In association with their participation in an 8-month physical activity program, improvements in memory were observed in older cognitively-normal adults with a family history of Alzheimer’s disease.
Keywords: Exercise, APOE, Genetic risk, Executive function, Memory, Information processing
Abstract
Background
Alzheimer’s disease is a progressive disease that degrades cognitive functioning and ultimately results in death. Currently, there is no cure for Alzheimer’s disease and, hence, the identification of preventative strategies is important. Physical activity (PA) is a behavioral intervention that holds promise with respect to delaying the onset of Alzheimer’s disease.
Purpose
The purpose of this study was to explore the differential cognitive benefits achieved in response to PA as a function of a person’s genetic risk for AD.
Methods
Older cognitively normal adults (50–65 years) with a family history of AD (FHxAD) participated in an 8-month PA program. Cognitive performance was measured at baseline, pretest, midtest, and posttest and changes over time were assessed as a function of apolipoprotein E (APOE) status (carriers: 1–2 copies of the ɛ4 allele; noncarriers: 0 copies of the ɛ4 allele).
Results
Improvements in memory were associated with PA participation irrespective of APOE ɛ4 carrier status.
Conclusions
Future experimental studies are needed to confirm that PA causes improvements to cognitive performance in older cognitively normal adults with a FHxAD and that these improvements are equivalent for cognitively normal APOE ɛ4 carriers and noncarriers.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that afflicts approximately 5.4 million Americans [1] with expectations that its prevalence will triple from 2010 to 2050. Currently, there is no cure for AD, thus researchers are exploring preventive strategies that could delay its onset [2]. One preventive strategy that is receiving attention is physical activity (PA). The cognitive reserve hypothesis provides a rationale for why PA might positively influence cognition, slow age-related cognitive decline, and delay the onset of AD [3]. This hypothesis suggests that cognitive reserves may be passive (related to brain structure) or active (related to brain function) and that reserves are decreased with advancing age or brain pathology. However, the hypothesis also postulates that cognitive reserves can be increased through lifestyle behaviors including formal education, mental stimulation through one’s occupation, and PA [4]. Considered together, these two propositions suggest that persons who have increased their cognitive reserves will have a lesser risk of dementia [4–6]. In support of the cognitive reserve hypothesis, there is evidence that PA benefits cognitive performance and reduces the risk of AD and dementia.
When reviewed meta-analytically, prospective evidence shows that PA is predictive of less cognitive decline [7] and a reduced risk of AD and dementia [8, 9] with advancing age. There is also experimental evidence showing that PA results in improvements in cognitive performance by older cognitively normal adults [10]. One important question to consider, however, is the extent to which PA can be protective for individuals who have an increased risk for AD. Individuals who have a family history of AD (FHxAD) are at increased risk of cognitive decline and AD [11–13]. In addition, apolipoprotein E (APOE) is a susceptibility gene for AD [14–19]. There is a dose-response relationship between the APOE epsilon 4 (ɛ4) allele and the risk of AD, with one copy of the ɛ4 allele resulting in 3–4 times [20, 21] and 2 copies of the ɛ4 allele resulting in 5–18 times [22] greater risk as compared to persons without the ɛ4 allele (noncarriers). Hence, it is important to understand the extent to which PA is protective against AD in persons with a FHxAD and as a function of APOE genotype.
Evidence from cross-sectional [23, 24] and prospective studies [25–32] shows that the relationship between PA or aerobic fitness and cognitive performance is moderated by APOE genotype. In particular, results from cross-sectional studies and from six of the eight prospective studies [25, 27–29, 31, 32] indicate that the benefits of PA for cognitive performance are largest for those at greatest genetic risk for AD. However, to our knowledge, there are no human studies that have experimentally tested APOE genotype as a moderator of the effect of PA on cognitive performance. Hence, the goal of this study was to conduct a PA intervention with older adults with a FHxAD to assess the extent to which these individuals could benefit from PA and to compare the cognitive benefits observed as a function of APOE ɛ4 carrier status.
Methods
Detailed methods and a consort flow diagram for the Physical Activity and Alzheimer’s Disease (PAAD) study have been previously published [33]. Hence, the study methods are briefly described herein.
Participants
Older (50–65 years) cognitively normal adults with a FHxAD were recruited in three cohorts to participate in an 8-month PA program. Recruitment took place via newsletters, radio advertisements, presentations, news columns, and flyer distribution targeted toward older adults. Recruitment efforts resulted in 136 individuals completing a telephone interview to initially determine eligibility relative to inclusion criteria and exclusion criteria. To be included in the study, participants had to be between 50 and 65 years of age, speak English, and fail to meet PA recommendations (i.e., perform fewer than 150 min of moderate intensity PA per week over the previous 3 months) based upon the Guidelines of the American College of Sports Medicine [34]. During this interview, eligibility was also determined relative to exclusion criteria for cognitive performance (see Cognitive Tests) and major contraindications to exercise. Of the 136 individuals who completed the telephone interview, 20 decided not to participate after learning more about the required commitment and 50 were determined to be ineligible. Thus, 66 participants completed baseline testing. Additional exclusion criteria were assessed at baseline testing as follows: participants were excluded from the study if they had any additional contraindications to PA based upon ACSM guidelines and risk categorizations (high risk were excluded, moderate risk were included with signed permission from their physician), had any chronic illness (e.g., mild-cognitive impairment, depression) or medication use (e.g., medication for memory problems) that would be expected to influence cognitive performance, or had uncorrected vision or hearing that would preclude participation in cognitive testing. After baseline testing, nine individuals decided they did not want to participate and three were excluded for health reasons. Thus, 54 participants were ultimately enrolled in the PA program.
Cognitive Tests
The modified Telephone Interview for Cognitive Status (TICS-m) [35] and the Folstein Mini-Mental Status Exam (MMSE) [36] were used to screen out participants with cognitive impairment (mild-cognitive impairment, AD, or other forms of dementia). Participants were included in the study if TICS-m scores were ≥36 [35] and MMSE scores were ≥27 (including a score between one and three on the recall subtest) [36].
Cognitive performance relative to the PA intervention was assessed across cognitive domains including attention (Paced Auditory Serial Addition Test [PASAT]), memory (Rey Auditory Verbal Learning Test [AVLT], Rey-Osterrieth Complex Figure Test [CFT], Digit Span), information processing (Wechsler Adult Intelligence Scale III Digit Symbol Substitution Task [WAIS-DS], Trail-Making Test A [TMT A], Stroop Color Test, Stroop Word Test), and executive function (EF; Trail-Making Test B [TMT B], Stroop Color-Word Test, set-switching [TMT B – TMT A], interference [Color-Word – average of Color and Word], Tower of London [TOL]). These measures have well established psychometrics, were selected because they have been used to assess cognitive performance in cognitively normal older adults in studies focused on AD [37, 38], and are expected to be sensitive to the early stages of dementia [39] and/or the effects of PA [10]. Specific measures for each test included in the statistical analyses are shown in Fig. 1.
Fig. 1.
Cognitive domains assessed in the study and the specific cognitive tests that were used.
PA Intervention
Participants were asked to come to the University campus to participate in the PA program at least 3 days per week for 8 months. All exercise sessions were led by an American College of Sports Medicine certified exercise physiologist who was assisted by graduate students in Kinesiology. Each session consisted of aerobic exercise (walking around the perimeter of the gymnasium for 15–20 min) and strength training for 30–40 min (time increased over the course of the 8 months). Participants were encouraged to walk at a speed that kept their heart rate at 60% of estimated maximal heart rate reserve (recalculated at 8-week intervals) and heart rate was recorded after 10 min of walking at every session. The strength training portion consisted of exercises completed with TheraBand resistance bands. The resistance level of the band, the number of exercises, the number of repetitions, and the number of sets gradually and individually increased over the 8-month period in response to strength gains. Exercise sessions were offered on 3 days of the week at three different times of day and the number of participants who were present at a given session ranged from 1 to 22 with six being the most common number of participants present at a session. Relative to prescribed sessions, the average attendance rate for participants who completed all of the cognitive testing sessions was 76% (see [40] for additional details regarding adherence). There were not significant differences in adherence as a function of APOE carrier status, p >.05.
Genotyping
Genomic DNA was extracted from buccal cell preparations at the University of North Carolina at Greensboro Molecular Core Laboratory for single nucleotide polymorphism (SNP) testing. The SNPs associated with the two amino acid residues (codons 112 and 158) were used to identify participants as APOE ɛ4 carriers (one or two copies of ɛ4) or APOE ɛ4 noncarriers (0 copies of ɛ4). Experimenters were blinded to the participants’ genotype for all exercise and testing sessions.
Procedure
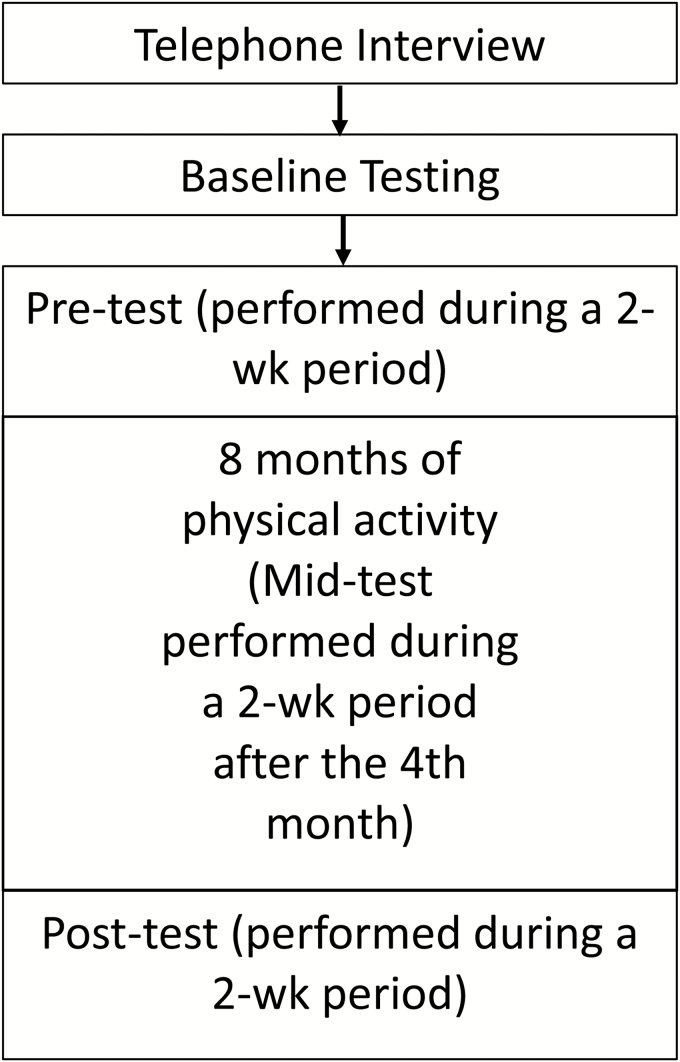
See Fig. 2 for an overview of the procedures. Screening for eligibility for the study took place in two parts. First, interested participants were interviewed over the telephone. This interview was used to more fully describe the study and to assess initial inclusion (50–65 years of age, FHxAD, not regularly physically active) and exclusion (contraindications to exercise, TICS-m score) criteria. Eligible participants were invited to baseline testing during which they were asked to sign a consent form approved by the University’s Institutional Review Board. At this time, depression (using the Geriatric Depression Scale), a medical health history, the American Heart Association/ACSM Health/Fitness Facility Preparticipation Screening Questionnaire, and the MMSE were completed to further assess eligibility, and baseline cognitive measures were taken. Cognitive measures were obtained at baseline to allow for a dual-baseline method whereby the most pronounced practice effects were expected to occur between baseline and pretest allowing for less substantial practice effects from pretest to midtest to posttest. Those participants who remained eligible and interested in participating after baseline screening were then assigned in three cohorts to begin the 8-month PA intervention. Because participants began the PA intervention in cohorts, variable amounts of time passed between baseline testing and the pretest, but for each cohort, each test (pre-, mid-, and post-) was performed within a 2-week period. Cognitive testing took place in a quiet laboratory space on the University campus and was conducted at the pretest (prior to beginning the intervention), midtest (following the 4th month of the intervention), and posttest (following the 8-month intervention). In addition, at pretest, midtest, and posttest, distance covered during a 6-min walk was assessed to provide an estimate of aerobic fitness [41].
Fig. 2.
Depiction of the study procedures.
Statistical Analysis
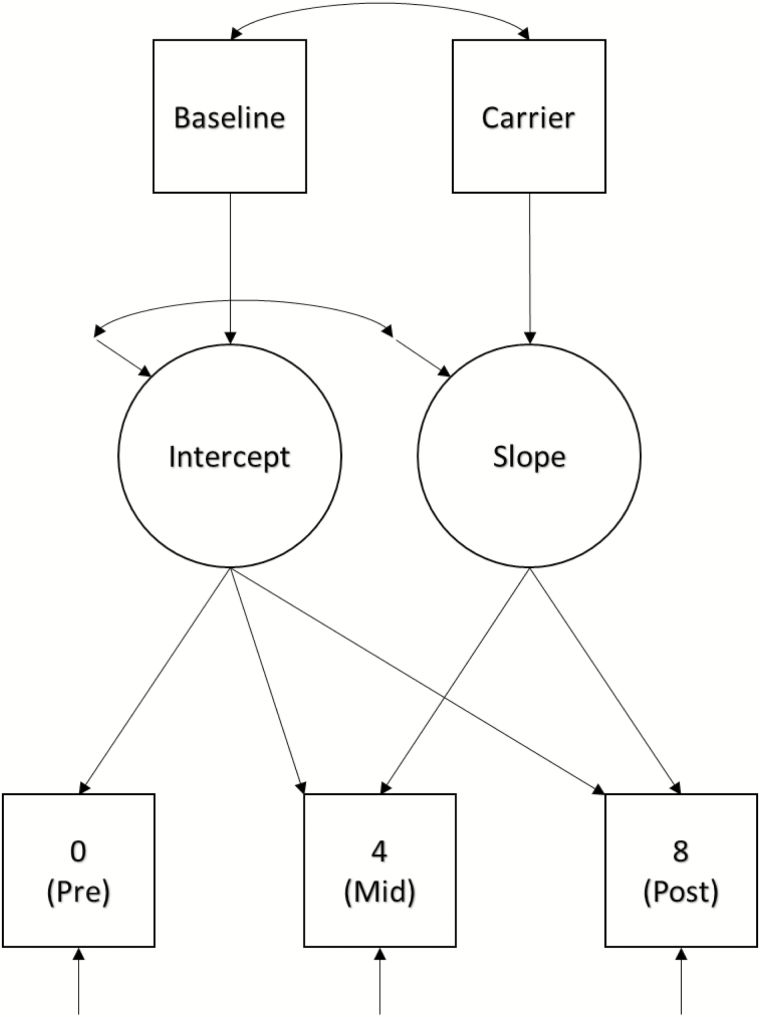
Descriptive information for the sample is presented in Table 1, and descriptive data for performance on the cognitive outcomes at each time point is presented in Table 2. Change in fitness across time was assessed using a repeated measures analysis of variance (ANOVA) with a Huynh-Feldt adjustment made in the case of violation of the sphericity assumption. Linear trajectories of change in cognitive performance across time were estimated using latent growth curve modeling (LGCM) [42]. Given the exploratory nature of this study, separate models were estimated for each cognitive outcome. This resulted in a total of 22 estimated models, grouped into four cognitive domains: Attention, Memory, Information Processing, and Executive Function. Time metrics were set at 0, 4, and 8 to model measures taken at pretest, midtest (4 months), and posttest (8 months) relative to the PA intervention. Cognitive performance at baseline and APOE ɛ4 carrier status (0 = noncarrier, 1 = carrier) were entered into the models as predictors of intercept and slope, respectively (see Fig. 3). Baseline cognitive performance was included as a predictor rather than as the first outcome time point to guard against the inflation of the slope coefficient due to practice or maturation effects [43], and because participants did not take part in the intervention during the time between baseline and pretest measures. Basic demographic variables (i.e., age, sex, and BMI) were also included as predictors of intercept and slope factors in initial models. However, they were only very sparsely associated with either growth factor, did not substantively alter associations between baseline performance and intercepts or between carrier status and slopes, and their inclusion did not improve model fit. Therefore, these covariates were excluded from the final models. Race and education variables were also excluded due to homogeneity of the sample (87% White, 88.9% higher education). Decisions involving inclusion or exclusion of covariates were held constant across all cognitive outcomes (n = 22) in order to facilitate interpretation of results. Had any of the basic demographics been associated with either of the latent growth factors for even a moderate portion of outcomes, they would have been retained in all models. The only decisions that were made on a model-by-model basis involved instances in which it had to be determined whether particular model parameters should be fixed or remain freely estimated. The most common example was a model that yielded a small, negative, nonsignificant residual variance estimate for the slope factor (i.e., nonpositive definite latent variable covariance matrix). In this case, the residual variance for slope was fixed to zero and the model re-estimated. These restrictions were only imposed when doing so substantively improved reliability of the parameter estimates and model fit.
Table 1.
Descriptive data for study participants (n = 54)
| M | SD | Range | |
|---|---|---|---|
| Age (years) | 56.98 | 4.61 | 50–65 |
| BMI | 28.13 | 4.12 | 20.3–35.6 |
| MMSE | 29.00 | 3.97 | 27–30 |
| n | % | ||
| Gender | |||
| Female | 43 | 79.6 | |
| Male | 11 | 20.4 | |
| Race | |||
| White | 47 | 87.0 | |
| Black | 6 | 11.1 | |
| Hispanic | 0 | 0.0 | |
| Native American | 0 | 0.0 | |
| Asian | 0 | 0.0 | |
| Other/unknown | 1 | 1.9 | |
| Education | |||
| Up to high school | 6 | 11.1 | |
| Up to Bachelor’s or Associate’s degree. | 28 | 51.9 | |
| Up to Graduate degree | 20 | 37.0 | |
| Genotype | |||
| Carrier | 23 | 43% | |
| Noncarrier | 31 | 57% | |
BMI body mass index; M mean; MMSE Mini-Mental State Examination; SD standard deviation.
Table 2.
Means and standard deviations for cognitive outcomes organized at each time point by cognitive domain
| Cognitive Domain (Test) | Baseline | Pretest | Midtest | Posttest |
|---|---|---|---|---|
| Attention | ||||
| PASAT3 | 44.56 (11.30) | 48.28 (11.26) | 50.33 (9.52) | 51.04 (9.38) |
| PASAT2 | 34.00 (9.50) | 37.80 (10.76) | 38.35 (10.16) | 42.29 (9.61) |
| Memory | ||||
| AVLT T1 | 5.87 (1.85) | 7.61 (2.02) | 8.72 (2.20) | 9.73 (2.27) |
| AVLT T6 | 9.94 (2.84) | 11.04 (2.60) | 11.54 (3.06) | 12.38 (2.24) |
| AVLT delayed recall | 9.41 (2.87) | 11.20 (2.81) | 11.63 (2.82) | 12.31 (2.35) |
| AVLT recognition | 13.57 (1.43) | 13.91 (1.66) | 14.19 (1.48) | 14.42 (2.24) |
| CFT recognition | 20.45 (1.79) | 20.94 (1.93) | 21.64 (1.46) | 21.48 (1.91) |
| Recall | 18.62 (6.61) | 23.35 (6.64) | 23.93 (5.47) | 26.21 (6.87) |
| CFT delayed recall | 18.36 (7.03) | 22.82 (5.55) | 23.69 (5.33) | 25.93 (6.18) |
| DS forward | 6.02 (1.22) | 6.52 (1.23) | 6.42 (1.51) | 6.42 (1.18) |
| DS backward | 4.72 (1.21) | 4.80 (1.04) | 4.98 (1.25) | 5.20 (1.15) |
| Information processing | ||||
| CFT copy | 33.94 (2.66) | 34.03 (2.62) | 34.10 (1.90) | 34.12 (1.82) |
| WAIS-DS | 51.59 (7.72) | 53.67 (7.90) | 54.44 (8.22) | 54.53 (8.21) |
| TMT A | 34.53 (7.61) | 33.83 (8.21) | 33.33 (10.68) | 31.57 (7.12) |
| Stroop Color | 65.85 (12.47) | 64.45 (11.75) | 65.01 (21.84) | 63.79 (10.92) |
| Stroop Word | 46.22 (6.85) | 48.27 (10.17) | 47.12 (8.91) | 45.98 (7.80) |
| Executive function | ||||
| TMT B | 55.24 (15.25) | 56.55 (16.96) | 53.45 (21.89) | 52.39 (13.78) |
| TMT exec function | 44.52 (15.29) | 46.89 (15.41) | 43.64 (21.29) | 43.51 (13.05) |
| Stroop Color-Word | 118.39 (27.92) | 110.92 (22.55) | 114.66 (29.31) | 107.74 (25.26) |
| Stroop Interference | 62.35 (22.71) | 54.56 (16.15) | 58.59 (27.38) | 52.86 (23.01) |
| TOL total moves | 82.08 (17.28) | 74.47 (12.42) | 74.23 (19.58) | 73.36 (13.76) |
| TOL total time | 363.89 (147.85) | 312.92 (113.12) | 312.45 (127.46) | 302.20 (96.61) |
AVLT Auditory Verbal Learning Test; CFT Complex Figure Test; DS digit span; PASAT Paced Auditory Serial Addition Test; TMT Trail-Making Test; TOL Tower of London; WAIS-DS Wechsler Adult Intelligence Scale III Digit Symbol Substitution Task.
Fig. 3.
Model representing the linear latent growth curve analyses.
There were two outcomes of primary interest in these models. One was the mean slope for each model, which was indicative of estimated monthly change in cognitive test performance. The other was the association of APOE ɛ4 carrier status with slope, which was indicative of whether test performance of APOE ɛ4 carriers changed at a rate different to noncarriers (i.e., moderation). Negative slopes and negative predictor associations were indicative of improvement for the TMT and the Stroop tasks, and for all measures of EF. For all other tasks, positive slopes and positive predictor associations with slope were indicative of improvement. Model fits were assessed by examining whether fit indices met commonly accepted criteria: chi-squared (p ≥ .05), root mean square error of approximation (<.05), comparative fit index (CFI; > .95), and Tucker Lewis index (TLI; > .95) and are presented in Table 3. Sample size limitations (n = 54) prevented estimation of higher-order (domain-level) models. Bonferroni corrections for multiple comparisons were determined too conservative; however, a correction factor of 10 (i.e., p < .005) was applied to address concerns over Type I error inflation. Further, results were interpreted in terms of consistency within each domain, rather than simply focusing on individual cognitive outcomes for which statistical significance was achieved.
Table 3.
Fit statistics for all latent growth models
| Attention | Χ 2 | df | p | RMSEA | CI90 | CFI | TLI |
|---|---|---|---|---|---|---|---|
| PASAT3 | 13.33 | 7 | 0.065 | 0.13 | 0.00–0.23 | 0.95 | 0.93 |
| PASAT2 | 13.15 | 7 | 0.069 | 0.13 | 0.00–0.23 | 0.96 | 0.95 |
| Memory | |||||||
| AVLT T1 | 4.59 | 5 | 0.469 | 0.00 | 0.00–0.18 | 1.00 | 1.00 |
| AVLT T6 | 4.25 | 5 | 0.515 | 0.00 | 0.00–0.17 | 1.00 | 1.00 |
| AVLT delayed recall | 4.77 | 6 | 0.574 | 0.00 | 0.00–0.16 | 1.00 | 1.00 |
| AVLT recognition | 4.80 | 5 | 0.440 | 0.00 | 0.00–0.19 | 1.00 | 1.00 |
| CFT delayed recognition | 10.84 | 7 | 0.146 | 0.10 | 0.00–0.21 | 0.81 | 0.76 |
| CFT immediate recall | 14.59 | 7 | 0.042 | 0.14 | 0.03–0.25 | 0.94 | 0.93 |
| CFT delayed recall | 7.67 | 5 | 0.176 | 0.10 | 0.00–0.23 | 0.98 | 0.97 |
| DS forward | 7.13 | 5 | 0.211 | 0.09 | 0.00–0.22 | 0.94 | 0.90 |
| DS backward | 2.46 | 7 | 0.930 | 0.00 | 0.00–0.05 | 1.00 | 1.00 |
| Information processing | |||||||
| CFT copy | 13.86 | 7 | 0.054 | 0.14 | 0.00–0.24 | 0.90 | 0.87 |
| WAIS-DS | 2.81 | 7 | 0.902 | 0.00 | 0.00–0.07 | 1.00 | 1.00 |
| TMT A | 5.96 | 7 | 0.544 | 0.00 | 0.00–0.15 | 1.00 | 1.00 |
| Stroop Color | 8.02 | 7 | 0.331 | 0.05 | 0.00–0.18 | 1.00 | 0.99 |
| Stroop Word | 4.92 | 5 | 0.426 | 0.00 | 0.00–0.19 | 1.00 | 1.00 |
| Executive function | |||||||
| TMT B | 9.86 | 6 | 0.131 | 0.11 | 0.00–0.23 | 0.96 | 0.94 |
| TMT set-switching | 10.40 | 6 | 0.109 | 0.12 | 0.00–0.24 | 0.94 | 0.91 |
| Stroop Color-Word | 8.48 | 7 | 0.292 | 0.06 | 0.00–0.19 | 0.99 | 0.98 |
| Stroop Interference | 6.57 | 5 | 0.255 | 0.08 | 0.00–0.22 | 0.97 | 0.95 |
| TOL total moves | 11.91 | 6 | 0.064 | 0.14 | 0.00–0.25 | 0.93 | 0.89 |
| TOL total time | 11.57 | 6 | 0.072 | 0.13 | 0.00–0.25 | 0.95 | 0.92 |
AVLT Auditory Verbal Learning Test; CFI comparative fit index; CFT Complex Figure Test; DS digit span; PASAT Paced Auditory Serial Addition Test; RMSEA root mean square error of approximation; TLI Tucker Lewis index; TMT Trail-Making Test; TOL Tower of London; WAIS-DS Wechsler Adult Intelligence Scale III Digit Symbol Substitution Task.
Results
Results of the repeated measures ANOVA indicated that there was a significant effect of time on fitness, F(1.79, 71.63) = 5.02, p = 0.01, with follow-up tests showing that fitness improved significantly from pretest (mean [M] = 556.79 ft, standard error [SE] = 10.26) to posttest (M = 594.85 ft, SE = 11.22). LGCM results are presented in Table 4 and are described below.
Table 4.
Results from linear growth curve models for each cognitive outcome organized by cognitive domain
| Intercept | p | Base (β) | p | Slope | p | Carrier | p | |
|---|---|---|---|---|---|---|---|---|
| Attention | ||||||||
| PASAT3 | 18.171 | 0.000 | 0.676 | 0.000 | 0.256 | 0.239 | -0.026 | 0.912 |
| PASAT2 | 8.994 | 0.012 | 0.836 | 0.000 | 0.384 | 0.017 | 0.068 | 0.772 |
| Memory | ||||||||
| AVLT Trial 1 | 4.029 | 0.000 | 0.608 | 0.000 | 0.287 | 0.000 | -0.074 | 0.276 |
| AVLT Trial 6 | 5.214 | 0.000 | 0.582 | 0.000 | 0.144 | 0.000 | 0.054 | 0.259 |
| AVLT delayed recall | 6.078 | 0.000 | 0.538 | 0.000 | 0.150 | 0.004 | -0.016 | 0.795 |
| AVLT delayed recognition | 8.731 | 0.000 | 0.376 | 0.017 | 0.093 | 0.031 | -0.036 | 0.478 |
| CFT delayed recognition | 15.142 | 0.000 | 0.293 | 0.001 | 0.024 | 0.597 | 0.094 | 0.140 |
| CFT immediate recall | 11.275 | 0.000 | 0.635 | 0.000 | 0.335 | 0.001 | 0.012 | 0.930 |
| CFT delayed recall | 12.379 | 0.000 | 0.567 | 0.000 | 0.389 | 0.000 | -0.045 | 0.703 |
| DS forward | 4.350 | 0.000 | 0.357 | 0.007 | -0.002 | 0.930 | -0.042 | 0.341 |
| DS backward | 2.753 | 0.000 | 0.431 | 0.000 | 0.044 | 0.087 | -0.002 | 0.951 |
| Information processing | ||||||||
| CFT copy | 20.894 | 0.000 | 0.385 | 0.000 | 0.057 | 0.165 | -0.037 | 0.615 |
| WAIS-DS | 6.943 | 0.022 | 0.907 | 0.000 | 0.141 | 0.114 | -0.054 | 0.715 |
| TMT A | 8.786 | 0.019 | 0.725 | 0.000 | -0.061 | 0.682 | -0.322 | 0.118 |
| Stroop Color | 10.524 | 0.009 | 0.819 | 0.000 | -0.007 | 0.949 | -0.177 | 0.214 |
| Stroop Word | -2.172 | 0.662 | 1.088 | 0.000 | -0.218 | 0.009 | -0.034 | 0.778 |
| Executive function | ||||||||
| TMT B | 18.747 | 0.020 | 0.680 | 0.000 | -0.302 | 0.293 | -0.050 | 0.902 |
| TMT set-switching | 21.038 | 0.000 | 0.576 | 0.000 | -0.280 | 0.335 | 0.007 | 0.987 |
| Stroop Color-Word | 30.075 | 0.001 | 0.684 | 0.000 | -0.343 | 0.245 | 0.819 | 0.330 |
| Stroop interference | 21.933 | 0.000 | 0.526 | 0.000 | -0.253 | 0.508 | 0.839 | 0.271 |
| TOL total moves | 38.297 | 0.001 | 0.441 | 0.003 | -0.050 | 0.878 | -0.156 | 0.687 |
| TOL total time | 137.183 | 0.000 | 0.511 | 0.000 | -0.906 | 0.835 | -2.156 | 0.401 |
AVLT Auditory Verbal Learning Test; Base baseline; CFT Complex Figures Test; DS digit span; n/a indicates that the parameter was fixed to 0; PASAT Paced Auditory Serial Addition Test; TMT Trail-Making Test; TOL Tower of London.
Attention
Attention was assessed using two cognitive outcomes (PASAT2, PASAT3). Fit indices suggested poor-to-mediocre fit for both the PASAT2 and the PASAT3 models. Baseline performance was significantly positively predictive of the intercept for both measures of attention (p < .005) indicating that scores at baseline were associated with scores at pretest. Participants failed to demonstrate improvement across time on either the PASAT2 (p = .017) or the PASAT3 (p = .24) Carrier status was not significantly predictive of slope for either of the PASAT tasks (p’s = .77-.91).
Memory
Memory was assessed using nine cognitive outcomes: AVLT (Trial 1 & Trial 6, delayed recall, delayed recognition), CFT (immediate recall, delayed recall, delayed recognition), and Digit Span (Forward, Backward). Fit indices suggested good-to-excellent fit for AVLT Trial 1 and Trial 6, AVLT and CFT-delayed recall, AVLT delayed recognition, and Digit Span Backward models, poor-to-mediocre fit for the Digit Span Forward model, and poor fit for the CFT-delayed recognition and immediate recall models. Baseline performance was a significant positive predictor of intercept in all memory models (p < .001) with the exception of AVLT delayed recognition (p = .017), and Digit Span Forward (p = .007). Participants demonstrated significant improvement (i.e., significant slope factors) for AVLT Trial 1, Trial 6, delayed recall (p < .005) and for CFT immediate and delayed recall (p < .001). Slope factors did not reach significance for the AVLT delayed recognition model (p = .031), the CFT-delayed recognition model (p = .597), or either the Digit Span Forward (p = .930) or Backward (p = .087) models. Carrier status was again not significantly associated with the slope factors in any of the memory outcome models.
Information Processing
Information processing was assessed using five cognitive outcomes: CFT copy, WAIS-DS, TMT A, Stroop Color, and Stroop Word. Fit indices suggested excellent fit for all models except CFT copy, for which fit was poor. Baseline performance was significantly, positively predictive of intercept for all information processing outcomes (p < .001). Participants failed to demonstrate significant improvement for any of the information processing outcomes. Carrier status was again not significantly predictive of slope for any information processing outcomes (p = .12–.78).
Executive Function
EF was assessed using six cognitive outcomes: TMT B, TMT set-switching (TMT B-TMT A), Stroop Color-Word time, and Stroop interference scores, and TOL total moves and total time to complete. Fit indices suggested good-to-excellent fit for Stroop Color-Word and Stroop interference models, mediocre fit for the TMT B and TOL total moves models; and, poor fit for the TMT set-switching and TOL total time models. Baseline performance was significantly, positively predictive of intercept for all EF outcomes (p < .005). However, participants did not demonstrate significant change in performance (p = .24 – .99), and carrier status was not significantly predictive of slope for any EF outcomes (p = .27 – .99).
Discussion
The cognitive reserve hypothesis and evidence from past research support the expectation that participation in a PA program will benefit cognitive performance by older adults. There is also cross-sectional and prospective evidence suggesting that the effects of PA on cognitive performance are moderated by APOE ɛ4 carrier status. The purpose of this study was to assess the extent to which benefits that are associated with participation in an 8-month exercise program can be observed in persons with a FHxAD and to assess the extent to which these benefits differ as a function of one’s genetic risk for Alzheimer’s disease.
Results from this study partially support past research showing that PA by older adults improves cognitive performance [10]. In particular, in association with their participation in an 8-month PA program consisting of aerobic exercise and strength training, older cognitively normal adults improved on multiple measures of memory. It is important to point out that study participants all had a FHxAD, yet the sample achieved cognitive benefits from pretest to posttest that are similar to what has been observed in previous samples that do not have this familial risk of AD. This is an important finding because of the fact that a FHxAD is associated with a heightened risk of AD [11, 12] and there is no known cure for AD. Further, if PA helps to maintain cognitive performance over time in persons with an increased risk of AD due to their familial history, this could have important public health implications. This is because delaying the onset of AD by as little as 6 months can reduce the prevalence of AD by 100,000 people after 10 years [44].
Of additional importance is the fact that these improvements were generally not influenced by APOE ɛ4 carrier status. That is, these results suggest that cognitively normal older adults with a FHxAD can achieve cognitive benefits to memory that are associated with participation in a PA program and that these benefits are evident irrespective of whether or not they carry the APOE ɛ4 allele which also heightens their genetic risk for AD. Importantly, it must be emphasized that because of the lack of a control group, it is not possible to know for certain if these improvements over time are causally related to the PA program or if they reflect practice effects [45]. Although we used a dual-baseline method to minimize practice effects across the PA intervention, past research has shown that practice effects can occur with repeated trials on cognitive measures like those used in this study. Hence, the lack of a control group and the potential for practice effects is a primary limitation of this study.
As previously mentioned, the primary limitation of this study is the lack of a control group. However, the decision was made a priori to focus resources on a case–control study specifically aimed at exploring the differential effects of an 8-month PA intervention on cognitive performance relative to APOE ɛ4 status. While this design precludes our ability to determine causality, findings will facilitate the design of future randomized control trials (RCTs). Interpreting these results relative to previous literature is challenging because this is the first study in which PA was manipulated so that associated changes in cognitive performance could be observed. The most relevant previous literature consists exclusively of nonexperimental prospective studies in which researchers typically compared active individuals to inactive individuals by assessing changes in global cognitive performance (e.g., MMSE) or clinical cognitive impairment over several years. Although most of these studies suggest that the benefits of PA are greater for carriers of the APOE ɛ4 allele than for noncarriers, we do not believe our results to be inconsistent with this past literature. It is our interpretation that amongst cognitively normal inactive adults aged 50–65 years and with a FHxAD, both those with and without a heightened genetic risk for AD can achieve similar behavioral cognitive benefits from exercise. Subsequent research will be needed to assess the extent to which these cognitive gains slow age-related declines in cognitive performance and lessen the risk for clinical cognitive impairment, both of which would be expected to be greater for the carriers than for the noncarriers [46], as they progress past the age range observed in the present study.
One surprising finding in this study that should be acknowledged was the failure to observe improvements in EF in response to PA. In a meta-analytic review of RCTs with adults aged 50 years and over, Colcombe and Kramer [10] reported the largest effects for measures of EF (g = 0.68). Thus, we expected to see improvements in EF associated with participation in PA. However, Smith et al. [47]. meta-analytically reviewed RCTs with adults and reported that the average effect size for measures of EF was substantially smaller (g = 0.12). They suggested that Colcombe and Kramer’s report might have been inflated because of the inclusion of two studies with relatively large positive effects that were not actually RCTs. It is also possible that setting the upper age limit at 65 in the present study made detection of changes in EF and other cognitive domains more difficult. In the aforementioned meta-analysis, the reported effect sizes for studies with participants ranging in age from 50 to 65 years were significantly lower than those reported for studies with older participants [10]. This pattern is not surprising given the observation that the average age of onset for AD or age-related cognitive decline ranges from the late 60’s to 70’s depending upon APOE ɛ4 carrier status [17, 19].
In sum, this study provides initial evidence that participation in a PA program is associated with cognitive performance benefits to memory in older cognitively normal adults with a FHxAD and regardless of their APOE ɛ4 carrier status. This is consistent with past RCTs which have shown that PA results in improvements in cognitive performance as compared to control conditions [10, 47, 48], but also extends our understanding to an appreciation that these benefits can be obtained by persons with a FHxAD and that APOE ɛ4 carrier status does not moderate behavioral outcomes within this age range. Given that both persons with a FHxAD [11, 12] and APOE ɛ4 carriers are at a heightened risk for AD [14–19], this is important because increased cognitive reserves (as might be achieved through PA) may be protective against clinical cognitive impairment [4]. Further, prospective evidence indicates that APOE ɛ4 carriers who are physically active have a reduced risk of cognitive decline, dementia, and AD [25, 27, 28, 49]. If previously sedentary, older individuals can improve cognitive function through PA, the typical progression of cognitive decline may be sufficiently delayed to dramatically reduce an individual’s risk of AD and, at a population level, this could have an impact on world-wide prevalence [44]. Given that there is at this time no known cure for AD, further experimental research exploring the potential of PA as a preventive strategy is clearly warranted.
Compliance with Ethical Standards
Conflict of interest The authors declare that they have no conflict of interest.
Informed consent Informed consent was obtained from all individuals included in the study.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The researchers thank Michael Castellano, Lauren Williams, Devin Bueker, Valen Zhao, Megan Haas, and Melvin Gaddy for their assistance with the physical activity intervention and with data collection. Lastly, we thank the PAAD participants whose commitment to this study made the research possible.
Research reported in this publication was supported by the National Institute of Aging of the National Institutes of Health under award number R21 AG040310-01.
References
- 1. Alzheimer’s Association. Alzheimer’s Association Report: 2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7(2):208–244. [DOI] [PubMed] [Google Scholar]
- 2. Emery VO. Alzheimer disease: are we intervening too late? Pro. J Neural Transm (Vienna). 2011;118(9):1361–1378. [DOI] [PubMed] [Google Scholar]
- 3. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002; 8(3):448–460. [PubMed] [Google Scholar]
- 4. Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Res Rev. 2004;3(4):369–382. [DOI] [PubMed] [Google Scholar]
- 5. Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343–353. [DOI] [PubMed] [Google Scholar]
- 6. Scarmeas N, Stern Y. Cognitive reserve and lifestyle. J Clin Exp Neuropsychol. 2003;25(5):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sofi F, Valecchi D, Bacci D et al. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. 2011;269(1):107–117. [DOI] [PubMed] [Google Scholar]
- 8. Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39(1):3–11. [DOI] [PubMed] [Google Scholar]
- 9. Daviglus ML, Plassman BL, Pirzada A et al. Risk factors and preventive interventions for Alzheimer disease: state of the science. Arch Neurol. 2011;68(9):1185–1190. [DOI] [PubMed] [Google Scholar]
- 10. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. [DOI] [PubMed] [Google Scholar]
- 11. Green RC, Cupples LA, Go R et al. ; MIRAGE Study Group Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287(3): 329–336. [DOI] [PubMed] [Google Scholar]
- 12. Silverman JM, Ciresi G, Smith CJ, Marin DB, Schnaider-Beeri M. Variability of familial risk of Alzheimer disease across the late life span. Arch Gen Psychiatry. 2005;62(5): 565–573. [DOI] [PubMed] [Google Scholar]
- 13. Hayden KM, Zandi PP, West NA et al. ; Cache County Study Group Effects of family history and apolipoprotein E epsilon4 status on cognitive decline in the absence of Alzheimer dementia: the Cache County Study. Arch Neurol. 2009; 66(11):1378–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10(5):333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cedazo-Mínguez A. Apolipoprotein E and Alzheimer’s disease: molecular mechanisms and therapeutic opportunities. J Cell Mol Med. 2007;11(6):1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farrer LA, Cupples LA, Haines JL et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997; 278(16):1349–1356. [PubMed] [Google Scholar]
- 17. Gomez-Isla T, West HL, Rebeck GW et al. Clinical and pathological correlates of apolipoprotein E epsilon 4 in Alzheimer’s disease. Ann Neurol. 1996;39(1):62–70. [DOI] [PubMed] [Google Scholar]
- 18. Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63(3):287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Myers RH, Schaefer EJ, Wilson PW et al. Apolipoprotein E epsilon4 association with dementia in a population-based study: the Framingham study. Neurology. 1996;46(3):673–677. [DOI] [PubMed] [Google Scholar]
- 20. Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9(10):768–778. [DOI] [PubMed] [Google Scholar]
- 21. Kapur S, Sharad S, Kapoor M, Bala K. Apo E. Genotypes: risk factor for Alzheimer’s disease. J Indian Acad Clin Med. 2006;7(2):118–122. [Google Scholar]
- 22. Alzheimer’s Association Working Group. Apolipoprotein E genotyping in Alzheimer’s disease. Lancet. 1996;347(9008):1091–1095. [PubMed] [Google Scholar]
- 23. Deeny SP, Poeppel D, Zimmerman JB et al. Exercise, APOE, and working memory: MEG and behavioral evidence for benefit of exercise in epsilon4 carriers. Biol Psychol. 2008; 78(2):179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Etnier JL, Caselli RJ, Reiman EM et al. Cognitive performance in older women relative to ApoE-e4 genotype and aerobic fitness. Med Sci Sports Exerc. 2007;39(1): 199–207. [DOI] [PubMed] [Google Scholar]
- 25. Niti M, Yap KB, Kua EH, Tan CH, Ng TP. Physical, social and productive leisure activities, cognitive decline and interaction with APOE-epsilon 4 genotype in Chinese older adults. Int Psychogeriatr. 2008;20(2):237–251. [DOI] [PubMed] [Google Scholar]
- 26. Podewils LJ, Guallar E, Kuller LH et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161(7):639–651. [DOI] [PubMed] [Google Scholar]
- 27. Rovio S, Kåreholt I, Helkala EL et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705–711. [DOI] [PubMed] [Google Scholar]
- 28. Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33(5):772–777. [DOI] [PubMed] [Google Scholar]
- 29. Kivipelto M, Rovio S, Ngandu T et al. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med. 2008;12(6B): 2762–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tolppanen AM, Solomon A, Kulmala J et al. Leisure-time physical activity from mid- to late life, body mass index, and risk of dementia. Alzheimers Dement. 2015;11(4):434–443.e6. [DOI] [PubMed] [Google Scholar]
- 31. Luck T, Riedel-Heller SG, Luppa M et al. Apolipoprotein E epsilon 4 genotype and a physically active lifestyle in late life: analysis of gene-environment interaction for the risk of dementia and Alzheimer’s disease dementia. Psychol Med. 2014;44(6):1319–1329. [DOI] [PubMed] [Google Scholar]
- 32. Pizzie R, Hindman H, Roe CM et al. Physical activity and cognitive trajectories in cognitively normal adults: the adult children study. Alzheimer Dis Assoc Disord. 2014;28(1): 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Etnier JL, Labban JD, Karper WB et al. Innovative research design exploring the effects of physical activity and genetics on cognitive performance in community-based older adults. J Aging Phys Act. 2015;23(4):559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription (8th Ed). Philadelphia, PA: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 35. Cook SE, Marsiske M, McCoy KJ. The use of the Modified Telephone Interview for Cognitive Status (TICS-M) in the detection of amnestic mild cognitive impairment. J Geriatr Psychiatry Neurol. 2009;22(2):103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- 37. Reiman EM, Caselli RJ, Yun LS et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334(12): 752–758. [DOI] [PubMed] [Google Scholar]
- 38. Caselli RJ, Graff-Radford NR, Reiman EM et al. Preclinical memory decline in cognitively normal apolipoprotein E-epsilon4 homozygotes. Neurology. 1999;53(1):201–207. [DOI] [PubMed] [Google Scholar]
- 39. Lezak MD. Neuropsychological Assessment (3rd Ed). Oxford: Oxford University Press; 2004. [Google Scholar]
- 40. Etnier JL, Karper WB, Park SY, Shih CH, Piepmeier AT, Wideman L. Motivating mature adults to be physically active. J Aging Phys Act. 2017;25(2):325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Steele B. Timed walking tests of exercise capacity in chronic cardiopulmonary illness. J Cardiopulm Rehabil. 1996;16(1): 25–33. [DOI] [PubMed] [Google Scholar]
- 42. Bollen KA, Curran PJ.. Latent Curve Models: A Structural Equation Perspective. Hoboken, NJ: John Wiley and Sons; 2006. [Google Scholar]
- 43. Benedict RH, Zgaljardic DJ. Practice effects during repeated administrations of memory tests with and without alternate forms. J Clin Exp Neuropsychol. 1998;20(3):339–352. [DOI] [PubMed] [Google Scholar]
- 44. Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88(9): 1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Calamia M, Markon K, Tranel D. Scoring higher the second time around: meta-analyses of practice effects in neuropsychological assessment. Clin Neuropsychol. 2012;26(4):543–570. [DOI] [PubMed] [Google Scholar]
- 46. Caselli RJ, Dueck AC, Osborne D et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361(3):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith PJ, Blumenthal JA, Hoffman BM et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3): 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Etnier JL, Salazar W, Landers DM et al. The influence of physical fitness and exercise upon cognitive functioning: a meta-analysis. J Sport Exerc Psychol. 1997;19(3):249–277. [Google Scholar]
- 49. Kivipelto M, Helkala EL, Hänninen T et al. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology. 2001;56(12):1683–1689. [DOI] [PubMed] [Google Scholar]