Abstract
The term “original antigenic sin” was coined approximately 60 years ago to describe the imprinting by the initial first influenza A virus infection on the antibody response to subsequent vaccination. These studies did not suggest a reduction in the response to current antigens but instead suggested anamnestic recall of antibody to earlier influenza virus strains. Then, approximately 40 years ago, it was observed that sequential influenza vaccination might lead to reduced vaccine effectiveness (VE). This conclusion was largely dismissed after an experimental study involving sequential administration of then-standard influenza vaccines. Recent observations have provided convincing evidence that reduced VE after sequential influenza vaccination is a real phenomenon. We propose that such reduction in VE be termed “negative antigenic interaction,” given that there is no age cohort effect. In contrast, the potentially positive protective effect of early influenza virus infection later in life continues to be observed. It is essential that we understand better the immunologic factors underlying both original antigenic sin and negative antigenic interaction, to support development of improved influenza vaccines and vaccination strategies.
Keywords: Influenza, immune response.
BACKGROUND OF THE DOCTRINE
The term “original antigenic sin” (OAS) was coined by Thomas Francis Jr in the late 1950s to describe patterns of antibody response to influenza vaccination [1]. Francis’ father was a Presbyterian minister, likely a reason he used a theologically charged term to describe a biologic phenomenon. The basic concept was recently summarized as “first flu is forever,” indicating the continued relevance of OAS throughout life [2]. The authors of this perspective commented on the observation that the first infection in life may predetermine later protection from encounters with avian influenza viruses [3]. This is exactly the idea that Francis and others were describing in terms of antibodies 60 years ago. The immune response to first infection imprinted itself on subsequent responses to infections and particularly vaccination. The doctrine was based on age cohort–associated antibody patterns observed in sera collected from the community, as well as on differences in antibody responses to vaccination between children and adults. The result could be either positive or negative and could be corrected by vaccination with appropriate antigens, termed the “blessing of induced immunity” [1]. In addition to being appropriately used to describe imprinting, OAS has also been sometimes inappropriately used to suggest simply that vaccine receipt might be deleterious. It has also been used to describe observations that have little to do with imprinting of early infections, such as when, later in life, vaccination in combination or in sequence does not have the desired response. This phenomenon should be termed instead “antigenic interaction.” With influenza, that interaction has manifested itself in the possible reduced effectiveness of repeat vaccinations. We here review the original observations leading to the doctrine of OAS and how that term has been applied both appropriately and inappropriately to recent events of public health importance. We will confine much of the review to influenza, including the possibility of negative antigenic interaction, even though similar discussions could involve other viruses, particularly flaviviruses and alphaviruses, and the use of various vaccines in sequence.
EARLY INFLUENZA VACCINES AND IMMUNE RECAPITULATION OF OLDER STRAINS
The discovery that influenza viruses hemagglutinate led both to the ability to concentrate the viruses to produce vaccine and the development of the hemagglutination inhibition (HAI) test to measure antibody response. The first evaluations of today’s inactivated influenza vaccine started in 1943 in conjunction with the US military [4]. Measurements of antibody titers in those who were vaccinated and those who received placebo showed that the HAI titer correlated with protection, indicating that the response to vaccine at least in part mimicked the response to natural infection. The composition of the 1943 vaccine also indicated recognition, even then, that influenza virus was undergoing antigenic drift. Updates of composition to account for drift continued to be made, but in early 1947, the vaccine stopped being effective, and it was concluded that a new A subtype had evolved [5]. We now know that this was an example of intrasubtypic reassortment [6]. Updating the vaccine virus resumed thereafter.
At the time, with limited molecular techniques available, it was believed that the influenza virus strains that we now term type A(H1N1) viruses comprised 3 independent subtypes: the swine influenza virus subtype (ASw), known to be related to the virus that caused the 1918 pandemic; the A0 subtype, prevalent starting in the 1930s; and the A1 or A prime subtype, postulated when the A0 vaccine stopped being effective in 1946–1947. It was not until 1980 that the current nomenclature was adopted, in which these viruses were designated A(H1N1) strains, meaning in current terms that they would all be considered antigenically drifted variants.
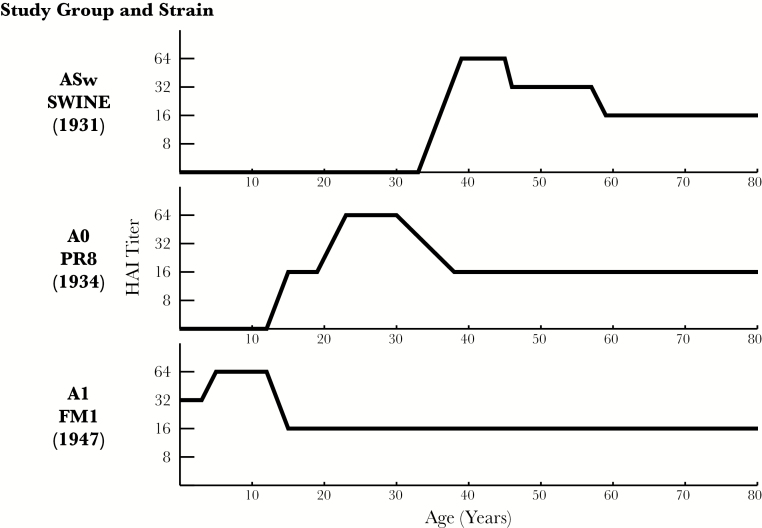
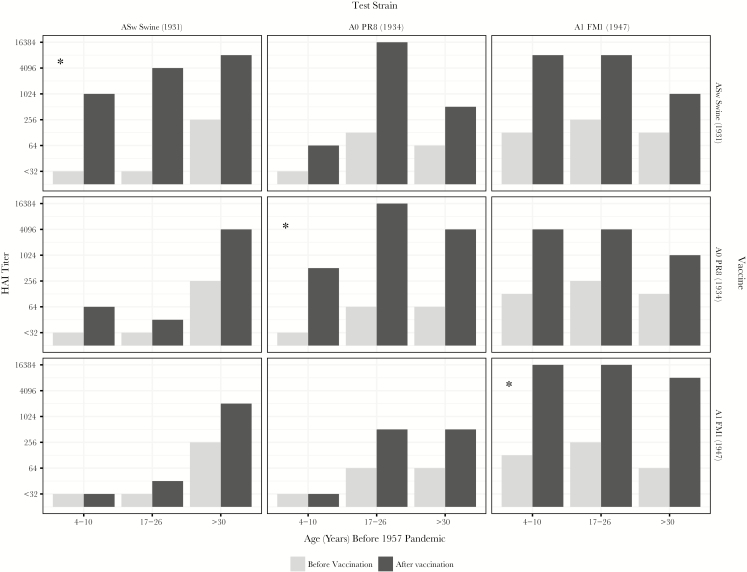
The concept of OAS was developed first on the basis of detection of residual antibodies from earlier infection with these strains and then by use of experimental vaccination with monovalent vaccines containing these inactivated viruses [7, 8]. Figure 1 is an illustration of antibodies left behind by first infections in life, based on HAI antibodies found in specimens collected before the 1957 pandemic [1]. ASw antibodies were present in the older individuals, in part from the 1918 pandemic, and they persisted. A0 antibodies were detected in middle-aged individuals but not in the youngest individuals, who only had A1 antibodies. The question was how vaccination would alter these patterns. Experimental monovalent vaccines were used, representing the 3 A variants shown in Figure 1. The individuals receiving the vaccines—before 1957—were children 4–10 years of age, military recruits aged 17–26 years, and persons aged >30 years [7]. In Figure 2, each panel represents a combination of a vaccine and an antigen used to identify antibody in the HAI test. It was observed that, when the children were vaccinated with 1946–1956 A1 virus vaccines, they produced antibody against these viruses but not antibodies against earlier strains. In contrast, when children were given the PR8-containing 1934 A0 virus monovalent vaccine or the ASw virus monovalent vaccine, they produced antibody not only against the virus specific to each vaccine, but also against the contemporary viruses, and the titer was higher against more-recent strains. When adults were given the vaccine containing a contemporary virus, however, they produced comparable antibody against both contemporary and older strains. Thus, the belief that OAS simply implies a diminished vaccine response to contemporaneous strains is at odds with this description of the phenomenon.
Figure 1.
Prevalence of antibody to type A influenza viruses in unvaccinated individuals to prior to the 1957 influenza A(H2N2) pandemic, demonstrating the absence of antibody in younger individuals to strains that circulated earlier and the persistence of antibody in older individuals. Data are adapted from findings in the report by Francis [1]. HAI, hemagglutination inhibition.
Figure 2.
Antibody response to monovalent adjuvant influenza virus vaccine in children, military recruits, and persons aged >30 years. Test antigens in the hemagglutination inhibition (HAI) test are those used in each of the vaccines. Homologous combinations of vaccine and antigen are indicated by an asterisk. Data are adapted from findings in the report by Davenport and Hennessy [8].
The meaning of these findings in terms of the immunologic theory of the time was examined using techniques such as antibody absorption [9]. This approach was intended to separate antibodies of varying avidity that were specific to the virus, either infecting or immunizing, from antibodies cross-reactive to the 3 strains under study. The results of these studies indicate the importance of using laboratory methods to understand epidemiologic observations [10, 11]. There was little focus on how well cross-reactive antibodies prevented infection, since vaccination against circulating strains, sometimes with adjuvanted antigens, had previously been demonstrated to be effective, especially in members of the US military who, because of their age, had been first infected with older strains [12].
Thus, the conclusion drawn, when OAS was first conceptualized, was that the first infection experience in life “orients” immunologic memory. When discussing the implications, the public health concern at the time was related not to any diminished response to contemporary viruses in different age groups, but to the fact that, although younger individuals did not produce antibody to older viruses, older people did, even in response to current strains. The heightened focus on population immunity to older viruses was rooted in the so-called recycling theory, which posited the existence of a limited number of influenza A virus subtypes that would each appear in succession. The concept was developed in the early 1950s, based in part on the serum survey data shown in Figure 1 and the belief that swine (1918), A0 (1934), and A1 (1947) viruses represented different subtypes. Under this theory, if antibodies to prior strains could be induced through vaccination, community spread would be limited when the strains later returned [1]. Belief in the recycling theory was strengthened following emergence in 1957 of the A(H2N2) viruses, termed at the time Asian influenza viruses [13]. It was found, first in the Netherlands, that sera collected from individuals ≥60 years of age prior to that pandemic had antibody to the A(H2N2) virus. The gap in antibody prevalence in younger individuals was viewed as increasing their susceptibility to future strains. In his classic article, “On the Doctrine of Original Antigenic Sin” [1], published after the A(H2N2) pandemic, Francis concluded that these gaps
in their immunity should be eliminated by providing early in life the antigenic stimuli to meet the known or anticipated recurrent strains. Natural exposures would then serve to enhance the broad immunity laid down by vaccination. It is our hope that such vaccines can be made from pools of chemically purified antigens – or even with strains experimentally devised. In this manner the original sin of infection could be replaced by an initial blessing of induced immunity.
THE HOSKINS PARADOX AND YEARLY VACCINATION
The issue of OAS remained in the background through the A(H3N2) pandemic of 1968. It was invoked by some as the explanation for what they termed the Hoskins paradox, after the first author of 3 articles on influenza VE, using data from an English boarding school for boys, Christ’s Hospital [14–16]. The first of these studies reported successful prevention of influenza A/England/42/72(H3N2) infection by an A/Hong Kong/x31/68(H3N2) vaccine. In the second, those protected in the first study were more likely to be infected in a later year. However, questions remained as to how many of those infected in the previously vaccinated group had actually been vaccinated in the current year [17]. Hoskins et al concluded at that time that prior infection is more effective than vaccination in preventing subsequent infection, an observation that remains undisputed. There was no mention about sequential, repeated vaccination as a concern for future protection.
It was the final article that concluded that the Christ’s Hospital study found no benefit of revaccination [16]. The reported attack rates were 13% in those with current-year vaccination only, 22% in those with prior- and current-year vaccination, and 21% in those who were unvaccinated. While these results can be questioned in terms of the role of major antigenic drift of that year’s strain (A/Victoria/3/75[H3N2]) and of use of serologic testing to confirm infection, a method known to introduce bias in vaccinated individuals, they suggest a decreasing effect of prior vaccination on VE [18, 19]. However, it is also clear that, since most of the boys in question were born in and therefore had their first influenza infections during the A(H2N2) era, the observations cannot be attributed to OAS as originally defined.
REACTION TO THE HOSKINS PARADOX
The issue of repeat vaccination brought up by the Hoskins articles prompted concern over annual vaccination policies and prompted a number of reviews. Many simply analyzed serologic responses to vaccination, a reflection of the common practice at the time of considering an HAI titer of 1:40 to be seroprotective and treating the presence of HAI antibody as equivalent to actual protection [20]. A notable exception to this approach was a 5-year experimental investigation by Keitel et al that examined sequential vaccination with licensed whole virus vaccines in adults [21, 22], with outcomes determined by virus isolation and/or an increase in antibody titer. While year-to-year variation in the actual VE was observed, the overall conclusion was that there was no clear evidence of an effect of repeat vaccination.
Subsequently, Beyer et al produced an extensive analysis of repeat vaccination, which evaluated the trials that determined actual efficacy in preventing laboratory-confirmed influenza [23]. While there was heterogeneity in the effect of repeat vaccination, overall there was no consistent negative effect [23]. Smith et al, in reviewing this meta-analysis, noted the heterogeneity and attributed the variability to antigenic distance between vaccine strains and circulating strains (ie, the greater the antigenic distance the less likely that prior-year vaccination would have an effect on VE) [24].
RETURN OF THE REPEAT VACCINATION ISSUE
Despite these cautionary observations, the consensus conclusion for years was that there was no significant prior-year interaction effect reducing VE. Even with the questions raised by Smith et al, this conclusion persisted until recently, when the development of the polymerase chain reaction (PCR) technique made it much easier to confirm that VE estimates were truly specific to influenza. The first clear demonstration of a reduction in VE estimates with prior vaccination was a longitudinal household study using real-time reverse-transcription PCR–defined infection that was conducted during 2010–2011, when there was a minor change in A(H3N2) vaccine composition [25]. This was followed by confirmation in a larger study using the test-negative design, currently the most common observational design for VE studies, during 2011–2012, a year in which there was no change in vaccine strains from the prior year [26]. The negative effect of prior-year vaccination has been repeatedly observed in many subsequent years, particularly in years with heavy A(H3N2) circulation [27–29]. In most studies, even when there was a reduction in VE among those vaccinated over 2 years, the infection risks in that group were lower than among those not vaccinated either year, unlike the report from the study by Hoskins et al. In addition, there was often considerable residual VE in those vaccinated in the prior year only, which may suggest that within-season waning of protection was overstated. The immunologic basis for this phenomenon, including whether it has anything to do with antigenic distance, remains unclear.
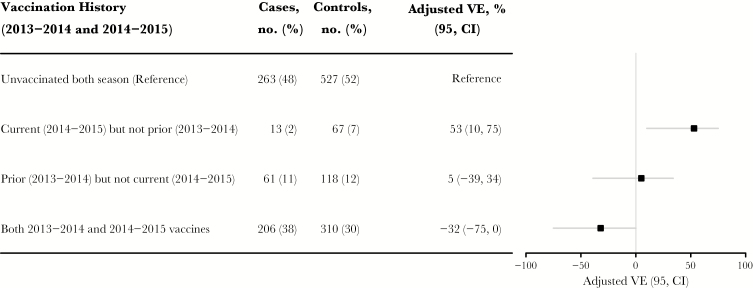
There is a new element that has now been added to the debate on the effects of repeat vaccination. VE estimates for A(H3N2) reported by Skowronski et al for 2014–2015 among those vaccinated in Canada in that year and for 2013–2014 are shown in Figure 3. The A(H3N2) virus circulating in 2014–2015 was a major drift variant of the one contained in that year’s vaccine. The point estimate for those vaccinated in both years was negative (–32%; 95% confidence interval, –75%–0%). This negative VE was interpreted by the authors to indicate that prior influenza vaccination can, in certain situations, produce actual increased susceptibility to infection, not simply a VE that was less than the expected value. This followed a somewhat similar Canadian observation during the 2009 pandemic of increased susceptibility among those who previously received the 2008 trivalent vaccine containing seasonal A(H1N1) [30]. That observation did not involve subsequent vaccination but was interpreted as increased susceptibility induced by vaccination with previously circulating strains. However, in terms of OAS, none of these studies reported an age cohort effect, which would be necessary for this to be termed OAS. Instead, this seems to be another demonstration of negative antigenic interaction [29, 30].
Figure 3.
Effect of prior 2013–2014 season influenza vaccine receipt on current 2014–2015 adjusted influenza vaccine effectiveness (VE) for A(H3N2) in Canada, comparing those not vaccinated in either year (reference) to those vaccinated in both years and, separately, those vaccinated in only 1 of 2 years. Data are adapted from findings in the report by Skowronski et al [29]. Abbreviation: CI, confidence interval.
OAS IN THE 21ST CENTURY
Only rarely was the actual concept of OAS with immunologic imprinting considered in the late 20th century, even after the return of the A(H1N1) viruses in 1977, when those >25 years of age were largely protected [31]. An exception was the work of Powers and Belshe, who, in studying an older population, sought to separate the effects of aging and OAS on immune response [32]; they were interpreting OAS to mean a possible reduction in antibody response to new viruses among individuals first exposed to older viruses. They examined rises in antibody titer to the original A(H1N1) variants, ASw and A0, after receipt of 1990–1991 seasonal vaccine and their relation to the ages of the individuals involved. Older individuals had smaller responses than younger individuals overall, but both age groups had better responses to newer viruses than to older viruses. Thus, OAS was not responsible for a reduced antibody response in older persons and the problem was attributed to immune senescence.
Pandemics are generally associated with a revisiting and reexamination of prior concepts. The 2009 A(H1N1) pandemic was no exception. Unlike the situation in 1977, the evidence of overall protection among older individuals was recognized in the context of the effects of prior infection with older A(H1N1) viruses. A commentary entitled “The Wages of Original Antigenic Sin” was written in response to a letter entitled “Original Antigenic Sin and Pandemic (H1N1) 2009” [33, 34]. As in 1977, nearly all older individuals who had lived through the previous period of A(H1N1) virus circulation were protected [31, 35, 36]. Response to the 2009 monovalent vaccine was good in all age groups, even in those that did not previously have experience with older strains of A(H1N1) [37]. A possible exception, cited in several reports, was that those who had previously received seasonal A(H1N1)-containing vaccines had reduced antibody responses to the pandemic vaccine, a phenomenon that also was referred to by the authors as a version of OAS but clearly involved negative antigenic interaction, rather than imprinting [38–41].
Recent years have seen a number of reports proposing OAS as an explanation for findings or as motivation for specific studies. A problem of many of these studies was that they were looking for potential negative effects of antigenic interactions, which were said to represent OAS. One of these investigations used sera collected during the 5-year study by Keitel et al, the trial that concluded that there was no deleterious effect of multiyear vaccination [42]. Amounts and avidities of preexisting antibody and antibody raised by the strains used for vaccination during that investigation were studied, and there were better responses to the vaccine virus itself when that vaccine strain was at greater antigenic distance from the prior one, in support of the hypothesis of Smith et al [24].
These results were said to represent OAS, again demonstrating the incorrect use of the term, even though the age of vaccinees was not considered; in response, a follow-up study was designed in mice [43]. The underlying assumption by Kim et al was that OAS resulted in a “severely diminished” response to the current strains [43]. The A(H1N1) viruses originally involved in the OAS studies were used after undergoing mouse adaptation for lethal challenge. While the negative effects were modest, the authors concluded that OAS exists.
In contrast, a more current study, by O’Donnell et al, using older A(H1N1) and 2009 pandemic A(H1N1) viruses, did not report evidence of OAS in ferrets or humans; they too focused on possible deleterious effects [44]. They explained conflicting findings from previous studies as being due to differences in the definition of what constituted a negative effect. Finding no evidence of deleterious effects agreed with another ferret study designed to explore the suggestion that prior receipt of seasonal trivalent inactivated influenza vaccine (TIV) led to an increased frequency of 2009 pandemic A(H1N1) infection [30, 45]. In contrast, these authors found actual protection against pandemic A(H1N1) infection in ferrets previously vaccinated with seasonal TIV.
A recent ferret study by Bodewes et al has extended the issue to heterosubtypic protection. The authors did not use the term OAS, but, unlike the study by O’Donnell et al, their conclusions imply imprinting [46]. Ferrets were either vaccinated or infected with an A(H3N2) virus (Table 1), and all were subsequently challenged with the lethal A(H5N1) virus; ferrets initially infected with A(H3N2) were protected. Those that had only been twice vaccinated but not infected were as susceptible as those that had not received any intervention. Ferrets that were initially vaccinated and then infected with A(H3N2) had intermediate levels of protection, indicating the greater influence of infection on protection, compared with vaccination. The authors generalized these results to critique the US policy of vaccinating children ≥6 months old, speculating that this may negatively affect subsequent development of heterosubtypic immunity through T cells. Although some data have been presented to support the relevance of animal results to the human situation [47], the debate continues. Nevertheless, there is clearly a need to balance the prevention of severe influenza in young children, who are at high risk of complications, and the possible benefits of allowing the first infection in life to take place without modification by prior vaccination. In contrast, the case for the positive effects of the first seasonal infection, rather than immunization, resulting in heterosubtypic protection against A(H5N1) or A(H7N9) infection through anti– hemagglutinin antibody, was recently made by Gostic et al [3]. This resulted in the editorial entitled “First Flu Is Forever,” a restatement of OAS [2].
Table 1.
Experimental Groups
| Group | Vaccinationa | Primary Infectionb |
|---|---|---|
| 1 | Mock | A/H3N2 virus |
| 2 | A(H3N2) vaccine | A/H3N2 virus |
| 3 | A(H3N2) vaccine | Mock |
| 4 | Mock | Mock |
Data are adapted from findings in the report by Bodewes et al [46]. All groups were challenged with influenza A/Indonesia/5/05(H5N1).
aThe A(H3N2) vaccine was a subunit vaccine derived from influenza A/Uruguay/ 716/2007(NYMC X-175-C; H3N2) virus adjuvanted with Titermax Gold adjuvant.
bThe influenza A(H3N2) strain was influenza A/Brisbane/010/07(H3N2).
OAS AND ANTIGENIC INTERACTION: THE NEED FOR A NEW TERM
At this point, >60 years after the first description of OAS, most would expect that the debate about what it is and whether it exists would be settled. Yet the doctrine is still invoked to explain observations that may but often may not relate to its original description, such as reductions in the response to antigens encountered in sequence later, namely in annual influenza vaccination. This issue has recently attracted much attention, with editorials describing its history and policy implications [48, 49]. The underlying immune mechanisms involved may be similar to that of OAS, but reductions in the response to a second antigen should not be termed OAS without evidence of a birth cohort effect. Such age specificity is still relevant and seems to be playing a role currently with A(H1N1) vaccine protection, in which there appears to be a cohort effect both overall and with respect to a single mutation [50]. In the absence of an age effect, we propose use of a modified term when describing a reduced response to repeated vaccination. Smith et al used the term “negative interference” to describe the phenomenon for vaccine strains without sufficient antigenic distance from those in a prior vaccine [24]. A better term might now be “antigenic interaction,” since it is not clear that interference is occurring.
The classic description of OAS did not report any “impaired” response to subsequent vaccination, only a strong anamnestic response to the original infecting strain, which was absent in younger individuals not so infected. It was said that the sin of original infection could be remedied by the blessing of vaccination [1]. That may also apply to modifying the phenomenon that we now refer to as negative antigenic interaction. Before that can happen, we need a better understanding of the immune mechanisms involved, which may vary based on the observed effect. Without that understanding, the remedy will be hard to prescribe. The work will require the use of modern immunologic techniques applied not only to animal models but also to observations in humans. Only by understanding the concept of OAS—now over half a century old—and the new concept of negative antigenic interaction may we be able to improve protection against the ever-changing threat of influenza.
Notes
Acknowledgment. We thank the Centers for Disease Control and Prevention, which provides financial support to the World Health Organization Initiative for Vaccine Research (U50 CK000431).
Financial support. This work was support by the Initiative for Vaccine Research, World Health Organization.
Potential conflicts of interest. A. S. M. has received grant support from Sanofi Pasteur and consultancy fees from Sanofi, Novartis, and Novavax for work unrelated to this report. R. E. M. and A. S. M. have received grant support from the Multiparty Group for Advice on Science Foundation for work unrelated to this report. E. T. M. has received grant support from Merck and Pfizer for work unrelated to this report. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Francis T. On the doctrine of original antigenic sin. Proc Am Philos Soc 1960; 104:572–578. [Google Scholar]
- 2. Viboud C, Epstein SL. First flu is forever. Science 2016; 354:706–7. [DOI] [PubMed] [Google Scholar]
- 3. Gostic KM, Ambrose M, Worobey M, Lloyd-Smith JO. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 2016; 354:722–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salk JE, Menke WJ, Francis T. A clinical, epidemiological and immunological evaluation op vaccination against epidemic influenza. Am J Epidemiol 1945; 42:57–93. [Google Scholar]
- 5. Francis T Jr, Salk JE, Quilligan JJ Jr. Experience with vaccination against influenza in the spring of 1947; a preliminary report. Am J Public Health Nations Health 1947; 37:1013–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nelson MI, Viboud C, Simonsen L et al. . Multiple reassortment events in the evolutionary history of H1N1 influenza A virus since 1918. PLOS Pathog 2008; 4:e1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davenport FM, Hennessy AV. A serologic recapitulation of past experiences with influenza A; antibody response to monovalent vaccine. J Exp Med 1956; 104:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davenport FM, Hennessy AV. Predetermination by infection and by vaccination of antibody response to influenza virus vaccines. J Exp Med 1957; 106:835–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jensen KE, Davenport FM, Hennessy AV, Francis T Jr. Characterization of influenza antibodies by serum absorption. J Exp Med 1956; 104:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fazekas De St G, Webster RG. Disquisitions of original antigenic sin. I. Evidence in man. J Exp Med 1966; 124:331–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Webster RG. Original antigenic sin in ferrets: the response to sequential infections with influenza viruses. J Immunol 1966; 97:177–83. [PubMed] [Google Scholar]
- 12. Davenport FM. Protective efficacy of inactivated influenza vaccines, 1943–1969. Med J Aust 1973; :33–8.4758153 [Google Scholar]
- 13. Mulder J, Masurel N. Pre-epidemic antibody against 1957 strain of Asiatic influenza in serum of older people living in the Netherlands. Lancet 1958; 1:810–4. [DOI] [PubMed] [Google Scholar]
- 14. Hoskins TW, Davies JR, Allchin A, Miller CL, Pollock TM. Controlled trial of inactivated influenza vaccine containing the a-Hong Kong strain during an outbreak of influenza due to the a-England-42-72 strain. Lancet 1973; 2:116–20. [DOI] [PubMed] [Google Scholar]
- 15. Hoskins TW, Davies JR, Smith AJ, Allchin A, Miller CL, Pollock TM. Influenza at Christ’s Hospital: March, 1974. Lancet Lond Engl 1976; 1:105–108. [DOI] [PubMed] [Google Scholar]
- 16. Hoskins TW, Davies JR, Smith AJ, Miller CL, Allchin A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ’s Hospital. Lancet 1979; 1:33–5. [DOI] [PubMed] [Google Scholar]
- 17. Beyer WE, De Bruijn IA, Palache AM, Westendorp RG, Osterhaus AD. The plea against annual influenza vaccination? ‘The Hoskins’ Paradox’ revisited. Vaccine 1998; 16:1929–32. [DOI] [PubMed] [Google Scholar]
- 18. Smith DJ, Lapedes AS, De Jong JC et al. . Mapping the antigenic and genetic evolution of influenza virus. Science 2004; 305:371–6. [DOI] [PubMed] [Google Scholar]
- 19. Petrie JG, Ohmit SE, Johnson E, Cross RT, Monto AS. Efficacy studies of influenza vaccines: effect of end points used and characteristics of vaccine failures. J Infect Dis 2011; 203:1309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beyer WE, Palache AM, Sprenger MJ et al. . Effects of repeated annual influenza vaccination on vaccine sero-response in young and elderly adults. Vaccine 1996; 14:1331–9. [DOI] [PubMed] [Google Scholar]
- 21. Keitel WA, Cate TR, Couch RB. Efficacy of sequential annual vaccination with inactivated influenza virus vaccine. Am J Epidemiol 1988; 127:353–64. [DOI] [PubMed] [Google Scholar]
- 22. Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997; 15:1114–22. [DOI] [PubMed] [Google Scholar]
- 23. Beyer WE, De Bruijn IA, Palache AM, Westendorp RG, Osterhaus AD. Protection against influenza after annually repeated vaccination: a meta-analysis of serologic and field studies. Arch Intern Med 1999; 159:182–8. [DOI] [PubMed] [Google Scholar]
- 24. Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A 1999; 96:14001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohmit SE, Petrie JG, Malosh RE et al. . Influenza vaccine effectiveness in the community and the household. Clin Infect Dis 2013; 56:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohmit SE, Thompson MG, Petrie JG et al. . Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mclean HQ, Thompson MG, Sundaram ME et al. . Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis 2014; 59:1375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Valenciano M, Kissling E, Reuss A et al. . Vaccine effectiveness in preventing laboratory-confirmed influenza in primary care patients in a season of co- circulation of influenza A(H1N1)pdm09, B and drifted A(H3N2), I-MOVE Multicentre Case-Control Study, Europe 2014/15. Euro Surveill 2016; 21:pii=30139. [DOI] [PubMed] [Google Scholar]
- 29. Skowronski DM, Chambers C, Sabaiduc S et al. . A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin Infect Dis 2016; 63:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skowronski DM, De Serres G, Crowcroft NS et al. . Association between the 2008–09 seasonal influenza vaccine and pandemic H1N1 illness during spring-summer 2009: four observational studies from Canada. PLoS Med 2010; 7:e1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Monto AS, Koopman JS, Longini IM Jr. Tecumseh study of illness. XIII. Influenza infection and disease, 1976–1981. Am J Epidemiol 1985; 121:811–22. [DOI] [PubMed] [Google Scholar]
- 32. Powers DC, Belshe RB. Vaccine-induced antibodies to heterologous influenza A H1N1 viruses: effects of aging and “original antigenic sin”. J Infect Dis 1994; 169:1125–9. [DOI] [PubMed] [Google Scholar]
- 33. Adalja AA, Henderson DA. Original antigenic sin and pandemic (H1N1) 2009. Emerg Infect Dis 2010; 16:1028–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morens DM, Burke DS, Halstead SB. The wages of original antigenic sin. Emerg Infect Dis 2010; 16:1023–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hardelid P, Andrews NJ, Hoschler K et al. . Assessment of baseline age-specific antibody prevalence and incidence of infection to novel influenza A/H1N1 2009. Health Technol Assess 2010; 14:115–92. [DOI] [PubMed] [Google Scholar]
- 36. Hancock K, Veguilla V, Lu X et al. . Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361:1945–52. [DOI] [PubMed] [Google Scholar]
- 37. Greenberg ME, Lai MH, Hartel GF et al. . Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med 2009; 361:2405–13. [DOI] [PubMed] [Google Scholar]
- 38. Choi YS, Baek YH, Kang W et al. . Reduced antibody responses to the pandemic (H1N1) 2009 vaccine after recent seasonal influenza vaccination. Clin Vaccine Immunol 2011; 18:1519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ohfuji S, Fukushima W, Deguchi M et al. . Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine among pregnant women: lowered antibody response by prior seasonal vaccination. J Infect Dis 2011; 203:1301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Uno S, Kimachi K, Kei J et al. . Effect of prior vaccination with a seasonal trivalent influenza vaccine on the antibody response to the influenza pandemic H1N1 2009 vaccine: a randomized controlled trial. Microbiol Immunol 2011; 55:783–9. [DOI] [PubMed] [Google Scholar]
- 41. Jackson LA, Chen WH, Stapleton JT et al. . Immunogenicity and safety of varying dosages of a monovalent 2009 H1N1 influenza vaccine given with and without AS03 adjuvant system in healthy adults and older persons. J Infect Dis 2012; 206:811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gulati U, Kumari K, Wu W, Keitel WA, Air GM. Amount and avidity of serum antibodies against native glycoproteins and denatured virus after repeated influenza whole-virus vaccination. Vaccine 2005; 23:1414–25. [DOI] [PubMed] [Google Scholar]
- 43. Kim JH, Skountzou I, Compans R, Jacob J. Original antigenic sin responses to influenza viruses. J Immunol 2009; 183:3294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O’donnell CD, Wright A, Vogel L, Boonnak K, Treanor JJ, Subbarao K. Humans and ferrets with prior H1N1 influenza virus infections do not exhibit evidence of original antigenic sin after infection or vaccination with the 2009 pandemic H1N1 influenza virus. Clin Vaccine Immunol 2014; 21:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Laurie KL, Carolan LA, Middleton D, Lowther S, Kelso A, Barr IG. Multiple infections with seasonal influenza A virus induce cross-protective immunity against A(H1N1) pandemic influenza virus in a ferret model. J Infect Dis 2010; 202:1011–20. [DOI] [PubMed] [Google Scholar]
- 46. Bodewes R, Kreijtz JH, Geelhoed-Mieras MM et al. . Vaccination against seasonal influenza A/H3N2 virus reduces the induction of heterosubtypic immunity against influenza A/H5N1 virus infection in ferrets. J Virol 2011; 85:2695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bodewes R, Fraaij PL, Geelhoed-Mieras MM et al. . Annual vaccination against influenza virus hampers development of virus-specific CD8⁺ T cell immunity in children. J Virol 2011; 85:11995–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Petrie JG, Monto AS. Untangling the effects of prior vaccination on subsequent influenza vaccine effectiveness. J Infect Dis 2017; 215:841–3. [DOI] [PubMed] [Google Scholar]
- 49. Treanor JJ. Flu vaccine—too much of a good thing? J Infect Dis 2017; 215:1017–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Petrie JG, Parkhouse K, Ohmit SE, Malosh RE, Monto AS, Hensley SE. Antibodies against the current influenza A(H1N1) vaccine strain do not protect some individuals from infection with contemporary circulating influenza A(H1N1) virus strains. J Infect Dis 2016; 214:1947–51. [DOI] [PMC free article] [PubMed] [Google Scholar]