Abstract
When Zika virus (ZIKV) emerged in the Americas, little was known about its biology, pathogenesis, and transmission potential, and the scope of the epidemic was largely hidden, owing to generally mild infections and no established surveillance systems. Surges in congenital defects and Guillain-Barré syndrome alerted the world to the danger of ZIKV. In the context of limited data, quantitative models were critical in reducing uncertainties and guiding the global ZIKV response. Here, we review some of the models used to assess the risk of ZIKV-associated severe outcomes, the potential speed and size of ZIKV epidemics, and the geographic distribution of ZIKV risk. These models provide important insights and highlight significant unresolved questions related to ZIKV and other emerging pathogens.
Keywords: Zika, mathematical modeling, epidemiology
Since its discovery in Uganda in 1947, the biology, pathogenesis, and transmission potential of Zika virus (ZIKV) has been characterized by uncertainty. Six years passed before the virus was identified as a human pathogen, and 6 decades passed before the first outbreak of ZIKV infection was documented [1–3]. That outbreak, on the island of Yap, demonstrated the explosive transmission potential of ZIKV, in which it infected an estimated 68%–77% of the population. Yet, it raised limited public health concern because most infections were asymptomatic or mild [3]. An increase in the incidence of Guillain-Barré syndrome (GBS) during a subsequent outbreak in French Polynesia gave the first indication that severe complications were possible [4]. In 2015, an association with congenital defects was noted in the months following large outbreaks in Brazil [5, 6]. The World Health Organization declared a public health emergency of international concern on 1 February 2016 in response to the uncertainties and concerns surrounding serious complications from this rapidly spreading and poorly understood pathogen [7].
The need to better understand ZIKV’s pathogenesis and transmission dynamics was immediate, but clinical and epidemiological data were extremely limited. As of early 2016, few outbreaks had been described, most cases remained undetected owing to mild and nonspecific symptoms, and broadly deployable diagnostic tests that could distinguish Zika from dengue (a common, related arboviral disease) were not available. While clinical data from cohorts and case-control studies were months away, countries were grappling with how to control the spread of ZIKV, develop surveillance and testing capacity, prepare for a surge in GBS cases, and protect pregnant women.
Quantitative models have long been used to provide insight into emerging epidemics, especially when data are limited and uncertainty is high [8]. Throughout the ZIKV epidemic, mathematical, statistical, and ecological models have been used to address key public health questions related to both the biology and the spread of ZIKV. Here, we review some of the critical roles mathematical modeling played in the response to the ZIKV epidemic. First, we focus on the role of models in characterizing the risk of severe consequences of infection, particularly GBS and microcephaly. We then review research on the epidemic dynamics of ZIKV, including estimates of how quickly these largely invisible epidemics grow and how many people get infected. Finally, we address estimates of large-scale epidemic spread, including both the global landscape of transmission risk and the dynamics of virus introduction. For each of these, we review relevant models in the context of available epidemiological data, identifying key insights, remaining questions, and implications for the future.
WHAT IS THE RISK OF GBS DUE TO ZIKV INFECTION?
GBS is a rare but serious neurological condition that can lead to hospitalization, long-term rehabilitation, and death [9]. By early 2016, there was evidence of an increased GBS incidence temporally associated with outbreaks of ZIKV infection across multiple countries, and a causal link was suspected [10–13]. As the ZIKV epidemic spread, there was widespread concern over the impact of GBS, particularly the resources needed to care for affected patients.
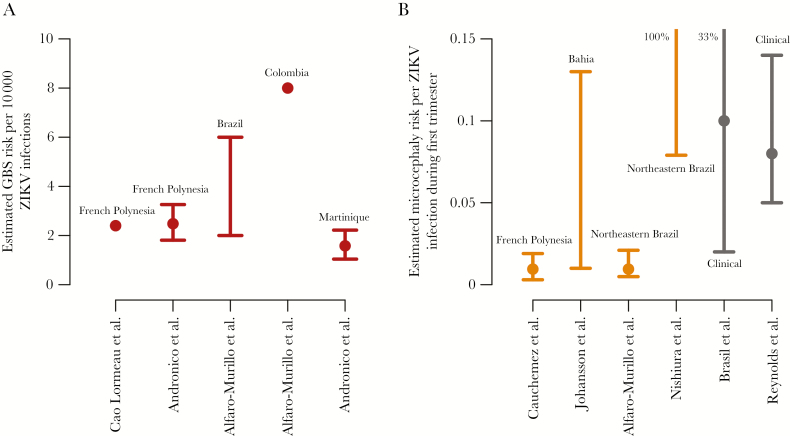
To use GBS case data to characterize the risk of GBS for people infected by ZIKV, the underlying number of ZIKV infections (ie, the denominator of the risk calculation) must be estimated. This task is complicated by limited seroprevalence data and high rates of asymptomatic and mild ZIKV infections. In French Polynesia (population, approximately 270000), a serosurvey found a ZIKV seroprevalence of 66% among 476 children [14] and 42 confirmed GBS cases among adults with laboratory evidence of ZIKV infection [10]. Applying the attack rate among children in a single location to the entire population, Cao-Lormeau et al estimated the risk of GBS to be 2.4 cases per 10000 infections (Figure 1A) [10]. While this estimate had limitations, even fewer data were available from other locations.
Figure 1.
Estimates of Guillain-Barré syndrome (GBS) BS and microcephaly risk. Estimated risk of GBS resulting from ZIKV infection (A) and microcephaly resulting from first trimester ZIKV infection (B). Lines indicate ranges or 95% confidence intervals, and points represent point estimates, means, or medians.
Estimating the true incidence of ZIKV infection from reported cases is a challenge for which epidemiological models are well suited. For example, statistical models can estimate incidence on the basis of limited and possibly biased sampling, while dynamic models can estimate incidence on the basis of other observable patterns, such as the shape of the epidemic curve. In a simple approach, Alfaro-Murillo et al adjusted the reported incidence in Colombia upward on the assumption that 18% of infections were reported (the estimated proportion of infections in Yap that were symptomatic), estimating a GBS risk of 8 cases per 10000 ZIKV infections [15]. In Brazil, the same group used ZIKV infection prevalence estimates from the Brazilian Ministry of Health to estimate a GBS risk of 2–6 cases per 10000 infections. Methods for those ZIKV infection estimates have not been published. Andronico et al developed dynamic epidemic models for French Polynesia and Martinique to estimate infection prevalence, using both Zika and GBS data from each country, and then calculated risks of 1.8–3.3 and 1.0–2.2 GBS cases per 10000 infections, respectively [16].
Although data were limited and approaches differed, risk estimates ranged from 1 to 8 GBS cases per 10000 infections. These estimates explain the relative scarcity of GBS during large ZIKV epidemics and why GBS may not be observed in smaller epidemics, such as the Yap outbreak, where an estimated 4700–5300 people were infected [3].
Estimates of GBS risk were important to the ZIKV response, informing forecasts of healthcare resource needs [15–17]. Over the course of the outbreak in Martinique, Andronico et al compared predictions of hospitalization, intensive care unit, and ventilator requirements with observed needs and found good agreement [16]. These estimates rapidly improved as more data became available and were incorporated [16]. While progress has been made, uncertainties remain, particularly regarding variation between countries, which may reflect differences in ZIKV incidence [10, 15, 16], surveillance [11], or subpopulation-specific risk (eg, by age or sex [18]) and the possibility of other neurological complications resulting from infection. Clinical studies and epidemiological data alone are unlikely to resolve all of these questions because GBS is a rare outcome and underlying ZIKV prevalence is difficult to measure. Hence, models that account for asymptomatic infections and underreporting will continue to play an important role in understanding GBS risk.
WHAT IS THE RISK OF MICROCEPHALY?
In November 2015, the Brazilian Ministry of Health reported >141 cases of infants born with microcephaly in the State of Pernambuco, where normally an average of 10 cases occur per year [19]. Microcephaly, abnormally small head size, is indicative of potentially severe developmental defects, and reports quickly climbed as other states reported hundreds of additional suspected cases [6]. Again, there was high uncertainty early on: a causal association with ZIKV infection was plausible but unproven, definitions of microcephaly were nonstandard and changing, surveillance data for Zika were sparse, and diagnostic tools were limited. Moreover, clinical studies would take time, especially given the need to follow women through pregnancy and beyond. Meanwhile, resolving these uncertainties was a top priority, as pregnant women were becoming infected in Brazil and other ZIKV-affected countries.
The first clinical cohort data from Brazil showed abnormalities in 12 of 42 pregnancies (29%) with laboratory-confirmed symptomatic ZIKV infection, predominantly in the second trimester [20]. Meanwhile, Cauchemez et al retrospectively identified 8 microcephaly cases in French Polynesia and built a model relating the timing of these pregnancies to the timing and magnitude of the ZIKV epidemic [21]. The model identified an association between microcephaly and ZIKV infection early in pregnancy, with an estimated 0.3%–1.9% risk of microcephaly if infected in the first trimester (Figure 1B). As with estimates of the risk of GBS, this and other projections of microcephaly risk relied on an assumed infection risk among pregnant women that was based on limited data. In this case, the authors assumed that the infection risk was 66% among pregnant women during the months of the outbreak, based on a serosurvey among school children [21]. Sensitivity analyses highlighted the importance of this assumption. Johansson et al developed a similar model with data from Bahia, Brazil [22]. Using data from previous chikungunya and ZIKV outbreaks to estimate the incidence of infection, they estimated a microcephaly risk of 1%–13% when mothers were infected in the first trimester. These models provided early and strong evidence of an elevated risk of microcephaly for ZIKV infections occurring early in pregnancy. It took many months before clinical data were available to substantiate these findings [23, 24].
Highlighting the challenge in these estimates, Nishiura et al estimated a lower ZIKV infection risk, based on the assumptions that many suspected Zika cases were reported as suspected dengue cases and that the microcephaly risk was limited to the first trimester [25]. This led to a highly uncertain microcephaly risk estimate of 7.9%–100% when mothers were infected during the first trimester in northeast Brazil. Also assuming that the microcephaly risk is confined to the first trimester, Alfaro-Murillo et al estimated a risk of 0.5%–2.1% in northeast Brazil, based on an infection prevalence of 23.5%–77% derived from previous chikungunya and ZIKV outbreaks [15]. In a simulation model, Saad-Roy et al [26] estimated microcephaly risk on the basis of the interval between ZIKV introduction and the first detection of microcephaly. Given the possibility that ZIKV was introduced to South America in 2013 and the timing of the first detected microcephaly cases in Colombia, they estimated a risk of 1%–10% when mothers were infected during the first trimester.
These models began to converge on risk estimates for microcephaly while clinical data on prevalence were being collected. In late 2016 and early 2017, clinical studies provided additional information on these risks. In December 2016, Brasil et al reported microcephaly in 2 of 20 births (10%; 95% confidence interval [CI], 2%–33%) to Brazilian women with confirmed ZIKV infection during the first trimester [23]. And in April 2017, brain abnormalities and/or microcephaly were reported in the US Zika Pregnancy Registry in 13 of 157 pregnancies (8%; 95% CI, 5%–14%) in which women had symptom onset or exposure to ZIKV in their first trimester, with the majority of these infections acquired outside of the United States [24]. More than a year after the first indication of microcephaly risk, these cohort-based estimates still have low precision because of the limited numbers of ZIKV-affected pregnancies under clinical observation. Data are also limited for other complications of congenital ZIKV infection and risk in other trimesters, although these risks clearly extend beyond microcephaly [23, 24].
Population-level epidemic models can offer insights into risk that may be difficult to rapidly capture by using clinical studies, such as risks associated with asymptomatic infection or infection early during pregnancy. The models presented here provided the first clear evidence of the timing and magnitude of the microcephaly risk and directly supported assessments of causality [13, 27]. Moreover, these results were used by simulation models to forecast microcephaly cases [28, 29] and assess possible interventions [15, 30].
HOW MANY PEOPLE WILL BE INFECTED AND HOW QUICKLY?
The ZIKV epidemics in Yap and French Polynesia demonstrated the ability of ZIKV to spread quickly through a population. An estimated 68%–77% of the residents of Yap were infected within 4 months [3], and 42%–57% of the residents of French Polynesia were infected within 7 months [16]. Explosive outbreaks were also apparent in northeast Brazil in early 2015 [31], but the scope of the outbreaks was largely invisible because of limited surveillance and the high proportion of infections that were asymptomatic or mild in severity. It was unclear how many people were getting infected, how quickly ZIKV was spreading through the affected populations, and how these dynamics differed across environments, particularly when comparing large metropolitan areas in the Americas to the islands in the Pacific where previous outbreaks had occurred.
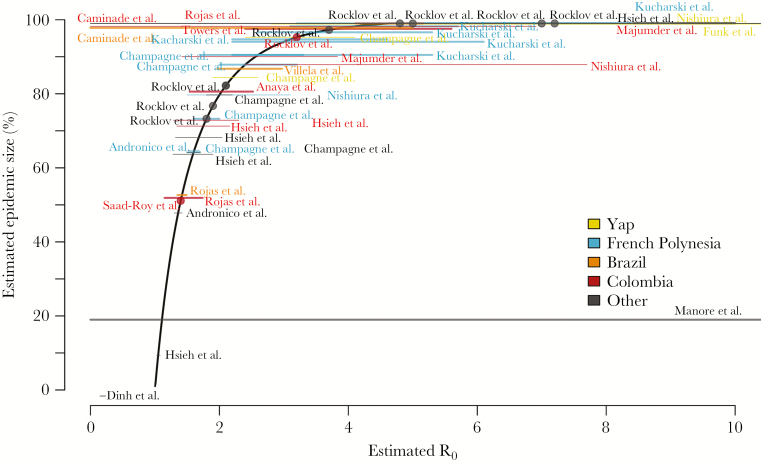
The size and speed of epidemics can be characterized by the number of individuals infected by a single infected individual in an immunologically naive population (characterized by the basic reproductive number [R0]) and the time between successive generations of infections (ie, the generation time). A variety of models used these principles to use time-series data from the Pacific Islands and several locations in the Americas to estimate R0 (Figure 2) [16, 32–43]. Estimates of R0 ranged from 1.3–1.4 in Martinique [16] to 4–12 for Yap [34], reflecting variability between outbreaks, model assumptions, and estimation methods. These estimates and the key determinants of variability are difficult to validate because of the limited available data. Estimates of the serial interval, which approximates the generation time, varied between 10 and 30 days, with most studies yielding considerable uncertainty around this parameter [40, 41].
Figure 2.
R0 estimates and theoretical epidemic size. Location-specific R0 estimates are shown superimposed on the theoretical relationship between R0 and final epidemic size in a perfectly mixed population. Horizontal lines represent the reported range or 95% confidence interval of estimates when reported and are placed vertically at the epidemic size corresponding to the mean or median R_0 estimate. Thick lines represent national-level estimates, and thin lines represent estimates for subnational areas (eg, states or cities). Points represent estimates for which only point estimates were reported.
R0 values for ZIKV were similar to those estimated for other arboviruses, especially dengue viruses, which are also transmitted by Aedes (Stegomyia) mosquitoes [34, 35, 37]. These estimates provided an early indication that ZIKV introductions into areas where dengue spreads efficiently would likely lead to explosive outbreaks. R0 estimates also form a basis for estimating final epidemic size. In a perfectly mixed population, R0 is mathematically related to the proportion of the population that is infected before an epidemic dies out because of herd immunity (Figure 2). Extrapolating from this relationship, the R0 estimates for northeast Brazil imply that the early outbreaks were likely much larger than suggested by the limited number of cases reported in 2015 [31]. However, the magnitude of that difference is unclear, as the actual relationship between R0 and epidemic size is complicated by spatial heterogeneity, stochastic transmission, and seasonal variations in transmissibility, leading to epidemics that are generally smaller than predicted by R0 alone [44]. This difference is reflected in epidemic size estimates for Yap [34] and French Polynesia [33], which exceeded the observed seroprevalence in studies of those outbreaks [3, 45]. A comparison of model-based projections of ZIKV attack rates for Martinique to seroprevalence estimates from blood donor data suggests that fully stochastic models may be better able to estimate epidemic size [16], but data to validate such models are rare.
Sexual transmission also complicates ZIKV transmission dynamics. Relatively few sexually transmitted cases have been reported, but differentiating sexual transmission from vector-borne transmission is next to impossible in the midst of epidemics. Hence, instances of sexual transmission have been documented almost exclusively among travelers [46]. In areas where vector-borne transmission is ongoing, models suggest that sexual transmission accounts for 0.12%–47% of all infections but is insufficient to cause or maintain ZIKV epidemics on its own [36, 47, 48]. However, the effect may be large enough to contribute to higher infection risk among women [49].
As noted, estimates of key ZIKV transmission characteristics vary substantially because of differences in epidemics, data, model assumptions, and estimation methods. Even with these uncertainties, estimates of R0 provide essential information on ZIKV’s ability to cause rapid, intense epidemics and inform estimates of disease burden [16]. They can also help parameterize simulation models used to evaluate the impact of potential interventions [32] and form a basis for estimates of transmission risk in areas where ZIKV has not been introduced.
WHAT AREAS ARE AT RISK OF ZIKV INTRODUCTION?
ZIKV had been moving through Pacific islands for several years prior to the epidemic in Brazil, yet its arrival in the Americas was a surprise. Once in Brazil, rapid expansion to other regions seemed inevitable. This assumption was confirmed when the first ZIKV case in Colombia was diagnosed in October 2015 [50]. However, it was unclear how quickly ZIKV would spread and which countries it would invade. Successful invasion requires 2 things: a suitable environment for transmission and importation of virus into that environment. Understanding both of these components can support preparedness, prevention, and control activities, informing travel or surveillance guidelines.
Two approaches were taken to characterize the spatial extent of ZIKV transmission risk. Phenomenological niche models identified areas with characteristics similar to those in other areas where ZIKV or the mosquitoes that transmit ZIKV had been observed [51–56]. Others calculated R0 under different environmental conditions, using biological relationships to external factors, such as the relationships between temperature and mosquito mortality or between population density and biting rates [44, 57–59]. These models were then used to make statistical or mechanistic estimates of the global distribution of ZIKV transmission risk. Most estimates from both types of models agreed that the range of ZIKV would not exceed the geographical distributions of dengue and chikungunya viruses (both of which are transmitted by Aedes mosquitoes).
There was also strong agreement that most populated tropical areas are highly suitable for autochthonous ZIKV transmission, with the exception of high altitude and desert locations. Some models went further, aiming to elucidate not just the presence of risk, but how risk may change seasonally [44, 53, 58–60]. These results supported year-round risk in tropical areas but were less clear for temperate regions. In Europe and the United States, for example, areas predicted to have seasonal or year-round transmission risk were extensive and varied across models [51, 61–65]. However, through March, 2017 no vector-borne transmission had been documented in Europe [66], and just over 200 locally acquired mosquito-transmitted cases reported in the United States occurred in small regions of southern Florida and Texas [67].
Fewer models have aimed to predict importation risk. Most estimates have focused on the number of travelers arriving from areas with documented transmission, identifying risk in urban areas that receive many such travelers [51, 53, 62, 64, 65, 68]. Limited validation has shown these estimates to be useful in identifying areas where travel-associated cases were most likely to arrive [64]. Ogden et al also found that importations were more likely to originate from locations with a higher estimated R0 [42]. Zhang et al adapted an existing spatially structured stochastic model that uses sociodemographic, climate, and human mobility data to model ongoing epidemics and spread between geographic regions [29].
Models of the spatial distribution of risk confirmed that the threat of ZIKV epidemics was highest in tropical areas, and they bolstered efforts to raise awareness and build surveillance capacity in areas where transmission had not yet been reported. For temperate regions, models identified places where imported cases were mostly likely to arrive and provided important insight to the possibility of introduced transmission. However, assessing the magnitude of risk in these areas is challenging. For example, while the United States and Europe have high travel volume to tropical areas, the geographical and seasonal distribution of Aedes mosquitoes is not well characterized. For both tropical and nontropical locations, predictions have generally focused on determining where introductions are most likely and where local transmission is possible. Integration of these models with real-time estimates of ZIKV transmission intensity would have potential to provide up-to-date projections of the risk of a ZIKV introduction, thereby directly informing preparedness activities in real time.
DISCUSSION
Once obscure, ZIKV has rapidly emerged as a pathogen of global importance. The pace of scientific discovery has also been rapid but has faced large challenges. Introduction into the Americas was unexpected, little was known about severe consequences of ZIKV infection, and the scale of initial epidemics was unknown because of the mild nature of most infections and limited surveillance. These challenges have thrust epidemiological models into the forefront of the response to ZIKV.
We have highlighted several areas where quantitative models were fundamental to better understanding Zika, including the rapid deployment of dynamic models to help illuminate the link between ZIKV and rare, but severe outcomes [16, 21, 22]. These models provided risk estimates for GBS and microcephaly that otherwise would have been difficult to rapidly obtain. Additionally, models were used to estimate R0, which suggested that large epidemics could be expected in areas suitable for transmission [16, 32–41]. Geostatistical and ecological niche models confirmed that this risk extended globally, finding strong evidence of suitability throughout the tropics [44, 51–59] and more limited, seasonal risk in temperate regions [44, 51, 57–59, 62].
Nonetheless, many uncertainties remain. There are still few estimates of the risk of acquiring GBS due to ZIKV infection, and associated risk factors are not well characterized. As more data are collected, estimates of the timing and magnitude of GBS and congenital defects can be further refined. Despite a clearer understanding of microcephaly risk, epidemiological questions remain, such as why rates of microcephaly in Brazil have decreased faster than expected [69]. Furthermore, much work remains to be done to determine the timing and magnitude of risk for other congenital defects [23, 24].
Estimates of R0 indicated that large epidemics were likely to occur in tropical areas where ZIKV was introduced. Where those epidemics have already occurred, many people will be immune to future infection, and there may not be another large epidemic for many years [40]. However, the accuracy of epidemic size estimates is difficult to validate, and heterogeneity and stochasticity will continue to complicate inferences [44]. Furthermore, in Africa and Asia, ZIKV has been previously documented, but the size, timing, and location of past outbreaks and the potential for future outbreaks remain unknown [53, 70]. Predicting Zika risk in temperate areas is also challenging; when risk is low, local transmission and the detection of transmission will depend on importation, chance, and the quality of surveillance systems. In particular, seasonality is likely to play an important role, but limited seasonal data make models difficult to develop and validate.
For many of these remaining questions, clinical and epidemiological data will not suffice on their own. Future risk is dependent on population-level immunity, the risk of local transmission, and the risk of importation. While none of these quantities can be directly observed, epidemic models can be used to leverage the data that are available and reduce uncertainties. Although we may be coming to the end of the first wave of the ZIKV epidemic in the Americas, these questions remain critical. Surveillance strategies, testing recommendations, and guidelines for travelers are all being further developed and refined. Vaccines and therapeutics are in the pipeline, and both clinical trials and implementation plans will be helped by a priori risk assessments. Many of these challenges are not unique to Zika but are general challenges in emerging epidemics. Often decisions need to be made when there are few data, disease severity is not well characterized, and spread is ongoing [71]. In the Zika epidemic and beyond, epidemic models will be central to drawing on available data and knowledge to address these challenges.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant R01 AI102939 to L. T. K. and J. L.).
Supplement sponsorship. This work is part of a supplement sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–20. [DOI] [PubMed] [Google Scholar]
- 2. Macnamara F. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg 1954; 48:139–45. [DOI] [PubMed] [Google Scholar]
- 3. Duffy MR, Chen TH, Hancock WT et al. . Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–43. [DOI] [PubMed] [Google Scholar]
- 4. Oehler E, Watrin L, Larre P et al. . Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Euro Surveill 2014; 19:20720. [DOI] [PubMed] [Google Scholar]
- 5. Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 2015; 110:569–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Oliveira WK. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy—Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016; 65. [DOI] [PubMed] [Google Scholar]
- 7. Chan MK. WHO Director General summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome. Geneva: World Health Organization, 2016. [Google Scholar]
- 8. Castillo-Chavez C, Blower S, Driessche P, Kirschner D, Yakubu A-A.. Mathematical approaches for emerging and reemerging infectious diseases: models, methods, and theory. Springer, 2002. [Google Scholar]
- 9. Yuki N, Hartung HP. Guillain-Barré syndrome. N Engl J Med 2012; 366:2294–304. [DOI] [PubMed] [Google Scholar]
- 10. Cao-Lormeau VM, Blake A, Mons S et al. . Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016; 387:1531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dos Santos T, Rodriguez A, Almiron M et al. . Zika virus and the Guillain-Barré syndrome - case series from seven countries. N Engl J Med 2016; 375:1598–601. [DOI] [PubMed] [Google Scholar]
- 12. Paploski IA, Prates AP, Cardoso CW et al. . Time lags between exanthematous illness attributed to Zika virus, Guillain-Barré syndrome, and microcephaly, salvador, Brazil. Emerg Infect Dis 2016; 22:1438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krauer F, Riesen M, Reveiz L et al. ; WHO Zika Causality Working Group Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barré syndrome: systematic review. PLoS Med 2017; 14:e1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aubry M, Teissier A, Roche C, Brouit J. Serosurvey of dengue, Zika and other mosquito-borne viruses in French Polynesia. Presented at: 64th Annual Meeting of the American Society of Tropical Medicine and Hygiene, Philadelphia, Pennsylvania, 26–29 October 2015. [Google Scholar]
- 15. Alfaro-Murillo JA, Parpia AS, Fitzpatrick MC et al. . A Cost-Effectiveness Tool for Informing Policies on Zika Virus Control. PLoS Negl Trop Dis 2016; 10:e0004743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andronico A, Dorléans F, Fergé J-L et al. . Real-time assessment of health-care requirements during the Zika virus epidemic in Martinique. Am J Epidemiol 2017. doi:10.1093/aje/kwx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dirlikov E, Kniss K, Major C et al. . Guillain-Barré syndrome and healthcare needs during Zika virus transmission, Puerto Rico, 2016. Emerg Infect Diseases 2017; 23:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology 2011; 36:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brazil Ministry of Health. Ministério da Saúde investiga aumento de casos de microcefalia em Pernambuco. Ministry of Health Brazil; http://portalsaude.saude.gov.br/index.php/cidadao/principal/agencia-saude/20629-ministerio-da-saude-investiga-aumento-de-casos-de-microcefalia-em-pernambuco. Accessed 4 April 2017. [Google Scholar]
- 20. Brasil P, Pereira J Jr, Raja Gabaglia C. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report. N Engl J Med. http://www.nejm.org/doi/suppl/10.1056/NEJMoa1602412/suppl_file/nejmoa1602412_prelim.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cauchemez S, Besnard M, Bompard P et al. . Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet 2016; 387:2125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly. N Engl J Med 2016; 375:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brasil P, Pereira JP Jr, Moreira ME et al. . Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 2016; 375:2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reynolds MR, Jones AM, Petersen EE et al. . Vital signs: update on Zika virus–associated birth defects and evaluation of all U.S. infants with congenital Zika Virus exposure — U.S. Zika pregnancy registry, 2016. MMWR Morb Mortal Wkly Rep 2017; 66:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishiura H, Mizumoto K, Rock KS, Yasuda Y, Kinoshita R, Miyamatsu Y. A theoretical estimate of the risk of microcephaly during pregnancy with Zika virus infection. Epidemics 2016; 15:66–70. [DOI] [PubMed] [Google Scholar]
- 26. Saad-Roy CM, van den Driessche P, Ma J. Estimation of Zika virus prevalence by appearance of microcephaly. BMC Infect Dis 2016; 16:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects–reviewing the evidence for causality. N Engl J Med 2016; 374:1981–7. [DOI] [PubMed] [Google Scholar]
- 28. Ellington SR, Devine O, Bertolli J et al. . Estimating the number of pregnant women infected with Zika virus and expected infants with microcephaly following the Zika virus outbreak in Puerto Rico, 2016. JAMA Pediatr 2016; 170:940–5. [DOI] [PubMed] [Google Scholar]
- 29. Zhang Q, Sun K, Chinazzi M et al. . Projected spread of Zika virus in the Americas. Proc Natl Acad Sci U S A. 2017; 114:E4334-E4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ndeffo-Mbah ML, Parpia AS, Galvani AP. Mitigating Prenatal Zika Virus Infection in the Americas. Ann Intern Med 2016; 165:551–9. [DOI] [PubMed] [Google Scholar]
- 31. Cardoso CW, Paploski IAD, Kikuti M et al. . Outbreak of exanthematous illness associated with Zika, chikungunya, and dengue viruses, Salvador, Brazil. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Champagne C, Salthouse DG, Paul R, Cao-Lormeau V-M, Roche B, Cazelles B. Structure in the variability of the basic reproductive number (R0) for Zika epidemics in the Pacific islands. eLife 2016; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kucharski AJ, Funk S, Eggo RM, Mallet HP, Edmunds WJ, Nilles EJ. transmission dynamics of Zika virus in island populations: a modelling analysis of the 2013-14 french polynesia outbreak. PLoS Negl Trop Dis 2016; 10:e0004726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Funk S, Kucharski AJ, Camacho A et al. . Comparative Analysis of Dengue and Zika Outbreaks Reveals Differences by Setting and Virus. PLoS Negl Trop Dis 2016; 10:e0005173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Villela DA, Bastos LS, De Carvalho LM et al. . Zika in Rio de Janeiro: Assessment of basic reproduction number and comparison with dengue outbreaks. Epidemiol Infect 2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Towers S, Brauer F, Castillo-Chavez C, Falconar AKI, Mubayi A, Romero-Vivas CME. Estimate of the reproduction number of the 2015 Zika virus outbreak in Barranquilla, Colombia, and estimation of the relative role of sexual transmission. Epidemics 2016; 17:50–5. [DOI] [PubMed] [Google Scholar]
- 37. Nishiura H, Kinoshita R, Mizumoto K, Yasuda Y, Nah K. Transmission potential of Zika virus infection in the South Pacific. Int J Infect Dis 2016; 45:95–7. [DOI] [PubMed] [Google Scholar]
- 38. Nishiura H, Mizumoto K, Villamil-Gómez WE, Rodríguez-Morales AJ. Preliminary estimation of the basic reproduction number of Zika virus infection during Colombia epidemic, 2015-2016. Travel Med Infect Dis 2016; 14:274–6. [DOI] [PubMed] [Google Scholar]
- 39. Rojas DP, Dean NE, Yang Y et al. . The epidemiology and transmissibility of Zika virus in Girardot and San Andres island, Colombia, September 2015 to January 2016. Eurosurveillance 2016; 21:30283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ferguson NM, Cucunubá ZM, Dorigatti I et al. . Countering Zika in Latin America. Science 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Majumder MS, Santillana M, Mekaru SR, McGinnis DP, Khan K, Brownstein JS. Utilizing Nontraditional Data Sources for Near Real-Time Estimation of Transmission Dynamics During the 2015-2016 Colombian Zika Virus Disease Outbreak. JMIR Public Health Surveill 2016; 2:e30 https://www.ncbi.nlm.nih.gov/pubmed/?term=Utilizing+Nontraditional+Data+Sources+for+Near+Real-Time+Estimation+of+Transmission+Dynamics+During+the+2015-2016+Colombian+Zika+Virus+Disease+Outbreak [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ogden NH, Fazil A, Safronetz D et al. . Risk of travel-related cases of Zika virus infection is predicted by transmission intensity in outbreak-affected countries. Parasit Vectors 2017; 10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hsieh YH. Temporal patterns and geographic heterogeneity of Zika virus (ZIKV) outbreaks in French Polynesia and Central America. PeerJ 2017; 5:e3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perkins TA, Siraj AS, Ruktanonchai CW, Kraemer MU, Tatem AJ. Model-based projections of Zika virus infections in childbearing women in the Americas. Nat Microbiol 2016; 1:16126. [DOI] [PubMed] [Google Scholar]
- 45. Aubry M, Teissier A, Huart M et al. . Zika Virus Seroprevalence, French Polynesia, 2014–2015. Emerg Infect Dis 2017; 23:669–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Walker WL, Lindsey NP, Lehman JA et al. . Zika Virus Disease Cases - 50 States and the District of Columbia, January 1-July 31, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:983–6. [DOI] [PubMed] [Google Scholar]
- 47. Gao D, Lou Y, He D et al. . Prevention and Control of Zika as a Mosquito-Borne and Sexually Transmitted Disease: A Mathematical Modeling Analysis. Sci Rep 2016; 6:28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yakob L, Kucharski A, Hue S, Edmunds WJ. Low risk of a sexually-transmitted Zika virus outbreak. Lancet Infect Dis 2016; 16:1100–2. [DOI] [PubMed] [Google Scholar]
- 49. Coelho FC, Durovni B, Saraceni V et al. . Higher incidence of Zika in adult women than adult men in Rio de Janeiro suggests a significant contribution of sexual transmission from men to women. Int J Infect Dis 2016; 51:128–32. [DOI] [PubMed] [Google Scholar]
- 50. Colombia Ministry of Health. MinSalud confirma primeros nueve casos de zika en Colombia. Ministry of Health, 2015. https://www.minsalud.gov.co/Paginas/Confirmados-primeros-casos-de-virus-del-zika-en-Colombia.aspx. Accessed 4 April 2017. [Google Scholar]
- 51. Bogoch II, Brady OJ, Kraemer MUG et al. . Anticipating the international spread of Zika virus from Brazil. Lancet 2016; 387:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Messina JP, Kraemer MU, Brady OJ et al. . Mapping global environmental suitability for Zika virus. eLife 2016; 5:569–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bogoch II, Brady OJ, Kraemer MUG et al. . Potential for Zika virus introduction and transmission in resource-limited countries in Africa and the Asia-Pacific region: a modelling study. Lancet Infect Dis 2016; 16:1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Samy AM, Thomas SM, Wahed AA, Cohoon KP, Peterson AT. Mapping the global geographic potential of Zika virus spread. Mem Inst Oswaldo Cruz 2016; 111:559–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Carlson CJ, Dougherty ER, Getz W. An Ecological Assessment of the Pandemic Threat of Zika Virus. PLoS Negl Trop Dis 2016; 10:e0004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Santos J, Meneses BM. An integrated approach for the assessment of the Aedes aegypti and Aedes albopictus global spatial distribution, and determination of the zones susceptible to the development of Zika virus. Acta Trop 2017; 168:80–90. [DOI] [PubMed] [Google Scholar]
- 57. Caminade C, Turner J, Metelmann S et al. . Global risk model for vector-borne transmission of Zika virus reveals the role of El Niño 2015. Proc Natl Acad Sci USA 2017; 114:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mordecai EA, Cohen JM, Evans MV et al. . Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl Trop Dis 2017; 11:e0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang Q, Sun K, Chinazzi M et al. . Spread of Zika virus in the Americas. Proc Natl Acad Sci 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Caminade C, Turner J, Metelmann S et al. . Global risk model for vector-borne transmission of Zika virus reveals the role of El Niño 2015. Proc Natl Acad Sci 2017; 114:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Manore CA, Ostfeld RS, Agusto FB, Gaff H, LaDeau SL. Defining the Risk of Zika and Chikungunya Virus Transmission in Human Population Centers of the Eastern United States. PLoS Negl Trop Dis 2017; 11:e0005255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Monaghan AJ, Morin CW, Steinhoff DF et al. . On the Seasonal Occurrence and Abundance of the Zika Virus Vector Mosquito Aedes Aegypti in the Contiguous United States. PLoS currents 2016; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dinh L, Chowell G, Mizumoto K, Nishiura H. Estimating the subcritical transmissibility of the Zika outbreak in the State of Florida, USA, 2016. Theor Biol Med Model 2016; 13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Massad E, Tan S-H, Khan K, Wilder-Smith A. Estimated Zika virus importations to Europe by travellers from Brazil. Glob Health Action 2016; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rocklöv J, Quam MB, Sudre B et al. . Assessing seasonal risks for the introduction and mosquito-borne spread of Zika virus in Europe. EBioMedicine 2016; 9:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus disease epidemic. Stockholm, 2017:13. [Google Scholar]
- 67. Centers for Disease Control and Prevention. Zika virus: case counts in the US. https://www.cdc.gov/zika/geo/united-states.html. Accessed April 12 2017.
- 68. Gardner LM, Chen N, Sarkar S. Global risk of Zika virus depends critically on vector status of Aedes albopictus. Lancet Infect Dis 2016; 16:522–3. [DOI] [PubMed] [Google Scholar]
- 69. de Oliveira WK, Carmo EH, Henriques CM et al. . Zika virus infection and associated neurologic disorders in Brazil. N Engl J Med 2017; 376:1591–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med 2016; 374:1552–63. [DOI] [PubMed] [Google Scholar]
- 71. Lipsitch M, Finelli L, Heffernan RT, Leung GM, Redd SC; 2009 H1N1 Surveillance Group. Improving the evidence base for decision making during a pandemic: the example of 2009 influenza A/H1N1. Biosecur Bioterror 2011; 9:89–115. [DOI] [PMC free article] [PubMed] [Google Scholar]