We found that KSHV seroprevalence and antibody titers in this long-standing rural Ugandan cohort are the highest yet reported and changed little over time, perhaps reflecting frequent viral reactivation and persistently elevated transmission and risk of developing Kaposi sarcoma.
Keywords: KSHV, HIV, epidemiology, rural population, Uganda
Abstract
Background
The prevalence and titers of antibodies against Kaposi sarcoma–associated herpesvirus (KSHV) in rural Africa are not completely understood, nor are their trends over time in populations in which human immunodeficiency virus (HIV) is also endemic. We examined prevalence, titers, temporal trends, and determinants of anti-KSHV antibodies in each of 3 time periods (1990–1991, 1999–2000, and 2007–2008) within a long-standing, rural population-based cohort in southwestern Uganda.
Methods
For each period, we measured antibodies to the K8.1 and ORF73 KSHV antigens in approximately 3000 people of all ages (1:1 sex ratio).
Results
In all periods, KSHV prevalence increased rapidly through childhood to approximately 90% by age 15 years, plateauing at approximately 95% thereafter. Similarly, antibody titers, particularly against the lytic antigen K8.1, were among the highest seen and increased significantly with age, suggesting sustained viral replication in this population. Male sex was also independently associated with higher prevalence, whereas HIV coinfection was not. A modest reduction in prevalence among children was noted in the most recent period.
Conclusions
KSHV seroprevalence and antibodies titers in this rural Ugandan population are the highest yet reported, perhaps reflecting frequent viral reactivation and persistently elevated transmission.
Kaposi sarcoma–associated herpesvirus (KSHV), also known as human herpesvirus type 8 (HHV-8) is the causative agent of Kaposi sarcoma (KS) [1, 2]. Unlike other human herpesviruses, KSHV is not ubiquitous in human populations, but varies in prevalence geographically and in human immunodeficiency virus (HIV) risk groups [3]. Incidence of KS in both HIV-infected and uninfected populations largely reflect these differences in KSHV prevalence.
KS was a relatively common malignancy in parts of sub-Saharan Africa prior to the AIDS epidemic and its incidence increased dramatically as HIV spread, becoming the commonest malignancy in countries such as Uganda [4]. The epidemiology of KSHV and KS in Uganda has been studied by ourselves [5–7] and others [8–10] but much remains to be elucidated. Specifically, few studies have addressed the effects of the changing HIV epidemic on KSHV over time, and most studies have been based in urban hospitals and clinics, rather than in rural populations.
The General Population Cohort (GPC) was established in rural southwestern Uganda as a HIV natural history study [11]. It is a population-based cohort study in which individuals of all ages and both sexes in a defined geographical community have been followed (with yearly visits and blood sampling) for over 20 years. We sought to investigate the prevalence and determinants of KSHV infection in this cohort and any potential changes over time.
METHODS
The GPC in Kyamulibwa, southwest Uganda, was established by the UK Medical Research Council and the Uganda Virus Research Institute in 1989 to study the dynamics of HIV in a typical rural Ugandan population [12]. More recently, research activity has broadened to include the epidemiology and genetics of other communicable and of noncommunicable diseases, including cancer, cardiovascular disease, and diabetes [11].
In brief, the GPC is a community-based open cohort study of residents of neighboring villages within a subcounty, lying about 40 km from the shores of Lake Victoria. The population is scattered across the countryside in villages defined by administrative boundaries with a few concentrated in small trading centers. A population of approximately 10000 people in a cluster of 15 adjacent villages was studied from 1989 to 1999. In 2000 the GPC was expanded to cover a further 10 villages. The cohort is dynamic with new births, deaths, and migration reported at each round of follow-up, and the current population under survey includes approximately 22000 people. Data are collected though an annual census, questionnaire, and serological survey. Details of sexual behavior, medical, sociodemographic, and geographic factors are recorded. Blood specimens are obtained at each annual survey. Serum is tested for HIV-1 and the remainder is stored at –80°C in Entebbe (Uganda Virus Research Institute). Since the start of the study, the seroprevalence of HIV in the study population has remained relatively stable at about 8% [11].
We estimated KSHV seroprevalence over the last 20 years from 3 cross-sectional samples of participants, at 1 each of 3 time points. Census rounds 3 (1991–1992), 11 (1999–2000), and 19 (2007–2008) were chosen, based on the availability of samples for testing and the inclusion of children in those rounds. At each time point, we randomly sampled individuals from 4 age classes: 0–14, 15–24, 25–44, and ≥45; 1200, 600, 600, and 600 people, respectively (males and females in equal ratio) were selected. We restricted our selection to people living in the 15 villages included the study from its inception. In these villages, the population size and the age and sex structure has remained stable during the study period.
We selected a total of 9112 samples: 3112 in round 3, and 3000 each in rounds 11 and 19. Sampling was independent in each of the rounds, and because there were no restrictions on resampling, overall we sampled 7601 individuals; 1101 were randomly sampled twice and 205 were sampled 3 times. For a small number of selected individuals, serum specimens were unavailable; in such cases, another participant was randomly selected from the remaining individuals of the same round, age class, and sex.
Antibodies to KSHV K8.1 and ORF73 (LANA) were determined by recombinant protein enzyme-linked immunosorbent assay (ELISA) as previously described [13]. Samples were considered seropositive if they were positive for either antigen. A random selection of 521 samples was also tested using previously described peptide enzyme immunoassays (EIAs) for K8.1 and ORF73 [14, 15]. A total of 1100 samples from HIV- uninfected individuals in round 19 were selected for titration based on the K8.1 optical density distribution: 300, 250, 150, 150, and 150 samples from the highest to lowest quintiles. KSHV K8.1 and ORF73 antibodies were titrated by recombinant assays as previously described [16].
Multivariate hierarchical logistic regression models were utilized when examining prevalence; for antibody levels (titers as number of doubling dilutions and log-transformed optical density [OD]), linear models were used. Statistical significance was determined using likelihood ratio tests; all P values were 2-sided. Nonparametric correlations were assessed between OD obtained with protein ELISA and peptide EIA, and between OD and titers. Assay agreement was evaluated with the Cohen κ statistic. Analyses were carried out using StataSE version 13 software (StataCorp LP, College Station, Texas).
Ethical approval for this study was granted by the Uganda Virus Research Institute Research Ethics Committee and by the Uganda National Council for Science and Technology.
RESULTS
Characteristics of the study sample are shown in Table 1. Overall, median age was 18.7 years (interquartile range [IQR], 10.7–39.5 years) and was slightly but significantly lower in 2000 and 2008 than in 1992; there were also statistically significant, albeit minor differences between rounds in the number of participants per village (median, 218 [IQR, 191–263]) and per household (median, 2 [IQR, 2–4]).
Table 1.
Characteristics of the Study Sample
| Characteristic | Round | |||||
|---|---|---|---|---|---|---|
| 1990–1991 | 1999–2000 | 2007–2008 | ||||
| Sample size | 3112 | 3000 | 3000 | |||
| Age, y | 18.8 | (11.14–39.6) | 18.5 | (11.1–39.2) | 18.5 | (9.5–39.6) |
| Male sex | 1495 | (48%) | 1500 | (50%) | 1500 | (50%) |
| HIV infected | 195 | (6.3%) | 184 | (6.4%) | 191 | (6.5%) |
| No. of villagesa | 15 | 15 | 15 | |||
| No. per villagea | 220 | (198–259) | 197 | (181–239) | 214 | (199–293) |
| Households sampleda | 1340 | 1418 | 1425 | |||
| No. of sampled per householda | 3 | (2–5) | 3 | (2–4) | 3 | (2–4) |
Data are presented as No. (%) or median (interquartile range).
Abbreviation: HIV, human immunodeficiency virus.
aThe same 15 villages were sampled in each round; however, the population within each village and household may have changed.
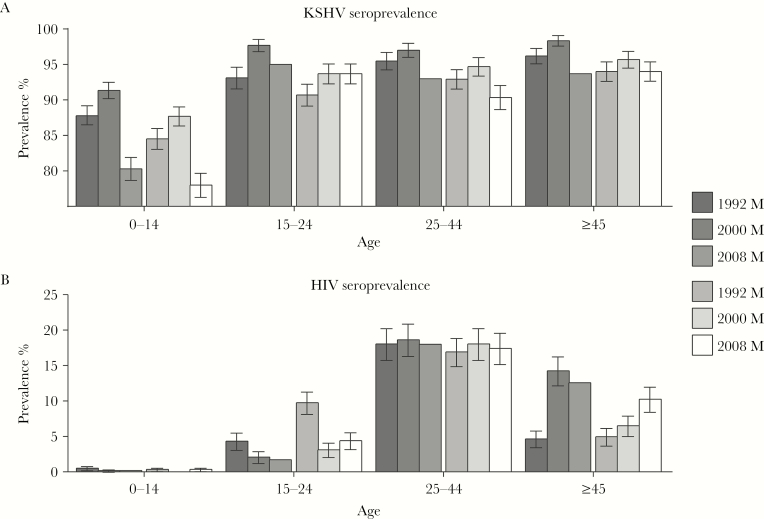
In each round, crude KSHV prevalence rose rapidly in childhood reaching about 90% by age 15 years in all rounds (Figure 1A). Crude HIV prevalence was very low among children; over time, it tended to decrease in young adults, while in older adults it was essentially stable or tended to increase (Figure 1B). Adjusting for age, sex, HIV serostatus, and round, KSHV prevalence increased with age (P for trend < .001) and was significantly higher in males than in females (P < .001), regardless of age. There was no significant interaction between sex and age group. Compared to 1992, prevalence was higher in 2000 and lower in 2008 in adjusted models (Table 2). The decline in crude KSHV prevalence in the last period was most pronounced in children (Figure 1A; Supplementary Table 1); Supplementary Table 2 presents results of adjusted analyses by narrowed age categories in children. HIV seropositivity was not independently associated with KSHV seroprevalence in adjusted models.
Figure 1.
Crude seroprevalence of Kaposi sarcoma–associated herpesvirus (KHSV) (A) and human immunodeficiency virus (HIV) (B) by age category, sex, and round.
Table 2.
Risk Factors for Prevalent Kaposi Sarcoma–Associated Herpesvirus Infectiona (N = 9077b)
| Characteristic | OR c | (95% CI) | P Value |
|---|---|---|---|
| Age, y | |||
| 0–14 | Ref. | <.0001 | |
| 15–24 | 3.73 | (2.70–5.16) | |
| 25–44 | 4.02 | (2.84–5.67) | |
| ≥45 | 5.31 | (3.61–7.82) | |
| Sex | |||
| Female | Ref. | ||
| Male | 1.56 | (1.26–1.92) | <.0001 |
| Year | |||
| 1992 | Ref. | ||
| 2000 | 1.64 | (1.26–2.13) | <.0001 |
| 2008 | 0.61 | (.48–.77) | <.0001 |
| HIV infected | 0.74 | (.47–1.16) | .192 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio.
aHierarchical model accounting for individual and village clustering.
bThirty-five biospecimens not available.
cAdjusted for the other factors.
The prevalence of KSHV in this study was higher than in several previous reports. To exclude potential assay-related misclassification, we repeated KSHV serology on a subsample, utilizing peptide based EIAs used by various research groups [14, 15]. We have previously observed that, for US populations, peptide EIAs are less sensitive than protein ELISAs (Whitby and Dollard, unpublished observations), especially for ORF73, which is a complex protein with many nonlinear epitopes [17]. In this subset, the seropositivity for K8.1 was 89% by recombinant protein ELISA and 92% by peptide EIA, while the seropositivity for ORF73 was 89% by recombinant protein ELISA and 69% by peptide EIA (Supplementary Figure 1). Intertest agreement was 95% for K8.1 and 72% for ORF73 (κ = 0.7 and κ = 0.3, respectively). Interassay correlation was good for ORF73 (κ = 0.51) and excellent for K8.1 (κ = 0.80). These confirmatory data with alternative assays provide reassurance that our data represent valid prevalence estimates for this population.
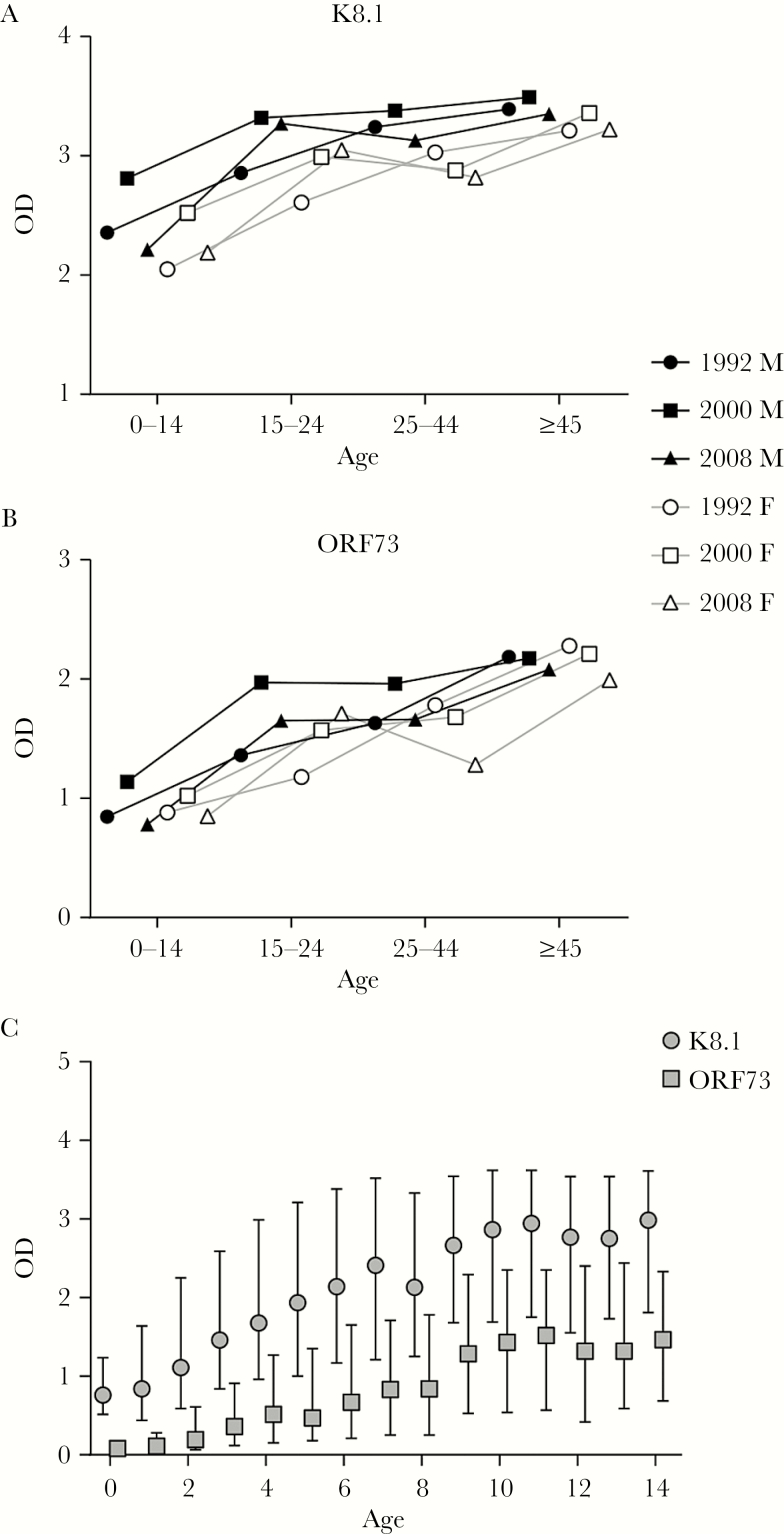
We examined the distribution of antibody levels, as measured by OD, across the study population and saw that the pattern was strikingly different from any other population we had observed, with considerably more subjects having high OD levels for both ORF 73 and K8.1. Supplementary Figure 2 compares OD distributions from this study to those observed in a prior large multinational population-based study conducted using the same ELISAs [18]. The pattern was most dramatic for K8.1. In multivariate models including age, sex, round, and HIV infection, OD increased significantly with age for both K8.1 and ORF73 (P for trend < .001; Table 3; Figure 2). ODs were also significantly higher for both antigens in males compared with females (P < .001) and in 2000 compared with 1992, and tended toward a decrease in 2008. K8.1 and ORF73 OD were slightly but significantly lower in HIV-seropositive individuals compared with HIV-negative persons.
Table 3.
Antibody Levels (Optical Density) as a Function of Age, Sex, Human Immunodeficiency Virus Serostatus and Calendar Timea (N = 9077c)
| Characteristic | K8.1 Log10 (OD) | ORF73 Log10 (OD) | ||||
|---|---|---|---|---|---|---|
| Coeff b | (95% CI) | P Value | Coeffb | (95% CI) | P Value | |
| Age, y | ||||||
| 0–14 | <.001 | <.001 | ||||
| 15–24 | 0.39 | (.33–.44) | 0.43 | (.37–.49) | ||
| 25–44 | 0.43 | (.37–.49) | 0.53 | (.47–.60) | ||
| ≥45 | 0.55 | (.49–.62) | 0.79 | (.73–.86) | ||
| Sex | ||||||
| Female | Ref | Ref | ||||
| Male | 0.18 | (.13–.23) | <.001 | 0.09 | (.04–.13) | <.001 |
| Year | ||||||
| 1992 | Ref | Ref | ||||
| 2000 | 0.17 | (.13–.22) | <.001 | 0.18 | (.13–.23) | <.001 |
| 2008 | –0.04 | (–.09 to .00) | .067 | –0.04 | (–.08 to .01) | .151 |
| HIV infected | –0.14 | (–.24 to –.05) | .004 | –0.15 | (–.25 to –.06) | .002 |
Abbreviations: HIV, human immunodeficiency virus; OD, optical density.
aHierarchical model accounting for individual and village clustering.
bCoefficient adjusted for the other factors.
cThirty-five biospecimen not available.
Figure 2.
Mean anti-K8.1 (A) and anti-ORF73 (B) enzyme-linked immunosorbent assay optical densities (ODs), by age category, sex, and round. C, Mean ODs in children, by age (in years).
To further examine antibody levels, we selected another subsample of 1100 HIV-seronegative specimens from 2008 with a wide range of ODs and performed ELISA titrations for ORF73 and K8.1. As expected, OD was an imperfect surrogate measure for titer, underestimating the antibody level at the highest titers because of the limited dynamic range of ELISA (Supplementary Figure 3). However, analyzing titers yielded findings that differed little from the results obtained with OD (Supplementary Tables 3 and 4; Supplementary Figure 4), except that ORF73 titers did not differ between sexes after adjusting for age.
DISCUSSION
Our study demonstrates that in a rural population in southwest Uganda, KSHV prevalence is higher than in any other population reported to date and that this population also has strikingly high levels of KSHV antibodies. Because both the prevalence and the antibody levels seen in this population are so much higher than has been previously reported for Uganda [19–21], we corroborated our findings on subsets of samples using peptide EIAs and ELISA titration. These confirmatory assays reinforce the validity of our data.
The disparity between the results of the current study (prevalence >90%) and previously reported prevalence estimates (approximately 40%–60%) can possibly be explained in part by differences in antigens and assay formats (recombinant ELISA and peptide EIA vs immunofluorescent assays) or cutoffs, but also are likely to reflect the study population and design. Most previous studies have been conducted in clinics or hospitals and in urban communities and have recruited participants in a relatively narrow age range and selected socioeconomic and health status [9, 22, 23], or have included only a small sample size [8]. Strengths of our study design include the large sample size (>9000 specimens in total) and the complete enumeration of this rural population, which has been followed for >2 decades. This allowed for the unbiased recruitment of participants of both sexes and all ages and health conditions, rather than selected groups that can be studied in antenatal clinics or other health centers.
KSHV transmission is known to vary geographically even within a region; for example, in northern Italy’s Po Valley, significant differences were observed in KSHV prevalence in elderly people living in the district in which the Po and Oglio rivers converged compared to an adjacent district without a major river [24]. Similarly, in the Gauteng province of South Africa, KSHV prevalence varied considerably between antenatal clinics, reflecting local geographic variations [25]. Ecological and lifestyle factors may underlie these local variations. In a country such as Uganda, geographic differences [26, 27] are likely to be greatest between urban and rural settings. It should be noted that 75% of the population of Uganda lives in rural areas, according to the 2014 national census. Previously reported co-factors for KSHV transmission include malaria and other parasitic infections [7, 28, 29], use of surface water [6], and HIV infection [28]. We did not have complete data on exposures other than HIV for the entire study duration. Further detailed studies of KSHV prevalence according to local geography are warranted, to elucidate the effect of these exposures and of additional hitherto unidentified ecological and lifestyle factors.
Previous reports have observed KSHV infection occurring during childhood in sub-Saharan Africa, consistent with our data [21, 30–32]. However, our data show a more rapid increase in prevalence in early childhood compared with previous studies. We also observed significantly higher prevalence in males than in females, contrary to previous studies that have not observed a significant difference in prevalence by sex [8, 21]. This finding, which needs to be further investigated, might be in part mediated by higher antibody levels in males, which can minimize false negatives in serodiagnosis.
We observed changes in KSHV prevalence over time: The overall trend was an increase in prevalence from 1992 to 2000 followed by a decrease in 2008, even below 1992 levels. It is likely that multiple factors contributed to these changes in prevalence. Changes in the risk of KSHV acquisition in children may reflect changes in KSHV immune control, reactivation, and shedding in mothers, siblings, and other individuals potentially transmitting the virus. Thus, changes in HIV prevalence and the introduction of combination antiretroviral therapy are potential contributing co-factors. All GPC participants are provided with routine free medical care, including HIV/AIDS treatment, which have been accompanied by substantial improvements in life expectancy [33]. Although in our study HIV infection per se did not influence the risk of KSHV seropositivity, HIV has been shown to be a risk factor for virus replication and shedding. Moreover, we have demonstrated that malaria and certain parasitic infections are a risk factor for KSHV acquisition in Uganda [7, 28, 29]; thus, recent increases in effective malaria control measures, periodic deworming campaigns, and possibly other changes in the health or behavior of this population, often centered on children, may play a role. This is consistent with our observation of a recent decrease in prevalence in childhood, during which most individuals acquire KSHV. To further elucidate the contribution of these and others co-factors, prospective studies of incident infection and transmission will be needed, with particular focus on children.
The high levels of antibodies in this population, especially anti-K8.1 antibodies, are more comparable to levels seen in patients with KSHV-related diseases than in healthy persons. One interpretation is that high K8.1 levels reflect persistent or frequent KSHV reactivation, which is an important risk factor for KS. Examining KS incidence was not an objective of the present work; we previously published a retrospective KS case-control study nested in the same cohort, spanning approximately the same time period [16]. In that study, KS cases already had significantly higher titers of anti-KSHV antibodies years before presentation and their titers further rose throughout follow-up to diagnosis. Consistent with this notion, in the present study ODs were higher in older participants and among males; both factors were associated with an increased risk of KS. The sex disparity in antibody levels (particularly of anti-K81 antibodies, which is confirmed by titers) is present since childhood, suggesting that sex-specific factors make both boys and men less able to control KSHV replication. Such a phenomenon needs to be investigated further in different settings to examine generalizability and to generate hypotheses about possible mechanisms. More generally, the very high prevalence of KSHV in this population, combined with the high levels of antibodies, is consistent with the high incidence of KS in this region both prior to and during the AIDS pandemic. Our findings may suggest that prevention and treatment of KS in this population may be considerably more challenging than in other populations.
Further studies are needed to understand these findings. Specifically, studies of the correlates of high OD are warranted, including KSHV viral load in peripheral blood mononuclear cells, and clinical and environmental factors associated with viral replication. In summary, our study provides new and exciting insights into the epidemiology of KSHV in rural Uganda in relation to changes over the past 2 decades and opens new avenues of study to help understand the epidemiology of KS.
Supplementary Material
Notes
Acknowledgments. We thank Joseph Meyer for helping produce the figures. We are especially grateful to all study participants.
Disclaimer. The study sponsors had no role in study design, collection, analysis, interpretation of the data, or in writing or submitting this report for publication.
Financial support. This work was supported by US federal funds from the National Cancer Institute, National Institutes of Health (contract number HHSN261200800001E) and the Intramural Research Program. The GPC is funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 19th International Workshop on KSHV and Related Agents, Los Angeles, California, 19–22 July 2016.
References
- 1. Chang Y, Cesarman E, Pessin MS et al. . Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994; 266:1865–9. [DOI] [PubMed] [Google Scholar]
- 2. Whitby D, Howard MR, Tenant-Flowers M et al. . Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi’s sarcoma. Lancet 1995; 346:799–802. [DOI] [PubMed] [Google Scholar]
- 3. Uldrick TS, Whitby D. Update on KSHV epidemiology, Kaposi sarcoma pathogenesis, and treatment of Kaposi sarcoma. Cancer Lett 2011; 305:150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wabinga HR, Parkin DM, Wabwire-Mangen F, Mugerwa JW. Cancer in Kampala, Uganda, in 1989-91: changes in incidence in the era of AIDS. Int J Cancer 1993; 54:26–36. [DOI] [PubMed] [Google Scholar]
- 5. Newton R, Ziegler J, Bourboulia D et al. . Uganda Kaposi’s Sarcoma Study Group The sero-epidemiology of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in adults with cancer in Uganda. Int J Cancer 2003; 103:226–32. [DOI] [PubMed] [Google Scholar]
- 6. Mbulaiteye SM, Biggar RJ, Pfeiffer RM et al. . Water, socioeconomic factors, and human herpesvirus 8 infection in Ugandan children and their mothers. J Acquir Immune Defic Syndr 2005; 38:474–9. [DOI] [PubMed] [Google Scholar]
- 7. Wakeham K, Webb EL, Sebina I et al. . Parasite infection is associated with Kaposi’s sarcoma associated herpesvirus (KSHV) in Ugandan women. Infect Agent Cancer 2011; 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wawer MJ, Eng SM, Serwadda D et al. . Prevalence of Kaposi sarcoma-associated herpesvirus compared with selected sexually transmitted diseases in adolescents and young adults in rural Rakai District, Uganda. Sex Transm Dis 2001; 28:77–81. [DOI] [PubMed] [Google Scholar]
- 9. Hladik W, Dollard SC, Downing RG et al. . Kaposi’s sarcoma in Uganda: risk factors for human herpesvirus 8 infection among blood donors. J Acquir Immune Defic Syndr 2003; 33:206–10. [DOI] [PubMed] [Google Scholar]
- 10. Biryahwaho B, Dollard SC, Pfeiffer RM et al. . Sex and geographic patterns of human herpesvirus 8 infection in a nationally representative population‐based sample in Uganda. J Infect Dis 2010; 202:1347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asiki G, Murphy G, Nakiyingi-Miiro J et al. . GPC Team The general population cohort in rural south-western Uganda: a platform for communicable and non-communicable disease studies. Int J Epidemiol 2013; 42:129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nunn AJ, Kengeya-Kayondo JF, Malamba SS, Seeley JA, Mulder DW. Risk factors for HIV-1 infection in adults in a rural Ugandan community: a population study. AIDS 1994; 8:81–6. [DOI] [PubMed] [Google Scholar]
- 13. Mbisa GL, Miley W, Gamache CJ et al. . Detection of antibodies to Kaposi’s sarcoma-associated herpesvirus: a new approach using K8.1 ELISA and a newly developed recombinant LANA ELISA. J Immunol Methods 2010; 356:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lam LL, Pau CP, Dollard SC, Pellett PE, Spira TJ. Highly sensitive assay for human herpesvirus 8 antibodies that uses a multiple antigenic peptide derived from open reading frame K8.1. J Clin Microbiol 2002; 40:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Engels EA, Whitby D, Goebel PB et al. . Identifying human herpesvirus 8 infection: performance characteristics of serologic assays. J Acquir Immune Defic Syndr 2000; 23:346–54. [DOI] [PubMed] [Google Scholar]
- 16. Wakeham K, Johnston WT, Nalwoga A et al. . Trends in Kaposi’s sarcoma-associated herpesvirus antibodies prior to the development of HIV-associated Kaposi’s sarcoma: a nested case-control study. Int J Cancer 2015; 136:2822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kellam P, Bourboulia D, Dupin N et al. . Characterization of monoclonal antibodies raised against the latent nuclear antigen of human herpesvirus 8. J Virol 1999; 73:5149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Sanjose S, Mbisa G, Perez-Alvarez S et al. . Geographic variation in the prevalence of Kaposi sarcoma-associated herpesvirus and risk factors for transmission. J Infect Dis 2009; 199:1449–56. [DOI] [PubMed] [Google Scholar]
- 19. Chang JT, Shebl FM, Pfeiffer RM, Biryahwaho B, Graubard BI, Mbulaiteye SM. A population-based study of Kaposi sarcoma-associated herpesvirus seropositivity in Uganda using principal components analysis. Infect Agent Cancer 2013; 8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shebl FM, Emmanuel B, Bunts L et al. . Population-based assessment of Kaposi sarcoma-associated herpesvirus DNA in plasma among Ugandans. J Med Virol 2013; 85:1602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Butler LM, Dorsey G, Hladik W et al. . Kaposi sarcoma-associated herpesvirus (KSHV) seroprevalence in population-based samples of African children: evidence for at least 2 patterns of KSHV transmission. J Infect Dis 2009; 200:430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mbulaiteye SM, Biggar RJ, Bakaki PM et al. . Human herpesvirus 8 infection and transfusion history in children with sickle-cell disease in Uganda. J Natl Cancer Inst 2003; 95:1330–5. [DOI] [PubMed] [Google Scholar]
- 23. Hladik W, Dollard SC, Mermin J et al. . Transmission of human herpesvirus 8 by blood transfusion. N Engl J Med 2006; 355:1331–8. [DOI] [PubMed] [Google Scholar]
- 24. Tanzi E, Zappa A, Caramaschi F et al. . Human herpesvirus type 8 infection in an area of northern Italy with high incidence of classical Kaposi’s sarcoma. J Med Virol 2005; 76:571–5. [DOI] [PubMed] [Google Scholar]
- 25. Malope-Kgokong BI, Macphail P, Mbisa G et al. . Kaposi’s sarcoma associated-herpes virus (KSHV) seroprevalence in pregnant women in South Africa. Infect Agent Cancer 2010; 5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dollard SC, Butler LM, Jones AM et al. . Substantial regional differences in human herpesvirus 8 seroprevalence in sub-Saharan Africa: insights on the origin of the “Kaposi’s sarcoma belt.” Int J Cancer 2010; 127:2395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pfeiffer RM, Wheeler WA, Mbisa G et al. . Geographic heterogeneity of prevalence of the human herpesvirus 8 in sub-Saharan Africa: clues about etiology. Ann Epidemiol 2010; 20:958–63. [DOI] [PubMed] [Google Scholar]
- 28. Wakeham K, Webb EL, Sebina I et al. . Risk factors for seropositivity to Kaposi sarcoma-associated herpesvirus among children in Uganda. J Acquir Immune Defic Syndr 2013; 63:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nalwoga A, Cose S, Wakeham K et al. . Association between malaria exposure and Kaposi’s sarcoma-associated herpes virus seropositivity in Uganda. Trop Med Int Health 2015; 20:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dedicoat M, Newton R, Alkharsah KR et al. . Mother-to-child transmission of human herpesvirus-8 in South Africa. J Infect Dis 2004; 190:1068–75. [DOI] [PubMed] [Google Scholar]
- 31. Malope BI, Pfeiffer RM, Mbisa G et al. . Transmission of Kaposi sarcoma–associated herpesvirus between mothers and children in a South African population. J Acquir Immune Defic Syndr 2007; 44:351–5. [DOI] [PubMed] [Google Scholar]
- 32. Brayfield BP, Phiri S, Kankasa C et al. . Postnatal human herpesvirus 8 and human immunodeficiency virus type 1 infection in mothers and infants from Zambia. J Infect Dis 2003; 187:559–68. [DOI] [PubMed] [Google Scholar]
- 33. Asiki G, Reniers G, Newton R et al. . Adult life expectancy trends in the era of antiretroviral treatment in rural Uganda (1991–2012). AIDS 2016; 30:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.