Carbon concentrating mechanism (CCM) systems, associated with evolutionarily diverse aquatic photosynthetic organisms, make a major contribution to global net primary productivity and marine carbon sequestration. Here, an overview of these global contributions is presented from their evolutionary origins, including a possible trigger for their diversification when the aqueous O2/CO2 ratio rose above parity, and a re-definition of the paradox of phytoplankton. The reviews and research in the special issue also include molecular physiology and ecology of CCMs, through to future potential applications for sustaining carbon sequestration and supporting terrestrial crop productivity.
The inorganic carbon substrate supply needed for photosynthesis in the aquatic milieu is limited by inorganic carbon solubility and diffusion across the boundary layer, cell wall and multiple membranes to the primary carboxylase Rubisco. Various biophysical carbon concentrating mechanism (CCM) systems are found in many aquatic phytoplankters and have overcome these chemical and physical limitations. Such CCMs deliver the appropriate inorganic carbon species demanded by Rubisco (CO2), at an enhanced concentration which compensates for the enzyme’s low substrate affinity and competitive inhibition from oxygen.
Despite this adversity, aquatic organisms clearly punch above their weight of biomass relative to terrestrial plants. The instantaneous standing biomass crop of aquatic plants (primarily microorganisms) is 3 PgC (i.e. 1015 g carbon) relative to the 610 PgC usually quoted for terrestrial plant above-ground biomass. The paradox of how phytoplankton deliver an annual net primary productivity of 47.5 PgC, relative to the 56.4 PgC of their terrestrial counterparts (Field et al., 1998), has long intrigued researchers. In addition, the oceanic sink for net carbon sequestration is equal to that of land plants (2.3 PgC per year), such that marine organisms also facilitate the absorption of over 25% of annual anthropogenic CO2 emissions (Pan et al., 2011).
The original paradox of the phytoplankton was thought to reflect phylogenetic diversity in competition for limiting light and inorganic resources. The high net primary productivity, identified by Field et al. (1998), could be explained by the interaction between ecological and environmental factors across space and time to prevent the dominance of any one phytoplankton group. Despite this contention, it is with some amusement we note that each authority tends to claim pre-eminence for the contribution made by their particular phytoplankton clade to net primary productivity!
However, the past few decades have seen several historical paradigms overturned – such as photosynthetic acclimation to light increasing the depth of the photic zone (Richardson et al., 1983; Raven et al., 2017), the breadth of productivity across oceanic gyres (Johnson et al., 2006; Partensky and Garczarek, 2010), and the molecular basis of niche differentiation found within cyanobacterial and eukarotic picoplankton populations in coastal and equatorial waters (Not et al., 2012; Biller et al., 2015). Additionally, we now recognize that more than 80% of marine primary productivity will be facilitated by some form of CCM (Raven and Beardall, 2016; Raven et al., 2017).
This special issue provides a comprehensive update on aquatic carbon concentrating mechanisms, as well as reflection on how the field has progressed since the 1980s (Kaplan, 2017) together with the latest new research (see Box 1).
Box 1. Pioneering contributions over 40 years
The diversity of papers presented in this special issue reflects the range of contributions made at CCM9 in 2016, the ninth International Symposium on Inorganic Carbon Uptake by Aquatic Photosynthetic Organisms (Cambridge, UK; a satellite meeting following the 17th International Congress on Photosynthesis in Maastricht, The Netherlands). At the meeting, we were able to celebrate pioneering contributions over the past 40 years in person with Joe Berry, Aaron Kaplan and John Raven, and also recognize the outstanding technical and theoretical innovations made throughout this period by Murray Badger. A series of special publications has historically accompanied previous CCM Symposia, starting with the pioneering ASPP (American Society of Plant Physiologists) ‘Green Book’ proceedings from the first meeting in Asilomar (CA, USA) (Lucas and Berry, 1985), and through to that summarized by Moroney and Wee (2014). In the current special issue, we capture this progression with the highly personalized account by Aaron Kaplan of CCM research developments during those early years (Kaplan, 2017).
Palaeohistorical and environmental drivers for CCM origins and diversity
We have more certainty about the timeline for the diversification of prokaryotic and eukaryotic clades, and their contrasting endosymbiotic exchanges, than for the origins and diversity of CCMs. Using projections based on the current Rubisco content and kinetic properties of extant cyanobacteria, Raven et al. (2017) suggest that the atmospheric CO2 concentrations ranging from ×10 current to ×4.5 current, between 1.6 to 0.6 Ga (i.e. 109 years ago), could have been associated with CCM activity (see also Riding, 2006). Raven et al. (2017) also speculate that were Gloeobacter to be representative of a basal cyanobacterium, as accorded by some phylogenetic studies, then the CCM could even extend back to the Great Oxidation Event at 2.4 Ga.
Eukaryotic CCMs are generally thought to be homoplastic, with independent origins in each lineage (Raven et al., 2017). The primary endosymbiosis which led to the earliest oxygenic eukaryotes was likely to have been 1.0–1.6 Ga, with green, red and glaucophyte lineages subsequently diversifying via secondary and tertiary endosymbioses (Leliaert et al., 2012). The green algal lineage leading to the Chlorophyta is thought to have diverged via the Prasinophyceae from the Streptophyta by some 0.5–0.75 Ga. The red lineage (Rhodophyta) gave rise to Chromist algae (e.g. Haptophyta, Cryptophyta and Dinophyta), with haptophytes diversifying around 0.5 Ga and diatoms around 0.2 Ga (Heureux et al., 2017; Young and Hopkinson, 2017). Whilst the Rubisco large subunit has provided key phylogenetic insights for this progression (Badger and Price, 2003; Price et al., 2013), the co-evolution of Rubisco variants, their kinetic properties and responsiveness to CO2 and O2 remain a critical element in CCM evolution.
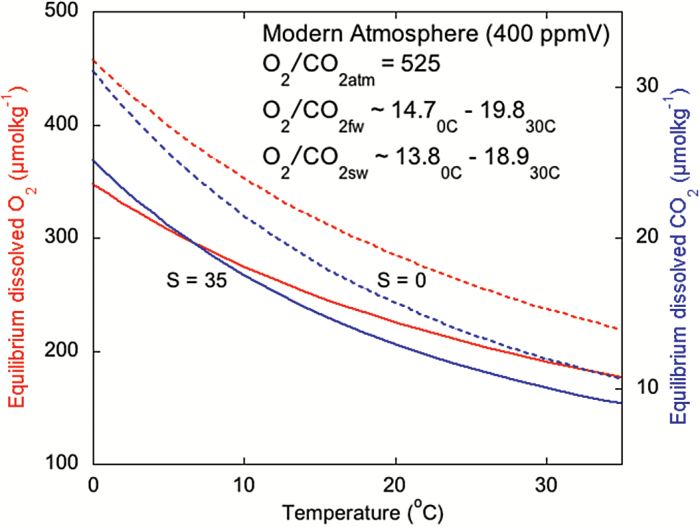
Rubisco is not only sensitive to CO2, rather the CO2/O2 ratio at its active site, and there is some evidence that oxygen exclusion may be an important component in many biophysical CCM systems (Meyer and Griffiths, 2013; Heureux et al., 2017; Meyer et al., 2017). Against the backdrop of generally declining CO2 and increasing O2 over geological history, it is an interesting thought-experiment to explore at what point rising aqueous O2 overtook CO2 to become more dominant in seawater. It is worth noting that such a chemical event would occur at different times in the atmosphere and ocean. CO2 is approximately 30 times more soluble than O2 in seawater (Fig. 1), so atmospheric CO2 can be 30 times less concentrated than O2 in the atmosphere, but the two species will be equimolar in the ocean.
Fig. 1.
(a) The sensitivity of equilibrium dissolved CO2 (blue; Weiss, 1974) and O2 (red; Benson and Krause, 1984) concentrations to temperature and salinity (S; 0, dashed line, and 35 ppt, solid line) and the modern range (with an atmosphere of 400 ppmV) of dissolved O2/CO2 ratios for freshwater (fw) and seawater (sw) between 0 and 30 °C.
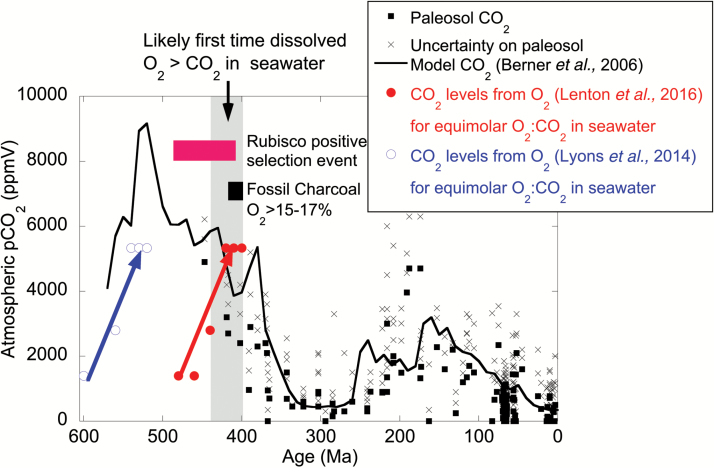
In the early stages of the oxygenation of the atmosphere during the Great Oxidation Event (2.4 Ga), oxygen is estimated to have risen to between 10–2 and 10–1 PAL (present atmospheric level), i.e. between 210 ppmV and 2100 ppmV (Lyons et al., 2014). The best estimates of CO2 at this time suggest that it was probably in excess of 100 PAL (i.e. 35 000 ppmV) (Young et al., 2012), so orders of magnitude more abundant than O2. Even if oxygen levels persisted at 0.1 to 0.2 PAL (21 000 ppmV) through the ‘boring billion’ (approximately 2 to 1 Ga) until close to the Precambrian/Cambrian boundary, CO2 levels would need to be 2–4 PAL (i.e. 7–1400 ppmV) to be equimolar with oxygen in the ocean, much lower than current estimates for this time. The timing then of parity between O2 and CO2 concentrations in the oceans is determined by the point at which O2 rose from around 0.2 PAL to close to modern values, and CO2 was sufficiently low to be equimolar. The most recent estimates of this O2 rise through 0.5 PAL (10.5%), around 450 Ma (Lenton et al., 2016), requires an atmospheric concentration of 10 PAL CO2 (3500 ppm) to provide equimolar dissolved CO2 and O2 in marine waters, a CO2 level which is well within range of coincident atmospheric estimates (Fig. 2). Therefore, the environmental threshold of O2 overtaking CO2 in surface waters, driving marine organisms to provide a mechanism to boost the CO2/O2 ratio at the site of Rubisco, is likely to date to the invasion of land by the earliest plants around the late Silurian/early Devonian. Such a timing seems to agree well with an analysis of a limited number of Rubisco large subunit sequences which also finds a number of events of positive selection up to 410 Ma. This indicates that emergence of CCMs around this time indeed left a footprint in the Rubisco protein (Young et al., 2012). Other, more conservative estimates, put the origins of cyanobacterial and eukaryotic CCMs following the ‘Devonian Drop’ and during the Carboniferous (Badger and Price, 2003).
Fig. 2.
An estimate of the threshold when the concentration of dissolved aqueous O2 rose to a higher concentration than CO2 in seawater as a trigger for the emergence of CCMs (grey bar). The CO2 concentrations that would be equimolar with O2 reconstructions (blue open circles – Lyons et al., 2014; red closed circles – Lenton et al., 2016) are based on an approximate 30-fold difference in solubilities. The Phanerozoic history of atmospheric CO2 is compiled from paleosols (black squares with crosses to show the uncertainty; Royer 2006) and the GEOCARB III model (solid black line; Berner and Kothavala, 2001). Also shown are the first appearance of fossil charcoal evidence that O2>15–17% (black bar, Glasspool et al., 2004), and a Rubisco positive selection event (magenta bar, Young et al., 2012).
There appears to be an evolutionary progression of a higher Rubisco specificity factor (selectivity for CO2 over O2) from cyanobacterial, chlorophyte and then to higher plant Form 1B Rubisco (Meyer and Griffiths, 2013). Considering the Form 1D in the marine algae, and Rhodophyta as the endo symbiont, the evolutionary trend is towards decreased Rubisco specificity factor and lower carbon affinity (higher Kc). This has been interpreted as an evolutionary response to improved CCM activity that allows a relaxation of substrate affinity and faster catalytic turnover of the enzyme (Tcherkez et al., 2006) in rhodophytes Young et al., 2012; Heureux et al., 2017) as well as in cyanobacteria and chlorophytes (Meyer and Griffiths, 2013). An apparent breakdown in the canonical trade-off between affinity for carbon and turnover in modern diatom Form 1D Rubisco (Young et al., 2016) challenges our understanding of this evolutionary progression to lower carbon affinities. It may be necessary to consider the impact of other Rubisco catalytic parameters on Rubisco performance.
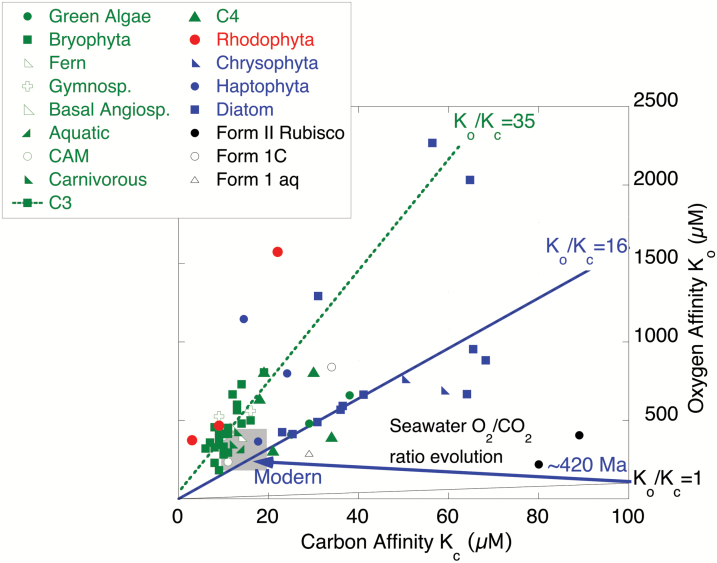
As well as the correlations between Rubisco affinity and turnover rate for both substrates, O2 and CO2 (Heureux et al., 2017), the Kc and Ko of many Rubiscos also appear to be linked (Fig. 3). The trade-off of a relaxed affinity for oxygen (higher Ko), which accompanies a more efficient CCM and a higher Kc, could improve Rubisco performance in an increasingly oxygenated environment. Whether this relationship between Kc and Ko is a constraint imposed by the structure of the enzyme (Savir et al., 2010), or a response to the O2/CO2 ratio at the active site of the Rubisco remains an open question. What is curious is that the ratio of the affinities of Rubisco for O2 and CO2 (Ko/Kc = ~16) of many Form 1D-containing marine algal Rubiscos, including most diatoms, and the Form 1B-containing green algae (e.g. Chlamydomonas), appears to match the modern dissolved O2/CO2 ratio of natural waters at equilibrium with an atmosphere containing 400 ppmV CO2 (16 at a representative sea surface temperature of 12 °C; see Fig. 3, and Heureux et al., 2017). The affinity of these Rubiscos for O2 is 16 times lower than that for CO2. This implies that the activity of a CCM to elevate the Rubisco Kc values above the environmental availability of CO2 also compensates perfectly for the environmental excess of O2 over CO2. Yet there is a bimodality to the Rubisco Ko/Kc ratio. The C3 plants and some C4 plants tend to fall on a line with a Ko/Kc gradient of 35. Such a ratio is much higher than the dissolved O2/CO2 ratio of any modern natural waters (see Fig. 1). However, during Pleistocene glacial periods, atmospheric CO2 fell to ~ 180 ppmV (Lüthi et al., 2008), but atmospheric O2 remained constant so the environmental dissolved O2/CO2 ratio was more than doubled relative to the modern (i.e. ~35). Modern Rubiscos in plants and algae, therefore, appear to be tuned in terms of O2/CO2 affinities to compensate for either glacial or modern O2/CO2 such that the Rubisco experiences a 1:1 competition between O2 and CO2 at the active site. This underpins the concept that it was the rising of environmental dissolved O2/CO2 above 1 that triggered the emergence of a CCM. It further demonstrates that the Rubisco enzyme, traditionally thought to be an inefficient relic of ancient environments, is highly dynamic. Rubisco appears to evolve its kinetics in response to environmental change over timescales of at least tens of kyrs, if not hundreds of years. The Ko/Kc data appear better tuned to anthropogenic conditions rather than average interglacial atmospheric compositions. At any event, we urgently need additional analyses of Rubisco kinetic properties and sequence specificity for Chromists (Young et al., 2016, 2017) as well as across the green algal lineages (Goudet, 2016) to fully understand the evolutionary history, rate of change and current diversity of carbon handling across the photosynthesizers.
Fig. 3.
Compiled Rubisco Kc (µmol kg–1) versus Ko (µmol kg–1) (Galmés et al., 2014; Young et al., 2016; Heureux et al., 2017) for a range of plant and algal species with Form 1B Rubisco-containing organisms in green, and Form 1D Rubisco-containing organisms in red (rhodophyta) and blue (diatoms, chrysophytes and haptophytes). Lines are plotted that indicate a Kc/Ko ratio of 1 (black), 16 (blue, equivalent to modern dissolved O2/CO2 ratio of seawater) and 35 (an apparent line of best fit to the C3 data). The range of environmental aqueous concentrations is indicated by the grey box. The evolution of the dissolved concentrations and O2/CO2 ratio from ~1 at 420 Ma (see Fig. 2) to 16 today (‘modern’) is indicated by the blue arrow. Note that the O2:CO2 dissolved ratio of natural waters at glacial maxima of the Pleistocene was ~35.
A final consideration is that the origin and maintenance of CCMs might reflect environmental limitations, in addition to external inorganic carbon supply. Interactions between nitrogen availability may relate to a reduced requirement for catalytic protein following CCM induction in Chlorella (Beardall et al., 1982), although other evidence is equivocal (Ruan et al., 2017). Consistent with the earlier observation, the proportion of Rubisco of total soluble protein is low in cells with a higher CCM efficiency in chromists, as evidenced by raised Kc of the Rubisco (Young et al., 2016; Heureux et al., 2017). Low energetic availability (as light or P) tends to reduce CCM activity (Maberly and Gontero, 2017), and interactions with low temperature may also have been significant, whether directly in terms of survival during the Cryogenian snowball earth, or at higher latitudes in terrestrial and marine algae (Raven et al., 2017). One additional driver for a CCM could also occur when inorganic carbon becomes locally depleted within a dense algal bloom, and so may be to some extent independent of equilibration with ambient air (Maberly and Gontero, 2017).
Convergence in CCM form and function
The three pillars usually invoked to support a CCM (Meyer and Griffiths, 2013) include:
(i) biophysical inorganic transporters, operating in parallel across adjacent membranes, raising the inorganic carbon pool by some 40-fold (Chlorophyte) to 400-fold (Cyanobacteria) and determining overall affinity and effectiveness of the CCM;
(ii) a suite of strategically placed carbonic anhydrases (CA) and CA-like moieties, adjacent to the inorganic transporters, to assist in bicarbonate interconversion or regeneration (or recapture) of CO2 close to Rubisco;
(iii) a microcompartment within which Rubisco aggregates, and from which CO2 leakage is minimized, such as the carboxysome in cyanobacteria and pyrenoid associated with most eukaryotic CCM systems.
Papers in this special issue report on the latest developments in identifying key cyanobacterial and chlorophyte CCM components (Rae et al., 2017), albeit in the context of their potential for introduction into higher plants to augment productivity (see also Price et al., 2013). Cyanobacterial α- and β-carboxysomes regulate the influx of bicarbonate and other metabolite exchanges via pore structures in the proteinaceous shell, with CO2 converted internally by CA systems (for details see Rae et al., 2017). The detailed variations between the two cyanobacterial lineages were described by Price et al. (2008), but in α-carboxysomes, Form-1A Rubisco is attached to the highly disordered CsoS2 protein, and in β-carboxysomes Form IB Rubisco is integrated via small subunit substitutions to the full-length CcmM protein in an ordered array (Rae et al., 2017).
Additional insights for the carboxysome shell proteins are provided by Sommer et al. (2017), who have undertaken a bioinformatic survey of β-carboxysome shell proteins, which suggest that variations in carboxysome structure allow plasticity in response to changing environmental conditions. Meanwhile, Larsson et al. (2017) provide crystallographic structural insights for regulation of metabolite exchange by gating of the CcmP protein in the β-carboxysome shell of Synechococcus elongatus PCC7942.
The diversity of CCM systems in most photosynthetic eukaryotics mostly requires all three physiological pillars indicated above, and those few which lack an identifiable pyrenoid show reduced capacity for carbon accumulation (Giordano et al., 2005). In this special issue, the comparative evolution of pyrenoids in chlorophytes and chromists is discussed in terms of the commonalities seen in mode of Rubisco aggregation, usually in association with some specialized thylakoid membrane organization (Meyer et al., 2017), although others may be stalked, and in some dinoflagellates Rubisco aggregation is more transient, forming centrally under circadian control (Nassoury et al., 2001).
For chlorophytes, the CCM in Chlamydomonas is the best-defined system from a molecular perspective (Meyer and Griffiths, 2013), with hierarchical models presented for regulatory processes leading to CCM induction (Mitchell et al., 2017). Various mutagenic screens and genetic manipulations have helped to characterize components of the Chlamydomonas CCM (Li et al., 2016; Machingura et al., 2017), including recent observations on the protein elements associated with Rubisco aggregation (Mackinder et al., 2016; Mitchell et al., 2017), and a new potential thylakoid bicarbonate transporter (Machingura et al., 2017). The observation that specific elements of Rubisco small subunits (SSU) were integral to the aggregation mechanism (Meyer et al., 2012) has led to insights into the function of a possible linker protein (EPYC1, formerly LCI5: Mackinder et al., 2016), and also now for the hierarchical organization of the pyrenoid (Meyer et al., 2017). The Chlamydomonas SSU mutants retain the knotted thylakoid tubules that intersect at the heart of the usual pyrenoid location, with growth and photosynthesis restored under elevated CO2 supply (Caspari et al., 2017). Such observations suggest that the pyrenoid-associated starch sheath and additional external regulatory elements (LCIB/C) are dependent upon Rubisco aggregation, and the spatial segregation of PSII (normally excluded from within the pyrenoid matrix) does not compromise overall energetic efficiency (Caspari et al., 2017).
For diatoms, whilst the specific details of CCM processes are less well understood than for Chlamydomonas, we have more detailed comparative insights into contrasting CCM systems for a wider range of species across the clade. Here, the four layers of thylakoid membranes, associated with secondary plastid endosymbiosis in the Dinophyceae, offer a range of options for concentrating inorganic carbon. Young and Hopkinson (2017) highlight the contrasting trade-offs which seem to have occurred in terms of investment in Rubisco relative to altered Rubisco kinetic properties for contrasting marine habitats. Matsuda et al. (2017; see also Tsuji et al., 2017) outline the contrasting modes of carbon uptake and conversion thought to operate in diatoms, dependent on either diffusive entry of CO2 or active transport, with one mechanism supported by the more detailed observations seen for the role of CAH1 in Nannochloropsis oceanica (Gee et al., 2017).
Further insights are provided by the co-evolution of inorganic carbon transporters (SLC4) in diatoms, used in combination with contrasting CA species (Shen et al., 2017), and also by the use of an intra-thylakoid CA to regenerate CO2 adjacent to the aggregated Rubisco (Tsuji et al., 2017), suggesting convergence with the mechanism also proposed for Chlorophytes (Meyer and Griffiths, 2013).
From molecular diversity to overcoming ecological adversity
The ecological implications of CCM systems are also addressed from an experimental perspective in a number of papers in this special issue. Evolutionary origins (Raven et al., 2017) are complemented by a more detailed comparison of ecological drivers in marine, freshwater and terrestrial habitats by Maberly and Gontero (2017). This leads to a highly original analysis of competitive interactions between cyanobacterial and chlorophyte cells (Ji et al., 2017; see also the Insight article by Beardall and Raven, 2017). These observations are consistent with notions that CCMs help to overcome adversity, as defined above in terms of nutrient availability or local depletion of inorganic carbon within blooms. Thus, chlorophyte algae endure under low ambient CO2 equilibration, relative to cyanobacteria, despite their ‘less effective’ CCM; whilst cyanobacteria may thrive under future elevated CO2 conditions (Ji et al., 2017; Beardall and Raven, 2017).
The major contribution made by diatoms to biogeochemical cycles, as reviewed by Young and Hopkinson (2017), is further characterized experimentally by a comparison of the effectiveness of the various inorganic carbon accumulation mechanisms (Clement et al., 2017). And finally, although macrophytes make a relatively small contribution to marine net primary productivity (1 PgC per year: Field et al., 1998), the issue contains papers analyzing the mechanisms of inorganic carbon uptake in seagrasses (Larkum et al., 2017) and Antarctic macrophytes (Iñiguez et al., 2017).
The future
The papers in this special issue convey a renewed sense of excitement and impetus in the field of aquatic carbon concentrating mechanisms, and include contributions from many young scientists with an astonishing breadth of skills, encompassing structural biology, novel molecular manipulations and bioinformatic approaches which are now augmenting traditional physiological and ecological experimentation. Globally, we may face uncertainty, but the potential for CCM systems to enhance marine carbon sequestration (Heureux et al., 2017; Raven et al., 2017; Young and Hopkinson, 2017) or terrestrial crop productivity (Rae et al., 2017), informed by ongoing cutting-edge research programmes, provide some hope, and much promise, for the future.
References
- Badger MR, Price GD. 2003. CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. Journal of Experimental Botany 54, 609–622. [DOI] [PubMed] [Google Scholar]
- Beardall J, Griffiths H, Raven JA. 1982. Carbon isotope discrimination and the CO2 accumulating mechanism in Chlorella pyrenoidosa.Journal of Experimental Botany 33, 729–737. [Google Scholar]
- Beardall J, Raven J. 2017. Cyanobacteria vs green algae: which group has the edge?Journal of Experimental Botany 68, 3697–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson BB, Krause D. 1984. The concentration and isotopic fractionation of oxygen dissolved in freshwater and seawater in equilibrium with the atmosphere. Limnology and Oceanography 29, 620–632. [Google Scholar]
- Berner RA, Kothavala Z. 2001. GEOCARB III: a revised model of atmospheric CO2 over Phanerozoic time. Americal Journal of Science 301, 182–204. [Google Scholar]
- Biller SJ, Berube PM, Lindell D, Chisholm SW. 2015. Prochlorococcus: the structure and function of collective diversity. Nature Reviews Microbiology 13, 13–27. [DOI] [PubMed] [Google Scholar]
- Caspari OD, Meyer MT, Tolleter D, Wittkopp TM, Cunniffe NJ, Lawson T, Grossman AR, Griffiths H. 2017. Pyrenoid loss in Chlamydomonas reinhardtii causes limitations in CO2 supply, but not thylakoid operating efficiency. Journal of Experimental Botany 68, 3903–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement R, Jensen E, Prioretti L, Maberly SC, Gontero B. 2017. Diversity of CO2 concentrating mechanisms and responses to CO2 concentration in marine and freshwater diatoms. Journal of Experimental Botany 68, 3925–3935. [DOI] [PubMed] [Google Scholar]
- Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. 1998. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240. [DOI] [PubMed] [Google Scholar]
- Galmés J, Kapralov MV, Andralojc PJ, Conesa MÀ, Keys AJ, Parry MA, Flexas J. 2014. Expanding knowledge of the Rubisco kinetics variability in plant species: environmental and evolutionary trends. Plant, Cell & Environment 37, 1989–2001. [DOI] [PubMed] [Google Scholar]
- Gee CW, Nyogi KK. 2017. The carbonic anhydrase CAH1 is an essential component of the carbon-concentrating mechanism in Nannochloropsis oceanica. Proceedings of the National Academy of Sciences, USA 114, 4537–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano M, Beardall J, Raven JA. 2005. CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annual Review of Plant Biology 56, 99–131. [DOI] [PubMed] [Google Scholar]
- Glasspool IJ, Edwards D, Axe L. 2004. Charcoal in the Silurian as evidence for the earliest wildfire. Geology 32, 381–383. [Google Scholar]
- Goudet MMMG. 2016. Evolutionary history of ribulose-1,5- biphosphate carboxylase/oxygenase and the origin of the algal pyrenoid. MPhil Thesis. University of Cambridge. [Google Scholar]
- Han X, Sun N, Xu M, Mi H. 2017. Co-ordination of NDH and Cup proteins in CO2 uptake in cyanobacterium Synechocystis sp. PCC 6803. Journal of Experimental Botany 68, 3869–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heureux AMC, Young JN, Whitney SM, Eason-Hubbard MR, Lee RBY, Sharwood RE, Rickaby REM. 2017. The role of Rubisco kinetics and pyrenoid morphology in shaping the CCM of haptophyte microalgae. Journal of Experimental Botany 68, 3959–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez C, Heinrich S, Harms L, Gordillo FJL. 2017. Increased temperature and CO2 alleviate photoinhibition in Desmarestia anceps: from transcriptomics to carbon utilization. Journal of Experimental Botany 68, 3971–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Verspagen JMH, Stomp M, Huisman J. 2017. Competition between cyanobacteria and green algae at low versus elevated CO2: who will win, and why?Journal of Experimental Botany 68, 3815–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZI, Zinser ER, Coe A, McNulty NP, Woodward EM, Chisholm SW. 2006. Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science 311, 1737–1740. [DOI] [PubMed] [Google Scholar]
- Kaplan A. 2017. On the cradle of CCM research: discovery, development, and challenges ahead. Journal of Experimental Botany 68, 3785–3796. [DOI] [PubMed] [Google Scholar]
- Larkum AWD, Davey PA, Kuo J, Ralph PJ, Raven JA. 2017. Carbon-concentrating mechanisms in seagrasses. Journal of Experimental Botany 68, 3773–3784. [DOI] [PubMed] [Google Scholar]
- Larsson AM, Hasse D, Valegård K, Andersson I. 2017. Crystal structures of β-carboxysome shell protein CcmP: ligand binding correlates with the closed or open central pore. Journal of Experimental Botany 68, 3857–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leliaert F, Smith DR, Moreau H, Herron MD, Verbruggen H, Delwiche CF, De Clerck O. 2012. Phylogeny and molecular evolution of the green algae. Critical Reviews in Plant Sciences 31, 1–46. [Google Scholar]
- Lenton TM, Dahl TW, Daines SJ, Mills BJ, Ozaki K, Salzmann MR, Porada P. 2016. Earliest land plants created modern levels of atmospheric oxygen. Proceedings of the National Academy of Sciences, USA 113, 9704–9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang R, Patena W et al. 2016. An indexed, mapped mutant library enables reverse genetics studies of biological processes in Chlamydomonas reinhardtii. The Plant Cell 28, 367–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas WJ, Berry JA. 1985. Inorganic carbon uptake by aquatic photosynthetic organisms. Rockville, MD: American Society of Plant Physiologists. [Google Scholar]
- Lüthi D, Le Floch M, Bereiter B et al. 2008. High-resolution carbon dioxide concentration record 650 000–800 000 years before present. Nature 453, 379–382. [DOI] [PubMed] [Google Scholar]
- Lyons TW, Reinhard CT, Planavsky NJ. 2014. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 506, 307–315. [DOI] [PubMed] [Google Scholar]
- Maberly SC, Gontero B. 2017. Ecological imperatives for aquatic carbon dioxide-concentrating mechanisms. Journal of Experimental Botany 68, 3797–3814. [DOI] [PubMed] [Google Scholar]
- Machingura MC, Bajsa-Hirschel J, Laborde SM, Schwartzenburg JB, Mukherjee B, Mukherjee A, Pollock SV, Förster B, Price GD, Moroney JV. 2017. Identification and characterization of a solute carrier, CIA8, involved in inorganic carbon acclimation in Chlamydomonas reinhardtii. Journal of Experimental Botany 68, 3879–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinder LC, Meyer MT, Mettler-Altmann T et al. 2016. A repeat protein links Rubisco to form the eukaryotic carbon-concentrating organelle. Proceedings of the National Academy of Sciences, USA 113, 5958–5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Griffiths H. 2013. Origins and diversity of eukaryotic CO2-concentrating mechanisms: lessons for the future. Journal of Experimental Botany 64, 769–786. [DOI] [PubMed] [Google Scholar]
- Meyer MT, Genkov T, Skepper JN et al. 2012. Rubisco small-subunit α-helices control pyrenoid formation in Chlamydomonas. Proceedings of the National Academy of Sciences, USA 109, 19474–19479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MT, Whittaker C, Griffiths H. 2017. The algal pyrenoid: key unanswered questions. Journal of Experimental Botany 68, 3739–3749. [DOI] [PubMed] [Google Scholar]
- Mitchell MC, Metodieva G, Metodiev MV, Griffiths H, Meyer MT. 2017. Pyrenoid loss impairs carbon-concentrating mechanism induction and alters primary metabolism in Chlamydomonas reinhardtii. Journal of Experimental Botany 68, 3891–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney JV, Wee JL. 2014. CCM8: the eighth international symposium on inorganic carbon uptake by aquatic photosynthetic organisms. Photosynthesis Research 121, 107–110. [DOI] [PubMed] [Google Scholar]
- Nassoury N, Fritz L, Morse D. 2001. Circadian changes in ribulose-1,5-bisphosphate carboxylase/oxygenase distribution inside individual chloroplasts can account for the rhythm in dinoflagellate carbon fixation. The Plant Cell 13, 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Not F, Siano R, Kooistra WHCF et al. 2012. Diversity and ecology of eukaryotic marine phytoplankton. Advances in Botanical Research 64, 1–53. [Google Scholar]
- Pan Y, Birdsey RA, Fang J et al. 2011. A large and persistent carbon sink in the world’s forests. Science 333, 988–993. [DOI] [PubMed] [Google Scholar]
- Partensky F, Garczarek L. 2010. Prochlorococcus: advantages and limits of minimalism. Annual Review of Marine Science 2, 305–331. [DOI] [PubMed] [Google Scholar]
- Price GD, Badger MR, Woodger FJ, Long BM. 2008. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. Journal of Experimental Botany 59, 1441–1461. [DOI] [PubMed] [Google Scholar]
- Price GD, Pengelly JJ, Forster B, Du J, Whitney SM, von Caemmerer S, Badger MR, Howitt SM, Evans JR. 2013. The cyanobacterial CCM as a source of genes for improving photosynthetic CO2 fixation in crop species. Journal of Experimental Botany 64, 753–768. [DOI] [PubMed] [Google Scholar]
- Rae BD, Long BM, Förster B, Nguyen ND, Velanis CN, Atkinson N, Hee WY, Mukherjee B, Price GD, McCormick AJ. 2017. Progress and challenges of engineering a biophysical carbon dioxide-concentrating mechanism into higher plants. Journal of Experimental Botany 68, 3717–3737. [DOI] [PubMed] [Google Scholar]
- Raven JA, Beardall J. 2016. The ins and outs of CO2. Journal of Experimental Botany 67, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA, Beardall J, Sánchez-Baracaldo P. 2017. The possible evolution, and future, of CO2-concentrating mechanisms. Journal of Experimental Botany 68, 3701–3716. [DOI] [PubMed] [Google Scholar]
- Richardson K, Beardall J, Raven JA. 1983. Adaptation of unicellular algae to irradiance: an annlysis of strategies. New Phytologist 93, 157–191. [Google Scholar]
- Riding R. 2006. Cyanobacterial calcification, carbon dioxide concentrating mechanisms, and Proterozoic–Cambrian changes in atmospheric composition. Geobiology 4, 299–316. [Google Scholar]
- Royer D. 2006. CO2-forced climate thresholds during the Phanerozoic. Geochimica et Cosmochimica Acta 70, 5665–5675. [Google Scholar]
- Ruan Z, Raven JA, Giordano M. 2017. In Synechococcus sp. competition for energy between assimilation and acquisition of C and those of N only occurs when growth is light limited. Journal of Experimental Botany 68, 3829–3839. [DOI] [PubMed] [Google Scholar]
- Savir Y, Noor E, Milo R, Tlusty T. 2010. Cross-species analysis traces adaptation of Rubisco toward optimality in a low-dimensional landscape. Proceedings of the National Academy of Sciences, USA 107, 3475–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H, Gontero B, Maberly SC, Jiang HS, Cao Y, Li W, Huang WM. 2017. Responses of Ottelia alismoides, an aquatic plant with three CCMs, to variable CO2 and light. Journal of Experimental Botany 68, 3985–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Dupont CL, Hopkinson BM. 2017. The diversity of carbon dioxide-concentrating mechanisms in marine diatoms as inferred from their genetic content. Journal of Experimental Botany 68, 3937–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Cai F, Melnicki M, Kerfeld CA. 2017. β-Carboxysome bioinformatics: identification and evolution of new bacterial microcompartment protein gene classes and core locus constraints. Journal of Experimental Botany 68, 3841–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez GG, Farquhar GD, Andrews TJ. 2006. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proceedings of the National Academy of Sciences, USA 103, 7246–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolleter D, Chochois V, Poiré R, Price GD, Badger MR. 2017. Measuring CO2 and HCO3− permeabilities of isolated chloroplasts using a MIMS-18O approach. Journal of Experimental Botany 68, 3915–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji Y, Mahardika A, Matsuda Y. 2017. Evolutionarily distinct strategies for the acquisition of inorganic carbon from seawater in marine diatoms. Journal of Experimental Botany 68, 3949–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji Y, Nakajima K, Matsuda Y. 2017. Molecular aspects of the biophysical CO2-concentrating mechanism and its regulation in marine diatoms. Journal of Experimental Botany 68, 3763–3772. [DOI] [PubMed] [Google Scholar]
- Weiss RF. 1974. Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Marine Chemistry 2, 203–215. [Google Scholar]
- Young JN, Heureux AM, Sharwood RE, Rickaby RE, Morel FM, Whitney SM. 2016. Large variation in the Rubisco kinetics of diatoms reveals diversity among their carbon-concentrating mechanisms. Journal of Experimental Botany 67, 3445–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JN, Rickaby RE, Kapralov MV, Filatov DA. 2012. Adaptive signals in algal Rubisco reveal a history of ancient atmospheric carbon dioxide. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 367, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JN, Hopkinson BM. 2017. The potential for co-evolution of CO2-concentrating mechanisms and Rubisco in diatoms. Journal of Experimental Botany 68, 3751–3762. [DOI] [PubMed] [Google Scholar]