Abstract
Background
As a safety and efficacy protocol, oocyte vitrification has been widely used in IVF treatment. The aim of this study was to evaluate the outcome of ICSI-ET utilizing vitrified oocytes with sperm obtained from non-obstructive azoospermia (NOA) patients via micro-TESE.
Material/Methods
A total of 150 NOA patients underwent micro-TESE. Ten patients were unable to ejaculate and refused to accept TESA at the time of oocyte retrieval; later, these patients underwent TESA. A total of 174 obstructive azoospermia (OA) patients underwent TESA. Vitrified oocytes were used with micro-TESE in 35 cycles (group 1), and TESA in 10 cycles (group 2). Fresh oocytes were used with micro-TESE in 38 cycles (group 3) and TESA in 174 cycles (group 4).
Results
The overall sperm retrieval rate of the 150 NOA patients was 48.7% (73/150). A total of 257 cycles of ICSI-ET were conducted with testicular spermatozoa; 212 cycles utilized fresh oocytes and 45 cycles utilized vitrified oocytes. No differences were observed with fertilization (73.8%, 77.2%,72.8%, 73.6%), implantation (33.3%, 34.7%, 33.8%, 37.5%), or clinical pregnancy rates (51.4%, 60%, 52.6%, 51.7%) for groups 1 through 4, respectively (P>0.05). Developmental competence was greatest among couples using sperm obtained via TESA rather than micro-TESE, not dependent on whether vitrified or fresh oocytes were utilized. Fertilization, implantation, and clinical pregnancy rates did not differ between using fresh vs. vitrified oocytes, nor did they differ between using testicular sperm derived from men with NOA vs. men with OA.
Conclusions
Vitrified oocytes combined with micro-TESE showed similar clinical efficacy when compared with fresh oocytes.
MeSH Keywords: Azoospermia; Microdissection; Sperm Injections, Intracytoplasmic; Sperm Retrieval; Vitrification
Background
Infertility refers to the inability of a couple to conceive after 12 months of regular unprotected sexual intercourse; it affects 10–15% of couples [1]. Male factor infertility is implicated in half of these couples [2,3]. The most severe form of male infertility, azoospermia, is present in 1% of males in the general population and accounts for 10–15% of male infertility. Azoospermia is divided into obstructive azoospermia (OA) and non-obstructive azoospermia (NOA). OA is due to a blockage anywhere along the path of sperm transit: efferent ducts to the epididymis, then vas deferens, followed by ejaculatory duct, and finally the urethra, before exiting from the urethral meatus. Depending on the level of obstruction, some men can undergo microsurgical reconstruction or ejaculatory duct resection, while others require sperm retrieval via the testicles or epididymis. NOA is due to defective spermatogenesis and can be classified as hypospermatogenesis (HS), maturation arrest (MA), or Sertoli cell only syndrome (SCOS) [4]. Defective spermatogenesis may be due to genetic abnormalities such as Y chromosome microdeletions or karyotype abnormalities in 15% of NOA patients, but is largely idiopathic [5]. Thus, men with NOA have fewer therapeutic options and reproduction relies on successful surgical sperm retrieval.
Micro-TESE is considered the criterion standard for sperm retrieval among NOA patients with the highest sperm retrieval rates while minimizing tissue loss [6–8]. However, sperm extracted from the testis have unique characteristics. The sperm obtained via retrieval in men with NOA are often immature and have not acquired proper motility. Therefore, they are not all viable and embryologists must rely on signs of potential success such as subtle twitching of the tails. Moreover, previous studies have identified a greater frequency of cytogenetically abnormal spermatozoa from men with NOA, despite their normal somatic karyotype [9,10]. Vozdova et al. [11] also demonstrated that testicular sperm samples of NOA patients show a higher incidence of numerical chromosomal abnormalities compared with ejaculated sperm of control donors as shown by multi-color fluorescence in situ hybridization. Although most relevant studies suggest that the rate of successful fertilization utilizing cryopreserved-thawed testicular spermatozoa is similar to that of fresh sperm, the freezing and thawing process can further damage sperm [12,13], and this can limit the number of viable sperm amenable to assisted reproduction technology. When available, use of fresh testicular sperm among NOA patients is preferred.
Oocyte cryopreservation is a viable option for fertility preservation among women undergoing cancer therapy and those with premature ovarian insufficiency [14,15]. Since human oocyte vitrification was first reported, this technology has evolved and is widely used clinically [16,17]. Oocyte cryopreservation has also become an important alternative to cryopreserved embryos for in vitro fertilization (IVF). More recent studies have demonstrated that vitrification of oocytes does not have a negative effect on oocyte physiology or the chromosomal status of the embryos derived [18–21]. Our reproductive center has been dedicated to the development of oocyte vitrification techniques and has achieved remarkable results.
In this study, we compared SRR among NOA patients stratified by the etiology of the NOA, and retrospectively compared fertilization and pregnancy outcomes of ICSI-ET cycles with fresh or frozen-thawed oocytes combined with testicular sperm derived from OA or NOA patients. We evaluated the feasibility of oocyte vitrification combined with micro-TESE.
Material and Methods
Subjects and treatment protocol
We retrospectively analyzed the medical records of NOA patients who underwent micro-TESE and OA patients who underwent TESA between December 2015 and February 2017 at the Department of Reproductive Medicine, Third Affiliated Hospital of Guangzhou Medical University, China. Azoospermia was confirmed by testing of at least 3 semen samples. All patients had a physical examination and assessment of sex hormones levels before surgical sperm retrieval. All 150 patients with NOA were assessed by a testicular volume clinical exam, serum studies (e.g., FSH, LH, and testosterone levels), and genetic studies (e.g., karyotyping and Y chromosome deletion analysis). We excluded patient with azoospermia secondary to testicular malignancy, any prior radiotherapy, and chemotherapy. The study protocol was approved by the Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University and all patients provided written informed consent before their micro-TESE and TESA.
Four groups were created based on the type of oocytes used (vitrified or fresh) and etiology of azoospermia (NOA, OA, or failure to ejaculate). Group 1 consisted of 70 NOA patients with fresh sperm obtained via micro-TESE. Those sperm underwent ICSI with vitrified and warmed oocytes. Group 2 was established to reduce the bias of only utilizing micro-TESE obtained sperm from NOA men with the vitrified oocytes. There were 10 men who were unable to provide an ejaculated sample and refused to accept TESA at the time of the oocyte retrieval. The oocytes were frozen. If the men still were unable to provide an ejaculated sample at a later time, they underwent testicular biopsy and ICSI-ET was performed with the sperm and the vitrified-then-warmed oocytes. Group 3 consisted of 80 NOA patients who underwent micro-TESE on the same day of oocyte retrieval for ICSI. Group 4 utilized sperm retrieved from 174 OA males either by TESA or with fresh oocytes. All couples were enrolled for final analysis (Figure 1).
Figure 1.
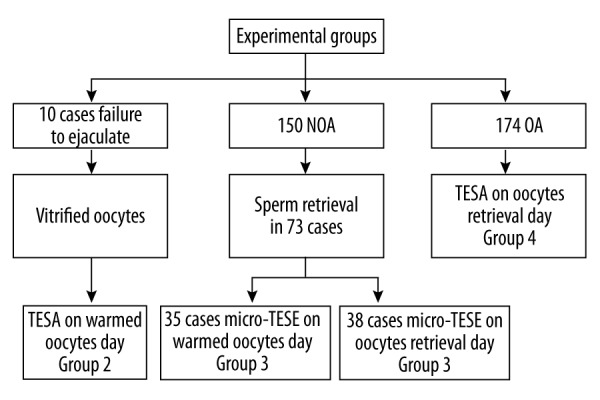
The grouping of all selected patients.
Micro-TESE and testicular sperm aspiration (TESA)
All surgical procedures of micro-TESE were performed according to the description of Schlegel and Li [22] with some modifications. All the surgeries were performed by a single surgeon. Briefly, under epidural anesthesia, a mid-line scrotal incision was made on the median raphe of the scrotum. A transverse tunical incision was performed. Following bipolar hemostasis, the testicular parenchyma was directly examined under an operating microscope (Leika M525 F40, Germany) at ×25 magnification. Opaque and more dilated tubules were removed and immediately placed into a sperm buffer (Vitrolife, Sweden). Retrieved tubules were ground into suspensions by microscopic tweezers and examined under bright-field microscopy (Olympus CKX41, Japan) concomitantly during the surgical procedure. When spermatozoa were identified from the tubules, additional seminiferous tubules from this region of sperm production were retrieved. If the sperm were not found on one side of the testis, we would open the other side of the testis. For all NOA patients, we sent some testicular tissue for histological analysis. In TESA, the aspirations were usually carried out using a needle attached to a syringe. The needle was inserted through the scrotal skin into the testis. The needle was usually inserted into the anterolateral portion of the superior testicular pole at an oblique angle toward the medium and lower poles. The testicular parenchyma was aspirated by creating negative pressure, and the specimen was sent to the laboratory.
The extracted testicular tissue was placed in a Petri dish with 1 ml G-MOPS-Plus (Vitrolife, Sweden) supplemented with 10% human albumin serum (HSA; Vitrolife, Sweden) and minced into testicular cell suspensions. Sperm were isolated and selected for use in ICSI.
Vitrification and warming of oocytes
The mature MII stage oocytes were placed in G-MOPS-plus (Vitrolife, Sweden) after rinsing and resting for 1 min. Oocytes were then placed in ES equilibrium solution (Kitazato Biophama, Japan) for 10 min. The oocytes first appeared to shrink, but then returned to their original size. The mature oocytes were transferred to the vitrification solution (VS solution), and then transferred to the frozen carrier and stored in liquid nitrogen. All procedures were performed at room temperature (~25°C).
Warming was performed on the day of sperm retrieval via surgical procedure. Briefly, the carrier equipped with frozen oocytes was removed from the liquid nitrogen tank and quickly placed in a liquid containing WS1 solution (Kitazato Biophama, Japan) in a thawing dish. Oocytes were transferred to the WS2 (Kitazato Biophama, Japan) and WS3 (Kitazato Biophama, Japan) solutions at room temperature (~25°C) for 3 min each. Then, oocytes were transferred into culture medium (SSM) and incubated for 3 h at 37°C (6% CO2 and 5% O2) before they were used for ICSI. Degenerated oocytes were removed from the Petri dishes.
Ovarian stimulation and ICSI
A single stimulation cycle was included for each group. The ovarian induction protocol was performed with the standard long protocol. The ovarian stimulation and oocyte retrieval procedure were conducted by ultrasound-guidance through the vagina, as well as embryo culture, as described in the literature [23]. Subsequently, ICSI was performed as previously described [24]. On day 1, at 16–18 h after insemination, all embryos were checked for fertilization. On day 2, all embryos in different groups were checked for cell number and fragmentation. On day 3, all embryos were checked for cell number, fragmentation, and cell symmetry, then embryos were scored according to the SART scoring system [25]. Embryo transfer was performed on day 3 after oocyte retrieval. The best embryos were selected for transfer. The remainder of the embryos were followed for blastocyst formation and subsequently frozen. Pregnancy was confirmed by the rise of serum b-human chorionic gonadotropin (hCG) concentration 14 days after ET. Clinical pregnancy was defined as a visible sac in the fifth gestational week. The miscarriage rate was considered the loss of a clinical pregnancy per cycle before 20 completed weeks of gestation. This information was reported to our clinic after communication with the couples by our follow-up staff.
Statistical analysis
All data was analyzed using the Statistical Package for the Social Sciences program (SPSS for Windows 16.0, SPSS Inc, USA). One-way ANOVA was used to assess the different outcomes of ICSI among the 4 groups. The data are expressed as the mean ±SEM. P-value <0.05 was considered statistically significant in all analyses.
Results
Patient characteristics
A total of 150NOA patients received micro-TESE treatment. Due to the differences in etiology and histopathology, we divided NOA patients into maturation arrest (MA), Sertoli cell only syndrome (SCOS), AZFc deletions, cryptorchidism, and Klinefelter syndrome (KS). For a variety of reasons, 174 OA and 10 failure-to-ejaculate patients received TESA treatment. Pre-operatively, sex hormones were checked for all the patients. Table 1 describes the pre-operative characteristics of patients in the different groups, including female age and endocrine lab results. There was a significant statistical difference in the FSH and testicular volume between OA and NOA patients. All the enrolled patients underwent micro-TESE or TESA, ICSI treatment, and embryo transfer.
Table 1.
Baseline characteristics of different groups.
| Characteristics | Group 1 micro-TESE on vitrified warmed | Group 2 TESA on vitrified warmed | p-Value | Group 3 micro-TESE on oocytes retrieval | Group 4 TESA on oocytes retrieval | p-Value |
|---|---|---|---|---|---|---|
| Male age | 31.2±3.3 | 32.9±3.8 | 0.783 | 31.7±3.6 | 31.9±3.9 | 0.653 |
| Female age | 27.8±3.2 | 32.8±4.6 | 0.674 | 29.4±3.3 | 28.6±3.5 | 0.573 |
| FSH (mIU/ml) | 15.9±5.1 | 5.4±1.4 | <.000* | 18.0±4.7 | 5.2±1.2 | <.000* |
| LH (mIU/ml) | 5.1±1.5 | 4.7±1.1 | 0.937 | 4.9±1.4 | 4.9±1.3 | 1.000 |
| T (ng/ml) | 7.3±3.7 | 13.2±8.7 | 0.201 | 6.8±3.8 | 12.4±5.2 | <.000* |
| PRL (ng/ml) | 9.1±4.4 | 7.5±2.0 | 0.935 | 7.8±2.7 | 8.8±4.4 | 0.283 |
| Testicular volume (ml) | 5.9±2.5 | 12.0±1.6 | <.000* | 6.2±2.9 | 12.2±1.7 | <.000* |
| KS (47,XXY) | 6 | – | 8 | – | NC | |
| Cryptorchidism | 7 | 5 | – | NC | ||
| AZFc deletion | 5 | 5 | – | NC | ||
| Histopathology | ||||||
| Maturation arrest | 24 | – | 33 | – | NC | |
| Hypospermatogenesis | 23 | – | 18 | – | NC | |
| SCOS | 6 | – | 10 | – | NC | |
| Obstructive azoospermia | 174 | NC | ||||
| Ejaculation difficulty | 10 | NC |
Statistically significant.
Micro-TESE and success rate of sperm retrieval
The overall SRR for NOA patients was 48.7% (73/150) (Table 2.). SRR was highest for those with a histological diagnosis of hypospermatogenesis (80.5%) and lowest in SCOS (25%), which agrees with results in the literature. Motile spermatozoa were successfully obtained in all 174 patients (100%) with OA.
Table 2.
The sperm retrieval rate (SRR) using Micro-TESE.
| Classification | Micro-TESE | Obtained sperm | SRR(%) |
|---|---|---|---|
| Histopathology | 114 | 55 | 48.2 |
| Maturation arrest | 57 | 18 | 31.6 |
| Hypospermatogenesis | 41 | 33 | 80.5 |
| SCOS | 16 | 4 | 25.0 |
| KS (47,XXY) | 14 | 4 | 28.6 |
| Cryptorchidism | 12 | 8 | 66.7 |
| AZFc deletion | 10 | 7 | 70.0 |
| Total | 150 | 73 | 48.7 |
Outcome of ICSI-ET cycles in NOA and OA
In group 1, among the 70 NOA patients, sperm retrieval via micro-TESE was successful in 35 patients (50%).Vitrified-warmed oocytes were used on the day of micro-TESE. In group 2, 10 patients underwent testicular biopsy because of the inability to produce an ejaculated specimen. In group 3, 80 NOA patients received micro-TESE on the day of oocyte retrieval and 38 patients (47.5%) had successful sperm retrieval enabling subsequent ICSI. In group 4, testicular sperm were successfully obtained in all 174 patients (100%) with OA.
The outcome of ICSI-ET cycles is presented in Table 3. Fertilization rates were similar among groups 1 to 4 (73.8%, 77.2%, 72.8%, and 73.6%, respectively). Implantation rates were 33.3%, 34.7%, 33.8%, and 37.5%, respectively. Clinical pregnancy rates were 51.4%, 60%, 52.6%, and 51.7%, respectively. There were no statistically significant differences between the different groups (p>0.05). Concerning the embryo developmental competence, rate of quality embryos on day 3 were observed among embryos derived from vitrified and fresh oocytes. With respect to the vitrified warmed oocytes (group 1 and 2), quality embryo rates were significantly lower in group 1 (29.0±8.3) compared to group 2 (43.1±10.9) (p<0.05). Similarly, D3 available embryo rates were lower in group 1 (31.3±9.0) compared to group 2 (46.5±8.7) (p<0.01). With respect to fresh oocytes (groups 3 and 4), quality embryo rates were lower in group 3 (30.5±8.7) when compared to group 4 (47.5±13.6), (p<0.01). D3 available embryos rate were also lower in group 3 (32.6±12.2) when compared to group 4 (47.7±13.4) (p<0.01).
Table 3.
Outcome of ICSI-ET cycles in different groups.
| Variable | Group 1 | Group 2 | P-value | Group 3 | Group 4 | P-value |
|---|---|---|---|---|---|---|
| Number of cycles | 35 | 10 | 38 | 174 | ||
| Number of oocytes | 11.1±2.8 | 11.4±2.8 | 1.000 | 12.2±3.5 | 11.5±3.1 | 0.838 |
| Oocyte survival rate (%) | 91.2±3.6 | 92.6±3.8 | 0.290 | – | – | – |
| Number of Injected | 8.8±2.5 | 9.2±1.8 | 0.996 | 9.1±2.2 | 8.9±2.2 | 1.000 |
| Fertilization rate (%) | 73.8±8.9 | 77.2±10.6 | 0.935 | 72.8±7.4 | 73.6±8.0 | 0.990 |
| Transferred embryos | 1.5±0.5 | 1.5±0.5 | 0.807 | 1.6±0.5 | 1.5±0.5 | 0.878 |
| Quality embryos rate (%) | 29.0±8.3 | 43.1±10.9 | 0.015* | 30.5±8.7 | 47.5±13.6 | <.000* |
| D3 available embryos | 31.3±9.0 | 46.5±8.7 | 0.001* | 32.6±12.2 | 47.7±13.4 | <.000* |
| Implantation rate (%) | 33.3±14.3 | 34.7±10.3 | 1.000 | 33.8±12.4 | 37.5±12.1 | 0.461 |
| Clinical pregnancies (%) | 51.4 (18/35) | 60.0 (6/10) | 52.6 (20/38) | 51.7 (90/174) | NC | |
| Miscarriage rates (%) | 11.1 (2/18) | 16.7 (1/6) | 10.0 (2/20) | 13.3 (12/90) | NC |
Statistically significant.
Discussion
It is established that TESE is the recommended method for sperm retrieval in men with testicular failure. Due to the fact that individual seminiferous tubules can be seen under the microscope, several authors have proposed that micro-TESE is a better method for sperm retrieval than conventional TESE [26–28]. However, those studies did not stratify SRR by the different etiologies of NOA. In this study, we analyzed our SRR using micro-TESE among 150 patients with NOA. Our SRR range, from 25% to 63%, was comparable to that in the reported literature [29–31]. Our study also demonstrates that the etiology of NOA is predictive of the corresponding SRR. While the overall SRR for the 150 NOA patients was 48.7%, it was least successful in Sertoli cell only syndrome (25%), followed by Klinefelter syndrome (28.6%), maturation arrest (31.6%), and AZFc deletion (70%), and was most successful in hypospermatogenesis (80.5%).
The development of sperm cryopreservation provides a guarantee to the preservation of male fertility. Testicular sperm can also be obtained and frozen before the initiation of controlled ovarian hyperstimulation for delayed ICSI. Karacan [32] reported that neither the timing of TESE (on the day of or the day before oocyte retrieval) nor the use of frozen-thawed testicular sperm affects the outcome of ICSI-ET cycle when motile spermatozoa are obtained in azoospermic men. However, for NOA patients undergoing micro-TESE, the amount of sperm retrieved is often small. Traditional freezing techniques do not guarantee the quantity and quality of thawed spermatozoa. Various methods of cryopreservation for very low numbers of spermatozoa, such as ICSI pipettes [33], cryoloop [34], LSL micro-straws [35], agarose capsules [36], and hyaluronan microcapsules [37] have been reported. However, each of these methods has its own problem. It is often difficult to find available sperm immediately due to the spermatozoa quantity being very low compared with the number of embryos needed. In addition, recovery rate is also a limiting factor. Therefore, selection of fresh sperm for fertilization is still the best option. However, new problems arise from this: in particular, when sperm is unable to be obtained by micro-TESE, the risk arises from necessity of canceling the oocyte harvest. Now that vitrification has proven to be a promising and popular alternative to cryopreserved oocytes in the field of assisted reproduction, our ideas could evolve accordingly. In this study, we explored the feasibility and clinical outcomes of vitrified oocyte cryopreservation combined with micro-TESE.
The outcomes of ICSI-ET fertilization rates and implantation rates were similar between the micro-TESE patients and TESA patients (group 1 and 3 vs. group 2 and 3). However, quality embryos rates and D3 available embryos rates in TESA patients were higher than in micro-TESE patients (group 1 vs. group 2). Our results demonstrate that the testicular sperm of males with normal sperm production may have better development potential than testicular sperm from men with NOA. Several studies have indicated that NOA patients produced increased numbers of cytogenetically abnormal testicular spermatozoa despite their normal somatic karyotype, and were at increased risk of producing aneuploid gametes and transmitting chromosome aneuploidy to the zygote [11,12,38]. These factors may lead to a reduced developmental potential of embryos derived from sperm of NOA males than in embryos derived from sperm of men with normal spermatogenesis. However, selecting the best embryos which result in a pregnancy is a challenge across all IVF laboratories. In our study, when choosing the best embryos for implantation according to the SART scoring system, clinical pregnancies (51.4%, 60%, 52.6%, and 51.7%) and miscarriage rates (11.1%, 16.7%, 10.0%, and 13.3%) were not statistically different among the groups.
Since the first development of oocyte vitrification, it is now possible to vitrify and warm unfertilized eggs at near maximal efficiency, resulting in high cell survival rates [39]. Many studies have suggested that vitrified oocytes preserve the potential to be fertilized and to develop into high-quality blastocysts. In addition, the success rates of ICSI using vitrified oocytes are similar to those obtained with fresh insemination with regards to fertilization, embryo developmental competence, pregnancy rates, and live birth [40–44]. Forman et al. [19] also demonstrated that oocyte vitrification does not increase the rate of aneuploidy or diminish the implantation potential of viable blastocysts. With the birth of healthy infants resulting from vitrified oocytes, this efficient and safe technique could become more widely available to women who are seeking fertility preservation [45–47]. In our study, we undertook a retrospective analysis of the data on success in combining vitrified oocytes with sperm obtained via micro-TESE of NOA males. In the group of vitrified oocytes, we observed a total survival rate of 91.5% after warming. Our results are similar to those reported in open vitrification system studies and a recent systematic review [48,49]. These data further illustrate the stability and feasibility of oocyte vitrification and the value of clinical applications.
Moreover, our study suggests that fertilization rate, implantation rate, and clinical pregnancies are not affected by the oocyte vitrification procedure between the 2 groups of NOA patients (group 1 and group 3). Similar to embryos derived from fresh oocytes, vitrified oocytes preserve the potential to be fertilized and to develop into high-quality blastocysts. Our results are also comparable to Forman’s report, which was a paired randomized study [21]. At present, due to the promising results and the safety of the oocyte vitrification as demonstrated by successful fertilization, embryo developmental competence, pregnancy rates, and live birth numbers, vitrification is an efficient, harmless, and safe technique that can be widened in use and proposed to women who are seeking fertility preservation for social reasons [40,50,51]. Our results support the use of oocyte vitrification as a feasible option for couples when the male has NOA and requires surgical sperm retrieval. Limitations to this study include the lack of live-birth outcomes and the necessity for more clinical samples, such as in group 2. However, fertilization rates, embryo developmental competence, and clinical pregnancy rates are all well recognized end points.
Conclusions
In this study, our results confirmed the hypothesis that ICSI outcomes utilizing vitrified oocytes and spermatozoa acquired from micro-TESE demonstrated similar fertilization rates, implantation rates, and clinical pregnancies compared with ICSI utilizing fresh oocytes. However, the testicular sperm of NOA patients leads to lower embryo developmental competence compared to that of OA patients. Therefore, the use of oocyte vitrification is a feasible management option for couples when the male presents with NOA and requires surgical sperm retrieval.
Footnotes
Conflict of interest
None.
Source of support: This work was funded by the Young Scientist Foundation Project of the National Natural Science Fund (No.8140060826), the Frederick J. and Theresa Dow Wallace Fund of the New York Community Trust, and the American Urology Association New York (Section E, Darracott Vaughan MD, Research Scholar Award
References
- 1.Evers JL. Female subfertility. Lancet. 2002;360(9327):151–59. doi: 10.1016/S0140-6736(02)09417-5. [DOI] [PubMed] [Google Scholar]
- 2.Ferlin A, Arredi B, Foresta C. Genetic causes of male infertility. Reprod Toxicol. 2006;22(2):133–41. doi: 10.1016/j.reprotox.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Sharlip ID, Jarow JP, Belker AM, et al. Best practice policies for male infertility. J Urol. 2002;77(5):873–82. doi: 10.1016/s0015-0282(02)03105-9. [DOI] [PubMed] [Google Scholar]
- 4.Mclachlan RI, Rajpert-De ME, Hoei-Hansen CE, et al. Histological evaluation of the human testis – approaches to optimizing the clinical value of the assessment: mini review. Hum Reprod. 2007;22(1):2–16. doi: 10.1093/humrep/del279. [DOI] [PubMed] [Google Scholar]
- 5.Dohle GR, Halley DJ, Van Hemel JO, et al. Genetic risk factors in infertile men with severe oligozoospermia and azoospermia. Hum Reprod. 2002;17(1):13–16. doi: 10.1093/humrep/17.1.13. [DOI] [PubMed] [Google Scholar]
- 6.Ramasamy R, Lin K, Gosden LV, et al. High serum FSH levels in men with nonobstructive azoospermia does not affect success of microdissection testicular sperm extraction. Fertil Steril. 2009;92(2):590–93. doi: 10.1016/j.fertnstert.2008.07.1703. [DOI] [PubMed] [Google Scholar]
- 7.Kalsi J, Thum MY, Muneer A, et al. In the era of micro-dissection sperm retrieval (m-TESE) is an isolated testicular biopsy necessary in the management of men with non-obstructive azoospermia? BJU Int. 2012;109(3):418–24. doi: 10.1111/j.1464-410X.2011.10399.x. [DOI] [PubMed] [Google Scholar]
- 8.Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14(1):131–35. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- 9.Bernardini L, Gianaroli L, Fortini D, et al. Frequency of hyper-, hypohaploidy and diploidy in ejaculate, epididymal and testicular germ cells of infertile patients. Hum Reprod. 2000;15(10):2165–72. doi: 10.1093/humrep/15.10.2165. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigo L, Rubio C, Peinado V, et al. Testicular sperm from patients with obstructive and nonobstructive azoospermia: Aneuploidy risk and reproductive prognosis using testicular sperm from fertile donors as control samples. Fertil Steril. 2011;95(3):1005–12. doi: 10.1016/j.fertnstert.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Vozdova M, Heracek J, Sobotka V, et al. Testicular sperm aneuploidy in non-obstructive azoospermic patients. Hum Reprod. 2012;27(7):2233–39. doi: 10.1093/humrep/des115. [DOI] [PubMed] [Google Scholar]
- 12.Onofre J, Baert Y, Faes K, et al. Cryopreservation of testicular tissue or testicular cell suspensions: A pivotal step in fertility preservation. Hum Reprod Update. 2016;22(6):744–61. doi: 10.1093/humupd/dmw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baert Y, Van Saen D, Haentjens P, et al. What is the best cryopreservation protocol for human testicular tissue banking? Hum Reprod. 2013;28(7):1816–26. doi: 10.1093/humrep/det100. [DOI] [PubMed] [Google Scholar]
- 14.Cobo A, Domingo J, Pérez S, et al. Vitrification: An effective new approach to oocyte banking and preserving fertility in cancer patients. Clin Transl Oncol. 2008;10(5):268–73. doi: 10.1007/s12094-008-0196-7. [DOI] [PubMed] [Google Scholar]
- 15.Oktay K, Rodriguezwallberg KA, Sahin G. Fertility preservation by ovarian stimulation and oocyte cryopreservation in a 14-year-old adolescent with Turner syndrome mosaicism and impending premature ovarian failure. Fertil Steril. 2010;94(2):15–19. doi: 10.1016/j.fertnstert.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 16.Kuleshova L, Gianaroli L, Magli C, et al. Birth following vitrification of a small number of human oocytes: Case report. Hum Reprod. 1999;14(12):3077–79. doi: 10.1093/humrep/14.12.3077. [DOI] [PubMed] [Google Scholar]
- 17.Kuwayama M, Vajta G, Kato O, et al. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11(3):300–8. doi: 10.1016/s1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 18.Chang CC, Lin CJ, Sung LY, et al. Impact of phase transition on the mouse oocyte spindle during vitrification. Reprod Biomed Online. 2011;22(2):184–91. doi: 10.1016/j.rbmo.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Forman EJ, Li X, Ferry KM, et al. Oocyte vitrification does not increase the risk of embryonic aneuploidy or diminish the implantation potential of blastocysts created after intracytoplasmic sperm injection: A novel, paired randomized controlled trial using DNA fingerprinting. Fertil Steril. 2012;98(3):644–49. doi: 10.1016/j.fertnstert.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Gardner DK, Sheehan CB, Rienzi L, et al. Analysis of oocyte physiology to improve cryopreservation procedures. Theriogenology. 2007;67(1):64–72. doi: 10.1016/j.theriogenology.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Gook DA, Osborn SM, Bourne H, et al. Fertilization of human oocytes following cryopreservation; normal karyotypes and absence of stray chromosomes. Hum Reprod. 1994;9(4):684–91. doi: 10.1093/oxfordjournals.humrep.a138572. [DOI] [PubMed] [Google Scholar]
- 22.Schlegel PN, Li PS. Microdissection TESE: Sperm retrieval in non-obstructive azoospermia. Hum Reprod Update. 1998;4(4):439. doi: 10.1093/humupd/4.4.439. [DOI] [PubMed] [Google Scholar]
- 23.Englert Y, Van den Bergh M, Rodesch C, et al. Comparative auto-controlled study between swim-up and Percoll preparation of fresh semen samples for in-vitro fertilization. Hum Reprod. 1992;7(3):399–402. doi: 10.1093/oxfordjournals.humrep.a137657. [DOI] [PubMed] [Google Scholar]
- 24.Hasani SA, Küpker W, Baschat AA, et al. Mini-swim-up: A new technique of sperm preparation for intracytoplasmic sperm injection. J Assist Reprod Genet. 1995;12(7):428–33. doi: 10.1007/BF02211143. [DOI] [PubMed] [Google Scholar]
- 25.Racowsky C, Stern JE, Gibbons WE, et al. National collection of embryo morphology data into Society for Assisted Reproductive Technology Clinic Outcomes Reporting System: Associations among day 3 cell number, fragmentation and blastomere asymmetry, and live birth rate. Fertil Steril. 2011;95(6):1985–89. doi: 10.1016/j.fertnstert.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Dabaja AA, Schlegel PN. Microdissection testicular sperm extraction: An update. Asian J Androl. 2013;15(1):35–39. doi: 10.1038/aja.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada H, Dobashi M, Yamazaki T, et al. Conventional versus microdissection testicular sperm extraction for nonobstructive azoospermia. J Urol. 2002;168(3):1063–67. doi: 10.1016/S0022-5347(05)64575-2. [DOI] [PubMed] [Google Scholar]
- 28.Tsujimura A, Matsumiya K, Miyagawa Y, et al. Conventional multiple or microdissection testicular sperm extraction: A comparative study. Hum Reprod. 2002;17(11):2924–29. doi: 10.1093/humrep/17.11.2924. [DOI] [PubMed] [Google Scholar]
- 29.Donoso P, Tournaye H, Devroey P. Which is the best sperm retrieval technique for non-obstructive azoospermia? A systematic review. Hum Reprod Update. 2007;13(6):539–49. doi: 10.1093/humupd/dmm029. [DOI] [PubMed] [Google Scholar]
- 30.Amer M, Ateyah A, Hany R, et al. Prospective comparative study between microsurgical and conventional testicular sperm extraction in non-obstructive azoospermia: Follow-up by serial ultrasound examinations. Hum Reprod. 2000;15(3):653–56. doi: 10.1093/humrep/15.3.653. [DOI] [PubMed] [Google Scholar]
- 31.Schiff JD, Palermo GD, Veeck LL, et al. Success of testicular sperm extraction and intracytoplasmic sperm injection in men with Klinefelter syndrome. J Clin Endocrinol Metab. 2005;90(11):6263–67. doi: 10.1210/jc.2004-2322. [DOI] [PubMed] [Google Scholar]
- 32.Karacan M, Alwaeely F, Erkan S, et al. Outcome of intracytoplasmic sperm injection cycles with fresh testicular spermatozoa obtained on the day of or the day before oocyte collection and with cryopreserved testicular sperm in patients with azoospermia. Fertil Steril. 2013;100(4):975–80. doi: 10.1016/j.fertnstert.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 33.Sohn JO, Jun SH, Park LS, et al. Comparison of recovery and viability of sperm in ICSI pipette after ultra rapid freezing or slow freezing. Fertili Steril. 2003;80(3):128. [Google Scholar]
- 34.Desai NN, Blackmon H, Goldfarb J. Single sperm cryopreservation on cryoloops: An alternative to hamster zona for freezing individual spermatozoa. Reprod Biomed Online. 2004;9(1):47–53. doi: 10.1016/s1472-6483(10)62109-8. [DOI] [PubMed] [Google Scholar]
- 35.Liu F, Zou SS, Zhu Y, et al. A novel micro-straw for cryopreservation of small number of human spermatozoon. Asian J Androl. 2017;19(3):326–29. doi: 10.4103/1008-682X.173452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Araki Y, Yao T, Asayama Y, et al. Single human sperm cryopreservation method using hollow-core agarose capsules. Fertil Steril. 2015;104(4):1004–9. doi: 10.1016/j.fertnstert.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 37.Tomita K, Sakai S, Khanmohammadi M, et al. Cryopreservation of a small number of human sperm using enzymatically fabricated, hollow hyaluronan microcapsules handled by conventional ICSI procedures. J Assist Reprod Genet. 2016;33(4):501–11. doi: 10.1007/s10815-016-0656-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burrello N, Calogero APA, Grazioso C, et al. Chromosome analysis of epididymal and testicular spermatozoa in patients with azoospermia. Eur J Hum Genet. 2002;10(6):362–66. doi: 10.1038/sj.ejhg.5200814. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J, Grudzinskas G, Johnson M. Welcome to the ‘100% club’! Reprod Biomed Online. 2012;24(4):375–76. doi: 10.1016/j.rbmo.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 40.García JI, Noriegaportella L, Noriegahoces L. Efficacy of oocyte vitrification combined with blastocyst stage transfer in an egg donation program. Hum Reprod. 2011;26(4):782–90. doi: 10.1093/humrep/der008. [DOI] [PubMed] [Google Scholar]
- 41.Trokoudes KM, Pavlides C, Zhang X. Comparison outcome of fresh and vitrified donor oocytes in an egg-sharing donation program. Fertil Steril. 2011;95(6):1996–2000. doi: 10.1016/j.fertnstert.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 42.Cobo A, Kuwayama MS, Ruiz A, et al. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. 2008;89(6):1657–64. doi: 10.1016/j.fertnstert.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 43.Herrero L, Pareja S, Aragonés M, et al. Oocyte versus embryo vitrification for delayed embryo transfer: An observational study. Reprod Biomed Online. 2014;29(5):567–72. doi: 10.1016/j.rbmo.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 44.Laura R, Stefania R, Laura A, et al. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: A prospective randomized sibling-oocyte study. Hum Reprod. 2010;25(1):66–73. doi: 10.1093/humrep/dep346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chian RC, Huang JY, Tan SL, et al. Obstetric and perinatal outcome in 200 infants conceived from vitrified oocytes. Reprod Biomed Online. 2008;16(5):608–10. doi: 10.1016/s1472-6483(10)60471-3. [DOI] [PubMed] [Google Scholar]
- 46.Cobo A, Serra V, Garrido N, et al. Obstetric and perinatal outcome of babies born from vitrified oocytes. Fertil Steril. 2014;102(4):1006–15. doi: 10.1016/j.fertnstert.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 47.Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online. 2009;18(6):769–76. doi: 10.1016/s1472-6483(10)60025-9. [DOI] [PubMed] [Google Scholar]
- 48.Potdar N, Gelbaya TA, Nardo LG. Oocyte vitrification in the 21st century and post-warming fertility outcomes: A systematic review and meta-analysis. Reprod Biomed Online. 2014;29(2):159–76. doi: 10.1016/j.rbmo.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 49.Cobo A, Meseguer M, Remohí J, et al. Use of cryo-banked oocytes in an ovum donation programme: A prospective, randomized, controlled, clinical trial. Hum Reprod. 2010;25(9):2239–46. doi: 10.1093/humrep/deq146. [DOI] [PubMed] [Google Scholar]
- 50.Practice Committees of American Society for Reproductive Medicine. Society for Assisted Reproductive Technology: Mature oocyte cryopreservation: A guideline. Fertil Steril. 2013;99(1):37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 51.Potdar N, Gelbaya TA, Nardo LG. Oocyte vitrification in the 21st century and post-warming fertility outcomes: A systematic review and meta-analysis. Reprod Biomed Online. 2014;29(2):159–76. doi: 10.1016/j.rbmo.2014.03.024. [DOI] [PubMed] [Google Scholar]


