We investigate cholera transmission using data from historical cholera epidemics. The results suggest short-cycle (household/institution level) transmission was important in early secondary transmission. This study of historical outbreaks can inform investigations in current cholera epidemic settings.
Keywords: cholera, epidemiology, historical, epidemics, R0, transmission patterns
Abstract
Background
Although cholera is considered the quintessential long-cycle waterborne disease, studies have emphasized the existence of short-cycle (food, household) transmission. We investigated singular Danish cholera epidemics (in 1853) to elucidate epidemiological parameters and modes of spread.
Methods
Using time series data from cities with different water systems, we estimated the intrinsic transmissibility (R0). Accessing cause-specific mortality data, we studied clinical severity and age-specific impact. From physicians’ narratives we established transmission chains and estimated serial intervals.
Results
Epidemics were seeded by travelers from cholera-affected cities; initial transmission chains involving household members and caretakers ensued. Cholera killed 3.4%–8.9% of the populations, with highest mortality among seniors (16%) and lowest in children (2.7%). Transmissibility (R0) was 1.7–2.6 and the serial interval was estimated at 3.7 days (95% confidence interval, 2.9–4.7 days). The case fatality ratio (CFR) was high (54%–68%); using R0 we computed an adjusted CFR of 4%–5%.
Conclusions
Short-cycle transmission was likely critical to early secondary transmission in historic Danish towns. The outbreaks resembled the contemporary Haiti outbreak with respect to transmissibility, age patterns, and CFR, suggesting a role for broader hygiene/sanitation interventions to control contemporary outbreaks.
Cholera remains a major cause of morbidity and mortality worldwide, with an estimated 2–3 million cases and >100000 deaths each year [1]. Some strains of toxigenic Vibrio cholerae can result in explosive outbreaks when introduced into immunologically naive populations with poor sanitary infrastructure, as was evident in the devastating 2010 cholera epidemic in Haiti after the earthquake disaster [2–4].
Mathematical modeling of the spread and health impact of cholera is a key effort to guide policy makers and intervention planners about the projected impact of interventions, such as vaccinations, in contemporary outbreaks [5–7]. Despite this public health importance, key aspects of cholera disease dynamics, such as the serial interval, transmissibility (R0), and the relative importance of short-cycle (locally mediate via food or household water) transmission vs long-cycle (environmentally mediated) transmission, contain a large amount of uncertainty or remain unresolved [8]. The high-quality epidemiological data needed to address these uncertainties are often lacking, especially in outbreak situations [6].
To fill this data void, we investigated data from an underutilized source: 19th-century cholera epidemics in Europe. Denmark provides an excellent source as its population was not exposed to cholera, likely due to a quarantine at the Danish coast [9]. Finally, in 1853, a year after the quarantine was lifted, a single and catastrophic outbreak hit the nation, including Copenhagen. The outbreaks were largely unmitigated, as contemporary physicians had no effective medical treatment and the miasmic theory was the dominant paradigm for cholera transmission.
Here we qualitatively and quantitatively analyze the Danish cholera experience. For a detailed analysis, we focused on the cholera experience in 2 cities in 1853 (Copenhagen and Aalborg) and 1 city in 1857 (Korsør), which experienced their singular epidemic with a delay. Copenhagen (population 138030) was a large city mostly confined behind city walls with a high population density, whereas Aalborg (population 8621) and Korsør (population 2258) were smaller towns. These were chosen because they all experienced substantial epidemics with large death tolls in the period of a few months and had daily morbidity and mortality counts, along with corresponding census data and information about water systems and other characteristics.
All 3 settings experienced 1 large unmitigated epidemic, providing a unique opportunity to characterize the natural course of cholera epidemics in populations that had never encountered cholera before. Additionally, narrative accounts of individual patients’ illnesses and transmission chains from towns across the country allow for an assessment of transmission pathways early in each outbreak setting and for empirical estimates of the serial interval.
METHODS
Cholera Surveillance, All-Cause Mortality, and Demographic Data
Time series of daily cholera morbidity and mortality counts by age and sex were obtained from datasets compiled by contemporary physicians in 3 towns and cities in Denmark: Copenhagen and Aalborg in 1853 and Korsør in 1857 [10–12]. The case definition used by the physicians at the time defined cholera cases as patients with rice-water diarrhea and evidence of severe dehydration [10]. This case definition is stricter than the one used by the World Health Organization that includes anyone with acute watery diarrhea in a cholera-infected area [2].
To provide epidemiological context to the outbreaks, we acquired cause-specific mortality data for the surrounding years for Copenhagen. We also obtained age-specific population data for Copenhagen, Aalborg, and Korsør (Supplementary Text 1).
Transmission Chains and Estimation of Serial Interval
Transmission chains, modes of transmission, seeding events, and risk factors in the first week of the outbreak were ascertained from physicians’ descriptions of the first generations of cases, including the arrival of the index cases, symptom onset, dates of symptom onset among secondary cases, cholera exposure history, and relationships between cases (Supplementary Text 2) [10, 13, 14]. To estimate the serial interval, we identified “pairs” of cholera cases for which we could establish a highly probable chain of transmission and for which we knew the symptom onset date. We computed observed serial intervals by subtracting the symptom onset date of the primary case from that of the secondary case(s). We fit a Weibull distribution to the observations to calculate the mean and used bootstrapping to estimate a 95% confidence interval (CI).
Transmissibility: Computing the Basic Reproductive Number
The basic reproductive number (R0) is defined as the expected number of secondary infections produced by a primary infection in a fully susceptible population. The R0 values and 95% CIs were estimated using 2 analytical methods: the exponential growth approach [15] and a maximum likelihood method [16]. These methods were implemented in R using the package “R0” [17] and the serial interval was derived from the transmission chain data. To estimate the period of the epidemic over which exponential growth took place, we visually inspected the data and used the deviance-based R2 statistic to choose the best time window [17]. These estimates were compared with an empirical estimate derived from the transmission chain narrative data.
Case Fatality Ratio
We estimated the case fatality ratio (CFR) in 2 ways. First, we estimated the CFRsevere as the reported number of deaths over the reported number of cases according to the strict case definition for cholera (severe dehydration and rice-water diarrhea) used during the outbreaks. As the case definition only included severe cases, we next computed the CFR for all cholera infections (CFRAR). We used the following relation between the R0 and the number uninfected, S, at the end of the epidemic, [18], to compute the overall population attack rates (ARs), including less severe and asymptomatic cholera infections. This relation assumes that nonreported infections, including mild and asymptomatic cases, conferred immunity for the duration of the outbreak [19], and the epidemics were limited by the depletion of susceptible hosts rather than intervention efforts.
Water Systems and Sanitation
To put the cholera epidemics in the context of water and sanitation at the time in each setting, we searched the Danish archives for information on drinking water supply and sanitation for Copenhagen, Aalborg, and Korsør (Supplementary Text 1). All houses/buildings in the 3 towns/cities had bucket latrines and cesspools for human waste, which was occasionally removed and used as agricultural fertilizer. The main water source for Copenhagen’s population was an extensive wooden-pipe network bringing water from both local and more distant lakes to the city. Neither of the 2 smaller cities had a water-pipe network; rather, residents of Aalborg obtained water from small local streams, while those in Korsør utilized a nearby lake. Nationwide infant mortality (<1 year old), as a proxy measure of sanitary conditions, was high, estimated at approximately 150 per 1000 births [20].
Data were tabulated from original sources and maintained in Excel files. All statistical analysis was done using R 3.3.1 software [21].
RESULTS
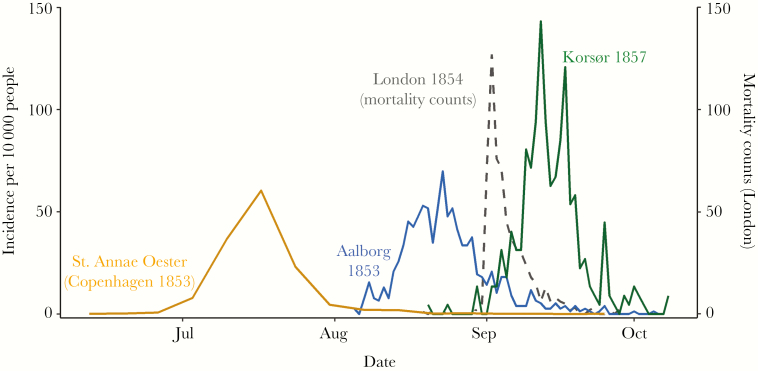
The Danish cholera epidemics were characterized by a singular late-summer severe outbreak in each town and city. The epidemic periods ranged from midsummer to midautumn in all locales (Figure 1). In both Korsør and Aalborg, the epidemic proceeded rapidly following the first known case in early August. For the larger city of Copenhagen, the epidemic appeared to last longer; however, individual neighborhoods experienced outbreaks of similar duration and intensity to Korsør and Aalborg (Supplementary Figure 1). The outbreak duration was around 1 month in all 3 settings, regardless of the existence of municipal water infrastructure. In terms of severity, the cholera experience was the deadliest epidemic for decades in Copenhagen, with 4663 deaths attributed to cholera, representing 68% of all deaths registered in 1853. The epidemic stands out as a catastrophic event in Copenhagen, which experienced almost a 10-fold increase in all deaths during the peak months of the outbreak (Supplementary Figure 2).
Figure 1.
Daily cholera case incidence and seasonality of outbreaks in 3 cities.
Copenhagen was an amalgamation of smaller, neighborhood-scale outbreaks, the most severe of which is shown here. Data for Copenhagen neighborhoods were aggregated to the weekly level and represent average daily incidence for each week. Mortality counts from the Broad Street outbreak in London (1854) are included as the dashed line as reference.
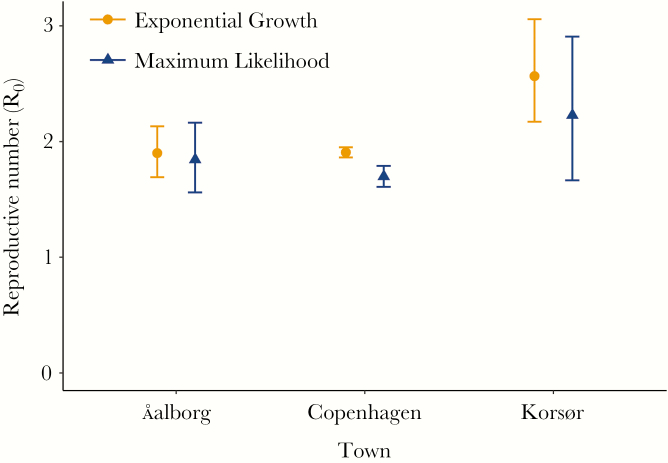
Point estimates of R0 were similar in the 3 cities, ranging from 1.7 in Copenhagen to 2.6 in Korsør (Figure 2). In all towns, the exponential growth method of R0 estimation produced slightly higher point estimates. While the CIs for the 2 methods overlapped in Aalborg and Korsør, in Copenhagen they did not. The results align with the empirically derived R0 estimate of 1.8 (95% CI, 1.3–2.3).
Figure 2.
The reproductive number (R0) in 3 Danish cities.
Based on the strict case definition for cholera (severe dehydration and rice-water diarrhea), the incidence rate of severe cholera ranged from 5.2% (95% CI, 5.1%–5.4%) in Copenhagen to 11.2% (95% CI, 10.1%–12.5%) in Korsør (Table 1). The cumulative mortality ranged from 3.4% in Copenhagen to 8.9% in Korsør. The case fatality ratio among severe cases (CFRsevere) was 66% (95% CI, 64%–67%) in Copenhagen and 68% (95% CI, 59%–78%) in Korsør. Using the R0 estimates above, we calculate the final epidemic size for Copenhagen to be 65%–78% of the total population and a resulting CFRAR of 4%–5%.
Table 1.
Summary Statistics
| Characteristic | Copenhagen | Aalborg | Korsør |
|---|---|---|---|
| Year | 1853 | 1853 | 1857 |
| Population | 138030 | 8621 | 2258 |
| Cumulative cases | 7219 | 759 | 294 |
| Cumulative incidence, % (95% CI) | 5.2 (5.1–5.4) | 8.8 (8.2–9.4) | 13.0 (11.5–14.5) |
| Cumulative deaths | 4737 | 409 | 201 |
| Cumulative mortality, % | 3.4 | 4.7 | 8.9 |
| CFR, % (95% CI) | 66 (64–67) | 54 (49–59) | 68 (59–78) |
Abbreviations: CFR, case fatality ratio; CI, confidence interval.
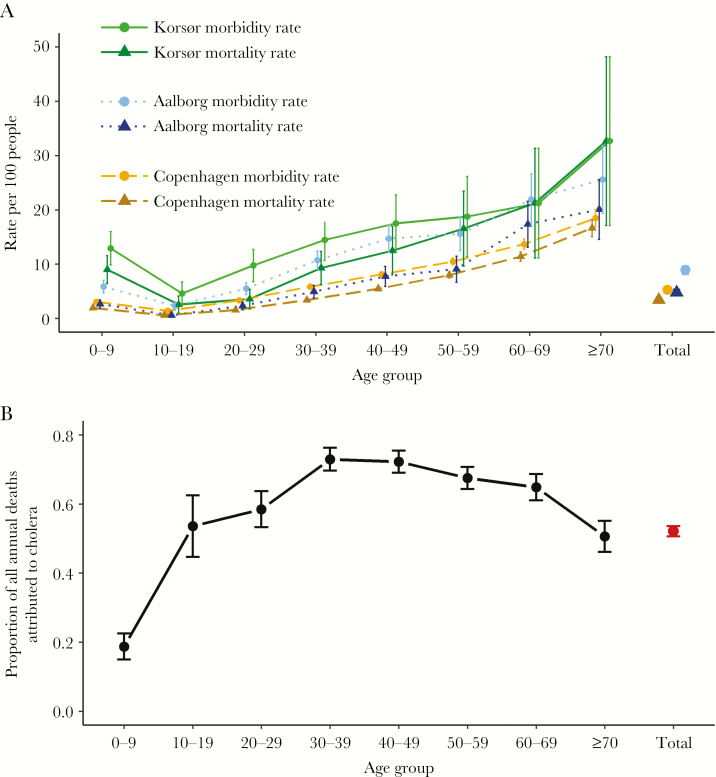
In terms of age breakdown, most registered cholera cases and deaths were in adults. Increasing age was associated with increasing cholera morbidity and mortality rates in all 3 locations. In Copenhagen, 16.7% of all seniors aged ≥70 years died of cholera, while in Aalborg and Korsør this figure was 20.1% and 32.7%, respectively (Figure 3A). Conversely, only 2.7% of children <5 years old in Copenhagen died. Although younger age groups had lower morbidity and mortality rates, cholera was still the leading cause of death in 1853 for all age groups ≥10 years, explaining 73% of all deaths that year in the age group 30–39 years, for example (Figure 3B). Across the 3 cities, sex was not consistently a risk factor in any age group (Supplementary Figure 3A and B).
Figure 3.
Cholera morbidity and mortality (A) disaggregated by age for 3 Danish cities and the proportion of all deaths (B) in Copenhagen, 1853, attributed to cholera disaggregated by age.
In addition to age, we found that socioeconomic status (SES) was a risk factor for cholera. In an analysis of data from 4 neighborhoods of Korsør (population 2013), the area of lowest SES had higher rates of morbidity and mortality than the wealthiest SES quarter. Compared to the wealthiest quarter of town, the odds of cholera illness and death in the lowest SES quarter were 3.0 (95% CI, 2.0–4.3) and 3.2 (95% CI, 2.2–4.7), respectively (Table 2).
Table 2.
Socioeconomic Status and Cholera Outcomes by Neighborhood
| SES Grouping | Population | Mean House Valuea | No. of Cases | No. of Deaths | Morbidity Rate (95% CI) | Mortality Rate (95% CI) | CFR, % | OR for Infection | P Value |
|---|---|---|---|---|---|---|---|---|---|
| High | 717 | 8647 | 58 | 35 | 0.08 (.06–.10) | 0.05 (.03–.06) | 60 | Ref | NA |
| Middle-high | 589 | 2264 | 80 | 56 | 0.14 (.11–.17) | 0.10 (.07–.12) | 70 | 1.8 (1.2–2.6) | .0015 |
| Middle-low | 348 | 1807 | 59 | 42 | 0.17 (.13–.21) | 0.12 (.08–.16) | 71 | 2.3 (1.6–3.4) | <.0001 |
| Low | 359 | 992 | 74 | 51 | 0.21 (.16–.25) | 0.14 (.10–.18) | 69 | 3.0 (2.0–4.3) | <.0001 |
| Totals | 2013 | NA | 271 | 184 | 0.13 (.12–.15) | 0.09 (.08–.10) | 68 | NA | NA |
Abbreviations: CFR, case fatality ratio; CI, confidence interval; NA, not available; OR, odds ratio; SES, socioeconomic status.
House value listed in 1853 Danish krone.
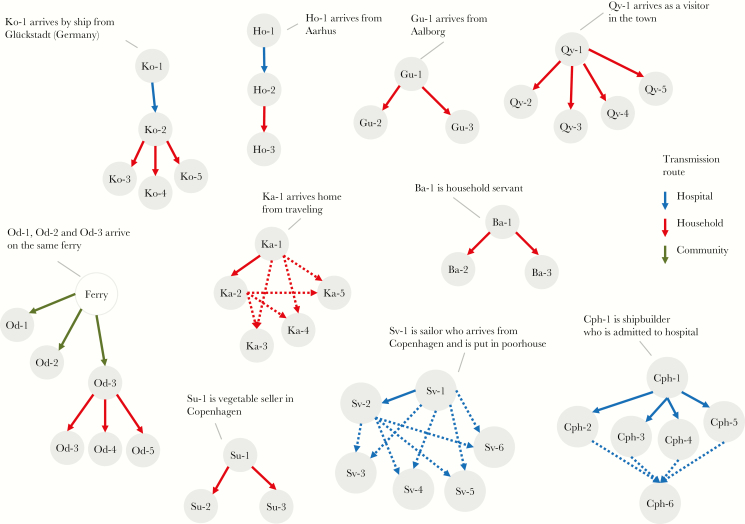
From the physician’s narratives, we identified 44 transmission events and 13 index cases with known travel history and disease onset in 13 towns across Denmark. The index case in each town was typically a sailor, merchant, or other traveler arriving by ship or land from another cholera-affected country or Danish city. Secondary cases occurred in immediate contacts, such as household members, hospital attendants, and other caretakers. Of the 44 transmission events, transmission within households accounted for 24 (55%) events, within hospitals for 16 (36%), within the communities for 3 (7%), and 1 (2%) could not be determined (Figure 4). A total of 22 events contained enough information to establish a serial interval; these ranged from 1 to 10 days (Supplementary Table 1). The serial interval distribution had a mean of 3.7 days (95% CI, 2.9–4.7 days).
Figure 4.
Transmission chains and modes. Solid lines indicate known transmission paths. Dotted lines indicate all possible transmission paths if one single path was not determinable.
As reported in final health reports, the cases clustered in some households while other households avoided infection. Contemporary physicians were divided; many were miasmatics and believed in the dangers of bad air/smells and the “spontaneous” eruption of cholera; this was the case in Aalborg, Korsør, and Copenhagen. Other physicians believed cholera was contagious and practiced isolation of cholera patients. No effective medical treatment, such as oral rehydration salts, was available at the time. Rather, it is likely that some of the treatments, including steam baths, administration of opiates, and/or recommendations to take laxatives, exacerbated patients’ conditions [22].
DISCUSSION
In our analysis of unique epidemiological data from 19th-century Danish cholera outbreaks, we have delineated key aspects of cholera transmission dynamics. While others have conducted detailed epidemiological studies of 19th-century cholera outbreaks in several European cities [23–25], ours is the first to do so in an immunologically naive population, similar to populations in the wake of modern disaster settings such as the cholera outbreak in Haiti in 2010. What we found in the historic Danish data is likely representative of what took place in European and North American cities and towns during the 19th century, at least in their first encounter with cholera (as most of these settings had multiple cholera outbreaks during 1830–1900). We further postulate that short-cycle (local) transmission was extensive in the early stages of the outbreaks and played an important role in kindling the epidemics. While long-cycle (environmentally mediate) transmission undoubtedly plays a role in cholera transmission, our contribution joins previous research in highlighting the importance of looking broader than long-cycle transmission in epidemic settings [26–30].
Regarding primary transmission, we have demonstrated the cholera index case was typically a traveler arriving by ship or foot from an outbreak area where he or she was recorded as recently caring for cholera-infected relatives in other towns. This is in line with the mechanism of intercatchment transport of cholera described in some contemporary settings [31–33], but stands in contrast to environmental aquatic reservoirs others have proposed [34]. This highlights the need to distinguish transmission due to human mobility from environmental mechanisms when investigating primary transmission in contemporary outbreaks.
In terms of secondary transmission, we have shown that close personal contact was a risk factor in the early phase of each epidemic across multiple towns and cities in Denmark. We base this assessment on the following findings: First, narrative reports of the first generations of transmission chains in multiple settings describe transmission from patient to caregivers or household members. This is very different from the point-source epidemic pattern of waterborne cholera outbreaks—for example, in Broad Street, London in 1854 [23]. Rather, the Danish transmission chains and evidence of household clustering are more reminiscent of epidemiology of Ebola reported in the 1976 and 2014 outbreaks, where transmission occurred following close contact with very ill or dead Ebola patients or their belongings [35]. Second, we found the epidemic curves to be similar in all settings despite differing sources of fresh water in each locale (municipal-wide water pipes in Copenhagen city neighborhoods and streams and lakes for Aalborg and Korsør). These stood in contrast to the epidemic curve for the Broad Street epidemic, in which the peak was reached in a matter of a few days (Figure 1) [23]. The Danish cholera epidemics further resembled each other regarding age pattern, outbreak duration, and transmissibility parameter R0.
Comparing the historic Danish with the contemporary Haitian cholera outbreak experiences reveals that apparent differences in clinical severity are largely explained by differing case definitions and modern treatment programs. Both populations were immunologically comparable prior to the outbreaks as no previous population exposure was recorded. The crude CFR estimate of 66% in Copenhagen and 42%–65% in other European settings [36, 37] would at first suggest that the historic epidemics were clinically far more severe than Haiti, where the cumulative CFR over the first 4 months was measured at approximately 2% [2] (Table 3). Indeed, there is evidence the historic classical O1 Vibrio cholerae type circulating in the 1850s [38] was more virulent than the contemporary El Tor biotypes in Haiti [39]; however, this alone is unlikely to explain the dramatic difference seen in the CFR between the 2 settings. Importantly, the Danish physicians used a stricter case definition than what was used in Haiti. Thus, to get a comparable estimate we used our measured R0 to estimate the total size of the epidemic, and from that computed the CFR based on the estimated attack rates (CFRAR) to be 4%–5% in the Danish historic setting, suggesting a similar magnitude of clinical severity in both settings. The effectiveness of modern medical treatment, including oral rehydration salts, intravenous resuscitation, and the use of antibiotics, is evidenced by comparing the CFR of severe cases in Copenhagen (66%) with the CFR of hospitalized cases in Haiti (~4% initially and dropping to ~0.5% after 1 year) [40].
Table 3.
Comparison of Key Metrics in Cholera Outbreaks in Historic and Contemporary Settings
| Location | Stockholm [36] | Aalborg | Copenhagen | Oslo [37] | Korsør | Haiti |
|---|---|---|---|---|---|---|
| Year | 1834 | 1853 | 1853 | 1853 | 1857 | 2010–2011 |
| R0 (95% CI)a | NA | 1.9 (1.7–2.1) | 1.9 (1.9–2.0) | NA | 2.6 (2.2–3.1) | 1.8 (1.6–2.0)b [41] |
| Reported morbidity rate, % of population (95% CI) | 8.1 (7.9–8.2) | 8.8 (8.2–9.4) | 5.2 (5.1–5.4) | 5.1 (4.9–5.3) | 13.0 (11.5–14.5) | 6 (5.1–5.4)c, d [2] |
| Adjusted morbidity rate, % population using R0 | NA | 62–84 | 65–79 | NA | 67–94 | 64e [3] |
| Excess mortality, % of population (95% CI) | NA | NA | 2.4 | NA | NA | 0.4 (.3–.7)f [2] |
| Reported CFR, % (95% CI) | 42 (40–43) | 54 (49–59) | 66 (64–67) | 65 (62–58) | 68 (59–78) | ~2g [2] |
| Adjusted CFR, % using R0 | NA | 6–8 | 4–5 | NA | 9–13 | <2g |
| % of cases in patients <5 y | NA | NA | 8.4 | NA | NA | 13.1g, h [2] |
| Water source | Local streams | Municipal pipes | NA | Local lake | Often untreated well/surface water [4] | |
| Population | 97952 | 8621 | 138030 | ~48000 | 2258 | 2723538c [2] |
Abbreviations: CFR, case fatality ratio; CI, confidence interval; NA, not available.
aCalculated using exponential growth method.
bArtibonite-adjacent communes.
cPort-au-Prince.
dFirst 4 months.
eVibriocidal titer ≥80 in Artibonite basin.
fGonaives.
gNationwide.
hFirst 2 years.
To calculate the CFRAR, we needed estimates of R0 values. A previous analysis of 19th-century European cholera outbreak data reported R0 values ranging from 1.9 to 550.9 [25], a range that likely reflects poor data quality rather than the true variability in cholera transmissibility. The unique availability of daily morbidity counts of cholera cases from surveillance efforts in 3 Danish cities allowed for more accurate R0 estimates, so that our point estimates ranged from 1.7 to 2.6 (Figure 2). These estimates are in good agreement with R0 estimates from the most affected regions of the Haitian outbreak of 1.8 (1.64–2.00) [41]. From the R0 values, and assuming homogeneous mixing within each city’s population, we estimated the likely final epidemic size (true attack rate) to range from 64% to 96%, which includes milder and asymptomatic cholera cases. This reassessment demonstrates that the majority of the population in the Danish cities was infected with cholera (and presumably gained immunity) following their singular outbreak experience in the 1850s.
We next looked at the age-specific risk of contracting cholera and found that for both the Haitian and historic Copenhagen epidemics, the majority of cholera cases were in people >5 years of age. In Haiti, 13.1% of cases [2] were in children <5 years, compared to 8.4% in Copenhagen (Table 3). Both settings had a similar population age structure, dominated by children and young adults [42, 43]. The availability of denominators in the Copenhagen setting allowed for comparison of attack rates between age groups, showing that the majority of both cholera cases and deaths were in older adults. This differs from cholera in endemic settings such as Bangladesh, where most cases occur in young children, probably due to partial or complete immunity in adults [44].
One limitation of our comparison of the Danish and Haitian epidemics is that we are comparing country-level data in Haiti with city-level data in Denmark, and we can only infer, from a recent phylogenetic study of the 1849 Philadelphia cholera outbreak [38], that it was presumably classical O1 cholera which caused the epidemic in Copenhagen. Furthermore, although both settings had high infant (<1 year old) mortality rates, the mortality in Denmark (~150 per 1000) [20] was higher than in Haiti (50.9 per 1000) [42]. Additionally, the Haitian epidemic continues to produce new cases 7 years after introduction, while the Danish epidemics were of short duration in each location (<4 months), possibly due to the lack of an environmental reservoir in the Danish setting. However, the similarities in the epidemics show that cholera of modern outbreak settings can be considered comparable to that of 19th-century Europe from an epidemiological perspective.
For the benefit of improved parameterization for mathematical models, we have provided the first estimate of a serial interval for cholera in an outbreak setting. Previous estimates from household studies in endemic settings show a range for the serial interval of 0–9 days [45]. Our point estimate of 3.7 days is, however, likely biased downward because the nature of our data made it less likely to capture long-cycle transmission. Furthermore, the data did not allow us to ascertain transmission chains after the first few generations in each chain, meaning this assessment is best suited to identifying pairs of human-to-human transmission events in the early generations of transmission. Other research investigating epidemics over longer time scales have concluded that long-cycle transmission contributes significantly to epidemic transmission [5, 46]. To fully explore the incongruity between these results and ours requires higher-resolution data of the subpopulations of the Danish cities over the full course of the epidemic.
Taken together, our quantitative and qualitative study of the singular 19th-century Danish cholera experience shows that short-cycle human-to-human transmission following close contact with cholera patients played a critical role, at least in the seeding and the initial stages of the outbreaks. Accordingly, addressing early cholera transmission in contemporary outbreak settings would benefit from including a focus on domestic and institutional infection control measures, such as safe water collection, handling, treatment, and storage, as well as limiting transmission via food or contact with bodily fluids from patients. Additionally, some interventions, such as water chlorination, affect both long-cycle and short-cycle transmission. Specifically, tackling household- and hospital-based transmission early in an outbreak, as was done in the recent 2014 Ebola outbreak, could be important in preventing larger-scale cholera epidemics.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank University of Copenhagen Public Health students Emma Davidsen and Laila Skrowny for help with editing earlier versions of the manuscript and for assistance identifying primary historical statistical data, respectively. We also thank Jes Clauson-Kaas of HOFOR (Hovedstadsområdets Forsyningsselskab) for excellent discussion about the municipal water systems in Copenhagen, and to our colleagues at the Dutch National Institute for Public Health and the Environment (RIVM) and Virginia Pitzer’s group at Yale University for helpful discussions following seminars there.
Financial support. This work was supported by the University of Copenhagen Changing Disasters 2016 project (to M. D. P), the European Commission H2020 Marie Sklodowska-Curie Actions (grant number 659437 to L. S.) and the US National Institutes of Health (grant number R01 AI112970 to V. E. P.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ali M, Lopez AL, You YA et al. . The global burden of cholera. Bull World Health Organ 2012; 90:209–218A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barzilay EJ, Schaad N, Magloire R et al. . Cholera surveillance during the Haiti epidemic—the first 2 years. N Engl J Med 2013; 368:599–609. [DOI] [PubMed] [Google Scholar]
- 3. Jackson BR, Talkington DF, Pruckler JM et al. . Cholera Serosurvey Working Group. Seroepidemiologic survey of epidemic cholera in Haiti to assess spectrum of illness and risk factors for severe disease. Am J Trop Med Hyg 2013; 89:654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Connor KA, Cartwright E, Loharikar A et al. . Risk factors early in the 2010 cholera epidemic, Haiti. Emerg Infect Dis 2011; 17:2136–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rinaldo A, Bertuzzo E, Mari L et al. . Reassessment of the 2010–2011 Haiti cholera outbreak and rainfall-driven multiseason projections. Proc Natl Acad Sci U S A 2012; 109:6602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chao DL, Longini IM Jr, Morris JG Jr. Modeling cholera outbreaks. Curr Top Microbiol Immunol 2014; 379:195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mukandavire Z, Smith DL, Morris JG Jr. Cholera in Haiti: reproductive numbers and vaccination coverage estimates. Sci Rep 2013; 3:997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grad YH, Miller JC, Lipsitch M. Cholera modeling: challenges to quantitative analysis and predicting the impact of interventions. Epidemiology 2012; 23:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonderup G.Kolera i 1800-tallet—med særlig henblik på Danmark. Tidsskrift for Forskning i Sygdom og Samfund [in Danish]. 2008.http://ojs.statsbiblioteket.dk/index.php/sygdomogsamfund/article/viewArticle/579 Accessed 5 January 2017. [Google Scholar]
- 10. Holst E.Meddelelser om Koleraepidemien i Korsør i 1857 især i hygiejnisk og statistisk Henseende, samt Bidrag til en medicinsk Topographi af Korsør, med et Kort over Korsør [in Danish]. Copenhagen: Schwartz, 1859. [Google Scholar]
- 11. Bricka T.Cholera-Epidemien i Kongeriget Danmark i Aaret 1853, efter de til det kongelige Sundhedscollegium indsendte Lægeberetninger [in Danish]. Copenhagen: C. A. Reitzell, 1855. [Google Scholar]
- 12. Hübertz JR.Beretning om Cholera-Epidemien i Kjøbenhavn, 12 Juni-1. October 1853 [in Danish]. Copenhagen: i Commission hos Jacob Lund, 1855. [Google Scholar]
- 13. Sommer AG. Cholera’s Udbredelsesmaade i Kongeriget Danmark (med Undtagels af Kjöbenhavn) i Aaret 1853 [in Danish]. Bibliotek for Læger 1854; 14:286–377. [Google Scholar]
- 14. Panum PL.Om Cholera-Epidemien i Bandholm 1850 [in Danish]. Hospitals-Meddelelser. Copenhagen: Bianco Luno, 1850; 3. [Google Scholar]
- 15. Wallinga J, Lipsitch M. How generation intervals shape the relationship between growth rates and reproductive numbers. Proc Biol Sci 2007; 274:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White LF, Pagano M. A likelihood-based method for real-time estimation of the serial interval and reproductive number of an epidemic. Stat Med 2008; 27:2999–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Obadia T, Haneef R, Boëlle PY. The R0 package: a toolbox to estimate reproduction numbers for epidemic outbreaks. BMC Med Inform Decis Mak 2012; 12:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kermack WO, McKendrick AG. A contribution to the mathematical theory of epidemics. Proc Royal Soc London A: Math Phys Eng Sci 1927; 115:700–21. [Google Scholar]
- 19. King AA, Ionides EL, Pascual M, Bouma MJ. Inapparent infections and cholera dynamics. Nature 2008; 454:877–80. [DOI] [PubMed] [Google Scholar]
- 20. Løkke A.Døden i barndommen: spædbørnsdødelighed og moderniseringsprocesser i Danmark 1800 til 1920 [in Danish]. Copenhagen: Gyldendal, 1998:478. [Google Scholar]
- 21. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. https://www.R-project.org/. Accessed 20 March 2015. [Google Scholar]
- 22. Larsen KF.Smitstof: kampen mod sygdom i 1800-tallets Danmark [in Danish]. Copenhagen: Munksgaard, 2014. [Google Scholar]
- 23. Snow J.On the mode of communication of cholera. London: John Churchill, 1855. [Google Scholar]
- 24. Tien JH, Poinar HN, Fisman DN, Earn DJ. Herald waves of cholera in nineteenth century London. J R Soc Interface 2011; 8:756–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chan CH, Tuite AR, Fisman DN. Historical epidemiology of the second cholera pandemic: relevance to present day disease dynamics. PLoS One 2013; 8:e72498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tauxe RV, Holmberg SD, Dodin A, Wells JV, Blake PA. Epidemic cholera in Mali: high mortality and multiple routes of transmission in a famine area. Epidemiol Infect 1988; 100:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goh KT, Teo SH, Lam S, Ling MK. Person-to-person transmission of cholera in a psychiatric hospital. J Infect 1990; 20:193–200. [DOI] [PubMed] [Google Scholar]
- 28. Ruiz-Moreno D, Pascual M, Emch M, Yunus M. Spatial clustering in the spatio-temporal dynamics of endemic cholera. BMC Infect Dis 2010; 10:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mukandavire Z, Liao S, Wang J, Gaff H, Smith DL, Morris JG. Estimating the reproductive numbers for the 2008–2009 cholera outbreaks in Zimbabwe. Proc Natl Acad Sci U S A 2011; 108:8767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koch R. An address on cholera and its bacillus. Br Med J 1884; 2:403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mari L, Bertuzzo E, Righetto L et al. . Modelling cholera epidemics: the role of waterways, human mobility and sanitation. J R Soc Interface 2012; 9:376–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rebaudet S, Sudre B, Faucher B, Piarroux R. Environmental determinants of cholera outbreaks in inland Africa: a systematic review of main transmission foci and propagation routes. J Infect Dis 2013; 208(Suppl 1):S46–54. [DOI] [PubMed] [Google Scholar]
- 33. Finger F, Genolet T, Mari L et al. . Mobile phone data highlights the role of mass gatherings in the spreading of cholera outbreaks. Proc Natl Acad Sci U S A 2016; 113:6421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jutla A, Whitcombe E, Hasan N et al. . Environmental factors influencing epidemic cholera. Am J Trop Med Hyg 2013; 89:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dowell SF, Mukunu R, Ksiazek TG, Khan AS, Rollin PE, Peters CJ. Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis 1999; 179(Suppl 1):S87–91. [DOI] [PubMed] [Google Scholar]
- 36. Zacke B.Koleraepidemien i Stockholm 1834, En socialhistorisk studie [in Swedish]. Stockholm: B. Zacke, 1971. [Google Scholar]
- 37. Actstykker angaaende Cholera-Epidemien i Norge i 1853, besørgede ved Medicinal-Committeen [in Norwegian]. Christiania (Oslo), Norway: Carl C. Werner & Co, 1854. [Google Scholar]
- 38. Devault AM, Golding GB, Waglechner N et al. . Second-pandemic strain of Vibrio cholerae from the Philadelphia cholera outbreak of 1849. N Engl J Med 2014; 370:334–40. [DOI] [PubMed] [Google Scholar]
- 39. Woodward WE, Mosley WH. The spectrum of cholera in rural Bangladesh. II. Comparison of El Tor Ogawa and classical Inaba infection. Am J Epidemiol 1972; 96:342–51. [DOI] [PubMed] [Google Scholar]
- 40. Tappero JW, Tauxe RV. Lessons learned during public health response to cholera epidemic in Haiti and the Dominican Republic. Emerg Infect Dis 2011; 17:2087–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lewnard JA, Antillón M, Gonsalves G, Miller AM, Ko AI, Pitzer VE. Strategies to prevent cholera introduction during international personnel deployments: a computational modeling analysis based on the 2010 Haiti outbreak. PLoS Med 2016; 13:e1001947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andersen O.The population of Denmark. Odense, Denmark: Andelsbogtrrykkeriet, 1977. [Google Scholar]
- 43. World Bank. Life expectancy at birth, total (years). 2016. http://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=HT Accessed 29 December 2016. [Google Scholar]
- 44. Harris JB, LaRocque RC, Chowdhury F et al. . Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis 2008; 2:e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chunara R, Andrews JR, Brownstein JS. Social and news media enable estimation of epidemiological patterns early in the 2010 Haitian cholera outbreak. Am J Trop Med Hyg 2012; 86:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eisenberg MC, Kujbida G, Tuite AR, Fisman DN, Tien JH. Examining rainfall and cholera dynamics in Haiti using statistical and dynamic modeling approaches. Epidemics 2013; 5:197–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.