Abstract
Background
Impaired delivery of antifungals to hyphae within necrotic lesions is thought to contribute to therapeutic failure in invasive pulmonary aspergillosis (IPA). We hypothesized that transfusion of leukocytes loaded ex vivo with the lipophilic antifungal posaconazole could improve delivery of antifungals to the sites of established infection and improve outcome in experimental IPA.
Methods
The HL-60 leukemia cell line was differentiated to a neutrophil-like phenotype (differentiated HL-60 [dHL-60] cells) and then exposed to a range of posaconazole concentrations. The functional capacity and antifungal activity of these cells were assessed in vitro and in a mouse model of IPA.
Results
Posaconazole levels in dHL-60 cells were 265-fold greater than the exposure concentration. Posaconazole-loaded cells were viable and maintained their capacity to undergo active chemotaxis. Contact-dependent transfer of posaconazole from dHL-60 cells to hyphae was observed in vitro, resulting in decreased fungal viability. In a neutropenic mouse model of IPA, treatment with posaconazole-loaded dHL-60 cells resulted in significantly reduced fungal burden in comparison to treatment with dHL-60 cells alone.
Conclusions
Posaconazole accumulates at high concentrations in dHL-60 cells and increases their antifungal activity in vitro and in vivo. These findings suggest that posaconazole-loading of leukocytes may hold promise for the therapy of IPA.
Keywords: posaconazole, Aspergillus fumigatus, invasive pulmonary aspergillosis, neutrophil, HL-60
The saprophytic mold Aspergillus fumigatus is a common cause of pulmonary infection in immunocompromised patients. The incidence of invasive pulmonary aspergillosis (IPA) has risen in recent decades, reflecting the increasing number of immunosuppressive medical interventions, such as chemotherapy, hematopoietic stem cell transplantation, and solid organ transplantation [1–7]. Even with appropriate antimicrobial therapy, the mortality rate of IPA remains as high as 50% [8, 9].
One factor underlying the failure of antifungal agents is the inability of these agents to penetrate foci of infection to reach their intracellular targets within fungi. Infection with A. fumigatus is characterized by the presence of filamentous hyphae, which invade and damage tissues, leading to extensive necrosis at foci of infection. Hyphae are also angiotropic and can invade blood vessels, causing thrombosis and subsequent tissue infarction [10–14]. Infarcted and necrotic tissue surrounding pulmonary fungal lesions provides a barrier to antifungal penetration, significantly undermining the clinical efficacy of antifungal drugs [15–18]. Enhancing antifungal penetration into these necrotic lesions is therefore an attractive strategy to improve outcomes in IPA.
Neutrophils exhibit potent anti-Aspergillus activity, and the rapid resolution of IPA following recovery of chemotherapy-induced neutropenia indicates that these cells can penetrate pulmonary lesions to reach invading hyphae [19, 20]. Studies in mouse models of IPA have confirmed that neutrophils efficiently migrate to the site of pulmonary infection following transfusion [21, 22]. Despite these findings, neutrophil transfusions have not proven highly effective in patients with IPA, in part because of the short half-life and poor fungicidal activity of these transfused cells [23, 24].
Posaconazole is a broad-spectrum triazole that is highly active against Aspergillus species [25, 26]. This lipophilic antifungal drug concentrates within the membranes of human cells, including neutrophils and other leukocytes [27–29]. We therefore hypothesized that the ex vivo loading of leukocytes with posaconazole could be used to enhance their ability to kill A. fumigatus and that transfusion with these cells will improve outcomes in a mouse model of IPA. In this study, we used differentiated HL-60 leukemia cells as a model system to investigate the effects of posaconazole loading on the activity of leukocytes against A. fumigatus in vitro and in vivo.
MATERIALS AND METHODS
Fungal Strains
A. fumigatus strain Af293 (a kind gift from P. Magee, University of Minnesota) was grown on YPD agar (Fisher) for 6 days at 37°C. Conidia were harvested by gently washing the plate with phosphate-buffered saline (PBS) containing 0.1% (weight/volume) Tween 80 (PBS-T). Conidia suspensions were passed through a cell strainer (pore diameter, 40 μm), centrifuged at 3000 ×g for 10 minutes, and resuspended in fresh PBS-T. The red fluorescent protein (RFP)–expressing Af293 mutant was generated as described elsewhere [30].
HL-60 Cell Line
HL-60 cells obtained from ATCC were cultured at 37°C in 5% CO2 in Iscove's modified Dulbecco medium (IMDM; Life Technologies) supplemented with 10% fetal bovine serum (FBS; Wisent), 1% penicillin-streptomycin (Life Technologies), and 0.3% sodium bicarbonate (Sigma-Aldrich). Cells were differentiated toward a neutrophil-like phenotype (differentiated HL-60 [dHL-60] cells) following incubation for 3 days with IMDM supplemented with 1.3% (v/v) dimethyl sulfoxide (DMSO; Bioshop) and 2.5 μM all-trans retinoic acid (Sigma-Aldrich). Cell viability was determined by trypan blue staining.
Antifungal Preparation, Minimum Inhibitory Concentration Testing, and Antifungal Loading of Cells
Posaconazole powder (Merck Canada) was dissolved in DMSO and stored at −80°C. For each experiment, final working concentrations were prepared in IMDM. Antifungal susceptibility testing was performed according to the CLSI broth microdilution reference method [31]. The dHL-60 cells were loaded with posaconazole by incubating cells at 37°C in 5% CO2 for 1 hour in IMDM containing the indicated concentrations of posaconazole. Following incubation, cells were washed twice by centrifugation and resuspended in fresh medium.
High-Performance Liquid Chromatography–Tandem Mass Spectrometry of Cell-Associated Posaconazole
Following loading of dHL-60 cells with posaconazole, cell samples were frozen at −80°C for storage. Samples were thawed and deproteinated with acetonitrile solution containing 1 mg/mL ketoconazole as an internal standard. Following centrifugation, 300 μL of the organic phase of each sample was added to 300 μL of LC/MS-grade H2O. An Agilent 6410 triple-quadrupole mass spectrometer equipped with an electrospray ionization (ESI) source (Agilent) was used. Chromatography was performed using an Infinity 1290 (Agilent) system and a Zorbax Eclipse Plus C18 column (internal diameter, 2.1 mm; height, 50 mm; particle size, 1.8 µm; Agilent) under a gradient elution with water containing 0.1% formic acid and acetonitrile. 3PLUS1 Multilevel Plasma Calibrator Set (ChromSystems) was used for calibration. The method was linear in the range of 0.3–5.9 µg/mL. The lower limit of quantification was 0.3 µg/mL. Mean cell-associated posaconazole concentration was calculated as a function of the number of cells per sample and using an estimated dHL-60 cell volume of 300 μm3 [32].
Chemotaxis of dHL-60 Cells
Transwell permeable supports with 5.0-μm-diameter pores (Corning) were coated with human plasma fibrinogen (hFb; Sigma-Aldrich) by incubating wells with 2.5 μM hFb in PBS for 1 hour at 37°C in 5% CO2. A total of 500 μL of IMDM containing 10% FBS as a chemoattractant was added to the lower wells of the 24-well plate, and 100 μL containing 3 × 105 HL-60/dHL-60 cells prepared in serum-free IMDM was added to the upper compartment of each well. The plate was then incubated for 3 hours at 37°C in 5% CO2 to allow for migration. The lower wells were then treated with 0.5 M ethylenediaminetetraacetic acid to promote detachment from the permeable support. Permeable supports were then removed, and the contents of each lower compartment were transferred to microfuge tubes. Cells were collected by centrifugation and frozen at −80°C. The concentration of DNA in the cell pellets was quantified using the CyQuant (Molecular Probes) DNA binding fluorescence dye per the manufacturer's instructions and was read using a fluorometer (Infinite M1000, Tecan) with excitation at 480 nm and emission at 520 nm.
Imaging Studies of BDP-PCZ Transfer
BODIPY fluorophore–tagged posaconazole (BDP-PCZ) was prepared as described previously [33]. A total of 3 × 104 RFP-expressing Af293 conidia were inoculated in an 8-well imaging chamber (Lab-Tek) in IMDM and grown for 7–9 hours at 37°C in 5% CO2. Wells were then washed twice with PBS, and 1 × 106 dHL-60 cells that had been exposed to 4 μg/mL of BDP-PCZ were added to the wells. At each experimental time point, medium was aspirated and then wells were fixed with 4% paraformaldehyde for imaging with a Ziess LSM780 laser scanning confocal microscope.
dHL-60–Mediated Inhibition of A. fumigatus Hyphal Growth
For coincubation, 3 × 104A. fumigatus conidia in 300 μL of IMDM were grown for 7–9 hours at 37°C in 5% CO2 in 24-well tissue culture treated plates and washed with PBS, and 500 μL of IMDM containing the indicated number of dHL-60 cells was added. Plates were incubated for 12 hours at 37°C in 5% CO2, and then the medium was removed and the wells washed twice with PBS. 500 μL of ice-cold sterile ddH2O was added to each well and the plate was incubated for 30 minutes at room temperature to promote lysis of dHL-60 cells. Wells were then washed with PBS, and fungal metabolic activity was measured via reduction of the tetrazolium reagent XTT (Bioshop) as described previously [34].
In Vivo Studies
Female Balb/c mice aged 6–8 weeks underwent immunosuppression via subcutaneous injection of 250 mg/kg cortisone acetate (Sigma) and intraperitoneal injection of 230 mg/kg cyclophosphamide (Baxter) 2 days prior to infection. For infection, mice were anesthetized with inhaled isoflurane and then underwent oral cannulation and received 5 × 103A. fumigatus conidia in PBS-T via intratracheal instillation. Enrofloxacin (Baytril) was added to drinking water to prevent bacterial superinfection. For experimental treatment, 250 μL of PBS containing 1.5 × 107 dHL-60 cells or posaconazole-loaded dHL-60 cells was administered intravenously via the tail vein 12 and 36 hours after infection. Control mice received injections of PBS only. Mice were euthanized 72 hours after infection, by CO2 asphyxiation followed by cervical dislocation, and lungs were removed for subsequent histopathological analysis. All procedures involving mice were approved by the McGill University Animal Care Committee and followed the guidelines established by the Canadian Council on Animal Care.
Histopathological Analysis
Lungs were removed from mice, perfused, and immersed in PBS with 10% formalin for 24 hours for fixation. Fixed samples were then embedded in paraffin, and serial step sections of 5 μm were collected at 80-μm intervals and stained with periodic acid-Schiff. Fungal lesions were then detected via blinded analysis with an inverted light microscope. Five sections of each lung, at minimum, were examined for all animals in each experiment, to ensure that 100 lesions were detected in the group displaying the highest level of infection.
Statistical Analysis
All statistical analysis and production of graphs was performed using GraphPad Prism, version 5.0.
RESULTS
Intracellular Concentrations of Posaconazole in dHL-60 Cells
dHL-60 leukocytes have been used as a model system for the study of neutrophil transfusions for treatment of fungal infections in a number of studies [35–37]. Although posaconazole has been reported to accumulate within primary neutrophils [29], the effects of posaconazole exposure on dHL-60 cells have not been reported. Following in vitro exposure of dHL-60 cells to posaconazole, high-performance liquid chromatography revealed the posaconazole concentrations within dHL-60 cells to be >265-fold greater than the exposure concentration (Figure 1A). There was a linear relationship between exposure and intracellular levels of posaconazole between exposure concentrations of 1 and 8 μg/mL. Beyond an exposure concentration of 8 μg/mL, the intracellular posaconazole levels increased only minimally, likely indicating that saturation of the membranes of dHL-60 cells had occurred. Posaconazole-loaded dHL-60 cells showed near normal viability for up to 96 hours after azole exposure, compared with untreated cells, as determined by trypan blue staining (Figure 1B). Together, these results suggest that loading dHL-60 cells with high concentrations of posaconazole does not significantly affect their viability.
Figure 1.
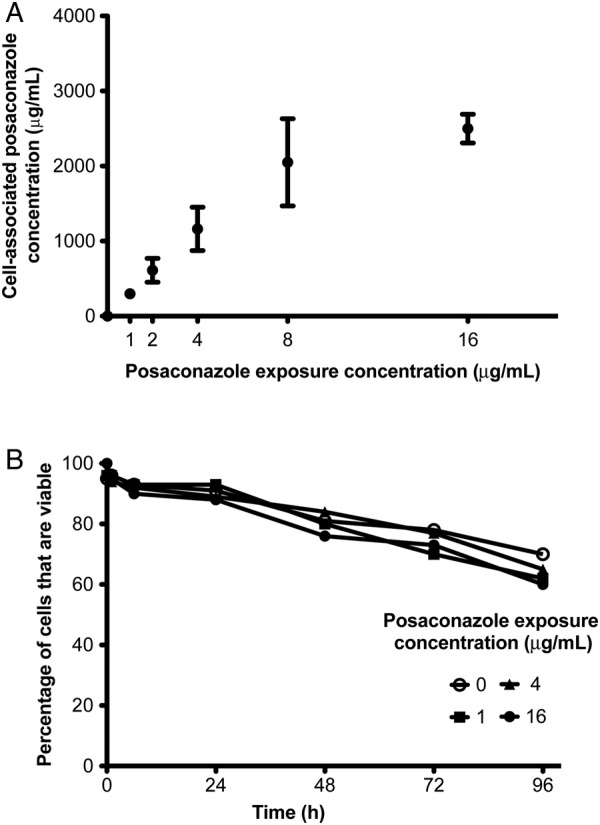
Cell-associated posaconazole concentrations within differentiated HL-60 (dHL-60) cells. Cells were exposed to varying concentrations of posaconazole for 1 hour and then washed to remove extracellular drug. A, High-performance liquid chromatography of cell-associated posaconazole levels as compared to exposure concentrations. B, Viability of dHL-60 cells following loading with posaconazole for up to 96 hours. Results of 3 independent experiments are shown. Error bars indicate standard errors of the mean.
Chemotaxis of Posaconazole-Loaded dHL-60 Cells
For posaconazole-loaded dHL-60 cells to act upon fungi within pulmonary lesions, dHL-60 cells must undergo chemotaxis to the site of infection. We used a Transwell system to evaluate the effects of posaconazole loading on chemotaxis of dHL-60 cells. Because chemotaxis of dHL-60 cells declined dramatically after 3 days of differentiation (Supplementary Figure S1), 3 days of differentiation was used for all studies. Exposure of dHL-60 cells to posaconazole concentrations of 16 μg/mL, placed in the upper wells, had no significant effect on the ability of these cells to migrate across Transwell membranes to the bottom wells in response to a chemoattractant (Figure 2). Migration of dHL-60 cells to the lower chamber was not observed in the absence of a chemoattractant or with undifferentiated HL-60 cells. These results indicate that posaconazole loading of dHL-60 cells does not impair their ability to undergo chemotaxis.
Figure 2.
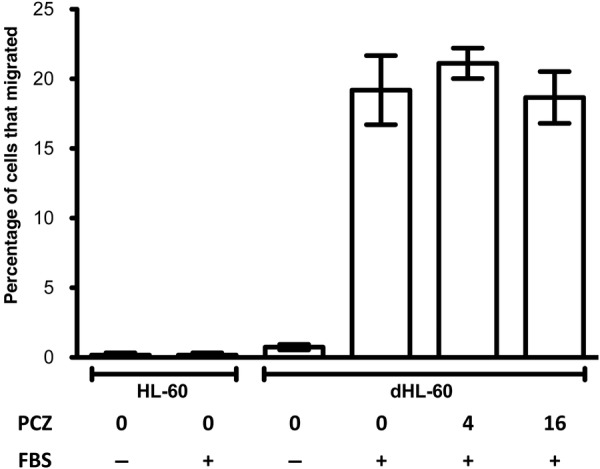
Chemotaxis of posaconazole (PCZ)–loaded differentiated HL-60 (dHL-60) cells. dHL-60 and HL-60 cells loaded at the indicated PCZ concentrations were subjected to a Transwell migration assay. Bars indicate the mean migration levels following incubation for 3 hours. Error bars indicate standard errors of the mean from at least 3 independent experiments. Numerical values indicate PCZ exposure concentration in micrograms/milliliter. Abbreviation: FBS, fetal bovine serum.
Transfer of Posaconazole From dHL-60 Cells to A. fumigatus Hyphae
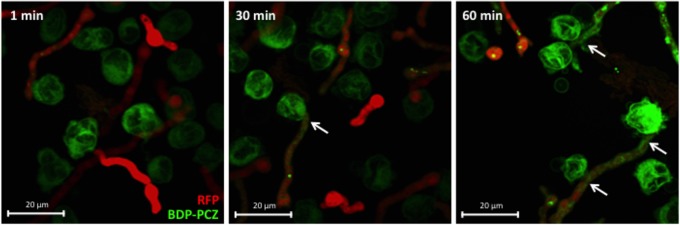
For cell-associated posaconazole to mediate an antifungal effect, it must be transferred from dHL-60 cells to fungal hyphae. To test whether posaconazole can transfer from dHL-60 cells to fungal hyphae, we used our previously described BODIPY-tagged posaconazole molecule (BDP-PCZ) to monitor intercellular trafficking of this drug. BDP-PCZ-–loaded dHL-60 cells were coincubated with RFP-expressing A. fumigatus hyphae and imaged by confocal microscopy. As has been described with pulmonary epithelial cells, BDP-PCZ localized predominantly to cell membranes within dHL-60 cells. Upon contact of dHL-60 cells with A. fumigatus, transfer of BDP-PCZ from leukocytes to hyphae was observed (Figure 3). Fungal accumulation of BDP-PCZ was time dependent, with increasing hyphal fluorescence observed over time. These results demonstrate that dHL-60 cells are able to deliver posaconazole to A. fumigatus hyphae and suggest the possibility that loading these cells with posaconazole may enhance their antifungal activity.
Figure 3.
Transfer of BODIPY fluorophore-tagged posaconazole (BDP-PCZ) from differentiated HL-60 (dHL-60) cells to Aspergillus fumigatus hyphae. Hyphae of red fluorescent protein (RFP)–expressing A. fumigatus were cocultured with BDP-PCZ–exposed dHL-60 cells for the indicated times and imaged by confocal microscopy. Arrows indicate the presence of BDP-PCZ within hyphae at the point of contact with dHL-60 cells.
Posaconazole-Loaded dHL-60–Mediated Inhibition of A. fumigatus Hyphal Growth
To test the effects of posaconazole loading on the ability of dHL-60 to kill pregrown A. fumigatus hyphae, we used the XTT-metabolic assay to quantify fungal metabolic activity following exposure of hyphae to posaconazole-loaded dHL-60 cells. Non–drug-exposed dHL-60 cells exhibited minimal activity against mature hyphae and required a multiplicity of infection (MOI) of >1:100 to mediate any reduction in fungal metabolic activity (Supplementary Figure S2). Posaconazole exposure greatly enhanced the antifungal activity of dHL-60 cells. The antifungal activity of posaconazole-loaded dHL-60 cells was dependent both on the posaconazole exposure concentration, as well as the MOI. Exposure of dHL-60 cells to 4 μg/mL of posaconazole prior to coincubation with hyphae resulted in a reduction of hyphal metabolic activity to 76%, 22%, and 5% of untreated controls at MOIs of 1:4, 1:8, and 1:16, respectively. Coculture of hyphae to dHL-60 cells exposed to 16 μg/mL of posaconazole before infection resulted in reductions of hyphal metabolic activity to 18%, 3%, and 0% of untreated controls at MOIs of 1:4, 1:8, and 1:16, respectively (Figure 4). These findings strongly suggest that cell-associated posaconazole can augment the antifungal activity of dHL-60 cells in a dose-dependent manner.
Figure 4.
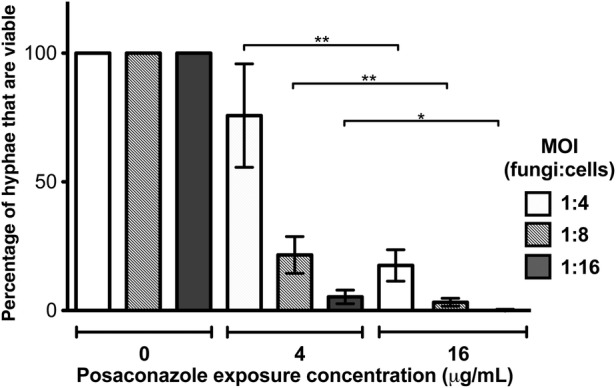
Posaconazole-loaded dHL-60 cell mediated inhibition of Aspergillus fumigatus. Differentiated HL-60 (dHL-60) cells exposed to the indicated posaconazole concentrations were cocultured with pregrown A. fumigatus hyphae for 12 hours. Bars represent mean fungal metabolic activity normalized to that of untreated fungi, as measured by the XTT assay. Error bars indicate standard errors of the mean (SEM) from at least 4 independent experiments. *P < .02 and **P < .005 compared to the same multiplicity of infection (MOI) at different posaconazole concentrations, using a paired t test.
Treatment of Invasive Aspergillosis With Posaconazole-Loaded dHL-60 Cells
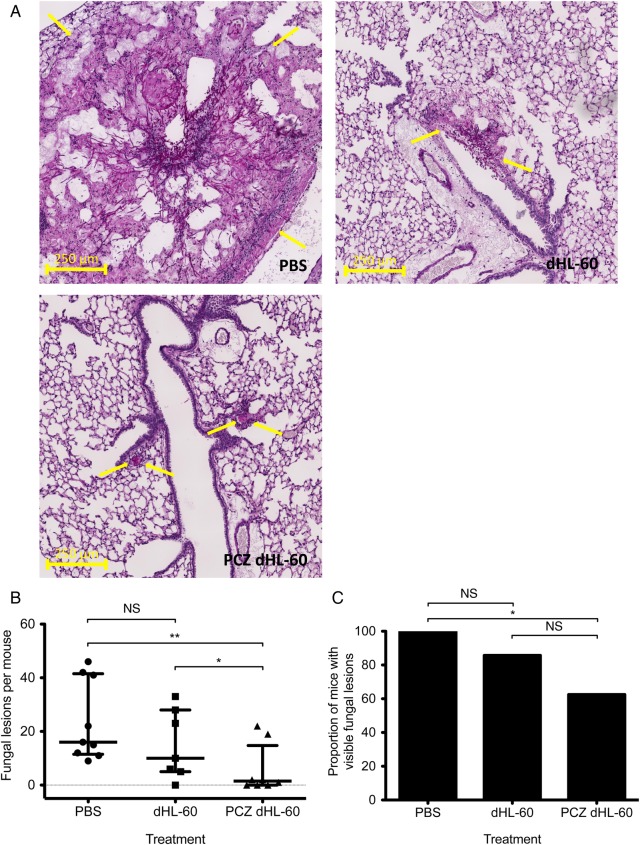
In light of the activity of posaconazole-loaded dHL-60 cells against A. fumigatus in vitro, we hypothesized that transfusion of posaconazole-loaded dHL-60 cells may improve outcomes in experimental IPA. To test this hypothesis, we performed a proof-of-concept study to evaluate the antifungal activity of non–drug-exposed and posaconazole-loaded dHL-60 cells in a neutropenic mouse model of invasive aspergillosis. Neutropenic mice were infected with A. fumigatus by intratracheal inoculation and, 12 and 36 hours after infection, were administered 1.5 × 107 untreated dHL-60 cells or dHL-60 cells exposed to 16 μg/mL. At 72 hours after infection, mice were euthanized and then the degree of pulmonary infection was quantified via blinded histopathological examination. Mice treated with PBS alone exhibited large fungal lesions characterized by extensive hyphal growth and tissue damage (Figure 5A). In contrast, only small lesions with minimal fungal growth were observed in mice treated with posaconazole-loaded dHL-60 cells. Mice treated with non–drug-exposed dHL-60 cells displayed a phenotype intermediate to those observed in mice treated with PBS and those treated with posaconazole-loaded dHL-60 cells. Mice treated with posaconazole-loaded dHL-60 cells had significantly fewer fungal lesions as compared to untreated mice or mice receiving dHL-60 cells alone (Figure 5B). The median number of fungal lesions detected per mouse was 16, 10, and 1.5 for mice treated with PBS, with dHL-60 cells, and with posaconazole-loaded dHL-60 cells, respectively. While fungal lesions were observed in all mice treated with PBS, 33% of the mice treated with posaconazole-loaded leukocytes were found to have no detectable fungal infection on histopathological examination (Figure 5C). Overall, these findings suggest that posaconazole loading enhances the antifungal activity of dHL-60 cells in vivo, as well as in vitro.
Figure 5.
Treatment of experimental invasive aspergillosis with posaconazole-loaded differentiated HL-60 cells (PCZ dHL-60). A, Histopathological staining of lung sections from neutropenic mice infected with Aspergillus fumigatus and administered the indicated treatments. Images are representative sections of periodic acid Schiff–stained lung tissue. Arrows indicate the perimeters of fungal lesions. B, Morphometric analysis of fungal burden in mice administered the indicated treatments. The median and interquartile range of the number of fungal lesions per mouse from 2 independent experiments, each consisting of at least 4 mice per treatment group, are shown. C, Proportions of infected mice following the indicated treatments. *P = .0398 and **P = .0079 (B) and *P = .0293 (C), by the Mann–Whitney test. Abbreviations: NS, not significant; PBS, sham treatment with phosphate buffered saline only.
DISCUSSION
Invasive pulmonary aspergillosis remains a leading cause of death among immunocompromised individuals. In the current study, we investigated the effects of posaconazole exposure on the ability of neutrophil-like dHL-60 cells to kill A. fumigatus. Our findings demonstrate that posaconazole concentrates to high levels within dHL-60 cells, with minimal effects on their viability or ability to undergo chemotaxis. These cells are able to transfer posaconazole to A. fumigatus hyphae upon contact and exhibit potent antifungal activity against A. fumigatus in vitro. Treatment with posaconazole-loaded dHL-60 cells in a neutropenic mouse model of IPA resulted in a reduced pulmonary fungal burden and even an absence of infection in several mice. These results suggest that neutrophils may be an effective posaconazole delivery system for the treatment of IPA.
Posaconazole loading had minimal effects on the function and viability of dHL-60 cells. Similar effects of posaconazole exposure on primary neutrophils have been reported [38]. In this study, the viability and chemotaxis of primary human neutrophils in vitro was preserved following exposure to posaconazole concentrations as high as 1.2 μg/mL [38]. In contrast to our findings, however, posaconazole-loaded primary human neutrophils did not exhibit enhanced killing of A. fumigatus conidia, as determined by quantitative culture [38]. It is likely that this observation reflects the fact that posaconazole is fungistatic rather than fungicidal. Thus, exposure of conidia to posaconazole-loaded neutrophils would be expected to prevent germination of conidia but would not reduce viable conidia counts as determined by quantitative culture, as was reported in the prior study [28]. In contrast, exposure of actively growing hyphae to posaconazole-loaded leukocytes results in a reduction of their metabolic activity, which is detectable using the XTT assay. This explanation is also consistent with our previous finding that, while conidial viability was not affected by 48 hours of coculture with posaconazole-loaded A549 pulmonary epithelial cells, fungal germination and hyphal growth was inhibited, suggesting that cell-associated posaconazole is primarily fungistatic against conidia [28].
The total amount of posaconazole that was administered by dHL-60 transfusions was extremely small. Extrapolation from the cell-associated posaconazole concentrations measured within dHL-60 cells revealed that the total dose of posaconazole administered to mice was 0.56 mg/kg/day. This concentration of posaconazole is almost 20-fold less than the dose of 10 mg/kg/day that has been reported to be required to reduce the fungal burden in a neutropenic mouse model of IPA [39, 40]. Thus, the antifungal effect of posaconazole-loaded cells observed in mice is highly likely due to delivery of posaconazole to fungal lesions and the development of high local concentrations of this antifungal at the site of infection, rather than a reflection of systemic antifungal therapy.
The ability of posaconazole to concentrate in leukocytes and be transferred to hyphae suggests the intriguing possibility that endogenous loading of neutrophils and delivery of posaconazole to fungal lesions may also occur during treatment of nonneutropenic patients with posaconazole. If endogenous neutrophil loading does occur, it is possible that posaconazole may be most active for the treatment of established IPA in nonneutropenic hosts, in whom cell-associated posaconazole would augment the impaired antifungal activity of neutrophils. Comparison of the efficacy of posaconazole in neutropenic and nonneutropenic patients in the ongoing randomized clinical trial of posaconazole for primary therapy of invasive aspergillosis may shed light on this question.
The results of our study provide proof of concept that posaconazole-loaded dHL-60 cells can improve outcome in fungal infection. However, further studies are required to optimize and translate this approach to clinical practice. Adapting this approach to leukocytes that are safe in humans will be a critical first step in this process, as the infusion of viable leukemia-derived dHL-60 cells is potentially dangerous. While primary neutrophils are an attractive approach, challenges in obtaining sufficient numbers and their susceptibility to undergo early apoptosis will need to be addressed. The use of modified cell lines, such as HL-60 cells that have been irradiated or engineered with a suicide trap [21, 41], may be a useful strategy to avoid the limitations of primary neutrophils. One interesting approach may be the combination of posaconazole loading with genetically engineered T cells, such as dectin-1 chimera-expressing D-CAR cells, which have been reported to exhibit anti-Aspergillus activity in vivo [42]. Further optimization of the use of posaconazole loading of cells in a clinically relevant leukocyte will also need to be performed, including dose optimization, validation of the effects on overall survival, and the efficacy of these cells at later stages of infection and against nonneutropenic, and extrapulmonary invasive aspergillosis. Although posaconazole loading is technically simple and simply requires coincubation of cells with the drug for <30 minutes, standard-operating procedures for clinical laboratories to perform posaconazole loading of leukocytes will need to be established and validated.
Drugs that display pharmacokinetics characterized by relatively low serum concentrations as a consequence of partitioning to intracellular compartments have traditionally been viewed as undesirable [43]. However, this study suggests that concentration of antimicrobials within leukocytes may facilitate drug delivery to infectious lesions or other compartments. This observation extends the results of previous studies of posaconazole in prophylaxis that suggest that concentration of this antifungal within pulmonary epithelial cells can enhance their resistance to primary infection [28]. The accumulation of antimicrobial agents within leukocytes has also been reported with macrolide antibiotics. These agents exhibit extensively high accumulation in a variety of cell types, particularly polymorphonuclear leukocytes. Intracellular to extracellular ratios of >500, 300, and 250 have been reported for the antibiotics cethromycin, telithromycin, and azithromycin, respectively, in human polymorphonuclear leukocytes following ex vivo exposure [44]. Intracellular levels of these antibiotics were up to 50-fold lower in macrophage and epithelial cell lines and also much lower in undifferentiated HL-60 cells as compared to differentiated HL-60 cells [44, 45], suggesting that neutrophils are particularly suited for antimicrobial loading. Moreover, these high levels of cell-associated antimicrobials suggest enhanced activity against intracellular pathogens. Collectively these studies suggest a need to reevaluate the utility of cell-associated antimicrobials for the treatment of other infections.
The results of the current study demonstrate that the accumulation of posaconazole within leukocytes enhances their antifungal ability both in vitro and in vivo. Future studies will be required to translate and optimize this approach to primary neutrophils or other leukocytes safe for human use. These proof-of-concept studies suggest that treatment with posaconazole-loaded leukocytes is a promising area for study as a novel therapeutic strategy and may have implications for the utility of posaconazole in the treatment of invasive aspergillosis in nonneutropenic hosts.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Supplementary Material
Notes
Acknowledgments. We thank Dr Hong Liu, for her assistance in supplying cyclophosphamide required for our animal model, and Dr David Perlin, for supplying BDP-PCZ used in imaging studies of posaconazole.
Financial support. This work was supported by Cystic Fibrosis Canada and the Canadian Institutes of Health Research (operating grants MOP-81361 and MOP-123306 to D. C. S.) and by the Fonds de Recherche Québec Santé (Chercheur-Boursier award to D. C. S.).
Potential conflicts of interest. D. C. S. has served on advisory boards, has been a paid consultant, and has received a grant from Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Taccone FS, Van den Abeele AM, Bulpa P et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care 2015; 19:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hajjeh RA. Epidemiology and prevention of invasive aspergillosis. Curr Infect Dis Rep 2001; 3:507–16. [DOI] [PubMed] [Google Scholar]
- 3. Manuel RJ, Kibbler CC. The epidemiology and prevention of invasive aspergillosis. J Hosp Infect 1998; 39:95–109. [DOI] [PubMed] [Google Scholar]
- 4. Warnock DW, Hajjeh RA, Lasker BA. Epidemiology and prevention of invasive aspergillosis. Curr Infect Dis Rep 2001; 3:507–16. [DOI] [PubMed] [Google Scholar]
- 5. Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis 1997; 175:1459–66. [DOI] [PubMed] [Google Scholar]
- 6. Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis 2001; 32:358–66. [DOI] [PubMed] [Google Scholar]
- 7. Alvarez-Lerma F, Alvarez-Rocha L, Blanquer J et al. Epidemiology, diagnosis and treatment of fungal respiratory infections in the critically ill patient. Rev Esp Quimioter 2013; 26:173–88. [PubMed] [Google Scholar]
- 8. Herbrecht R, Denning DW, Patterson TF et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002; 347:408–15. [DOI] [PubMed] [Google Scholar]
- 9. Cornely OA, Maertens J, Bresnik M et al. Liposomal amphotericin b as initial therapy for invasive mold infection: a randomized trial comparing a high–loading dose regimen with standard dosing (AmBiLoad Trial). Clin Infect Dis 2007; 44:1289–97. [DOI] [PubMed] [Google Scholar]
- 10. Segal BH, Walsh TJ. Current approaches to diagnosis and treatment of invasive aspergillosis. Am J Respir Crit Care Med 2006; 173:707–17. [DOI] [PubMed] [Google Scholar]
- 11. Sethi P, Saluja R, Jindal N, Singh V. Invasive aspergillosis in an immunocompetent host. J Oral Maxillofac Pathol 2012; 16:297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tochigi N, Okubo Y, Ando T et al. Histopathological implications of Aspergillus infection in lung. Mediators Inflamm 2013; 2013:809798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franquet T, Muller NL, Gimenez A, Guembe P, de La Torre J, Bague S. Spectrum of pulmonary aspergillosis: histologic, clinical, and radiologic findings. Radiographics 2001; 21:825–37. [DOI] [PubMed] [Google Scholar]
- 14. Filler SG, Sheppard DC. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog 2006; 2:e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ben-Ami R, Albert ND, Lewis RE, Kontoyiannis DP. Proangiogenic growth factors potentiate in situ angiogenesis and enhance antifungal drug activity in murine invasive aspergillosis. J Infect Dis 2013; 207:1066–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paterson PJ, Seaton S, Prentice HG, Kibbler CC. Treatment failure in invasive aspergillosis: susceptibility of deep tissue isolates following treatment with amphotericin B. J Antimicrob Chemother 2003; 52:873–6. [DOI] [PubMed] [Google Scholar]
- 17. Wezensky SJ, Cramer RA Jr. Implications of hypoxic microenvironments during invasive aspergillosis. Med Mycol 2011; 49(suppl 1):S120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ito JI. Enhancing angiogenesis in invasive aspergillosis: a novel therapeutic approach. J Infect Dis 2013; 207:1031–3. [DOI] [PubMed] [Google Scholar]
- 19. Mehrad B, Strieter RM, Moore TA, Tsai WC, Lira SA, Standiford TJ. CXC chemokine receptor-2 ligands are necessary components of neutrophil-mediated host defense in invasive pulmonary aspergillosis. J Immunol 1999; 163:6086–94. [PubMed] [Google Scholar]
- 20. Todeschini G, Murari C, Bonesi R et al. Invasive aspergillosis in neutropenic patients: rapid neutrophil recovery is a risk factor for severe pulmonary complications. Eur J Clin Invest 1999; 29:453–7. [DOI] [PubMed] [Google Scholar]
- 21. Lin L, Ibrahim AS, Baquir B, Palosaari A, Spellberg B. Luminescent-activated transfected killer cells to monitor leukocyte trafficking during systemic bacterial and fungal infection. J Infect Dis 2012; 205:337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin L, Ibrahim AS, Baquir B et al. Safety and efficacy of activated transfected killer cells for neutropenic fungal infections. J Infect Dis 2010; 201:1708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hubel K, Dale DC, Engert A, Liles WC. Current status of granulocyte (neutrophil) transfusion therapy for infectious diseases. J Infect Dis 2001; 183:321–8. [DOI] [PubMed] [Google Scholar]
- 24. doi: 10.1002/14651858.CD005339. Stanworth S, Massey E, Hyde C, Brunskill SJ, Navarette C, Lucas G, Marks D, Paulus U. Granulocyte transfusions for treating infections in patients with neutropenia or neutrophil dysfunction. Cochrane Database of Syst Rev 2005; 3:CD005339. doi:10.1002/14651858.CD005339. [DOI] [PubMed] [Google Scholar]
- 25. Keating GM. Posaconazole. Drugs 2005; 65:1553–67; discussion 68-9. [DOI] [PubMed] [Google Scholar]
- 26. Nagappan V, Deresinski S. Reviews of anti-infective agents: posaconazole: a broad-spectrum triazole antifungal agent. Clin Infect Dis 2007; 45:1610–7. [DOI] [PubMed] [Google Scholar]
- 27. Farowski F, Cornely OA, Vehreschild JJ et al. Intracellular concentrations of posaconazole in different compartments of peripheral blood. Antimicrob Agents Chemother 2010; 54:2928–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Campoli P, Al Abdallah Q, Robitaille R et al. Concentration of antifungal agents within host cell membranes: a new paradigm governing the efficacy of prophylaxis. Antimicrob Agents Chemother 2011; 55:5732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Conte JE, Golden JA, Krishna G, McIver M, Little E, Zurlinden E. Intrapulmonary pharmacokinetics and pharmacodynamics of posaconazole at steady state in healthy subjects. Antimicrob Agents Chemother 2009; 53:703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Campoli P, Perlin DS, Kristof AS, White TC, Filler SG, Sheppard DC. Pharmacokinetics of posaconazole within epithelial cells and fungi: Insights into potential mechanisms of action during treatment and prophylaxis. J Infect Dis 2013; 208:1717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clinical Laboratory Sciences Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard. CLSI document M38-A2. Wayne, PA: CLSI, 2008.
- 32. Ting-Beall HP, Needham D, Hochmuth RM. Volume and osmotic properties of human neutrophils. Blood 1993; 81:2774–80. [PubMed] [Google Scholar]
- 33. Pratt A, Garcia-Effron G, Zhao Y et al. Evaluation of fungal-specific fluorescent labeled echinocandin probes as diagnostic adjuncts. Med Mycol J 2013; 51:103–7. [DOI] [PubMed] [Google Scholar]
- 34. Pierce CG, Uppuluri P, Tristan AR et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 2008; 3:1494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hauert AB, Martinelli S, Marone C, Niggli V. Differentiated HL-60 cells are a valid model system for the analysis of human neutrophil migration and chemotaxis. Int J Biochem Cell Biol 2002; 34:838–54. [DOI] [PubMed] [Google Scholar]
- 36. Martin SJ, Bradley JG, Cotter TG. HL-60 cells induced to differentiate towards neutrophils subsequently die via apoptosis. Clin Exp Immunol 1990; 79:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shuto T, Furuta T, Cheung J et al. Increased responsiveness to TLR2 and TLR4 ligands during dimethylsulfoxide-induced neutrophil-like differentiation of HL-60 myeloid leukemia cells. Leuk Res 2007; 31:1721–8. [DOI] [PubMed] [Google Scholar]
- 38. Farowski F, Cornely OA, Hartmann P. High intracellular concentrations of posaconazole do not impact on functional capacities of human polymorphonuclear neutrophils and monocyte-Derived macrophages in vitro. Antimicrob Agents Chemother 2016; 60:3533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Howard SJ, Lestner JM, Sharp A et al. Pharmacokinetics and pharmacodynamics of posaconazole for invasive pulmonary aspergillosis: clinical implications for antifungal therapy. J Infect Dis 2011; 203:1324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cacciapuoti A, Loebenberg D, Corcoran E et al. In vitro and in vivo activities of SCH 56592 (posaconazole), a new triazole antifungal agent, against Aspergillus and Candida. Antimicrob Agents Chemother 2000; 44:2017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spellberg BJ, Collins M, Avanesian V et al. Optimization of a myeloid cell transfusion strategy for infected neutropenic hosts. J Leukoc Biol 2007; 81:632–41. [DOI] [PubMed] [Google Scholar]
- 42. Kumaresan PR, Manuri PR, Albert ND et al. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proc Natl Acad Sci U S A 2014; 111:10660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pizarro AM, McQuitty RJ, Mackay FS, Zhao Y, Woods JA, Sadler PJ. Cellular accumulation, lipophilicity and photocytotoxicity of diazido platinum(IV) anticancer complexes. Chem Med Chem 2014; 9:1169–75. [DOI] [PubMed] [Google Scholar]
- 44. Bosnar M, Kelneric Z, Munic V, Erakovic V, Parnham MJ. Cellular uptake and efflux of azithromycin, erythromycin, clarithromycin, telithromycin, and cethromycin. Antimicrob Agents Chemother 2005; 49:2372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Munić V, Bosnar M, Kelnerić Ž et al. Macrolide uptake and release by HL-60 and human polymorphonuclear (PMN) cells. In: International Conference on the Macrolides, Azalides, Streptogramins, Ketolides and Oxazolidinones (ICMAS-KO 6)(6; 2002). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.