with a ketamine (80mg/kg body weight)/xylazine (10mg/kg) mixture and transcardially exsanguinated with heparinized saline, followed by 4% paraformaldehyde perfusion. Each vagus nerve was identified adjacent to the carotid artery and gently separated from surrounding tissues and traced rostrally to the NG, which was excised using surgical microscissors and removed. Superior cervical ganglia were identified on both sides at the bifurcation of the common carotid artery and removed to be used as control tissue. In the same rats, following a dorsal spinal laminectomy and removal of the overlying dura mater, paired L1/L2 and L6/S1 dorsal root ganglia were dissected free. Upon removal, all NG and L1/L2/L6/S1 ganglia were placed immediately in individually labeled tubes containing a cryoprotectant solution of 30% sucrose/phosphate buffer with 1% sodium azide at 4°C for at least 24 hours. The abdominal cavity and viscera were inspected for potential tracer leakage, followed by the removal of the bladder and distal colon.
Antibody Characterization
The primary antibodies used for signal enhancement of the TMR and FD tracers in this study are described in Table 1 and have been documented previously in numerous tracing studies (Angelucci and Sainsbury, 2006; Saleem et al., 2008; Kowski et al., 2008; McNeal et al., 2010; Borra et al., 2010; deCampo and Fudge, 2013). The antibody against TMR has been analyzed previously for cross-reactivity through the dot-blotting method and was shown not to recognize FD or Cascade Blue dextran amines (Kaneko et al., 1996). As expected, both the polyclonal antibodies against TMR and FD did not recognize cells in control tissue from animals not receiving tracer injections in this study (DRG sections) as well as others (Kaneko et al., 1996; McNeal et al., 2010).
Table 1.
Summary of antibodies used
| Target | Immunogen | Host organism, clonality, manufacturer, catalog No., Research Resource Identifier | Dilution | Specificity controls |
|---|---|---|---|---|
| TMR | 5-carboxy- tetramethylrhodamine | Rabbit, polyclonal, Molecular Probes, Cat# A-6397, RRID: AB_1502299 | 1:6,000 | Tissue type controls did not produce perikarya labeling. |
| FD | Fluorescein isothiocyanate | Goat, polyclonal, Vector Labs, Cat# SP-0601, RRID: AB_2307446 | 1:2,000 | Tissue type controls did not produce perikarya labeling. |
TMR-tetramethylrhodamine, FD-Fluorescein Dextran
Cell Quantification
Ganglia coated in embedding medium were cross-sectioned at 12μm on a Leica CM 1850 cryostat and mounted onto gelatin-coated histological slides (Azer Scientific, Morgantown, PA). In the group that received dextran injections, signal amplification of slide-mounted sections was performed by overnight incubation in primary antisera (Rbt-anti-TMR, Molecular Probes, Inc., Eugene, OR, Cat# A-6397, RRID: AB_1502299 and Goat-anti-FD, Vector Labs, Burlingame, CA, Cat # SP-0601, RRID: AB_2307446 ) (see Table 1). Secondary antisera (Jackson Immuno ResearchLaboratory Inc., West Grove, PA) diluted 1:200 and 1:100, respectively, was applied for both primary antisera (anti-rbt-Texas Red and anti-goat-FITC).
To view the DiI/DiO labeled sections, imaging was performed using the Nikon Eclipse TiE inverted microscope with NIS Elements software. Initially, images were captured using a 10× lens (APO DIC N1 10×/0.45 NA, Nikon) with consistent exposure times and computationally stitched together to visualize whole ganglion sections. Fluorescent imaging utilized a mercury arc lamp filtered through either a Brightline Tritc-B bandpass filter (543/22nm excitation, 593/40nm emission, 562nm dichroic, Semrock) or through a GFP bandpass filter (470/40nm excitation, 525/50nm emission, 495nm dichroic, Nikon). As previously determined, the individual emission maxima of DiI and DiO are easily separated, facilitating two-color labeling, and were therefore selected as our tracers (Honig and Hume, 1986; Godement et.al, 1987; Ragnarson et.al, 1992). Across the entire ganglion, assembled by automated stitching, counts of all singly-, dually- as well as non-labeled (collectively comprising total neuronal counts) NG and DRG neurons with a visible nucleus and definable soma were made using Nikon Elements software in every 5th section (60 microns apart) to avoid double counting. Note that non-labeled cells were quantified if they met the above criteria and did not exhibit any evidence of punctate fluorescence distributed within the cytoplasm, disqualifying them as bladder-, colon- or convergent-specific neurons. Counts for regional differences (rostral, middle and caudal divisions) of the NG were determined using an anatomical division of the NG made by a laryngeal branch of the vagus nerve as described previously (Bielefeldt et al., 2006b). Positive neuron counts were expressed as a percentage of the total number of neurons (labeled and non-labeled) from within the entire stitched ganglion as well as a percentage of all labeled neurons. A Nikon Eclipse 90i confocal microscope (Plan Apo VC DIC N2 oil 60×, 1.4 NA, 488nm excitation/515 emission, 561nm excitation/605nm emission, 1μm step size, 13 passes) with EZ-C1 3.60 software and a Nikon A1R MP+ confocal microscope (Plan Apo λ 20x, 0.75 NA, 488nm excitation/525nm emission, 561nm excitation/595nm emission, 0.5μm step size, 47 passes) with Elements software were used to collect high resolution, serial optical sections of NG and S1 DRG neurons, respectively. All channels of the confocal images were contrast enhanced simultaneously for display purposes only.
Statistics
Analyses were performed using SPSS v19-20 (IBM, North Castle, NY). Levene's statistic was applied for homogeneity of variances and data are expressed as mean ± SD. Repeated measures analysis of variance (ANOVA) with a group factor was performed for analyzing side differences in the NG. After finding no significant differences between the right and left sides, bilateral ganglia were averaged (some ganglia were lost due to tissue processing errors). Next, these averaged values were analyzed via one-way ANOVA assessing regional differences in the NG. For cell quantifications and tracer comparisons, data were normalized as percentages of total NG cells and analyzed with a one-way ANOVA, followed by Tukey HSD post hoc t-tests. Two-way ANOVA and Tukey HSD post hoc t-tests were performed for the analysis of DRG ganglion differences and pelvic organ differences. One-way ANOVA followed by Tukey HSD post hoc t-tests were used for section sampling data. An independent t-test was performed for evaluating group differences (DiI vs DiO) in colon labeled NG neurons. Statistical significance was defined as p≤.05.
Results
Nodose Ganglion (NG) Labeling
Use of the retrograde lipophilic tracers, DiI and DiO, revealed a total count of 5516 either singly- or dually-labeled afferents supplying the rat bladder and distal colon in the NG (8 rats; 15 total ganglia). Tracer injections into these two different pelvic organs (Figure 1) resulted in significantly more neurons labeled only from bladder than either only from colon or convergent neurons and significantly more neurons labeled only from colon than convergent neurons (Table 2 and Figure 2). Specifically, bladder labeled afferents in the NG were approximately 40% or 1.7 times more numerous than those labeled from the distal colon and 66% or almost 3 times more numerous than those labeled from both organs. Colon labeled afferents (Figure 1B) were approximately 43% or 1.8 times more numerous than dually-labeled afferents (Figure 2). Of the total number of labeled afferents in the NG, 51.6 ± 1.9% were labeled only from the bladder, 31.2 ± 1.5% only from colon, while convergent neurons represented 17.2 ± 1.6%. Retrograde tracing from the bladder and colon did not reveal any obvious somatotopy in terms of side-to-side or intra-NG arrangement (Figures 3 and 4, respectively). A typical example showing labeling of NG neurons traced from the bladder and colon is provided in the Figure 5 confocal image and in Supplemental Figure 1(magenta-green copy). Note that percentages of NG labeling from bladder and colon in a female rat (n=2 ganglia) did not reveal significant differences from the male rats (unpublished observations).
Figure 1. Visceral organ tracer injections.
Hamilton microsyringe retrograde tracer injections (arrows) of FAST DiI™ were made into the wall of the urinary bladder (A) and FAST DiO™ into the distal colon wall (B).
Table 2.
Average (± SD) number of DiI/DiO-positive neurons counted per ganglion
| Vagal PNS | Spinal PNS | Spinal PNS | Spinal SNS | Spinal SNS | |
|---|---|---|---|---|---|
| NG | L6 DRG | S1 DRG | L1 DRG | L2 DRG | |
| Bladder | 188.5 ± 92.0 | 165.8 ± 64.7 | 42.9 ± 28.7 | 30.7 ± 42.2 | 30.1 ± 17.7 |
| Colon | 114.3 ± 52.8 | 87.9 ± 49.6 | 15.8 ± 9.4 | 23.9 ± 24.5 | 24.1 ± 11.9 |
| Convergent | 64.9 ± 38.6 | 8.1 ± 4.7 | 9.3 ± 6.1 | 5.0 ± 4.4 | 2.4 ± 1.9 |
| Total Labeled | 367.7 ± 162.1 | 261.8 ± 110.9 | 68.0 ± 33.0 | 59.6 ± 66.2 | 56.6 ± 24.9 |
| Overall Total (Labeled + Non-labeled) | 879.8 ± 365.8 | 955.9 ± 466.9 | 774.9 ± 302.3 | 1011.7 ± 228.5 | 1166.5 ± 160.3 |
NG, Nodose Ganglion; DRG, Dorsal Root Ganglion; PNS, Parasympathetic Nervous System; SNS, Sympathetic Nervous System
Figure 2. Percentage of bladder-, colon- and convergent-specific neurons in the nodose ganglion (NG).
The percentages are in relation to the total number of neurons counted. There were significantly more bladder labeled neurons than colon (21.4 ± 3.1% vs 12.7 ± 1.6%, *p<.001) and convergent (7.4 ± 2.8%, *p<.001) and significantly more colon labeled neurons than convergent (#p=.001). (Simple bar graph, data are expressed as mean ± SD, one-way AVOVA with Tukey HSD post hoc t-tests, 15 ganglia total)
Figure 3. Assessment of side differences between NG.
There were no significant differences between the left and right NG using DiI and DiO tracers. (Grouped bar graph, data are expressed as mean ± SD, part one of RM ANOVA with group factor, 15 ganglia total.)
Figure 4. Assessment of regional differences in the NG.
No significant differences were found in the percentage of NG labeled cells among rostral (proximal), middle and caudal (distal) regions of the NG using DiI/DiO. Rostral, middle, caudal divisions were demarcated anatomically by the laryngeal branch of the vagus nerve. White scale bar is 200 μm. (Stacked bar graph, data are expressed as mean ± SD, part two of RM ANOVA with group factor, 15 ganglia total.)
Figure 5. Fluorescent labeling in the NG.
A representative confocal image of the NG displayed in the orthogonal view is shown. A bladder-only labeled cell is indicated with an arrow. A colon-only labeled cell is indicated with an arrowhead. A non-labeled cell is indicated with a # sign. Orthogonal guides (red lines) are centered upon a double-labeled cell (yellow) and the corresponding XZ and YZ projections are indicated at the bottom and right side of the image, respectively. Only one example of each cell type is indicated above. The scale bar is 25μm (60X objective). Please refer to Supplemental Figure 1 for the magenta-green copy
L6/S1 DRG labeling
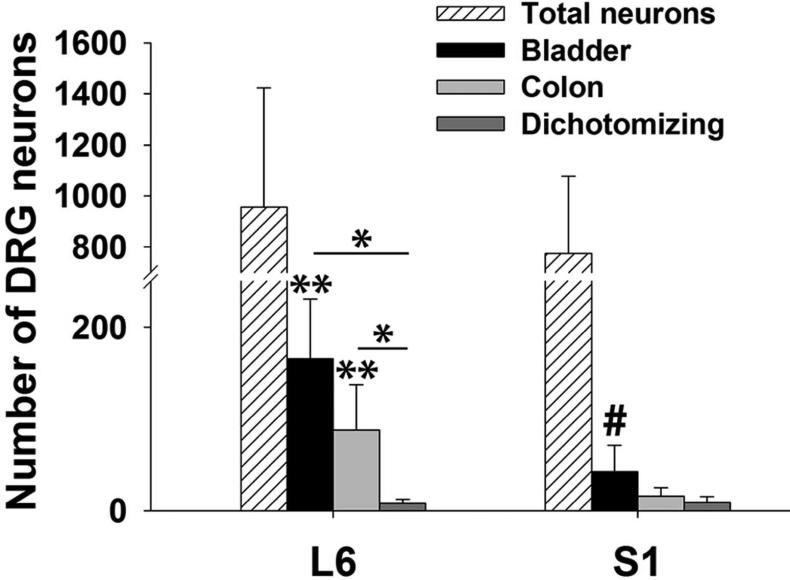
In the same set of male Wistar rats (n=8, 24 ganglia), injections of DiI into the wall of the bladder and DiO into the wall of the distal colon revealed singly- and dually-labeled spinal afferents in both L6 and S1 DRG. There were significantly more bladder labeled neurons than colon (p<.01) and convergent neurons (p<.01) and significantly more colon labeled than convergent neurons (p<.01) within the L6 DRG (Table 2). Specifically, bladder labeled afferents within the L6 DRG were approximately 47% or 1.9 times more numerous than those labeled from the L6 colon and 95% or 20 times more numerous than those labeled from both organs. Colon labeled afferents were approximately 91% or 11 times more numerous than dually-labeled afferents within the L6 ganglia (Figure 6). With respect to only the total number of labeled afferents in the L6 DRG, the bladder represented (64.3 ± 2.2%), the colon represented (32.5 ± 2.1%), while convergent neurons represented (3.2 ± 0.5%).
Figure 6. Bladder-, colon- and convergent-specific neurons in the L6 and S1 dorsal root ganglia (DRG).
Within L6, there were significantly more bladder neurons (165.8 ± 64.7) than colon (87.9 ± 49.6, *p<.01) and convergent (8.1 ± 4.7, *p<.01) neurons and significantly more colon than convergent (*p<.01) neurons. Within S1 there were significantly more bladder neurons (42.9 ± 28.7) than convergent neurons (9.3 ± 6.1, #p<.05). Across the ganglia, there were significantly more bladder and colon labeled neurons in L6 compared to S1 (S1 colon, 15.8 ± 9.3, ** p<.01). (Grouped bar graph, data are expressed as mean ± SD, two-way ANOVA with Tukey HSD post hoc t-tests, 10 ganglia (L6) and 14 ganglia (S1).)
In contrast to the L6 DRG, the numbers of bladder labeled afferents within the S1 DRG were not significantly different from the numbers of colon labeled afferents and, as a group, colon labeled afferents were not significantly different from convergent afferents (p>.05). However, bladder labeled afferents within the S1 DRG were approximately 78% or 4.6 times more numerous than those labeled from both organs (p<.05). With respect to only the total number of labeled afferents in the S1 DRG, the bladder represented (59.7 ± 3.8%), the colon represented (25.7 ± 2.9%), while convergent neurons represented (14.7 ± 1.9%). Examination of labeled neurons across the ganglia revealed that bladder-specific afferents (74.1% or 3.9 times, p<.01) and colon-specific afferents (82% or 5.6 times, p<.01) were more numerous in L6 than those labeled from S1. A typical example showing labeling of traced S1 neurons from the bladder and colon is provided in the Figure 7 confocal image and in Supplemental Figure 2 (magenta-green copy).
Figure 7. Fluorescent labeling in the S1 DRG.
A representative confocal optical section of the S1 DRG is shown revealing in A) a bladder-only labeled cell indicated by the red arrow, in B) a colon-only labeled cell indicated by the green arrow, and in C) a convergent neuron indicated by the yellow arrow. A 3D projection of the cells from A-C is shown in panel D. The white scale bars in the bottom images are 50μm (20X objective). Please refer to Supplemental Figure 2 for the magenta-green copy
Since the majority of labeled afferents were present in L6 compared to S1, specific aspects of the collected data were evaluated (i.e. sampling per section) to determine if there were differences between the two DRG levels. Specifically evaluating bladder afferents, there were significantly more DiI + cells per section in L6 (23.7 ± 7.2) versus S1 (6.8 ± 3.6, p<.05), but the total number of cells/section were similar (128.8 ± 25.9 versus 124.1 ± 34.2) between the two ganglia. Overall, L6 had a greater amount of DiI labeling compared to S1 and, therefore, a significantly greater percentage of bladder labeling throughout the ganglion (18.6 ± 5.2% vs 5.4 ± 2.2%, p<.001).
L1/L2 DRG Labeling
In a subset of the same male Wistar rats (n=4, 15 ganglia), injections of DiI into the wall of the bladder and DiO into the wall of the distal colon revealed singly- and dually-labeled spinal afferents in both L1 and L2 DRG. In contrast to the L6 and S1 DRG, bladder and colon labeling was uniform throughout the L1 and L2 DRG demonstrating no significant differences within and across the ganglia (Table 2). However, in the L2 DRG bladder and colon labeling was significantly greater than convergent labeling (bladder, 2.5 ± 1.3% vs 0.2 ± 0.6%, p<.001; colon, 2.1 ± 0.9% vs 0.2 ± 0.6%, p=.001). With respect to only the total number of labeled afferents in L1, the bladder represented (44.8 ± 19.5%), the colon (42.4 ± 19.0%) and convergent neurons (12.7 ± 16.6%). Similar results were found for L2, where the bladder represented (50.5 ± 17.3%), the colon (44.3 ± 15.6%) and convergent neurons (5.2 ± 4.5%).
Spinal versus non-spinal labeling
In terms of the total percentage of DiI labeled bladder sensory afferents, 49.8% are represented by a vagal source (NG) and 50.2% are represented by a spinal source (43.9% for L6/S1 and 6.3% for L1/L2). For example, in comparison with the L6 DRG, which contains the vast majority of bladder-labeled neurons, significantly more DiI + cells/section were found in the NG (51.3 ± 23.2 versus 23.7 ± 7.2 in L6) as well as total cells/section (232.5 ± 76.7 for NG versus 128.8 ± 25.9 for L6) (Table 3). However, the proportion of bladder labeled neurons relative to the total population of neurons was similar in the NG relative to L6 (21.6 ± 3.9% vs 18.6 ± 5.2%, Table 3).
Table 3.
Cell sampling data between the NG and L6 DRG
| Sampling Data | NG | L6 DRG |
|---|---|---|
| Dil+ cells/section (n) | 51.3 ± 23.3 | 23.7 ± 7.2 |
| Total cells/section (n) | 232.5 ± 76.7 | 128.8 ± 25.9 |
| Dil+ cells/ganglia (%) | 21.6 ± 3.9 | 18.6 ± 5.2 |
NG, Nodose Ganglion; DRG, Dorsal Root Ganglion
Comparisons with other tracers and controls
Comparisons with other widely used neuronal tracers were performed to assess the effectiveness of the lipophilic tracers DiI and DiO. We compared NG neuronal labeling following injections of dextrans (TMR–into bladder and FD–into colon) and CTB (-594–into bladder and -488–into colon). We did not find any significant differences in the percentage of bladder-specific, colon-specific, and convergent-specific neurons in the NG between dextrans TMR/FD and carbocyanines DiI/DiO. However, there were significantly fewer CTB-bladder labeled neurons in the NG than DiI and TMR and significantly fewer CTB-colon labeled neurons than DiO (Figure 8).
Figure 8. Tracer comparisons in the NG.
The percentage of bladder-specific, colon-specific and convergent neurons in the NG after injections of either TMR, CTB-594, or carbocyanine (DiI) into the bladder and FD, CTB-488 or carbocyanine (DiO) into the distal colon showed significantly fewer CTB-bladder and colon labeled cells than DiI (# bladder, p<.005), DiO (+ colon, p=.001) and TMR (* bladder, p<.05) in the NG. No significant differences were found between both dextrans and carbocyanine labeling nor between FD (colon) and CTB-488 (colon) (p>.05). (Grouped bar graph, data are expressed as mean ± SD, one-way ANOVA with Tukey HSD post hoc t-tests, Dextran 2 ganglia, CTB 2 ganglia, Carbocyanine 15 ganglia, TMR=tetramethylrhodamine, CTB=cholera toxin subunit B.)
Removal of the superior cervical ganglion, which lies in close proximity to the NG, did not reveal any evidence of punctate labeling in conjunction with any of our tracers (image not shown). Furthermore, results from two additional rats that received DiI injections into the distal colon did not reveal significant differences in colon labeled NG tafferents from those animals which received prior DiO injections in the distal colon (DiI, 11.0 ± 1.8% vs DiO, 12.9 ± 2.3%). As an additional control, the bladder was sectioned and imaged, to ensure there was no evidence of tracer cross-contamination from the distal colon and indicating that the tracer remained in that organ (image not shown).
Discussion
In the present study, we identified both singly- and dually-labeled vagal afferents supplying the rat bladder and distal colon. We found a uniform distribution of labeling throughout all regions of bilateral NG ganglia. Furthermore, considering the proportional distribution of labeled afferents between the NG and DRG, the percentages of bladder vagal afferents were similar to the percentages of bladder spinal afferents in the L6 DRG. This result demonstrates that the vagus nerve makes a substantial contribution to the anatomical connections of the male rat bladder. A summary diagram of the dual innervation to the bladder and colon from both spinal and vagal supplies is provided in Figure 9. Based on our comparisons with other types of tracers, the labeling efficacy of the retrograde tracers DiI and DiO, with no known selective tropism for different types of neurons, was appropriate for these experiments with regard to quantifying cells at the primary afferent level.
Figure 9. Summary diagram of spinal and vagal innervation to the bladder and colon.
The top pie graphs represent the percentage of bladder-, colon- and convergent-specific labeling from spinal (L1/L2 and L6/S1) and vagal (NG) sources with respect to only labeled neurons counted. The bottom pie graphs examine the percentage of bladder-, colon-, and convergent-specific labeling from spinal and vagal sources with respect to total neurons counted (labeled and non-labeled).
Nodose Ganglion Labeling
Injections of the retrograde tracers DiI and DiO revealed bladder-, colon- and convergent-specific neurons in the NG of the male Wistar rat. Anatomically, this suggests that a single vagal afferent can supply either the bladder or colon, but also can dichotomize and supply both organs, thereby providing a mechanism of communication between the two structures.
Evidence of vagal innervation to the male rat bladder provides a greater level of understanding to the complexity of the neural circuitry of the pelvis. These results are in line with previous animal studies demonstrating that the vagus nerve can indeed project below the transverse colon (splenic flexure), providing sensory innervation to the kidney (Gattone et al., 1986), ovary (Burden et al., 1983) and even portions of the female reproductive tract (uterus, cervix) (Ortega-Villalobos et al., 1990; Hubscher and Berkley, 1995; Collins et al., 1999). Additionally, indication of a possible vagal-bladder connection was noted when injection of horseradish peroxidase (HRP)- wheat germ agglutinin (WGA) into the bladder was performed, in which the authors report subsequently labeled neurons in the NG as well as spinal ganglia (Jancso and Maggi, 1987). Furthermore, our result of 21.4% of bladder neurons represented in the NG is similar to the percentage of NG neurons labeled from stomach (18%) (Sakurai et al., 2008), demonstrating that the vagus nerve provides a substantial degree of afferent innervation to the bladder.
The fact that 7.4% of the neurons in the NG were double labeled demonstrates that through the presence of vagal dichotomizing afferents, convergence of bladder and colon afferents are present at the primary afferent level. The percentage of convergent neurons falls within the range of other studies (DRG neurons) in multiple species (5-27%) (McNeill and Burden, 1986; Brumovsky and Gebhart, 2010). Dichotomizing spinal bladder/colon afferents have been shown at multiple DRG levels in both the rat, mouse (Keast and De Groat, 1992; Christianson et al., 2007) and cat (De Groat, 1987). The existence of these shared peripheral neural pathways may contribute to some of the bladder/colon co-morbid conditions seen clinically, such as interstitial cystitis and irritable bowel syndrome. Evidence of anatomically traced dichotomizing vagal axons has been reported with dually-labeled gastroduodenal (Zhong et al., 2008) and pancreatic (head and tail) afferents present in the NG (Fasanella et al., 2008). Since the bladder and colon are closely related (stemming from the same embryological origin, having the same spinal innervation peripherally, similar central processing areas, similar functions related to storage and elimination), it is perhaps not surprising that there is evidence of co-innervation and sensory neuron-level cross-talk. The functional relevance of communication between the bladder and colon is thought to aid in maintaining bodily homeostasis (Denny-Brown, 1933; Floyd et al., 1978, 1979, 1982, De Wachter and Wyndaele, 2003; Vilensky et al., 2004; De Wachter et al., 2007; Malykhina et al., 2012).
The existence of such shared pathways also provides a means by which pathology in one organ can affect the functionality of an adjacent, healthy organ. For instance, numerous studies in experimental models of different species and in clinical reports (Whorwell et al., 1986a; Whorwell et al., 1986b; Alagiri et al., 1997) demonstrate physiological evidence of cross-organ sensitization between both organs, including effects such as increased urinary frequency, urgency, nocturia, abnormal bladder detrusor muscle contractility and emptying or increased responses to colonic distention at lower than normal pressures (Floyd et al.; 1978, 1979, 1982, Bouvier et al., 1990; Malykhina et al., 2004; Pezzone et al., 2005; Qin et al., 2005; Bielefeldt et al., 2006a; Lamb et al., 2006; Malykhina et al., 2006; Ustinova et al., 2006; Noronha et al., 2007; Qiao and Grider, 2007; Rudick et al., 2007; Ustinova et al., 2007; Ustinova et al., 2010; Lei and Malykhina, 2012; Lei et al., 2013). It is important to note that convergence through both spinal and/or vagal afferents occurs centrally as well. Neurons in the solitary nucleus and medullary reticular formation, for example, have been shown to receive mechano-sensitive inputs from multiple pelvic organs, demonstrating convergence at the second order neuronal level and beyond (Hubscher and Berkley, 1995; Kaddumi and Hubscher, 2006). Central viscero-visceral convergence likely explains why multi-symptomatic patients experience referred pain or altered sensations in unaffected viscera (Berkley, 2005).
L6/S1 and L1/L2 Labeling
The quantitative results of singly- and dually-labeled spinal afferents supplying the male rat bladder and colon were similar to an earlier study (Christianson et al., 2007) assessing this same outcome for L6/S1 and L1/L2 spinal ganglia in the rat despite using different tracers and in a different strain (Sprague-Dawley). There were no significant differences from the Christianson et al. study in terms of labeling between the bladder, colon and convergent spinal afferents in the L6/S1 ganglia when considering only labeled afferents. Similar findings were also apparent for the L1 and L2 ganglia between studies. Although percentages for labeled afferents with respect to all neurons (both labeled and non-labeled) was not reported in that study, the current study indicates significant differences when considering the L6 and S1 separately for this measurement criterion. A similar pattern of labeling in the L6 DRG was found compared to the NG, where there were a greater number of bladder labeled afferents followed by colon and then convergent neurons. In S1, only significantly more bladder than convergent neurons was found. However, L6 still displayed a greater percentage of bladder and colon afferents than S1, indicating a larger degree of bladder/colon information travels through this DRG compared to S1. Even though this study focused on sensory afferents (NG via the abdominal branches of the vagus and L6/S1 via the pelvic nerve) of nerves containing parasympathetic fibers to the bladder, there also are some inputs to thoracolumbar DRG's via the hypogastric nerve which contains sympathetic innervation of the bladder. Note that the L1/L2 DRG results are consistent with a predominant sensory supply via L6/S1 pelvic afferents (Christianson et al., 2007) and even when combined the spinal sources and NG supply are in relatively similar proportions.
The general finding of significantly more bladder versus distal colon labeled afferents in both the NG and DRG may be attributed to surface area differences between the two organs. For example, even though the same circumferential region of the distal colon (1-3cm rostral to the anus) and bladder receive the same injection parameters across all animals, the actual injection area of the distal colon is smaller than the bladder with respect to the entire organ and therefore may explain the smaller percentage of distal colon afferents relative to the bladder.
The bladder afferent percentages in the NG were most similar to those found in the L6 ganglia. Despite the fact that the NG exhibited more bladder-specific cells per section as well as more total cells per section compared to L6, there were no significant differences in the overall representation of bladder labeling throughout the two ganglia. These findings are consistent with reports on the percentage of stomach-labeled afferents in the NG, which were also found on average to be similar to the percentage of DRG (T9/10) stomach labeled (18%) afferents (Sakurai et al., 2008). The proportional similarities of the dual supply of bladder afferents may relate to different functional roles of NG and DRG neurons. For example, it has been hypothesized that spinal afferents are responsible for conveying noxious mechanical information, while noxious chemical stimuli are conveyed via vagal afferents (Schuligoi et al., 1998; Michl et al., 2001; Page et al., 2002; Holzer, 2003; Lamb et al., 2003; Danzer et al., 2004; Holzer et al., 2004; Sugiura et al., 2005; Kaddumi and Hubscher, 2007). Aside from communicating differential stimuli from the periphery, both vagal and spinal projections likely converge in the brainstem. In particular, the midbrain PAG receives spinal afferent information from the lower urinary tract as well as vagal afferent visceral information via projections from the solitary nucleus (Herbert and Saper, 1992; Monnikes et al., 2003; Nishii et al., 2008). The PAG also receives input from various cortical regions which play a role in conscious control such as determining when voiding is appropriate (Blok et al., 1997a; Blok et al., 1998; Kuipers et al., 2006; Please refer to Beckel and Holstege, 2014 for a thorough review). Numerous levels of input and organization help provide fine tune control over complex neural pathways important for conscious control of continence and micturition.
We did not find significant differences between NG colon and convergent afferent labeling compared to that from DRG neurons. A large degree of convergent afferents may be expected for the DRG supply based upon their known roles in multiple eliminative functions, but this was not the case. It should be noted, however, that labeled DRG and NG neurons may not accurately reflect the full extent of the peripheral supply to these pelvic organs as there could be differences in the extent of branching. For example, sacral DRGs have been shown to have 2.3 times the number of peripheral fibers as there are cell bodies (Langford and Coggeshall, 1981).
Organization of the NG Neurons
Included in the quantification of NG neuronal labeling from the bladder and colon were assessments of potential rostral, middle and caudal regional differences. The NG may exhibit a specialized viscerotopic organization where, potentially, vagal afferents from one organ reside more frequently in one area compared to other regions (Browning and Mendelowitz, 2003; Bielefeldt et al., 2006b). A specific organized distribution is important for the coordination of events and reflexes as demonstrated in higher integrative centers (Broussard and Altschuler, 2000; Altschuler, 2001). The solitary nucleus, location of vagal afferent terminals, for example, exhibits a high degree of organization with a distinctive organotypic pattern of inputs. Gustatory information is localized rostrally, while cardiovascular, respiratory and gastrointestinal afferents terminate in the caudal two-thirds of the nucleus (Torvik, 1956; Cottle, 1964; Beckstead and Norgren, 1979; Kalia and Mesulam, 1980). Cell counts in sections from the rostral, middle and caudal NG were analyzed and no significant regional differences were found. The finding of a uniform distribution of labeled bladder/colon/convergent cells in the NG also has been demonstrated for upper digestive afferents in the mouse (Zhong et al., 2008) and rat (Altschuler et al., 1989) as well as for pancreatic afferents in the rat (Sharkey and Williams, 1983).
In addition, side to side comparisons between both NG's were made since the left and right vagus nerves are known to play different roles with regard to cardiac function (Randall et al., 1986, Saper et al., 1990; Schachter and Saper, 1998). Importantly, retrograde tracing from the heart (coronary sulcus and anterior interventricular groove) and pancreas reveal asymmetrical vagal distributions in the NG (Sharkey and Williams, 1983; Sharkey et al., 1984; Carobi, 1987; Fasanella et al., 2008; Hayakawa et al., 2011). However, despite the slight anatomical differences in the distribution of the thoraco-abdominal vagus, no side differences were present in the current study for the pelvic viscera supplied by the abdominal branches of the vagus.
Tracing Controls
One caveat related to the selection of neuroanatomical tracers is whether multiple fiber types take up and retrogradely transport the tracer, as the goal of this initial study was not only to determine if evidence of vagal innervation of the bladder/colon exists but also to quantify the extent of innervation by the abdominal branches of the vagus nerve. The selection of the carbocyanine retrograde tracers, Fast DiI™ and Fast DiO™, for the current study was based upon their capability of uptake in all cell types (small, medium, large, unmyelinated, lightly- and myelinated) and a well-documented method for tracing cells from the periphery (Honig and Hume, 1989; Su et al., 1999; Wang and Scott, 1999; Ohtori et al., 2001; Ueno et al., 2001; Gold et al., 2002; Deng et al., 2007; Wang et al., 2007). The additional assessments with other tracers, dextran amines and CTB, allowed us to examine the versatility of the lipophilic tracers we were interested in utilizing and ensure their effectiveness for the current study. Certain HRP conjugates, such as Biotinylated-HRP or WGA-HRP have been shown to have a preference towards larger diameter or smaller diameter, myelinated axons, whereas vagal afferents are comprised of primarily small to medium size, unmyelinated or lightly myelinated axons (Robertson and Arvidsson, 1985; Rivero-Melian and Grant, 1991; Maslany et al., 1992; Zhuo et al., 1997). Cholera toxin subunit B (CTB), a commonly used tracer, may be problematic since it requires the presence of the GM-1 ganglioside for uptake (Heyningen, 1974) and perhaps not all vagal bladder/colon afferents express this receptor. This consideration could provide an explanation for why we found significantly fewer CTB-labeled bladder and colon neurons in the NG compared to the other tracers used. It is important to note that the use of dextran amines (TMR/FD), while yielding similar NG cell counts as DiI/DiO, also utilize a different uptake mechanism (active transport) than the lipophilic type (passive diffusion) (Kobbert et al., 2000).
As a control, the superior cervical ganglia were processed for detection of the retrograde dyes. This ganglion is spatially close to the NG, with fibers that run relatively adjacent to the vagus nerve. There was no evidence of any dye transfer between the two ganglia in the present study. In addition, previous studies examining the use of these tracers did not find any complications with tracer leakage or the spread of dye to adjacent organs/neurons as well as spread within the ganglia itself (DRG in this example) (Dang et al., 2005; Dang et al., 2008).
To address any quantification concerns due to potential spectral overlap from the long fluorescent emission tail of DiO that may confound our image analyses, DiI was injected into the distal colon of two additional rats and counts of DiI positive neurons were compared with DiO positive colon neurons in the NG. In agreement with our initial colon counts, similar percentages of colon labeled afferents were found between the two groups, indicating that the tracers could be used reliably together and separated by the emission filter combinations used. Additionally, this finding corroborates the notion of convergent neurons in the NG. Overall, based on our controls, the lipophilic tracers incorporated in this study were effective for accurately labeling bladder and colon afferents as well as maintaining stable fluorescence for cell quantification in the NG.
Clinical Relevance
Current human anatomical texts report that vagal innervation to the viscera terminates at the left colic (splenic) flexure (Agur and Dalley, 2009). Complementary to this understanding, a 2013 review of the female abdominopelvic region indicates this caudal extension of the vagus based upon a drawing from the mid-1800s (Frankenhaüser, 1866), in which the authors additionally state that upon entry into the abdomen, the sympathetic and vagal fibers become “indistinguishably mixed” (Shoja M., 2013). Indirect evidence of a vagal connection to the pelvic viscera in humans is demonstrated from the fact that women with functionally “complete” spinal cord injury (SCI - American Spinal Injury Association criteria) are able to perceive sensations from mechano-stimulation to the vagina and cervix and even respond with orgasms (Komisaruk et al., 1997; Komisaruk et al., 2004). Additionally, patients with functionally “complete” SCI are able to perceive visceral pain from urinary and bowel distention as well as sensations of bladder distention and filling (Wyndaele, 1991, 1997; Ersoz and Akyuz, 2004). An unanswered question is whether additional tracing studies would reveal a further caudal extension of the vagus in humans. Although anatomical evidence of connections to the pelvic viscera by the vagus nerve is discussed in the clinical literature pertaining to chronic SCI cases, we hypothesize that the vagal pathways exist in the human pelvic cavity, but in terms of generating perception, the fibers are “silent” and re-organize following injury or under certain pathophysiological conditions and thus become evident. Given that the putative role of vagal afferents is relaying homeostatic information, much of which we are unaware, this hypothesis seems plausible. However, vagal afferents show the capability of detecting noxious stimuli, as seen in the lungs for example. Their responses to inflammatory insults evoke defensive responses such as a temporary cessation of breathing, bronchoconstriction, hypotension and coughing, thereby providing the organ with its own potential sense of injury, which fulfills Sherrington's role of a nociceptor (Sherrington, 1906; Coleridge and Coleridge, 1984; Undem et al., 2004).
Conclusion
This study provides evidence for a sensory anatomical connection of the vagus nerve to the male rat urinary bladder and distal colon. A similar degree of afferent labeling within the NG compared to the DRG as well as the presence of dichotomizing vagal afferents suggests that the vagus nerve likely is substantially involved with the lower urinary tract and gastrointestinal functions. Current studies are examining the electrophysiological and histochemical phenotype of NG neurons, which will aid in our functional understanding of these pelvic visceral afferents.
Acknowledgements
The authors thank Jim Armstrong, Jason Fell, Patricia Ward, Ann-Claude Rakotoniaina, Jason Beare, Amanda Pocratsky, Matthew Hamilton and Alice Shum-Siu for technical assistance. The authors also thank Drs. Robert Lundy, Martha Bickford and David Magnuson for tracer and antibody donations and Darlene Burke for statistical assistance. Supported by NIH NINDS Grant F31NS077750 (A.H.) and Intramural Research Incentive Grant from the University of Louisville (C.H.). This project also utilized KSCIRC Neuroscience Core facilities that are supported by the NIH/NCRR P30 8P30GM103507 grant.
Role of the Authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: A.H., K.R., J.P., C.H. Acquisition of data: A.H., C.H. Analysis and interpretation of data: A.H., K.R., J.P., D.S., C.H. Manuscript editing and approval: A.H., K.R., J.P., D.S., C.H. The authors report no conflict of interest with this project.
Literature Cited
- Agur A, Dalley A. Grant's Atlas of Anatomy. Lippincott Williams & Wilins; Bethesda: 2009. [Google Scholar]
- Angelucci A, Sainsbury K. Contribution of feedforward thalamic afferents and corticogeniculate feedback to the spatial summation area of macaque V1 and LGN. J Comp Neurol. 2006;498:330–51. doi: 10.1002/cne.21060. [DOI] [PubMed] [Google Scholar]
- Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology. 1997;49:52–57. doi: 10.1016/s0090-4295(99)80332-x. [DOI] [PubMed] [Google Scholar]
- Altschuler SM. Laryngeal and respiratory protective reflexes. Am J Med. 2001;111(Suppl 8A):90S–94S. doi: 10.1016/s0002-9343(01)00862-2. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Bao XM, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central orga8nization of the vagus nerve innervating the colon of the rat. Gastroenterology. 1993;104:502–509. doi: 10.1016/0016-5085(93)90419-d. [DOI] [PubMed] [Google Scholar]
- Basinski C, Fuller E, Brizendine EJ, Benson JT. Bladder-anal reflex. Neurourol Urodyn. 2003;22:683–686. doi: 10.1002/nau.10101. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Holstege G. Neuroanatomy of the lower urinary tract. Handb Exp Pharmacol. 2011a:99–116. doi: 10.1007/978-3-642-16499-6_6. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Holstege G. Neurophysiology of the lower urinary tract. Handb Exp Pharmacol. 2011b:149–169. doi: 10.1007/978-3-642-16499-6_8. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Holstege G. The lower urinary tract. In: Paxinos, editor. The rat nervous system. Elsevier; 2014. Ch. 12. [Google Scholar]
- Beckstead RM, Norgren R. An autoradiographic examination of the central distribution of the trigeminal, facial, glossopharyngeal, and vagal nerves in the monkey. J Comp Neurol. 1979;184:455–472. doi: 10.1002/cne.901840303. [DOI] [PubMed] [Google Scholar]
- Berkley KJ. A life of pelvic pain. Physiol Behav. 2005;86:272–280. doi: 10.1016/j.physbeh.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Lamb K, Gebhart GF. Convergence of sensory pathways in the development of somatic and visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2006a;291:G658–665. doi: 10.1152/ajpgi.00585.2005. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Zhong F, Koerber RH, Davis BM. Phenotypic characterization of gastric sensory neurons in mice. Am J Physiol Gastrointest Liver Physiol. 2006b;291:G987–997. doi: 10.1152/ajpgi.00080.2006. [DOI] [PubMed] [Google Scholar]
- Blok BF, de Weerd H, Holstege G. The pontine micturition center projects to sacral cord GABA immunoreactive neurons in the cat. Neurosci Lett. 1997a;233:109–112. doi: 10.1016/s0304-3940(97)00644-7. [DOI] [PubMed] [Google Scholar]
- Blok BF, Sturms LM, Holstege G. A PET study on cortical and subcortical control of pelvic floor musculature in women. J Comp Neurol. 1997b;389:535–544. [PubMed] [Google Scholar]
- Blok BF, Sturms LM, Holstege G. Brain activation during micturition in women. Brain. 1998;121(Pt 11):2033–2042. doi: 10.1093/brain/121.11.2033. [DOI] [PubMed] [Google Scholar]
- Blok BFM, Holstege G. The pontine micturition center in rat receives direct lumbosacral input. An ultrastructural study. Neurosci Lett. 2000;282:29–32. doi: 10.1016/s0304-3940(00)00833-8. [DOI] [PubMed] [Google Scholar]
- Borra E, Belmalih A, Gerbella M, Rozzi S, Luppino G. Projections of the hand field of macaque ventral premotor area F5 to the brainstem and spinal cord. J Comp Neurol. 2010;518:2570–91. doi: 10.1002/cne.22353. [DOI] [PubMed] [Google Scholar]
- Bouvier M, Grimaud JC, Abysique A. Effects of stimulation of vesical afferents on colonic motility in cats. Gastroenterology. 1990;98:1148–1154. doi: 10.1016/0016-5085(90)90327-w. [DOI] [PubMed] [Google Scholar]
- Broussard DL, Altschuler SM. Brainstem viscerotopic organization of afferents and efferents involved in the control of swallowing. Am J Med. 2000;108(Suppl 4a):79S–86S. doi: 10.1016/s0002-9343(99)00343-5. [DOI] [PubMed] [Google Scholar]
- Browning KN, Mendelowitz D. Musings on the wanderer: what's new in our understanding of vago-vagal reflexes?: II. Integration of afferent signaling from the viscera by the nodose ganglia. Am J Physiol Gastrointest Liver Physiol. 2003;284:G8–14. doi: 10.1152/ajpgi.00322.2002. [DOI] [PubMed] [Google Scholar]
- Brumovsky PR, Gebhart GF. Visceral organ cross-sensitization - an integrated perspective. Auton Neurosci. 2010;153:106–115. doi: 10.1016/j.autneu.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden HW, Leonard M, Smith CP, Lawrence IE., Jr. The sensory innervation of the ovary: a horseradish peroxidase study in the rat. Anat Rec. 1983;207:623–627. doi: 10.1002/ar.1092070410. [DOI] [PubMed] [Google Scholar]
- Carobi C. Capsaicin-sensitive vagal afferent neurons innervating the rat pancreas. Neurosci Lett. 1987;77:5–9. doi: 10.1016/0304-3940(87)90597-0. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Liang R, Ustinova EE, Davis BM, Fraser MO, Pezzone MA. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain. 2007;128:235–243. doi: 10.1016/j.pain.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Reviews of physiology, biochemistry and pharmacology. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Collins JJ, Lin CE, Berthoud HR, Papka RE. Vagal afferents from the uterus and cervix provide direct connections to the brainstem. Cell Tissue Res. 1999;295:43–54. doi: 10.1007/s004410051211. [DOI] [PubMed] [Google Scholar]
- Cottle MK. DEGENERATION STUDIES OF PRIMARY AFFERENTS OF IXTH AND XTH CRANIAL NERVES IN THE CAT. J Comp Neurol. 1964;122:329–345. doi: 10.1002/cne.901220304. [DOI] [PubMed] [Google Scholar]
- Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- Dang K, Bielfeldt K, Lamb K, Gebhart GF. Gastric ulcers evoke hyperexcitability and enhance P2X receptor function in rat gastric sensory neurons. J Neurophysiol. 2005;93:3112–3119. doi: 10.1152/jn.01127.2004. [DOI] [PubMed] [Google Scholar]
- Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol. 2008;99:49–59. doi: 10.1152/jn.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer M, Jocic M, Samberger C, Painsipp E, Bock E, Pabst MA, Crailsheim K, Schicho R, Lippe IT, Holzer P. Stomach-brain communication by vagal afferents in response to luminal acid backdiffusion, gastrin, and gastric acid secretion. Am J Physiol Gastrointest Liver Physiol. 2004;286:G403–411. doi: 10.1152/ajpgi.00308.2003. [DOI] [PubMed] [Google Scholar]
- deCampo DM, Fudge JL. Amygdala projections to the lateral bed nucleus of the stria terminalis in the macaque: comparison with ventral striatal afferents. J Comp Neurol. 2013;521:3191–216. doi: 10.1002/cne.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat WC, Kawatani M, Houston MB, Rutigliiano M, Erdman S. Identification of neuropeptides in afferent pathways to the pelvic viscera of the cat. In: Ciriello J, Calaresu F, Renaud L, Polosa C, editors. Organization of the autonomic nervous system: central and peripheral mechanisms. A.R. Liss; New York: 1987. pp. 81–90. [Google Scholar]
- De Wachter S, de Jong A, Van Dyck J, Wyndaele JJ. Interaction of filling related sensation between anorectum and lower urinary tract and its impact on the sequence of their evacuation. A study in healthy volunteers. Neurourol Urodyn. 2007;26:481–485. doi: 10.1002/nau.20384. [DOI] [PubMed] [Google Scholar]
- De Wachter S, Wyndaele JJ. Impact of rectal distention on the results of evaluations of lower urinary tract sensation. J Urol. 2003;169:1392–1394. doi: 10.1097/01.ju.0000053393.45026.4d. [DOI] [PubMed] [Google Scholar]
- Deng JB, Yu DM, Wu P, Li MS. The tracing study of developing entorhino-hippocampal pathway. Int J Dev Neurosci. 2007;25:251–258. doi: 10.1016/j.ijdevneu.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Denny-Brown D, Robertson EG. On the physiology of micturition. Brain. 1933;56:149–190. [Google Scholar]
- Ersoz M, Akyuz M. Bladder-filling sensation in patients with spinal cord injury and the potential for sensation-dependent bladder emptying. Spinal Cord. 2004;42:110–116. doi: 10.1038/sj.sc.3101525. [DOI] [PubMed] [Google Scholar]
- Fasanella KE, Christianson JA, Chanthaphavong RS, Davis BM. Distribution and neurochemical identification of pancreatic afferents in the mouse. J Comp Neurol. 2008;509:42–52. doi: 10.1002/cne.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd K, McMahon SB, Morrison JF. Inhibition of the micturition reflex by stimulation of pelvic nerve afferents from the colon [proceedings]. J Physiol. 1978;284:39P–40P. [PubMed] [Google Scholar]
- Floyd K, McMahon SB, Morrison JF. Inhibitory interactions between the colonic and vesical branches of the pelvic nerve in the cat [proceedings]. J Physiol. 1979;290:50P–51P. [PubMed] [Google Scholar]
- Floyd K, McMahon SB, Morrison JF. Inhibitory interactions between colonic and vesical afferents in the micturition reflex of the cat. J Physiol. 1982;322:45–52. doi: 10.1113/jphysiol.1982.sp014021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhaüser I. Nerven der weiblichen Geschlechtorgane. Jena Ztschr. 1866 [Google Scholar]
- Gattone VH, 2nd, Marfurt CF, Dallie S. Extrinsic innervation of the rat kidney: a retrograde tracing study. Am J Physiol. 1986;250:F189–196. doi: 10.1152/ajprenal.1986.250.2.F189. [DOI] [PubMed] [Google Scholar]
- Godement P, Vanselow J, Thanos S, Bonhoeffer F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development. 1987;101:697–713. doi: 10.1242/dev.101.4.697. [DOI] [PubMed] [Google Scholar]
- Gold MS, Zhang L, Wrigley DL, Traub RJ. Prostaglandin E(2) modulates TTX-R I(Na) in rat colonic sensory neurons. J Neurophysiol. 2002;88:1512–1522. doi: 10.1152/jn.2002.88.3.1512. [DOI] [PubMed] [Google Scholar]
- Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51(Suppl 1):i2–5. doi: 10.1136/gut.51.suppl_1.i2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Kuwahara-Otani S, Maeda S, Tanaka K, Seki M. Projections of calcitonin gene-related peptide immunoreactive neurons in the vagal ganglia of the rat. J Chem Neuroanat. 2011;41:55–62. doi: 10.1016/j.jchemneu.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Herbert H, Saper CB. Organization of medullary adrenergic and noradrenergic projections to the periaqueductal gray matter in the rat. J Comp Neurol. 1992;315:34–52. doi: 10.1002/cne.903150104. [DOI] [PubMed] [Google Scholar]
- Heyningen SV. Choleratoxin: interaction of subunits with ganglioside GM1. Science. 1974;183:656–657. doi: 10.1126/science.183.4125.656. [DOI] [PubMed] [Google Scholar]
- Holstege G, Kuypers HGJM, Boer RC. Anatomical evidence for direct brain stem projections to the somatic motoneuronal cell groups and autonomic preganglionic cell groups in cat spinal cord. Brain Res. 1979;171:329–33. doi: 10.1016/0006-8993(79)90337-8. [DOI] [PubMed] [Google Scholar]
- Holstege G, Kuypers HG. The anatomy of brain stem pathways to the spinal cord in cat. A labeled amino acid tracing study. Prog Brain Res. 1982;57:145–175. doi: 10.1016/S0079-6123(08)64128-X. [DOI] [PubMed] [Google Scholar]
- Holstege G, Griffiths D, de Wall H, Dalm E. Anatomical and physiological observations on supraspinal control of bladder and urethral sphincter muscles in the cat. J Comp Neurol. 1986;250:449–61. doi: 10.1002/cne.902500404. [DOI] [PubMed] [Google Scholar]
- Holstege G. Micturition and the soul. J Comp Neurol. 2005;493:15–20. doi: 10.1002/cne.20785. [DOI] [PubMed] [Google Scholar]
- Holzer P. Afferent signalling of gastric acid challenge. J Physiol Pharmacol. 2003;54(Suppl 4):43–53. [PubMed] [Google Scholar]
- Holzer P, Danzer M, Schicho R, Samberger C, Painsipp E, Lippe IT. Vagal afferent input from the acid-challenged rat stomach to the brainstem: enhancement by interleukin-1beta. Neuroscience. 2004;129:439–445. doi: 10.1016/j.neuroscience.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Honig MG, Hume RI. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J Cell Biol. 1986;103:171–187. doi: 10.1083/jcb.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig MG, Hume RI. Dil and diO: versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends Neurosci. 1989;12:333–335. 340–331. [PubMed] [Google Scholar]
- Hubscher CH, Berkley KJ. Spinal and vagal influences on the responses of rat solitary nucleus neurons to stimulation of uterus, cervix and vagina. Brain Res. 1995;702:251–254. doi: 10.1016/0006-8993(95)01121-8. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Kaddumi EG, Johnson RD. Brain stem convergence of pelvic viscerosomatic inputs via spinal and vagal afferents. Neuroreport. 2004;15:1299–1302. doi: 10.1097/01.wnr.0000128428.74337.ef. [DOI] [PubMed] [Google Scholar]
- Huynh HK, Willemsen AT, Lovick TA, Holstege G. Pontine control of ejaculation and female orgasm. The journal of sexual medicine. 2013;10:3038–3048. doi: 10.1111/jsm.12300. [DOI] [PubMed] [Google Scholar]
- Jancso G, Maggi CA. Distribution of capsaicin-sensitive urinary bladder afferents in the rat spinal cord. Brain Res. 1987;418:371–376. doi: 10.1016/0006-8993(87)90106-5. [DOI] [PubMed] [Google Scholar]
- Kaddumi EG, Hubscher CH. Convergence of multiple pelvic organ inputs in the rat rostral medulla. J Physiol. 2006;572:393–405. doi: 10.1113/jphysiol.2005.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddumi EG, Hubscher CH. Urinary bladder irritation alters efficacy of vagal stimulation on rostral medullary neurons in chronic T8 spinalized rats. J Neurotrauma. 2007;24:1219–1228. doi: 10.1089/neu.2007.0276. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980;193:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Saeki K, Lee T, Mizuno N. Improved retrograde axonal transport and subsequent visualization of tetramethylrhodamine (TMR) – dextran amine by means of an acidic injection vehicle and antibodies against TMR. J Neurosci Methods. 1996;65:157–65. doi: 10.1016/0165-0270(95)00162-x. [DOI] [PubMed] [Google Scholar]
- Keast JR, De Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- Klop EM, Mouton LJ, Kuipers R, Holstege G. Neurons in the lateral sacral cord of the cat project to periaqueductal grey, but not to thalamus. Eur J Neurosci. 2005;21:2159–2166. doi: 10.1111/j.1460-9568.2005.04039.x. [DOI] [PubMed] [Google Scholar]
- Kobbert C, Apps R, Bechmann I, Lanciego JL, Mey J, Thanos S. Current concepts in neuroanatomical tracing. Prog Neurobiol. 2000;62:327–351. doi: 10.1016/s0301-0082(00)00019-8. [DOI] [PubMed] [Google Scholar]
- Komisaruk BR, Gerdes CA, Whipple B. ‘Complete’ spinal cord injury does not block perceptual responses to genital self-stimulation in women. Arch Neurol. 1997;54:1513–1520. doi: 10.1001/archneur.1997.00550240063014. [DOI] [PubMed] [Google Scholar]
- Komisaruk BR, Whipple B, Crawford A, Liu WC, Kalnin A, Mosier K. Brain activation during vaginocervical self-stimulation and orgasm in women with complete spinal cord injury: fMRI evidence of mediation by the vagus nerves. Brain Res. 2004;1024:77–88. doi: 10.1016/j.brainres.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Kowski AB, Geisler S, Krauss M, Veh RW. Differential projections from subfields in the lateral preoptic area to the lateral habenular complex of the rat. J Comp Neurol. 2008;507:1465–78. doi: 10.1002/cne.21610. [DOI] [PubMed] [Google Scholar]
- Kuipers R, Mouton LJ, Holstege G. Afferent projections to the pontine micturition center in the cat. J Comp Neurol. 2006;494:36–53. doi: 10.1002/cne.20775. [DOI] [PubMed] [Google Scholar]
- Lamb K, Kang YM, Gebhart GF, Bielefeldt K. Gastric inflammation triggers hypersensitivity to acid in awake rats. Gastroenterology. 2003;125:1410–1418. doi: 10.1016/j.gastro.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Lamb K, Zhong F, Gebhart GF, Bielefeldt K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G451–457. doi: 10.1152/ajpgi.00353.2005. [DOI] [PubMed] [Google Scholar]
- Langford LA, Coggeshall RE. Branching of sensory axons in the peripheral nerve of the rat. J Comp Neurol. 1981;203:745–750. doi: 10.1002/cne.902030411. [DOI] [PubMed] [Google Scholar]
- Lei Q, Malykhina AP. Colonic inflammation up-regulates voltage-gated sodium channels in bladder sensory neurons via activation of peripheral transient potential vanilloid 1 receptors. Neurogastroenterol Motil. 2012;24:575–585. e257. doi: 10.1111/j.1365-2982.2012.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Q, Pan XQ, Villamor AN, Asfaw TS, Chang S, Zderic SA, Malykhina AP. Lack of transient receptor potential vanilloid 1 channel modulates the development of neurogenic bladder dysfunction induced by cross-sensitization in afferent pathways. Journal of neuroinflammation. 2013;10:3. doi: 10.1186/1742-2094-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R, Ustinova EE, Patnam R, Fraser MO, Gutkin DW, Pezzone MA. Enhanced expression of mast cell growth factor and mast cell activation in the bladder following the resolution of trinitrobenzenesulfonic acid (TNBS) colitis in female rats. Neurourol Urodyn. 2007;26:887–893. doi: 10.1002/nau.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhina AP. Neural mechanisms of pelvic organ cross-sensitization. Neuroscience. 2007;149:660–672. doi: 10.1016/j.neuroscience.2007.07.053. [DOI] [PubMed] [Google Scholar]
- Malykhina AP, Qin C, Foreman RD, Akbarali HI. Colonic inflammation increases Na+ currents in bladder sensory neurons. Neuroreport. 2004;15:2601–2605. doi: 10.1097/00001756-200412030-00008. [DOI] [PubMed] [Google Scholar]
- Malykhina AP, Qin C, Greenwood-van Meerveld B, Foreman RD, Lupu F, Akbarali HI. Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil. 2006;18:936–948. doi: 10.1111/j.1365-2982.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- Malykhina AP, Wyndaele JJ, Andersson KE, De Wachter S, Dmochowski RR. Do the urinary bladder and large bowel interact, in sickness or in health? ICI-RS 2011. Neurourol Urodyn. 2012;31:352–358. doi: 10.1002/nau.21228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslany S, Crockett DP, Egger MD. Organization of cutaneous primary afferent fibers projecting to the dorsal horn in the rat: WGA-HRP versus B-HRP. Brain Res. 1992;569:123–135. doi: 10.1016/0006-8993(92)90378-m. [DOI] [PubMed] [Google Scholar]
- McNeal DW, Darling WG, Ge J, Stilwell-Morecraft KS, Solon KM, Hynes SM, Pizzimenti MA, Rotella DL, Vanadurongvan T, Morecraft RJ. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J Comp Neurol. 2010;518:586–621. doi: 10.1002/cne.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill DL, Burden HW. Convergence of sensory processes from the heart and left ulnar nerve onto a single afferent perikaryon: a neuroanatomical study in the rat employing fluorescent tracers. Anat Rec. 1986;214:441–444. doi: 10.1002/ar.1092140416. [DOI] [PubMed] [Google Scholar]
- Michl T, Jocic M, Heinemann A, Schuligoi R, Holzer P. Vagal afferent signaling of a gastric mucosal acid insult to medullary, pontine, thalamic, hypothalamic and limbic, but not cortical, nuclei of the rat brain. Pain. 2001;92:19–27. doi: 10.1016/s0304-3959(00)00467-x. [DOI] [PubMed] [Google Scholar]
- Monnikes H, Ruter J, Konig M, Grote C, Kobelt P, Klapp BF, Arnold R, Wiedenmann B, Tebbe JJ. Differential induction of c-fos expression in brain nuclei by noxious and non-noxious colonic distension: role of afferent C-fibers and 5-HT3 receptors. Brain Res. 2003;966:253–264. doi: 10.1016/s0006-8993(02)04197-5. [DOI] [PubMed] [Google Scholar]
- Nishii H, Nomura M, Fujimoto N, Matsumoto T. Thalamic neural activation in the cyclophosphamide-induced visceral pain model in mice. Neuroscience research. 2008;60:219–227. doi: 10.1016/j.neures.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Noronha R, Akbarali H, Malykhina A, Foreman RD, Greenwood-Van Meerveld B. Changes in urinary bladder smooth muscle function in response to colonic inflammation. Am J Physiol Renal Physiol. 2007;293:F1461–1467. doi: 10.1152/ajprenal.00311.2007. [DOI] [PubMed] [Google Scholar]
- Ohtori S, Takahashi K, Yamagata M, Sameda H, Moriya H, Chiba T, Takahashi Y. Neurones in the dorsal root ganglia of T13, L1 and L2 innervate the dorsal portion of lower lumbar discs in rats. A study using diI, an anterograde neurotracer. J Bone Joint Surg Br. 2001;83:1191–1194. doi: 10.1302/0301-620x.83b8.11012. [DOI] [PubMed] [Google Scholar]
- Ortega-Villalobos M, Garcia-Bazan M, Solano-Flores LP, Ninomiya-Alarcon JG, Guevara-Guzman R, Wayner MJ. Vagus nerve afferent and efferent innervation of the rat uterus: an electrophysiological and HRP study. Brain Res Bull. 1990;25:365–371. doi: 10.1016/0361-9230(90)90221-k. [DOI] [PubMed] [Google Scholar]
- Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol. 2002;87:2095–2103. doi: 10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- Pan XQ, Gonzalez JA, Chang S, Chacko S, Wein AJ, Malykhina AP. Experimental colitis triggers the release of substance P and calcitonin gene-related peptide in the urinary bladder via TRPV1 signaling pathways. Exp Neurol. 2010;225:262–273. doi: 10.1016/j.expneurol.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128:1953–1964. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Qiao LY, Grider JR. Up-regulation of calcitonin gene-related peptide and receptor tyrosine kinase TrkB in rat bladder afferent neurons following TNBS colitis. Exp Neurol. 2007;204:667–679. doi: 10.1016/j.expneurol.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Malykhina AP, Akbarali HI, Foreman RD. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology. 2005;129:1967–1978. doi: 10.1053/j.gastro.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Ragnarson B, Bengtsson L, Haegerstrand A. Labeling with fluorescent carbocyanine dyes of cultured endothelial and smooth muscle cells by growth in dye-containing medium. Histochemistry. 1992;97:329–333. doi: 10.1007/BF00270034. [DOI] [PubMed] [Google Scholar]
- Randall WC, Milosavljevic M, Wurster RD, Geis GS, Ardell JL. Selective vagal innervation of the heart. Ann Clin Lab Sci. 1986;16:198–208. [PubMed] [Google Scholar]
- Rau KK, Jiang N, Johnson RD, Cooper BY. Heat sensitization in skin and muscle nociceptors expressing distinct combinations of TRPV1 and TRPV2 protein. J Neurophysiol. 2007;97:2651–2662. doi: 10.1152/jn.00840.2006. [DOI] [PubMed] [Google Scholar]
- Rivero-Melian C, Grant G. Choleragenoid horseradish peroxidase used for studying projections of some hindlimb cutaneous nerves and plantar foot afferents to the dorsal horn and Clarke's column in the rat. Exp Brain Res. 1991;84:125–132. doi: 10.1007/BF00231767. [DOI] [PubMed] [Google Scholar]
- Robertson B, Arvidsson J. Transganglionic transport of wheat germ agglutinin-HRP and choleragenoid-HRP in rat trigeminal primary sensory neurons. Brain Res. 1985;348:44–51. doi: 10.1016/0006-8993(85)90357-9. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Chen MC, Mongiu AK, Klumpp DJ. Organ cross talk modulates pelvic pain. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1191–1198. doi: 10.1152/ajpregu.00411.2007. [DOI] [PubMed] [Google Scholar]
- Sakurai J, Obata K, Ozaki N, Tokunaga A, Kobayashi K, Yamanaka H, Dai Y, Kondo T, Miyoshi K, Sugiura Y, Matsumoto T, Miwa H, Noguchi K. Activation of extracellular signal-regulated protein kinase in sensory neurons after noxious gastric distention and its involvement in acute visceral pain in rats. Gastroenterology. 2008;134:1094–1103. doi: 10.1053/j.gastro.2008.01.031. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol. 2008;506:659–93. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- Saper CB, Kibbe MR, Hurley KM, Spencer S, Holmes HR, Leahy KM, Needleman P. Brain natriuretic peptide-like immunoreactive innervation of the cardiovascular and cerebrovascular systems in the rat. Circ Res. 1990;67:1345–1354. doi: 10.1161/01.res.67.6.1345. [DOI] [PubMed] [Google Scholar]
- Schachter SC, Saper CB. Vagus nerve stimulation. Epilepsia. 1998;39:677–686. doi: 10.1111/j.1528-1157.1998.tb01151.x. [DOI] [PubMed] [Google Scholar]
- Schuligoi R, Jocic M, Heinemann A, Schoninkle E, Pabst MA, Holzer P. Gastric acid-evoked c-fos messenger RNA expression in rat brainstem is signaled by capsaicin-resistant vagal afferents. Gastroenterology. 1998;115:649–660. doi: 10.1016/s0016-5085(98)70144-1. [DOI] [PubMed] [Google Scholar]
- Sharkey KA, Williams RG. Extrinsic innervation of the rat pancreas: demonstration of vagal sensory neurones in the rat by retrograde tracing. Neurosci Lett. 1983;42:131–135. doi: 10.1016/0304-3940(83)90395-6. [DOI] [PubMed] [Google Scholar]
- Sharkey KA, Williams RG, Dockray GJ. Sensory substance P innervation of the stomach and pancreas. Demonstration of capsaicin-sensitive sensory neurons in the rat by combined immunohistochemistry and retrograde tracing. Gastroenterology. 1984;87:914–921. [PubMed] [Google Scholar]
- Sherrington SC. The Integrative Action of The Nervous System. Yale University Press; New Haven: 1906. [Google Scholar]
- Shoja MSA, Mirzayan N, Groat C, Watanabe K, Loukas M, Tubbs RS. Neuroanatomy of the female abdominopelvic region: A review with application to pelvic pain syndromes. Clinical Anatomy. 2013;26:66–76. doi: 10.1002/ca.22200. [DOI] [PubMed] [Google Scholar]
- Spyer KM. Annual review prize lecture. Central nervous mechanisms contributing to cardiovascular control. J Physiol. 1994;474:1–19. doi: 10.1113/jphysiol.1994.sp019997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Wachtel RE, Gebhart GF. Capsaicin sensitivity and voltage-gated sodium currents in colon sensory neurons from rat dorsal root ganglia. Am J Physiol. 1999;277:G1180–1188. doi: 10.1152/ajpgi.1999.277.6.G1180. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci. 2005;25:2617–2627. doi: 10.1523/JNEUROSCI.2894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torvik A. Afferent connections to the sensory trigeminal nuclei, the nucleus of the solitary tract and adjacent structures; an experimental study in the rat. J Comp Neurol. 1956;106:51–141. doi: 10.1002/cne.901060104. [DOI] [PubMed] [Google Scholar]
- Ueno T, Ueno S, Kakazu Y, Akaike N, Nabekura J. Bidirectional modulation of P2X receptor-mediated response by divalent cations in rat dorsal motor nucleus of the vagus neurons. J Neurochem. 2001;78:1009–1018. doi: 10.1046/j.1471-4159.2001.00473.x. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–917. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustinova EE, Fraser MO, Pezzone MA. Colonic irritation in the rat sensitizes urinary bladder afferents to mechanical and chemical stimuli: an afferent origin of pelvic organ cross-sensitization. Am J Physiol Renal Physiol. 2006;290:F1478–1487. doi: 10.1152/ajprenal.00395.2005. [DOI] [PubMed] [Google Scholar]
- Ustinova EE, Fraser MO, Pezzone MA. Cross-talk and sensitization of bladder afferent nerves. Neurourol Urodyn. 2010;29:77–81. doi: 10.1002/nau.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustinova EE, Gutkin DW, Pezzone MA. Sensitization of pelvic nerve afferents and mast cell infiltration in the urinary bladder following chronic colonic irritation is mediated by neuropeptides. Am J Physiol Renal Physiol. 2007;292:F123–130. doi: 10.1152/ajprenal.00162.2006. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Luppi PH, Zhu Y, Van Bockstaele E, Aston-Jones G. Evidence for widespread afferents to Barrington's nucleus, a brainstem region rich in corticotropin-releasing hormone neurons. Neuroscience. 1994;62:125–43. doi: 10.1016/0306-4522(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Mouton LJ, Blok BF, Holstege G. Distinct cell groups in the lumbosacral cord of the cat project to different areas in the periaqueductal gray. J Comp Neurol. 1996;376:361–385. doi: 10.1002/(SICI)1096-9861(19961216)376:3<361::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Vilensky JA, Bell DR, Gilman S. “On the physiology of micturition” by Denny-Brown and Robertson: a classic paper revisited. Urology. 2004;64:182–186. doi: 10.1016/S0090-4295(03)00341-8. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Brisson M, de Groat WC. Transneuronal labeling of neurons in the adult rat central nervous system following inoculation of pseudorabies virus into the colon. Cell Tissue Res. 2000;299:9–26. doi: 10.1007/s004419900128. [DOI] [PubMed] [Google Scholar]
- Wang G, Scott SA. Independent development of sensory and motor innervation patterns in embryonic chick hindlimbs. Dev Biol. 1999;208:324–336. doi: 10.1006/dbio.1999.9212. [DOI] [PubMed] [Google Scholar]
- Wang HZ, Li SR, Wen C, Xiao CG, Su BY. Morphological changes of cholinergic nerve fibers in the urinary bladder after establishment of artificial somatic-autonomic reflex arc in rats. Neurosci Bull. 2007;23:277–281. doi: 10.1007/s12264-007-0041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorwell PJ, Lupton EW, Erduran D, Wilson K. Bladder smooth muscle dysfunction in patients with irritable bowel syndrome. Gut. 1986a;27:1014–1017. doi: 10.1136/gut.27.9.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorwell PJ, McCallum M, Creed FH, Roberts CT. Non-colonic features of irritable bowel syndrome. Gut. 1986b;27:37–40. doi: 10.1136/gut.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyndaele JJ. Investigation of the afferent nerves of the lower urinary tract in patients with ‘complete’ and ‘incomplete’ spinal cord injury. Paraplegia. 1991;29:490–494. doi: 10.1038/sc.1991.67. [DOI] [PubMed] [Google Scholar]
- Wyndaele JJ. Correlation between clinical neurological data and urodynamic function in spinal cord injured patients. Spinal Cord. 1997;35:213–216. doi: 10.1038/sj.sc.3100391. [DOI] [PubMed] [Google Scholar]
- Zhong F, Christianson JA, Davis BM, Bielefeldt K. Dichotomizing axons in spinal and vagal afferents of the mouse stomach. Dig Dis Sci. 2008;53:194–203. doi: 10.1007/s10620-007-9843-z. [DOI] [PubMed] [Google Scholar]
- Zhuo H, Ichikawa H, Helke CJ. Neurochemistry of the nodose ganglion. Prog Neurobiol. 1997;52:79–107. doi: 10.1016/s0301-0082(97)00003-8. [DOI] [PubMed] [Google Scholar]