CBL-interacting protein kinase 6 (CIPK6) negatively regulates the PAMP-triggered and effector-triggered immune response in Arabidopsis.
Keywords: Arabidopsis thaliana, CIPK6, ETI, Pseudomonas syringae, PTI, salicylic acid
Abstract
Cytosolic calcium ion (Ca2+) is an essential mediator of the plant innate immune response. Here, we report that a calcium-regulated protein kinase Calcineurin B-like protein (CBL)-interacting protein kinase 6 (CIPK6) functions as a negative regulator of immunity against the bacterial pathogen Pseudomonas syringae in Arabidopsis thaliana. Arabidopsis lines with compromised expression of CIPK6 exhibited enhanced disease resistance to the bacterial pathogen and to P. syringae harboring certain but not all avirulent effectors, while restoration of CIPK6 expression resulted in abolition of resistance. Plants overexpressing CIPK6 were more susceptible to P. syringae. Enhanced resistance in the absence of CIPK6 was accompanied by increased accumulation of salicylic acid and elevated expression of defense marker genes. Salicylic acid accumulation was essential for improved immunity in the absence of CIPK6. CIPK6 negatively regulated the oxidative burst associated with perception of pathogen-associated microbial patterns (PAMPs) and bacterial effectors. Accelerated and enhanced activation of the mitogen-activated protein kinase cascade in response to bacterial and fungal elicitors was observed in the absence of CIPK6. The results of this study suggested that CIPK6 negatively regulates effector-triggered and PAMP-triggered immunity in Arabidopsis.
Introduction
Plants lack an acquired immune system and rely entirely on the innate immune response. The first line of defense operates by recognizing pathogen/microbe-associated molecular patterns (PAMPs/MAMPs) of the pathogens present at the apoplastic regions using transmembrane pattern recognition receptors (PRRs), and is known as PAMP/MAMP-triggered immunity (PTI/MTI). Well-characterized PRRs in Arabidopsis are Flagellin Sensing 2 (FLS2) and Elongation Factor EF-Tu receptor (EFR) that recognize PAMPs, namely flagellin (flg) and EF-Tu, and activate a common signaling pathway involving activation of the mitogen-activated protein kinase (MAPK) cascade and defense gene transcription (Asai et al., 2002; Boudsocq et al., 2010). Many Gram-negative bacteria inject various effector proteins mostly through the type III secretion system into the host cells to evade PTI. These effectors interfere with the signaling cascades initiated by PAMP recognition to suppress PTI (Alfano and Collmer, 2004; de Torres et al., 2006; He et al., 2006). The other branch, commonly known as effector-triggered immunity (ETI), acts by recognizing the effectors, with the proteins encoded by the plant resistance (R) genes (Belkhadir et al., 2004; Nimchuk et al., 2003; Alfano and Collmer, 2004; Jones and Dangl, 2006; de Torres et al., 2006; He et al., 2006). Pathogen effectors that activate the R proteins, and thereby the immune response, are called avirulent (Avr) proteins. Some of these R proteins have a central NB-LRR (nucleotide binding-leucine-rich repeat) domain with the N-termini having homology to Toll and interleukin receptors (TIR-NB-LRR), or have a coiled-coil motif (CC-NB-LRR) (Ting et al., 2008; Eitas and Dangl, 2010). Activation of R proteins results in induction of a strong immune response culminating in local and systemic changes in gene expression, increased salicylic acid (SA) level, NADPH-oxidase-dependent oxidative burst, and sometimes cell death, known as the hypersensitive response (HR) (Grant et al., 2000; Jones and Dangl, 2006; Torres et al., 2006; Hein et al., 2009).
The ubiquitous plant pathogen Pseudomonas syringae pv. tomato DC3000 (Pst DC3000), widely used as a surrogate for studying mechanism of various effector functions, secretes various effectors, including AvrPto and AvrPtoB, through the type III secretion system (Shan et al., 2008; Cheng et al., 2011). In a resistant host such as a resistant tomato variety, AvrPto and AvrPtoB are recognized by Pto, a serine-threonine kinase. Pto kinase is required for activation of Prf, an NB-LRR protein, leading to cell death and disease resistance (Mucyn et al., 2006; Eitas and Dangl, 2010; Oh and Martin, 2011). Enhanced disease susceptibility 1 (EDS1), a lipase like protein, is indispensable for immunity mediated by R proteins having TIR-NB-LRR domains such as RPS4. A type III effector AvrRps4 (from P. syringae pv. phaseolicola) disrupts the protein complex made by EDS1 and RPS4 by interacting with EDS1 (Bhattacharjee et al., 2011; Heidrich et al., 2011). Two type III effectors AvrRpm1 (from P. syringae pv. maculicola) and AvrB (from P. syringae pv. glycinea) target a host plasma membrane-associated protein RIN4 and induce phosphorylation of RIN4. This RIN4 modification activates a CC-NB-LRR protein RPM1 (Belkhadir et al., 2004). Similarly, another effector protein AvrRpt2 (from P. syringae pv. tomato JL1065), a cysteine protease, cleaves RIN4 and the cleaved products are sensed by another CC-NB-LRR protein RPS2. RIN4-mediated defense signaling requires NDR1, a RIN4-interacting membrane protein (Mackey et al., 2002; Axtell et al., 2003; Chisholm et al., 2005; Kim et al., 2005; Day et al., 2006). Signaling cascades mediated by R proteins of both CC-NB-LRR and TIR-NB-LRR types converge at EDS5, a MATE-family protein, ultimately causing elevation of SA accumulation. EDS5 expression in response to pathogen infection is dependent on EDS1 and NDR1, and its expression is essential for pathogen-mediated induction of the SA level in the host cell (Nawrath et al., 2002). Transcript of isochorismate synthase 1 (ICS1), a major SA biosynthetic gene, is quickly accumulated upon infection. Lesions in the ICS1 gene (sid2-1 and sid2-2) resulted in impairment of pathogen-induced SA accumulation (Nawrath and Métraux, 1999; Wildermuth et al., 2001).
A rapid and sustained increase in the cytosolic calcium level [Ca2+]cyt is necessary for pathogen response (Grant et al., 2000; Lecourieux et al., 2006). Direct regulation of the SA level by a Ca2+-binding protein has been demonstrated using AtSR1, which negatively regulated the pathogen-induced SA level (Du et al., 2009). The Calcineurin B-like protein (CBL) family is a group of Ca2+ sensors which is activated by Ca2+ to initiate various signaling processes. Recently, tomato CBL10 and its interacting partner SlCIPK6 were shown to function as a positive regulator of Pto/AvrPto-mediated programmed cell death in tomato through activation of Respiratory burst oxidase homolog B (RbohB) upon infection with Pst DC3000 (de la Torre et al., 2013). In contrast to tomato, AvrPto does not trigger ETI in Arabidopsis [Columbia-0 (Col-0)], which is a susceptible host to this pathogen (Hauck et al. 2003). In this study, we report that CIPK6 (AT4G30960), a Ca2+-regulated protein kinase functions as a negative regulator of PTI and the SA-mediated immune response against Pst DC3000 in Arabidopsis.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana T-DNA insertion lines cipk6kd (SALK_08095) and cipk6 (GK-448C12-CS442948) have been described earlier (Tripathi et al., 2009; Held et al., 2011). Loss-of-function mutants sid2-1 (Wildermuth et al., 2001) and eds1-2 (Parker et al., 1996) were procured from Dr V. Bonardi, University of North Carolina, Chapel Hill, USA and Dr Saikat Bhattacharjee, Regional Centre for Biotechnology, Faridabad, India. The CIPK6 gene (CIPK6 lacks an intron) without or with the 2.1 kb long promoter region was amplified by PCR to construct 35S::CIPK6 or PCIPK6::CIPK6 in pCAMBIA1305.1 with or without the Cauliflower mosaic virus (CaMV) 35S promoter, respectively. The Agrobacterium strain GV3101 harboring CIPK6 constructs was used to transform the wild-type (Col-0) and cipk6 plants by floral dip infiltration as described previously (Clough and Bent, 1998). T3/T4 single-insertion homozygous lines were used. The presence of the transgene and its expression were confirmed by PCR and reverse transcription–PCR (RT–PCR), respectively. Seeds of the wild type (Col-0), RNAi, T-DNA insertion, mutant, and overexpressing Arabidopsis lines were stratified for 2 d at 4 °C, sown on soil, and grown in controlled-environment chambers (Conviron, Winnipeg, Canada) set to 22–24 °C, 70% humidity with a 10 h light/14 h dark photoperiod (100 µmol µm−2 s−1 light) for 4–5 weeks.
Generation of the CIPK6 RNAi construct
To generate the CIPK6 hairpin RNAi transformation construct, a 409 bp fragment of the second intron of the BjMYB28-3 gene was amplified using specific primers having XbaI/HindIII sites and were cloned into pGEMT-easy vector (Augustine et al., 2013). To this construct, a 334 kb fragment covering the ORF and 3'-untranslated region (UTR) of CIPK6 (encompassing base pairs 1160–1493 of the CIPK6 gene) was amplified and cloned in both sense and antisense orientations to create an RNAi cassette. This cassette was excised and cloned directionally at NcoI/SpeI sites of the binary vector pCAMBIA1305.1 to develop the RNAi construct. The transformation and subsequent selection methods were followed as described previously. All the primers used in this study are listed in Supplementary Table S1 at JXB online.
Bacterial infection
The bacterial pathogen Pseudomonas syringae pv. tomato DC3000 (Pst DC3000) and its type III secretion-deficient mutant, ΔhrcC, were provided by Professor G.B. Martin, Boyce Thompson Research Institute, Ithaca, NY, USA. The bacteria carrying an empty vector (EV) or constructs encoding various type III effectors (AvrB, AvrRpt2, AvrRpm1, and AvrRps4) were grown on King’s medium B agar plates or in liquid medium supplemented with 50 µg ml–1 rifampicin and 50 µg ml–1 kanamycin at 28 °C. The bacterial culture was resuspended in 10 mM MgCl2 and manually infiltrated in leaves with an OD600 of 0.0005 for the virulent strain, Pst DC3000 (EV) and an OD600 of 0.001 for other strains. Crushed leaf samples were serially diluted with 10 mM MgCl2 and plated onto King’s B medium containing the appropriate antibiotics for bacterial counts. The values presented are the mean of at least three biological replicates. At least eight representative leaves for each plant type and five technical replicates were used to generate results. Statistical analyses were performed using two-way ANOVA (Fujikoshi, 1993).
Gene expression analysis
Gene-specific primers were used to detect transcripts by qRT–PCR. All primers were designed using PRIMER EXPRESS version 3.0 (Applied Biosystems, Foster City, CA, USA) with default parameters. The primers used are listed in Supplementary Table S1. qRT–PCRs were performed with 2× SYBR Power Green master mix using the ViiA 7 system (Applied Biosystems) according to the manufacturer’s instructions. The specificity of amplicons was verified by melting curve analysis. At least three biological replicates and three technical replicates for each sample were used. ACTIN 2 (At3g18780) and TUBULIN 4 (At5g44340) were used as the reference gene internal controls. Relative expression was calculated according to the ΔΔCt method.
Reactive oxygen species (ROS) detection and measurement
Production of hydrogen peroxide was visualized in situ by 3,3'-diaminobenzidine (Sigma-Aldrich) staining. Leaves of 4- to 5-week-old Arabidopsis plants were manually infiltrated with Pst DC3000 (EV) and related bacterial strains (OD600=0.02), and the plants were incubated for 5 h before DAB staining. Five to six leaves were vacuum-infiltrated with a solution containing 1 mg ml–1 DAB and incubated for 4 h. Leaves were then de-stained in a solution of 3:1:1 ethanol/lactic acid/glycerol for visualization. Leaf samples for each plant type were constituted of three leaves per plant from four independent plants. To perform ROS burst kinetics, the third, fourth, and fifth true leaves of 4 week-old Arabidopsis plants were sampled with a cork borer (1.1 cm2) and floated adaxial side up overnight on sterile water. Prior to elicitation, bacteria were scraped from plates and washed twice in sterile distilled water, and the final concentration of bacterial elicitation solution was adjusted to 1 × 108. Water was replaced with 100 µl of the elicitation solution containing 0.2 μM luminol (Sigma-Aldrich), 20 μg ml–1 horseradish peroxidase (HRP; Sigma-Aldrich), and the appropriate bacterial strain. Pst-induced ROS production was measured in vivo as luminescence using a POLARstar Omega (BMG Labtech, UK) 96-well microplate luminometer every 42 s up to 250 min. The values presented are the mean of six biological replicates.
Quantification of salicylic acid
Free SA and glucose-conjugated SA (SAG) measurements were performed using a biosensor system as described before (Defraia et al., 2008). In brief, leaves (100 mg) were collected at 24 hours post-infiltration (hpi; for avirulent effectors) or 9 hpi [Pst DC3000 and Pst DC3000 (ΔhrcC)] and frozen in liquid nitrogen. The tissue was homogenized in 200 μl of 0.1 M acetate buffer (pH 5.6). One aliquot (100 μl) of the supernatant was used for free SA measurements, and another was incubated with 4 U of β-glucosidase (Sigma-Aldrich) for 90 min at 37 °C for total SA measurement. A 20 μl aliquot of each plant extract and standard SA solutions (prepared in sid2-1 extract) were added to the assigned wells of a black cell culture plate. A 50 μl aliquot of Acinetobacter sp. ADPWH_lux (OD600 of 0.4) was added to each well, incubated at 37 °C for 1 h, and luminescence readings for each sample was taken using POLARStar Omega (BMG Labtech). Leaf samples for each plant type constitute three leaves per plant from six independent plants, and the values presented are the mean of three biological replicates.
PAMP treatment
Leaves of 4- to 5-week-old Arabidopsis plants were manually infiltrated with flg22 (1 µM) or water. Leaf samples were then taken at 0, 5, and 15 min time points for both Col-0 and atcipk6. Tissue samples were ground in liquid nitrogen and resuspended in 50 mM Tris–HCl (pH 7.5), 5 mM EDTA, 5 mM EGTA, 150 mM NaCl, 1 mM DTT, 10 mM Na3VO4, 10 mM NaF, 50 mM β-glycerolphosphate, 1 mM phenylmethylsulfonyl fluoride (PMSF), plant protease inhibitor cocktail (Sigma-Aldrich), 10% glycerol, 0.1% NP-40. After centrifugation, the protein supernatant was mixed with Laemmli sample buffer and boiled for 5 min. Active forms of MAPK3 and MAPK6 were then detected by western blotting using pTEpY antibody (Cell Signaling Technology, #A9101). Total MPK3 and MPK6 proteins were detected by western blotting using anti-AtMPK3 and anti-AtMPK6 (Sigma-Aldrich, #M8318, #A7104) as primary antibodies, respectively, and goat anti-rabbit–HRP (Amersham Biosciences) as secondary antibody. For gene expression analysis, seedlings were grown on a plate for 8 d and then transferred to liquid Murashige and Skoog (MS) medium (0.5× MS, 0.25% sucrose, and 1 mM MES pH 5.7) in 12-well plates for equilibration for 48 h to allow recovery from stress caused during the transfer, and then treated with flg22 (1 µM). Seedlings were treated similarly for other PAMPs, elf18 (1 µM), chitin (50 µg ml–1), or peptidoglycan (PGN; 50 µg ml–1). For MAPK activity assay, leaves of 4-week-old plants were manually infiltrated with 1 µM flg22.
Results
CIPK6 negatively regulates resistance to Pst DC3000 in Arabidopsis
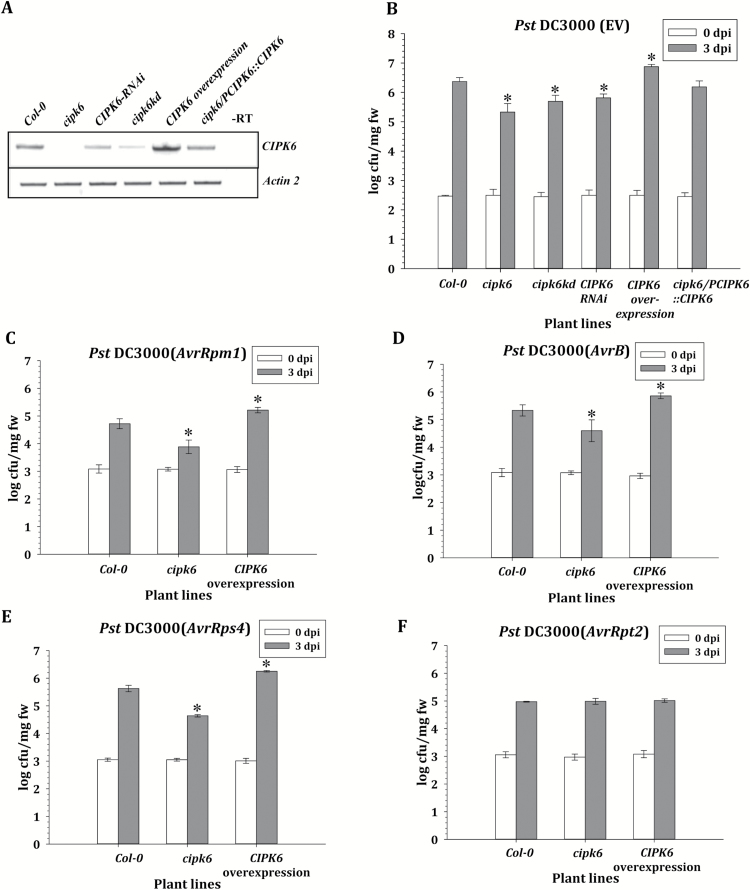
Pto, a tomato ser/thr kinase, recognizes Pst DC3000 effectors AvrPto and AvrPtoB, and elicits AvrPto/Pto-triggered immunity in resistant tomato varieties (Mucyn et al., 2006; Oh and Martin, 2011). In contrast, delivery of AvrPto does not show AvrPto/Pto-mediated immunity, but rather suppresses the defense response and promotes bacterial growth in Arabidopsis (Hauck et al., 2003; de Torres et al., 2006). Tomato CIPK6 was shown to regulate AvrPto/Pto-triggered immunity positively (de la Torre et al., 2013). To investigate the role of CIPK6 in the immune response of Arabidopsis, leaves of two Arabidopsis T-DNA insertion lines, a CIPK6 RNAi line, and a CIPK6-overexpressing line (CIPK6OX), which do not express (cipk6), partially express (cipk6kd, CIPK6-RNAi), or highly express CIPK6 (Fig. 1A), were infiltrated with Pst DC3000. Almost 10- and 5-fold lower bacterial counts were observed in cipk6 and cipk6kd lines, respectively, in comparison with that in the wild-type (Col-0) plants at 3 days post-infiltration (dpi), while the RNAi line also showed a similar bacterial count to the cipk6kd line (Fig. 1B). Lower bacterial growth in the Arabidopsis lines with no or low CIPK6 expression was also evident from less chlorosis in the infected leaves (Supplementary Fig. S1). Leaves of cipk6 plants transformed with the CIPK6 cDNA under the control of the native promoter (PCIPK6::CIPK6) did not show any significant difference in bacterial titer and chlorosis with respect to the wild-type (Col-0) plants. In contrast, an ~5-fold higher bacterial count and severe chlorosis were observed in the wild-type plants expressing CIPK6 under the CaMV 35S promoter (CIPK6OX) (Fig. 1B; Supplementary Fig. S1). Various other bacterial effectors such as AvrRps4, AvrRpt2, AvrRpm1, and AvrB induce ETI in Arabidopsis (Jones and Dangl, 2006; Xin and He, 2013). Therefore, Pst DC3000 strains carrying these individual effectors were infiltrated in the leaves of Col-0, cipk6, and CIPK6OX plants. The cipk6 line showed 6- to 10-fold reduced bacterial growth for Pst DC3000-AvrRps4, -AvrRpm1, and -AvrB as compared with the wild-type plants, while CIPK6OX plants displayed an ~5-fold higher bacterial count at 3 dpi. No significant difference in bacterial titer was observed for AvrRpt2 between the wild type and cipk6 line, suggesting that Arabidopsis CIPK6 is not a general negative regulator of plant defense, but rather functions differentially in distinct effector-driven signaling pathways (Fig. 1C–F).
Fig. 1.
CIPK6 is a negative regulator of plant defense. (A) Expression of CIPK6 in the wild type (Col-0) and different Arabidopsis lines used in this study as determined by RT–PCR. A lane with RNA from Col-0 without using reverse transcriptase (–RT) is shown to rule out genomic DNA contamination. Actin2 transcript was used as the control. (B) Bacterial growth content assay. Pst DC3000 (empty plasmid vector, EV) (OD600=0.0005) was manually infiltrated into leaves of the various Arabidopsis plants mentioned. Bacterial growth was assessed at 0 and 3 days post-infection (dpi) and was expressed as log of colony-forming unit per miligram of fresh weight (log cfu/mg fw). The asterisks indicate a significant difference following two-way ANOVA (α=0.05). (C–F) Pst DC3000 expressing AvrRpm1, AvrB, AvrRps4, or AvrRpt2 from plasmid vectors were manually infiltrated (OD600=0.001) into the rosette leaves of Col-0, cipk6, and CIPK6OX plant lines. Bacterial growth was assessed at 0 and 3 dpi and is presented similarly to as described above.
CIPK6 negatively regulates expression of the pathogenesis-related gene PR1
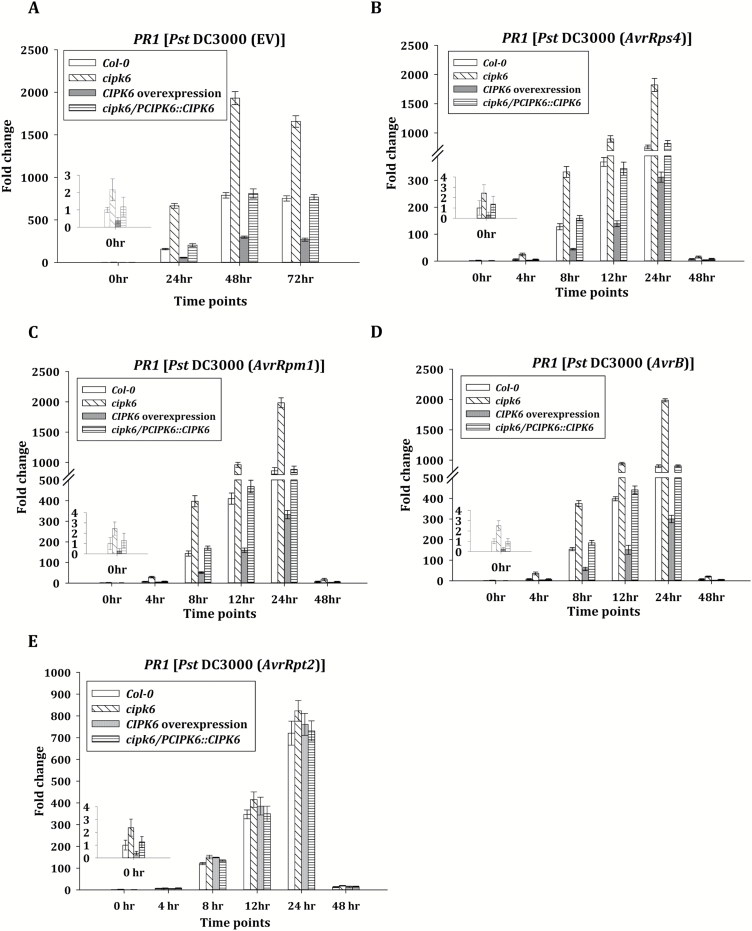
The CIPK6 gene expression level in response to Pst DC3000 infiltration decreased with time and was decreased by 3-fold at 12 hpi (Supplementary Fig. S2). This is in contrast to the CIPK6 expression pattern in tomato, in which the SlCIPK6 expression level rapidly increased by 4-fold within 4 h of infection (de la Torre et al., 2013). The CIPK6 expression pattern has been reported to vary in different plants, indicating its diverse functions in different species (Kim et al., 2007; Quan et al., 2007; Tripathi et al., 2009a). Resistance against bacterial pathogen is accompanied by the expression of the PR1 gene, a marker for activation of SA signaling (Cameron et al., 1999). In agreement with enhanced resistance, cipk6 mutant plants showed a markedly higher level of PR1 expression, while CIPK6OX plants exhibited a >3-fold lower expression level compared with wild-type and cipk6/PCIPK6::CIPK6 plants (Fig. 2A) Similar differential PR1 expression was observed in Col-0, cipk6, CIPK6OX, and cipk6/PCIPK6::CIPK6 plants in response to Pst DC3000-AvrRps4, -AvrB, and -AvrRpm1 infection, suggesting that CIPK6 negatively regulates effector-triggered defense signaling in Arabidopsis (Fig. 2B–D). In agreement with the bacterial titer described before, lack of expression or high expression of CIPK6 did not affect PR1 expression in the case of Pst DC3000-AvrRpt2 infection (Fig. 2E).
Fig. 2.
Expression of the defense marker gene PR1 was enhanced in the absence of CIPK6. (A–E) Time course of PR1 expression assessed by qRT–PCR in Col-0, cipk6, CIPK6 overexpression, and cipk6/PCIPK6::CIPK6 plant lines in response to Pst DC3000 (EV) and to Pst DC3000 (AvrRps4/AvrRpm1/AvrB/AvrRpt2) infiltration. Actin 2 and Tubulin 4 were used as internal controls.
Resistance in cipk6 plants is dependent on salicylic acid accumulation
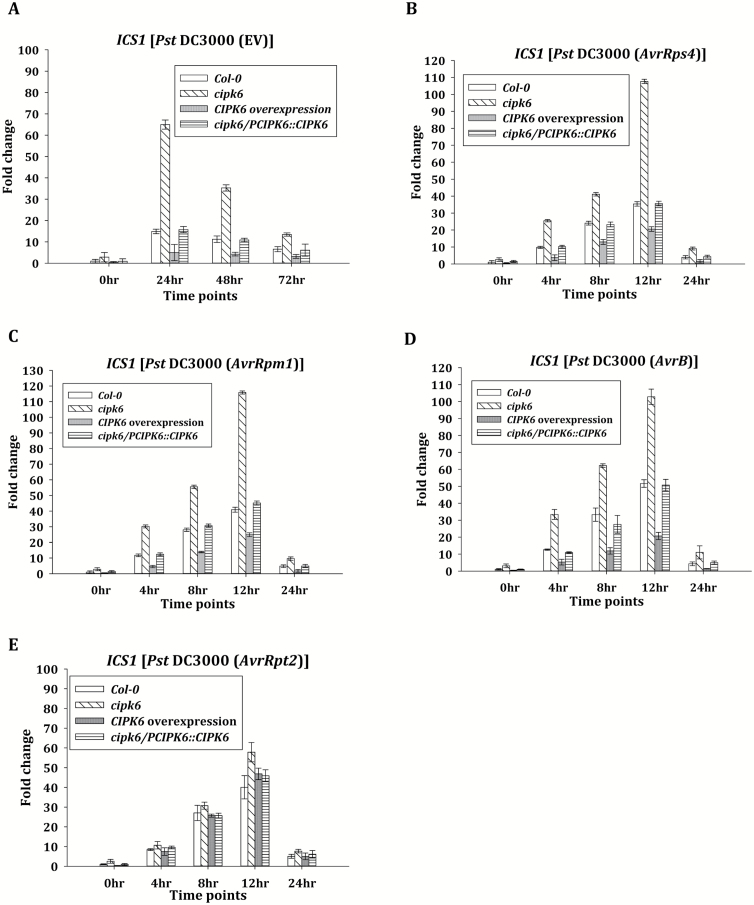
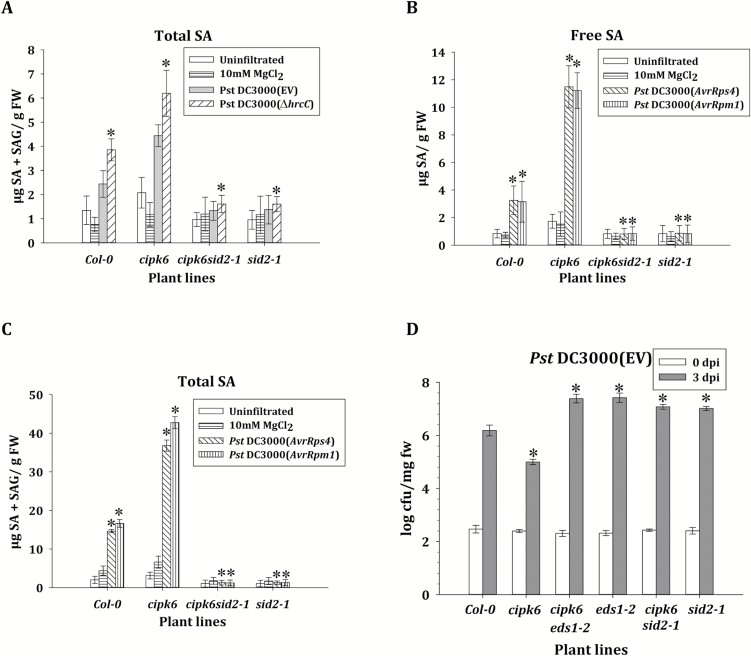
SA accumulation upon pathogen infection is a hallmark of the defense response in plants against biotrophic and hemibiotrophic pathogens. SA is also a key regulator of plant immunity. Therefore, the expression level of ICS1, an important SA biosynthetic gene, was monitored in all four plant lines mentioned above. ICS1 expression in response to Pst DC3000 was >3-fold higher in the cipk6 line and 2-fold lower in CIPK6OX plants than that in wild-type and CIPK6-complemented cipk6 plants from 2 dpi to 3 dpi (Fig. 3A). A similar difference in ICS1 expression was observed in response to Pst DC3000-AvrRps4, -AvrB, and -AvrRpm1 infection (Fig. 3B–D). Lack of expression or high expression of CIPK6 did not affect ICS1 expression in the case of Pst DC3000-AvrRpt2 infection, similar to PR1 expression (Figs 2E, 3E). SA undergoes various modifications to keep the balance between free active SA and total SA (Dempsey et al., 2011). Since CIPK6 was found to modulate ICS1 expression, total SA content was assayed in wild-type and cipk6 plants after pathogen infiltration. Pst DC3000 infiltration in the cipk6 line resulted in an ~2-fold higher accumulation of total SA than that in the wild-type (Col-0) plants (Fig. 4A). Infiltration with a Pst DC3000 strain with a disrupted type III secretion system, Pst DC3000 (ΔhrcC), resulted in accumulation of a much higher and differential level of SA in both the Col-0 and cipk6 plants, most probably because the mutated pathogen is incapable of secreting PTI-suppressing type III effectors. To investigate the role of ICS1 in enhanced SA accumulation in cipk6 plants, the total SA content was assayed in the cipk6sid2-1 double mutant line after pathogen infiltration. The double mutant did not show any increase in total SA content upon infiltration, indicating that enhanced SA accumulation in cipk6 plants was totally dependent on ICS1 (Fig. 4A). Similar enhanced and compromised accumulation of free and total SA was observed in cipk6 and cipk6sid2-1, respectively, when infected with Pst DC3000 carrying AvrRps4 or AvrRpm1 (Fig. 4B, C). Compromise in SA accumulation upon Pst DC3000 infiltration by combined loss of function of both CIPK6 and ICS1 (sid2-1) was also reflected in disease susceptibility. The double mutant cipk6sid2-1 exhibited an ~100-fold greater bacterial titer than cipk6 plants, completely abolishing the resistance observed in the absence of CIPK6 (Fig. 4D). Induction of PR1 expression was subsequently not observed in infected cipk6sid2-1 plants at 48 hpi (Supplementary Fig. S3), showing that CIPK6-regulated defense modulation was dependent on SA accumulation. Similar higher bacterial growth and absence of induction of PR1 expression were observed in Pst DC3000-infected cipk6eds1-2, suggesting that CIPK6 functions genetically upstream of EDS1 (Fig. 4D). External application of SA resulted in equivalent PR1 gene expression in Col-0 and cipk6 plants, indicating that CIPK6 does not function downstream of SA in Arabidopsis (Supplementary Fig. S4).
Fig. 3.
Expression of the SA biosynthetic gene ICS1 was enhanced in the absence of CIPK6. (A–E) Time course of ICS1 expression assessed by qRT–PCR in Col-0, cipk6, CIPK6 overexpression, and cipk6/PCIPK6::CIPK6 plant lines in response to Pst DC3000 (EV) and to Pst DC3000 (AvrRps4/AvrRpm1/AvrB/AvrRpt2) infiltration. Actin 2 and Tubulin 4 were used as internal controls.
Fig. 4.
Negative regulation of pathogen resistance by CIPK6 was dependent on salicylic acid (SA). (A) Col-0, cipk6, cipk6sid2-1, and sid2-1 leaves were manually infiltrated with MgCl2 and Pst DC3000 (EV) or Pst DC3000 (ΔhrcC). Total SA was measured at 9 hpi. (B, C) The same plant lines as above were infiltrated with MgCl2 and Pst DC3000 (AvrRps4/AvrRpm1). Total and free SA was measured at 9 hpi. (D) Bacterial growth was assessed at 3 dpi in Col-0, cipk6, eds1-2, cipk6eds1-2, sid2-1, and cipk6sid2-1 plants and was expressed as the log cfu/mg fresh weight. The asterisks indicate a significant difference following two-way ANOVA (α=0.05).
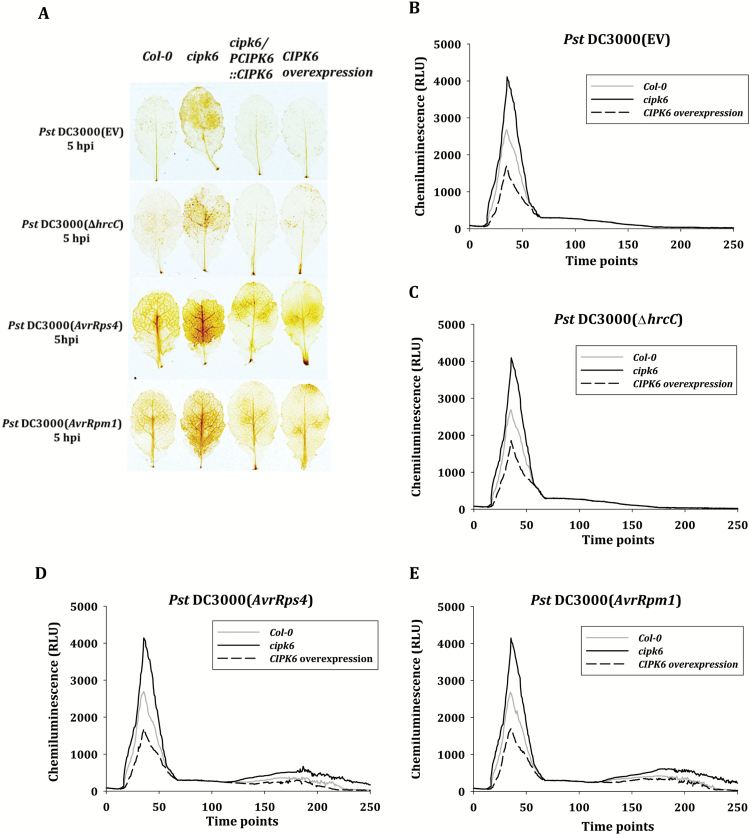
CIPK6 negatively regulates ROS generation
Production of ROS is a hallmark of early PTI and ETI responses, and calcium sensors are known to be involved in the generation of elicitor-induced ROS, critical for the onset of the defense mechanism and the HR (Harding et al., 1997). Hydrogen peroxide production was detected in situ using DAB in the leaves of Col-0, cipk6, cipk6 /PCIPK6::CIPK6 and CIPK6OX at 5 hpi with Pst DC3000 without and with AvrRps4/AvrRpm1. A relatively intense DAB stain showing production of hydrogen peroxide was observed in cipk6 as compared with that in Col-0, cipk6/PCIPK6::CIPK6, and CIPK6OX plants in the case of all the bacterial strains including Pst DC3000 (ΔhrcC) (Fig. 5A). DAB stain in the case of Pst DC3000 with AvrRps4 or AvrRpm1 was more intense as these effectors elicit an effector-triggered immune response in Arabidopsis. The time course of ROS generation was analyzed by in vivo luminol-based assay to determine the involvement of CIPK6 in PTI- and ETI-mediated ROS generation against the same bacterial strains. Pst DC3000 and Pst DC3000 (ΔhrcC) generated only one ROS peak corresponding to PTI at 35 min post-inoculation while Pst DC3000 (AvrRps4/ AvrRpm1) generated two ROS peaks at 35 min and 180 min post-inoculation, denoting PTI and ETI, respectively (Fig. 5B–E). All the ROS peaks were substantially increased in cipk6 plants as compared with Col-0 plants for all the pathogens, while ROS peaks were reduced to a much lower level in CIPK6OX plants. Further, the ETI-associated ROS peaks in Pst DC3000 (AvrRps4/AvrRpm1)-infected plants were prolonged in cipk6 plants. Collectively, these data suggested that CIPK6 functions as a negative regulator of ROS generation during both PTI and ETI.
Fig. 5.
CIPK6 is a negative regulator of PTI- and ETI-triggered ROS generation. (A) Leaves of the Arabidopsis lines mentioned were manually infiltrated with Pst DC3000 (EV) (OD600=0.001), Pst DC3000 (ΔhrcC), Pst DC3000 (AvrRps4), or Pst DC3000 (AvrRpm1) (OD600=0.02). Hydrogen peroxide accumulation was detected by 3',3'-diaminobenzidine (DAB) staining at 5 hpi. (B–E) Time-course of ROS production in response to Pst DC3000 (EV), Pst DC3000 (ΔhrcC), Pst DC3000 (AvrRps4), and Pst DC3000 (AvrRpm1). ROS were measured for 250 min. The values presented are the mean of at least six biological replicates, each with four technical replicates. (This figure is available in colour at JXB online.)
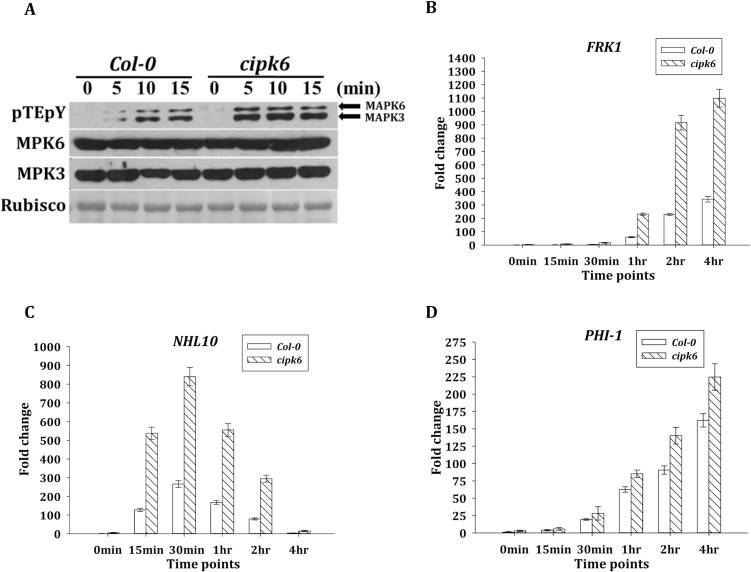
CIPK6 negatively regulates MAPK-mediated gene expression during PTI
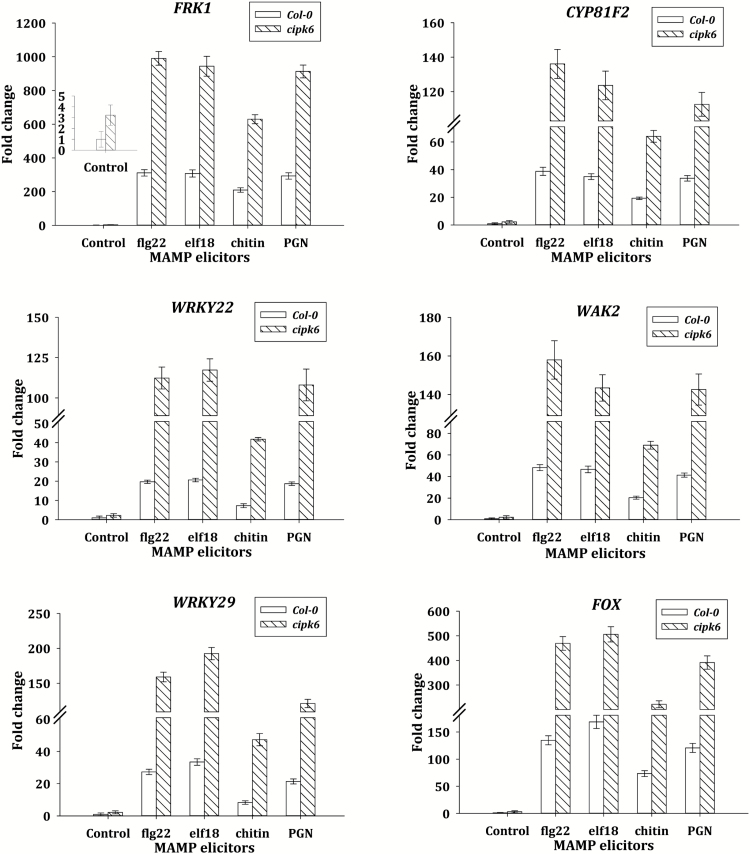
As CIPK6 negatively regulated PTI-mediated ROS generation, its role in PTI-mediated gene expression was investigated using a well-known elicitor of PTI, flg22. Activation of the MAPK cascade and phosphorylation in the activation loops of MPK3 and MPK6 are the hallmarks of PTI signaling (Yung et al., 1997; Asai et al., 2002; Li et al., 2007). While constitutive phosphorylation in the activation motifs of these MAPKs was not detectable in either of the plants, treatment with flg22 resulted in accelerated and enhanced phosphorylation of MPK3 and MPK6 in the cipk6 plants in comparison with the wild-type plants (Fig. 6A). The role of CIPK6 in the PTI-mediated transcriptional activation was investigated by analyzing the expression patterns of early flg22-responsive genes such as FRK1 (FLG22-induced Receptor Kinase1), PHI-1 (Phosphate Induced 1), and NHL10 (NDR1/Hin1-Like 10). Calcium-dependent protein kinase (CDPK) and MAPK cascade act differentially and synergistically to modulate transcriptional reprogramming. It was shown that flg22-induced expression of FRK1 was MAPK dependent (Asai et al., 2002), whereas that of PHI-1 was CDPK dependent (Boudsocq et al., 2010). NHL10 expression was shown to be equally activated by CDPK and MAPK cascades (Boudsocq et al., 2010). flg22 elicited >4-fold higher expression of FRK1 and NHL10 in cipk6 plants as compared with the wild-type plants, whereas no significant increase (<1.5-fold) was observed in PHI-1 expression (Fig. 6B–D). Expression patterns of MAPK-dependent PTI marker genes, FRK1, CYP81F2, WAK2, FOX, WRKY22, and WRKY29 (Boudsocq et al., 2010), in response to flg22 and various bacterial and fungal elicitors, flg22, elf18, PGN, and chitin (Kunze et al, 2004; Sharp RG, 2013; Ao et al., 2014), were analyzed. Expression levels of CYP81F2, WAK2, FOX, and FRK1 were >3-fold higher, and those of WRKY22 and WRKY29 were >5-fold higher in cipk6 plants as compared with their expression levels in Col-0 at 4 h post-treatment (Fig. 7). All these results suggested that CIPK6 negatively modulates the MAPK signaling pathway during PTI.
Fig. 6.
CIPK6 negatively regulated MAPK signaling during PTI. (A) Time course of MPK3 and MPK6 phosphorylation in Col-0 and cipk6 plants upon flg22 treatment was assessed by western blot using antibody (pTEpY) specific for phosphorylated MAPKs. Total MPK3 and MPK6 proteins were detected by the respective protein-specific antibodies. Ponceau S staining of Rubisco was used as a loading control. (B–D) Expression analysis of NHL10, FRK1, and PHI-1 in Col-0 and cipk6 plants by qRT–PCR. The Col-0 and cipk6 plants were treated with flg22 (1 µM). The samples were collected at the indicated time points for qRT–PCR analysis. Actin 2 and Tubulin 4 were used as internal controls. SDs were determined using three biological replicates and two technical replicates for each.
Fig. 7.
Expression analyses of PTI marker genes in response to various elicitors in the absence of CIPK6. qRT–PCR analysis of FRK1, CYP81F2, WRKY22, WAK2, WRKY29, and FOX expression in Col-0 and cipk6 plants. Plants (8 d old, 21–23 °C) were treated with water, flg22 (1 µM), elf18 (1 µM), chitin (50 µg ml–1), or peptidoglycan (PGN; 50 µg ml–1). The samples were collected after 4 h of treatment for qRT–PCR analysis. The error bars in the qRT–PCR analysis indicate the SD. Expression of Actin 2 and Tubulin 4 was used as internal controls. At least three biological replicates for each sample were used for qRT–PCR analysis and at least two technical replicates were analyzed for each biological replicate.
Discussion
Calcium is a universal second messenger involved in modulation of diverse developmental and adaptive process. A rapid and sustained increase in the cytosolic calcium level ([Ca2+]cyt) is necessary for pathogen response (Grant et al., 2000; Lecourieux et al., 2006). An increase in free [Ca2+]cyt is recognized by an array of Ca2+- sensors. Calmodulin (CaM) and calmodulin-like proteins (CMLs), a major group of Ca2+- sensors, are some of the key regulatory proteins of the plant immune response. CaM proteins were demonstrated to be transcriptional regulators and interactors of several plant immunity-associated proteins (Galon et al., 2010). Direct regulation of the SA level by a Ca2+-binding protein has been demonstrated using AtSR1/CAMTA3, which negatively regulated the pathogen-induced SA level (Du et al., 2009). Arabidopsis CML43 functions as an SA-inducible root-specific Ca2+- sensor (Bender et al., 2014). The role of another group of calcium sensors, the CDPKs, particularly CPK4, -5, -6, and -11, in early signaling during PTI has been well demonstrated (Boudsocq et al., 2010). Another group of Ca2+- sensors, CBLs, have been shown to play key roles in calcium-dependent processes in plants (Sanyal et al., 2015). Recently, the Arabidopsis CBL-interacting protein kinase CIPK26 was shown to phosphorylate RbohF in vitro, and co-expression of AtCBL1 or AtCBL9 with AtCIPK26 was shown to enhance ROS production by RbohF in a human cell line (Drerup et al., 2013). In tomato, the calcium sensor CBL10 and its interacting partner CIPK6 were shown to form a signaling module for ROS production and Pto-mediated resistance against P. syringae (de la Torre et al., 2013). OsCIPK14/15 were shown to regulate microbe-associated hypersensitive cell death in rice cell culture (Kurusu et al., 2010). In this study, we have shown that CIPK6, a component of the Ca2+ signaling pathway, functions as a negative regulator of the PAMP-triggered- and SA-mediated effector-triggered immune response in Arabidopsis. The CBL–CIPK signaling module functions as a Ca2+ decoding system (Batistič et al., 2010), and phosphorylation of CBL by the interacting CIPK is necessary for full activity of this complex (Hashimoto et al., 2012). Arabidopsis CIPK6 was shown to interact with CBL1, -2, -3, -4, and -9 in a yeast two-hybrid system and in plant cells (Lee et al., 2007; Held et al., 2011). Among these, AtCBL1 and -9 were shown to enhance RbohF phosphorylation by AtCIPK26 in a human cell line. The role of these CBL proteins in the plant immune response needs to be investigated.
Tomato and Arabidopsis show contrasting responses to AvrPto. While AvrPto triggers Pto-mediated defense in resistant tomato varieties, it suppresses PTI and enhances bacterial virulence by suppressing cell wall-based extracellular defense in Arabidopsis (Hauck et al., 2003; Xiang et al., 2008). Pathogen-induced expression patterns of CIPK6 genes differ in these two species. In silico comparison of their upstream regulatory sequence showed very good similarity with respect to the presence of pathogen-responsive cis-acting elements, such as the WRKY-binding element (GTCAACG/TTCAACG) at ~1.0 kb upstream, the EIN3/EIL-binding element (ATGCA/ATGTA) at ~0.95 kb upstream, and the ERF-binding elements (TAGCT/TAGAG/TAGAA) at several positions upstream of the transcription start sites of both genes. EIN3 and ERF transcription factors are expressed in response to ethylene (Chao et al., 1997). Tomato ERF proteins Pit4, Pit5, and Pit6 were shown to interact with Pto kinase and are involved in pathogen response (Zhou et al., 1997). In contrast, Arabidopsis does not show AvrPto/Pto kinase-mediated ETI. This might be the reason for contrasting expression patterns of CIPK6 genes in these two plant systems. However, our result demonstrated that CIPK6 negatively regulated defense against the bacterial effectors AvrRps4, AvrRpm1, and AvrB, which elicit ETI in Arabidopsis. Therefore, it appears that CIPK6 proteins perform distinct roles in different systems.
Unlike AvrRpm1 and AvrB, CIPK6 did not modulate resistance against AvrRpt2, although all of them target the common protein RIN4. However, AvrRpt2 is distinct from AvrRpm1 and AvrB as AvrRpt2 is a cysteine protease and functions by cleaving RIN4 (Chisholm et al., 2005; Kim et al., 2005), whereas the other two effectors induce phosphorylation of RIN4 (Mackey et al., 2002). Therefore, the role of CIPK6 in phosphorylation of RIN4 needs further investigation. CIPK6 appears to act genetically upstream of EDS1 and, therefore, probably functions during recognition of the effectors or during PTI, as suggested by negative regulation of PTI-associated ROS production. Enhanced activation of the MAPK cascade and subsequent MAPK-dependent gene expression suggested that CIPK6 functions during PTI, probably upstream of MAPK activation, although the role of CIPK6 in multiple steps cannot be ruled out. Ca2+ has been recognized as the primary mediator of plant defense. CDPK and MAPK cascades were shown to act differentially as well as synergistically in this regulatory program (Boudsocq et al., 2010). The absence of CIPK6 did not significantly alter the expression level of PHI-1, which is primarily regulated by CDPK after flg22 treatment. Further experiments are required in order to be able to comment on whether or not CIPK6 and CDPKs operate in different pathways.
Recently, Gutiérrez-Beltrán et al. (2017) have shown that SlCIPK6 interacts with and phosphorylates a universal stress protein, SlRd2. Co-expression of SlCIPK6 and SlRd2 in Nicotiana benthamiana resulted in reduced ROS generation. SlRd2 and Arabidopsis protein AtPHOS32 belong to the UspA protein family. AtPHOS32 was shown to be phosphorylated by MAPK3 and MAPK6 in response to flg22 treatment (Merkouropoulos et al., 2008). We have shown that CIPK6 negatively regulated activation of the MAPK cascade. Hence, a co-ordinated role for MAPKs, and CIPK6 in regulating ROS generation during early PTI signaling needs to be investigated.
Plant immunity is a well-balanced process of positive and negative regulation of the immune response. In the absence of negative regulators, constitutive activation or overactivation of the defense response after infection would cause retarded plant growth or, in extreme conditions, even death of the plant (Heil and Baldwin, 2002; Nimchuk et al., 2003). Previously, CaM and CaM-binding transcription factors were shown to regulate the plant immune response negatively (Kim et al., 2002; Du et al., 2009). Here, we show that a member of another major class of Ca2+-regulated proteins functions as a negative regulator of defense signaling in Arabidopsis.
Previously, we and others demonstrated that CIPK6 in Arabidopsis and Brassica napus functions as a positive regulator of abscisic acid (ABA) signaling and salinity tolerance (Tripathi et al., 2009; Chen et al., 2012; Tsou et al., 2012). CIPK6 was shown to modulate the activity and plasma membrane targeting of the potassium transporter AKT2 (Held et al., 2011). Dual and contrasting roles for AtCIPK6 in abiotic and biotic stress signaling illustrated its importance as a crosstalking node of two important plant signaling pathways.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Chlorosis in Pst DC3000-infected Arabidopsis lines.
Fig. S2. Time course of CIPK6 expression after infection with Pst DC3000.
Fig. S3. PR1 expression analysis in Col-0, cipk6, cipk6eds1-2, eds1-2, cipk6sid2-1, and sid2-1 plants by qRT––PCR.
Fig. S4. The expression levels of PR1 in Col-0 and cipk6 in response to exogenous SA treatment were similar.
Table S1. List of primers used in the current study
Supplementary Material
Acknowledgements
We acknowledge Dr J.W. Reed, Dr Vera Bonardi, and Dr Punita Nagpal of the Department of Biology, University of North Carolina, Chapel Hill (UNC) for their scientific and technical suggestions and materials. We also acknowledge Professor G.B. Martin, Cornel University, Ithaca, Dr V. Bonardi, Department of Biology, UNC, and Dr Saikat Bhattacharjee, Regional Centre for Biotechnology, Faridabad, India for plant lines and bacterial strains. Dr Alok K. Sinha of National Institute of Plant Genome Research, New Delhi is acknowledged for providing antibodies. AS acknowledges the Department of Biotechnology, Government of India for a fellowship.
References
- Alfano JR, Collmer A. 2004. Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annual Review of Phytopathology 42, 385–414. [DOI] [PubMed] [Google Scholar]
- Ao Y, Li Z, Feng D, et al. 2014. OsCERK1 and OsRLCK176 play important roles in peptidoglycan and chitin signaling in rice innate immunity. The Plant Journal 80, 1072–1084. [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. 2002. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415, 977–983. [DOI] [PubMed] [Google Scholar]
- Augustine R, Mukhopadhyay A, Bisht NC. 2013. Targeted silencing of BjMYB28 transcription factor gene directs development of low glucosinolate lines in oilseed Brassica juncea. Plant Biotechnology Journal 11, 855–866. [DOI] [PubMed] [Google Scholar]
- Axtell MJ, Chisholm ST, Dahlbeck D, Staskawicz BJ. 2003. Genetic and molecular evidence that the Pseudomonas syringae type III effector protein AvrRpt2 is a cysteine protease. Molecular Microbiology 49, 1537–1546. [DOI] [PubMed] [Google Scholar]
- Batistič O, Waadt R, Steinhorst L, Held K, Kudla J. 2010. CBL-mediated targeting of CIPKs facilitates the decoding of calcium signals emanating from distinct cellular stores. The Plant Journal 61, 211–222. [DOI] [PubMed] [Google Scholar]
- Belkhadir Y, Subramaniam R, Dangl JL. 2004. Plant disease resistance protein signaling: NBS-LRR proteins and their partners. Current Opinion in Plant Biology 7, 391–399. [DOI] [PubMed] [Google Scholar]
- Bender KW, Dobney S, Ogunrinde A, et al. 2014. The calmodulin-like protein CML43 functions as a salicylic-acid-inducible root-specific Ca(2+) sensor in Arabidopsis. Biochemical Journal 457, 127–136. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S, Halane MK, Kim SH, Gassmann W. 2011. Pathogen effectors target Arabidopsis EDS1 and alter its interactions with immune regulators. Science 334, 1405–1408. [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng SH, Sheen J. 2010. Differential innate immune signalling via Ca(2+) sensor protein kinases. Nature 464, 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron RK, Paiva NL, Lamb CJ, Dixon RA. 1999. Accumulation of salicylic acid and PR-1 gene transcripts in relation to the systemic acquired resistance (SAR) response induced by Pseudomonas syringae pv. Tomato in Arabidopsis. Physiological and Molecular Plant Pathology 55, 121–130. [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. 1997. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89, 1133–1144. [DOI] [PubMed] [Google Scholar]
- Chen L, Ren F, Zhou L, Wang QQ, Zhong H, Li XB. 2012. The Brassica napus calcineurin B-Like 1/CBL-interacting protein kinase 6 (CBL1/CIPK6) component is involved in the plant response to abiotic stress and ABA signalling. Journal of Experimental Botany 63, 6211–6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Munkvold KR, Gao H, et al. 2011. Structural analysis of Pseudomonas syringae AvrPtoB bound to host BAK1 reveals two similar kinase-interacting domains in a type III Effector. Cell Host and Microbe 10, 616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm ST, Dahlbeck D, Krishnamurthy N, Day B, Sjolander K, Staskawicz BJ. 2005. Molecular characterization of proteolytic cleavage sites of the Pseudomonas syringae effector AvrRpt2. Proceedings of the National Academy of Sciences, USA 102, 2087–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B, Dahlbeck D, Staskawicz BJ. 2006. NDR1 interaction with RIN4 mediates the differential activation of multiple disease resistance pathways in Arabidopsis. The Plant Cell 18, 2782–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre F, Gutiérrez-Beltrán E, Pareja-Jaime Y, Chakravarthy S, Martin GB, del Pozo O. 2013. The tomato calcium sensor Cbl10 and its interacting protein kinase Cipk6 define a signaling pathway in plant immunity. The Plant Cell 25, 2748–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defraia CT, Schmelz EA, Mou Z. 2008. A rapid biosensor-based method for quantification of free and glucose-conjugated salicylic acid. Plant Methods 4, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey DA, Vlot AC, Wildermuth MC, Klessig DF. 2011. Salicylic acid biosynthesis and metabolism. The Arabidopsis Book 9, e0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Torres M, Mansfield JW, Grabov N, et al. 2006. Pseudomonas syringae effector AvrPtoB suppresses basal defence in Arabidopsis. The Plant Journal 47, 368–382. [DOI] [PubMed] [Google Scholar]
- Drerup MM, Schlücking K, Hashimoto K, Manishankar P, Steinhorst L, Kuchitsu K, Kudla J. 2013. The Calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Molecular Plant 6, 559–569. [DOI] [PubMed] [Google Scholar]
- Du L, Ali GS, Simons KA, Hou J, Yang T, Reddy AS, Poovaiah BW. 2009. Ca(2+)/calmodulin regulates salicylic-acid-mediated plant immunity. Nature 457, 1154–1158. [DOI] [PubMed] [Google Scholar]
- Eitas TK, Dangl JL. 2010. NB-LRR proteins: pairs, pieces, perception, partners, and pathways. Current Opinion in Plant Biology 13, 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikoshi Y. 1993. Two-way ANOVA models with unbalanced data. Discrete Mathematics 116, 315–334. [Google Scholar]
- Galon Y, Finkler A, Fromm H. 2010. Calcium-regulated transcription in plants. Molecular Plant 3, 653–669. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Beltrán E, Personat JM, de la Torre F, Del Pozo O. 2017. A universal stress protein involved in oxidative stress is a phosphorylation target for protein kinase CIPK6. Plant Physiology 173, 836–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M, Brown I, Adams S, Knight M, Ainslie A, Mansfield J. 2000. The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. The Plant Journal 23, 441–450. [DOI] [PubMed] [Google Scholar]
- Harding SA, Oh SH, Roberts DM. 1997. Transgenic tobacco expressing a foreign calmodulin gene shows an enhanced production of active oxygen species. EMBO Journal 16, 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Eckert C, Anschütz U, et al. 2012. Phosphorylation of calcineurin B-like (CBL) calcium sensor proteins by their CBL-interacting protein kinases (CIPKs) is required for full activity of CBL–CIPK complexes toward their target proteins. Journal of Biological Chemistry 287, 7956–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck P, Thilmony R, He SY. 2003. A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants. Proceedings of the National Academy of Science, USA 100, 8577–8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Shan L, Lin NC, Martin GB, Kemmerling B, Nürnberger T, Sheen J. 2006. Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell 125, 563–575. [DOI] [PubMed] [Google Scholar]
- Heidrich K, Wirthmueller L, Tasset C, Pouzet C, Deslandes L, Parker JE. 2011. Arabidopsis EDS1 connects pathogen effector recognition to cell compartment-specific immune responses. Science 334, 1401–1404. [DOI] [PubMed] [Google Scholar]
- Heil M, Baldwin IT. 2002. Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends in Plant Science 7, 61–67. [DOI] [PubMed] [Google Scholar]
- Hein I, Gilroy EM, Armstrong MR, Birch PR. 2009. The zig–zag–zig in oomycete–plant interactions. Molecular Plant Pathology 10, 547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held K, Pascaud F, Eckert C, et al. 2011. Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Research 21, 1116–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. 2006. The plant immune system. Nature 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Kim BG, Waadt R, Cheong YH, Pandey GK, Dominguez-Solis JR, Schültke S, Lee SC, Kudla J, Luan S. 2007. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. The Plant Journal 52, 473–484. [DOI] [PubMed] [Google Scholar]
- Kim CK, Panstruga R, Elliot C, Schulze-Lefert P. 2002. Calmodulin interacts with MLO protein to regulate defence against mildew in barley. Nature 416, 447–450. [DOI] [PubMed] [Google Scholar]
- Kim HS, Desveaux D, Singer AU, Patel P, Sondek J, Dangl JL. 2005. The Pseudomonas syringae effector AvrRpt2 cleaves its C-terminally acylated target RIN4 from Arabidopsis membranes to block RPM1 activation. Proceedings of the National Academy of Sciences, USA 102, 6496–6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G. 2004. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. The Plant Cell 16, 3496–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu T, Hamada J, Nokajima H, et al. 2010. Regulation of microbe-associated molecular pattern-induced hypersensitive cell death, phytoalexin production, and defense gene expression by calcineurin B-like protein-interacting protein kinases, OsCIPK14/15, in rice cultured cells. Plant Physiology 153, 678–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourieux D, Ranjeva R, Pugin A. 2006. Calcium in plant defence-signalling pathways. New Phytologist 171, 249–269. [DOI] [PubMed] [Google Scholar]
- Lee SC, Lan WZ, Kim BG, Li L, Cheong YH, Pandey GK, Lu G, Buchanan BB, Luan S. 2007. A protein phosphorylation/dephosphorylation network regulates a plant potassium chanel. Proceedings of the National Academy of Sciences, USA 104, 15959–15964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Samaj J, Franklin-Tong VE. 2007. A mitogen-activated protein kinase signals to programmed cell death induced by self-incompatibility in Papaver pollen. Plant Physiology 145, 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey D, Holt BF, III, Wiig A, Dangl JL. 2002. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated disease resistance in Arabidopsis. Cell 108, 743–754. [DOI] [PubMed] [Google Scholar]
- Merkouropoulos G, Andreasson E, Hess D, Boller T, Peck SC. 2008. An Arabidopsis protein phosphorylated in response to microbial elicitation, AtPHOS32, is a substrate of MAP kinases 3 and 6. Journal of Biological Chemistry 283, 10493–10499. [DOI] [PubMed] [Google Scholar]
- Mucyn TS, Clemente A, Andriotis VM, Balmuth AL, Oldroyd GE, Staskawicz BJ, Rathjen JP. 2006. The tomato NBARC-LRR protein Prf interacts with Pto kinase in vivo to regulate specific plant immunity. The Plant Cell 18, 2792–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Heck S, Parinthawong N, Métraux JP. 2002. EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. The Plant Cell 14, 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Métraux JP. 1999. Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. The Plant Cell 11, 1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchuk Z, Eulgem T, Holt BF, III, Dangl JL. 2003. Recognition and response in the plant immune system. Annual Review of Genetics 37, 579–609. [DOI] [PubMed] [Google Scholar]
- Oh CS, Martin GB. 2011. Effector-triggered immunity mediated by the Pto kinase. Trends in Plant Science 16, 132–140. [DOI] [PubMed] [Google Scholar]
- Parker JE, Holub EB, Frost LN, Falk A, Gunn ND, Daniels MJ. 1996. Characterization of eds1, a mutation in Arabidopsis suppressing resistance to Peronospora parasitica specified by several different RPP genes. The Plant Cell 8, 2033–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan R, Lin H, Mendoza I, et al. 2007. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. The Plant Cell 19, 1415–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal SK, Pandey A, Pandey GK. 2015. The CBL–CIPK signaling module in plants: a mechanistic perspective. Physiology Plantarum 155, 89–108. [DOI] [PubMed] [Google Scholar]
- Shan L, He P, Li J, Heese A, Peck SC, Nürnberger T, Martin GB, Sheen J. 2008. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host and Microbe 4, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RG. 2013. A review of the application of chitin and its derivatives in agriculture to modify plant–microbial interactions and improve crop yields. Agronomy 3, 757–793. [Google Scholar]
- Ting JP, Willingham SB, Bergstralh DT. 2008. NLRs at the intersection of cell death and immunity. Nature Reviews. Immunology 8, 372–379. [DOI] [PubMed] [Google Scholar]
- Torres MA, Jones JD, Dangl JL. 2006. Reactive oxygen species signaling in response to pathogens. Plant Physiology 141, 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi V, Parasuraman B, Laxmi A, Chattopadhyay D. 2009. CIPK6, a CBL-interacting protein kinase is required for development and salt tolerance in plants. The Plant Journal 58, 778–790. [DOI] [PubMed] [Google Scholar]
- Tsou PL, Lee SY, Allen NS, Winter-Sederoff H, Robertson D. 2012. An ER-targeted calcium-binding peptide confers salt and drought tolerance mediated by CIPK6 in Arabidopsis. Planta 235, 539–552. [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. 2001. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414, 562–565. [DOI] [PubMed] [Google Scholar]
- Xiang T, Zong N, Zou Y, et al. 2008. Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Current Biology 18, 74–80. [DOI] [PubMed] [Google Scholar]
- Xin XF, He SY. 2013. Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annual Review of Phytopathology 51, 473–498. [DOI] [PubMed] [Google Scholar]
- Yung Y, Dolginov Y, Yao Z, et al. 1997. Detection of ERK activation by a novel monoclonal antibody. FEBS Letters 408, 292–296. [DOI] [PubMed] [Google Scholar]
- Zhou J, Tang X, Martin GB. 1997. The Pto kinase conferring resistance to tomato bacterial speck disease interacts with proteins that bind a cis-element of pathogenesis-related genes. EMBO Journal 16, 3207–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. 2006. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125, 749–760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.