Abstract
The emergence of Zika virus (ZIKV) as a major public health threat has focused research on understanding virus biology and developing a suite of strategies for disease intervention. Recent advances in cryoelectron microscopy have accelerated structure-function studies of flaviviruses and of ZIKV in particular. Structures of the mature and immature ZIKV have demonstrated its similarity with other known flaviviruses such as dengue and West Nile viruses. However, ZIKV’s unique pathobiology demands an explanation of how its structure, although similar to its flavivirus relatives, is sufficiently unique to address questions of receptor specificity, transmission, and antigenicity. Progress in defining the immunodominant epitopes and how neutralizing antibodies bind to them will provide great insight as vaccines progress through clinical trials. Identification of host receptors will substantially illuminate the interesting ZIKV tropism and provide insights into pathogenesis. Although the answers to all of these questions are not yet available, rapid progress in combining structural biology with other techniques is revealing the similarities and the differences in virion structure and function between ZIKV and related flaviviruses.
Keywords: cryo-EM, maturation, neutralizing antibodies, receptors, Zika virus
This review article describes the structure of mature and immature ZIKV in the context of related flaviviruses and provides insights into the antibody interactions and receptor usage of the virus.
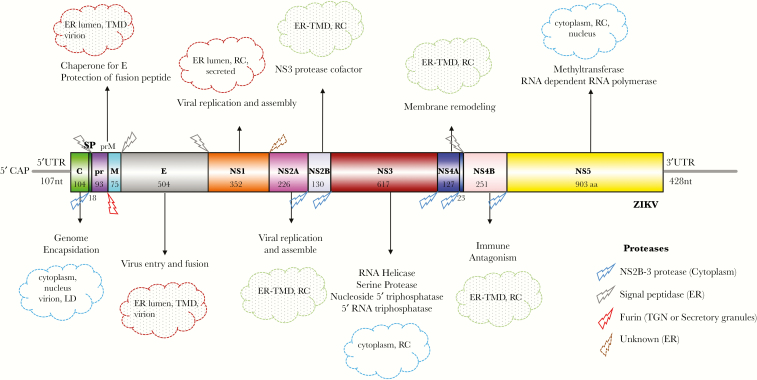
Zika virus has recently emerged as an important human pathogen. It is a member of the flavivirus genus within the family Flaviviridae. This group of approximately 70 viruses are primarily transmitted by mosquito or tick vectors and include members such as dengue virus (DENV), yellow fever virus (YFV), West Nile virus (WNV), Japanese encephalitis virus (JEV), and tick-borne encephalitis virus. Zika virus is a positive sense, single-strand ribonucleic acid (RNA) virus with a genome size of approximately 10.8 kilobases. The RNA is translated into a single polyprotein (3423 amino acids in length) encoding 3 structural proteins—capsid (C); membrane (M), which is generated from its precursor premembrane (prM); and envelope (E)—as well as 7 nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) (Figure 1). The structural proteins, as the name suggests, form the virus particle. The nonstructural proteins assist in replication and packaging of the genome as well as in subverting the host pathways in favor of the virus. The generation of the 10 individual proteins from the polyprotein is regulated by viral and host proteases, and the efficiency of one of the host proteases (furin) in cleaving the viral targets (prM) is variable and may play a role in pathogenesis (described in Assembly and maturation of Zika virus section).
Figure 1.
Architecture of Zika virus (ZIKV) genomic ribonucleic acid (RNA), translation, and cleavage of ZIKV polyprotein, function, and localization of ZIKV proteins. The ZIKV genomic RNA is capped but it lacks a poly A tail. The gray lines represent 5’- and 3’-untranslated regions (UTR). The viral RNA codes for a polyprotein that is cotranslationally cleaved to yield 10 proteins. The 3 structural proteins (C, prM/M, and E) and 7 nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) are displayed with the length of each protein (number of amino acids). The cleavage profile of the polyprotein, the proteases involved, as well as the role and the subcellular location of individual proteins are provided in the figure. Information shown in the figure is true for flaviviruses in general, but many aspects need to be specifically verified for ZIKV. The figure does not provide an exhaustive list of the functions of each protein. The viral proteins interact with several host proteins, which expands their functional repertoire. The figure was created using Illustrator for Biological Sequences [103]. Abbreviations: aa, amino acid; ER, endoplasmic reticulum; LD, lipid droplet; nt, nucleotide; RC, replication complex; SP, signal peptide; TGN, Trans-Golgi Network; TMD, transmembrane domain.
This review is focused on the structure of ZIKV and the proteins that constitute the virus particle. Our current understanding is restricted to the arrangement of surface glycoproteins prM or M and E on the lipid bilayer. The structure of the internal core, which is made up of C protein and genomic RNA, has not been determined to date. The surface glycoproteins show significant movement or rearrangement during the viral life cycle. Some of the states have been well captured by cryoelectron microscopy (cryo-EM), whereas others remain obscure and technically challenging but nonetheless the focus of current efforts. The structures of “defined” states of ZIKV, namely the mature and immature states, are discussed with reference to those of other flaviviruses. The maturation pathway that converts an immature noninfectious form to a mature infectious state is also described along with the complexity surrounding the inefficient maturation process seen in flaviviruses. Finally, insights on how the virus structure dictates antibody recognition and receptor usage are provided in the context of ZIKV to understand its unique host cell tropism, placental transmigration, and fetal neuronal progenitor cell infection.
STRUCTURE OF MATURE ZIKA VIRUS
The near atomic structure of ZIKV was solved using cryo-EM by 2 groups independently at 3.8 and 3.7 Å resolution and was found consistent with structures seen for other flaviviruses [1, 2]. Prior studies had established the structures of several of the DENV serotypes [3–7], WNV [8], and most recently JEV [9]. The structure of the so-called “mature virus” bears resemblance to a golf ball in that the major surface protein E lies parallel to the viral membrane, thus presenting a rather smooth surface (Figure 2A and B).
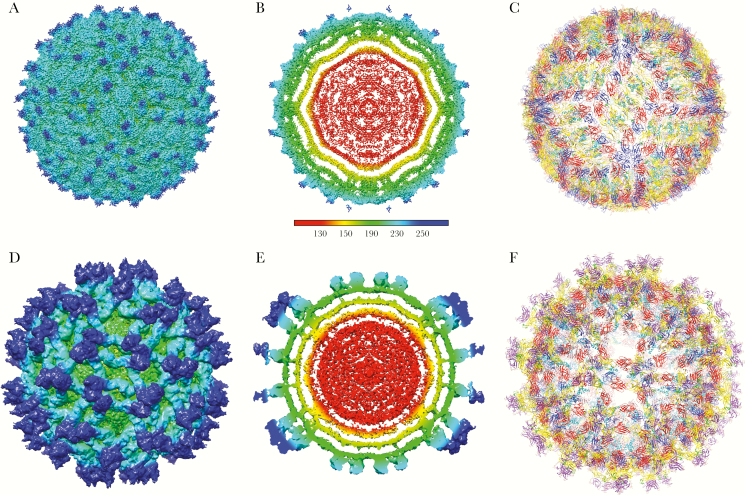
Figure 2.
Structure of Zika virus (ZIKV). A–C depict the mature form of ZIKV (3.8 Å resolution) [1], whereas D–F represent the immature form (9.1 Å) [23]. Both mature and immature structures of ZIKV are centered on the 2-fold axis. A and D are surface-shaded, radially colored views of mature (EMD-8116) and immature ZIKV (EMD-8508), respectively. B and E are the respective cross-sections of A and D. Color coding is based on the key provided: red, up to 130 Å; yellow, up to 150 Å; green, up to 190 Å; cyan, up to 230 Å; blue, 250 Å and beyond. C displays the icosahedral arrangement and Cα-backbone of the E and M proteins derived from the 3.8 Å density map of mature ZIKV (Protein Data Bank [PDB] 5IRE). Two asymmetric units related by 180° define the raft subunit of the virus consisting of 3 pairs of E and M homodimers. F shows the Cα-backbone of the DENV2 prM-E heterodimer (PDB 3C6E) and transmembrane domains of ZIKV E and M proteins (PDB 5IRE) fitted into the immature ZIKV map (PDB 5U4W). E protein is colored as follows: domain I (red), II (yellow) with fusion loop in green, III (blue) and stem-transmembrane helices (pink). pr peptide is shown in purple. The soluble region of M protein is displayed in magenta, and the stem-transmembrane helices are represented in cyan. The glycans projecting from the surface on prM and E proteins are highlighted. The molecular graphics of ZIKV were made using the Chimera package developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIH P41 RR-01081) [104].
The 50-nm mature ZIKV particle has 180 copies of the E and M proteins embedded in the viral membrane. The structures of both E and M proteins were determined from the cryo-EM structures that were reported [1, 2]. Like other flaviviruses, the ZIKV E protein consists of 4 domains: the stem-transmembrane domain pair, and 3 domains found outside of the membrane referred to as ectodomains I, II, and III. The E protein predominates on the surface of the particle with the smaller M protein residing underneath the larger E protein with its small extracellular region and stem-transmembrane domains. The basic organizational unit of E protein in mature flaviviruses is a dimer with each E monomer within the dimer related to its neighbor by 2-fold symmetry. Three E proteins dimers lie parallel to one another in the so-called raft configuration, with the virion having a total of 30 rafts (Figure 2C). The asymmetric unit of the virion contains a half of the raft, and thus there are 60 asymmetric units in the ZIKV particle. Although there are 180 E and M proteins arranged in the raft configuration, the virion does not exhibit T = 3 quasi-equivalence due to this unusual arrangement of E proteins. Unlike the E protein, the M protein has very few amino acids that are solvent exposed as it resides under the E protein, obeying similar icosahedral organization.
Comparison of the ZIKV structure with those of other flaviviruses demonstrates a common organization pattern with minor differences apparent only at the atomic level. The root mean square deviation between equivalent Cα atoms of the E and M proteins between ZIKV and DENV2 is only 1.8 Å despite the modest sequence identity of 54% in the E protein. This represents a remarkable level of structural similarity between these 2 viral E proteins and reflects the conformation required for function(s) of this protein. The most notable difference (up to 6 Å) between equivalent Cα atoms of the 2 viruses can be seen in the region near the glycosylation site (Asn153 and Asn154 in DENV and ZIKV, respectively). This region is 5 amino acids larger in ZIKV than in DENV, and it has high sequence variability when compared across flaviviruses or between ZIKV strains. The glycan loop is proximal to the immunodominant fusion loop of the neighboring E protein and may therefore influence accessibility of this epitope. Furthermore, the glycans are putative binding sites for lectin receptors (as discussed in Zika virus tropism and receptors section). These differences in ZIKV and DENV E proteins could influence receptor interactions, antibody response, and perhaps downstream biology of the virus and requires further investigation [1].
The organization between proteins within the asymmetric unit is very similar but not identical. This is especially apparent around the 5-fold axis where E protein domain III’s pack tightly together as well as in the angle between the domains. This difference stems from the quasi-equivalent positioning of E proteins on the 5-fold, 3-fold, or 2-fold axes. Tighter packing of E proteins at the 5-fold axis in ZIKV relative to DENV, presumably, due to an amino acid insertion and extra hydrogen bond interactions was suggested to be responsible for the observed high thermal stability of ZIKV. It was speculated that the high thermal stability was related to ZIKV’s unusual pathobiology [2]. However, a study by Goo et al [10] did not find ZIKV to be exceptionally stable; it’s half life was observed to be between that of WNV and DENV. Furthermore, mutations that disrupted the extra hydrogen bond interaction did not reduce the thermostability of ZIKV [10, 11]. The impact of thermal stability (or conversely thermal sensitivity) in virus biology and disease pathology is not well understood. Thermal sensitivity or loss of infectivity over time (also called intrinsic decay) could potentially be correlated with viral dynamics. Flavivirions are not static or closed entities, as originally anticipated. Evidence now suggests that the cryo-EM reconstructions are snapshots of virions at a given time under a defined set of conditions. Similar to other proteins and virus systems, flaviviral glycoproteins are in constant dynamic small-scale motion, a process termed as “breathing”. In doing so, the viruses sample different conformational and antigenic landscapes. Support for this conjecture mainly stems from antibody studies (reviewed extensively in [12–15]). Numerous studies have suggested that flaviviruses are dynamic, transiently revealing new epitopes [16] and also potentially providing access to the concealed lipid bilayer that might be exploited by lipid receptors implicated in both DENV and ZIKV entry (discussed in Zika virus tropism and receptors section). A more dynamic virus particle should have a higher rate of intrinsic decay and reduced thermal stability because there is theoretically a greater probability for it to enter a dead irreversible state during fast stochastic sampling of antigenic repertoire. Differences in thermal stability between ZIKV and other flaviviruses could imply differences in the dynamics of these viruses. How that translates to downstream biology is unknown. Furthermore, some strains of DENV2, but not other DENV serotypes, have been shown to exist as an expanded (~55 nm) state called “bumpy” at 37°C [17, 18]. Transition to a bumpy state was not seen for ZIKV when the particles were incubated at elevated temperatures of up to 40°C [2].
ASSEMBLY AND MATURATION OF ZIKA VIRUS
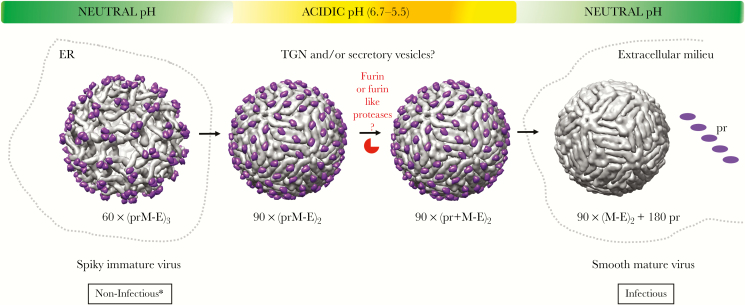
Zika virus genome RNA is synthesized within virus-induced replication vesicles that are derived and closely tied to the endoplasmic reticulum (ER) [19, 20]. The details of particle assembly and budding into the ER lumen are not well understood for any flavivirus. However, it is expected that the C protein in association with newly synthesized positive polarity genome RNA must coalesce on the cytoplasmic face of the ER membrane containing the prM-E heterodimers. Undefined interactions between these molecules lead to particle budding into the ER lumen, producing an immature virus particle. The structures of these 60-nm, noninfectious “immature viruses” have been solved [6, 21, 22], with the ZIKV immature virus (strain H/PF/2013) being recently published [23]. The immature ZIKV has 3 prM-E heterodimers that come together and project outward from the membrane with the distal fusion loop of E protein at its apex (Figure 2D–F and Figure 3, left panel). The pr domain of prM is positioned at the top of this spike and covers the fusion loop, preventing premature low pH-mediated E protein fusion. This spiky immature virus particle is glycosylated in the ER followed by transport to the Golgi and Trans-Golgi Network (TGN), where its glycans are trimmed and the particle encounters a slightly acidic environment. The pH of the TGN is sufficient to trigger a dramatic rearrangement of the trimeric prM-E spike such that the E protein homodimers are formed that lie parallel to the membrane (Figure 3, middle pannel) [24]. This rearrangement unveils the cleavage site for host furin to act on the pr-M junction, and the maturation cleavage can take place. Once cleaved, the pr domain remains bound to the distal end of the E protein until the particle is released in the neutral pH environment on the extracellular milieu (Figure 3, right panel) [25]. It is important to note that structures exist for the first and last states of this process for ZIKV, whereas the intermediate states (Figure 3, middle panel) have been shown only for DENV.
Figure 3.
The maturation pathway for flaviviruses. The orchestrated conformational changes in viral surface glycoproteins (prM/M, E) and the proteolytic cleavage of prM protein by host protease furin in the secretory pathway that converts an immature, spiky, and noninfectious virus to a mature, smooth, and infectious virus. The asterisk indicates exceptions to the idea that immature viruses are noninfectious. The immature viruses can be rendered infectious if the furin cleavage site is intact and the entry of these virions is assisted by nonneutralizing antibodies. The furin cleavage is inefficient for dengue virus (DENV) due to a suboptimal cleavage site leading to morphogenetic diversity in the virus population. The efficiency of prM cleavage for Zika virus (ZIKV) remains unknown. The figure was generated using University of California, San Francisco chimera package from the Computer Graphics Laboratory, San Francisco [104]. The figure was created using the Chimera package developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco. The Protein Data Bank identification for the neutral pH immature DENV2 on the left is 3C6D, the low pH immature DENV2 is 3C6R, furin cleaved but pr-bound DENV2 at low pH is 3IYA, and the mature smooth DENV2 on the right is 1THD. DENV, instead of ZIKV, was used for the schematic because only the structures for immature and mature forms of ZIKV are available. The maturation pathway for ZIKV is expected to be similar to that of DENV. Abbreviations: ER, endoplasmic reticulum; TGN, Trans-Golgi Network.
There is overwhelming structural [26], biochemical [27–29], and antibody data [30, 31], suggesting that flaviviruses are compromised in their maturation ability such that the cleavage of the precursor prM protein into M protein and pr peptide is inefficient (reviewed in [32]). Virions released from DENV-infected cells show a spectrum of morphologies from completely spiky (180prM/0M) to smooth (0prM/180M) and all things in between, subsequently referred to as mosaic (180-0prM/0-180M). Analysis of the distribution of ZIKV (strain H/PF/2013) particle morphologies released from infected Vero cells that overexpressed furin protease showed that the vast majority of particles displayed a smooth “mature” appearance. Although their appearance was smooth, many of the particles had small imperfections that limited their incorporation into the pool of particles used for the reconstruction [1]. These imperfections may be due to an improper number of incorporated glycoproteins, leading to errors in their surface arrangement and perturbations in the structure of individual E or M proteins, or they may contain a mixture of cleaved M and uncleaved precursor prM. The percentage of prM present in mature ZIKV has not been determined, but, in DENV2, the prM content can be as high as 50%, reflecting inefficient furin cleavage [33]. This results in a heterogeneous population of particles that presents an ensemble of epitopes to the immune system in far greater numbers than a well defined homogeneous particle. Whether this has important biological consequences for flaviviruses or whether there are additional advantages to such particle heterogeneity remains to be discerned.
The 9.1 Å structure of immature ZIKV revealed, for the first time, density from the C protein core that extended toward the inner leaflet of the bilayer in the vicinity of the prM and E transmembrane domains (Figure 2E) [23]. The close approach of these densities suggests that they may interact with one another. It also suggests that, at least in the immature virus, a portion of the core may have icosahedral symmetry, perhaps imposed by the E glycoproteins. Additional experiments are ongoing, but these results suggest that the C protein may be linked or associated with the transmembrane domains of the glycoproteins shortly after particle budding and that this association is abrogated upon low pH-mediated rearrangement of the glycoproteins (Figure 2B). How the signal for core rearrangement is transmitted across the lipid membrane is not known yet, but this signals an aspect of flavivirion assembly previously unknown.
EPITOPES FOUND ON ZIKA VIRUS
An exhaustive overview of the antibody response to ZIKV is beyond the scope of the review. A few antibodies for which structural information is available are discussed below. The first report of the structure of a broadly neutralizing murine antibody (2A10G6) Fab bound to the ZIKV E protein using x-ray crystallography revealed binding to the flavivirus highly conserved fusion loop [34]. It is expected that binding to the fusion loop will prevent the E protein from engaging with the membrane as required for fusion. Although this antibody could protect mice against ZIKV challenge, whether this is an epitope that elicits antibodies in ZIKV-infected individuals has not been shown. In another structural study, human monoclonal (C10) that was originally isolated from a DENV-infected patient that has potent neutralizing activity against ZIKV [35] was shown by cryo-EM to bind to all 180 E proteins. The Fab binds at both ends of the E protein dimer (domain II on one E monomer and domain I and III of the other E monomer) creating an intradimer lock. Furthermore, Fabs interact across the dimer interface with adjacent dimers. Thus, this antibody neutralizes virus infectivity by cross-linking the E proteins and preventing the required prefusion conformational changes rearranging the E homodimers to fusion-competent homotrimers [36].
A ZIKV-specific monoclonal (ZIKV-117) isolated from an infected patient was shown to be a highly potent neutralizing antibody with no cross-reactivity against other flaviviruses. The ZIKV-117 was shown to protect mice against a ZIKV challenge, which suggests its potential as a therapeutic reagent [37]. Analysis of ZIKV-117 Fab fragments complexed with ZIKV using cryo-EM demonstrated that the antibody cross-links monomers within the dimer as well as between neighboring dimers. The reconstruction demonstrated that only 60 Fabs bound per virion under saturating conditions due to steric occlusion and inaccessibility of the epitope around the 5-fold axis. Thus, just like the DENV antibodies described above, this ZIKV-specific antibody locks E proteins together, preventing the reorganization required for membrane fusion and virus infection [38]. Subunit or subviral particle (SVP)-derived vaccines will not elicit this type of antibody, and so it will be interesting to evaluate in these different vaccines which types of antibodies prove most efficacious for protection.
A comprehensive structural analysis of DENV antibodies isolated from infected patients was shown by Barba-Spaeth et al [35] to have a subset of antibodies that potently neutralize ZIKV. The potent neutralizers were usually directed at “E-dimer epitopes” and were capable of broadly neutralizing all 4 DENV serotypes as well as ZIKV. In contrast, maturation-sensitive fusion loop antibodies against DENV were weakly neutralizing against ZIKV but still neutralized mature DENV in spite of the epitope being inaccessible [35]. This suggests that the ZIKV epitope may not be as accessible as in DENV. This may be related to differences in breathing between DENV and ZIKV (DENV has a higher rate of intrinsic decay as described earlier) or due to the differences between ZIKV and DENV in the glycan 154 loop (also described above). The DENV loop may have greater dynamic activity than ZIKV, allowing more access to the underlying fusion loop of the adjacent E protein.
Mapping the epitopes on ZIKV that elicit potent neutralizing antibodies is a useful guide for the development of efficacious vaccines against the virus. With more than 45 vaccine candidates in the pipeline to control this pathogen, it is likely that many will have inherent bias in eliciting antibodies against specific epitopes. This is especially valid for subunit vaccines or for SVPs because they may not elicit antibodies that recognize quaternary epitopes. The SVPs are usually produced by expression of prM and E and result in the production of T = 1 icosahedral core-less particles with 60 copies of each of the 2 glycoproteins as opposed to 180 for the virus particle. The current deoxyribonucleic acid and mRNA vaccine candidates are designed to produce SVPs and will perhaps illicite a different repitiore of antibodies in human vaccines relative to the live attenuated or inactivated ZIKV vaccines. It will be of great interest to evaluate the protective efficacy of these different classes of vaccines.
ZIKA VIRUS TROPISM AND RECEPTORS
Despite the overwhelming similarity in the structure and the replication strategy of ZIKV with other flaviviruses, the downstream diseases caused by these viruses are remarkably different. Zika virus, unlike most other flaviviruses, traverses the placental barrier and causes robust infection in the fetal brains, resulting in teratogenicity or fetal demise [39, 40]. It has been suggested that ZIKV should be added to the list of TORCH agents [41]. A wide variety of cells have been shown to be permissive to ZIKV infection, including but not limited to cells of the central or peripheral nervous system, such as neural stem cells, oligodendrocyte precursor cells, cranial neural crest cells, neurons, and astrocytes; cells of the retina and optic nerve; placental cells, such as trophoblasts; immune cells, such as brain resident macrophages (microglia), placental macrophages (Hofbauer cells), and dendritic cells; endothelial cells serving as barriers to the brain and placental compartments; as well as skin keratinocytes and human dermal fibroblasts ([39] and included references). Furthermore, ZIKV has been shown to infect the testis and epididymis of male mice, which leads to damage and infertility [42–45]. In line with this observation, ZIKV has been shown to persist in human male seminal fluids up to ~12 weeks after the onset of symptoms [46] and is capable of male-to-female [47] and male-to-male sexual transmission [48] during this period. Zika virus can also be detected for a prolonged period in vaginal secretions [49] and, as such, has been in implicated in female-to-male sexual transmission [50]. The observation is supported by animal studies in which ZIKV has been shown to readily infect the vaginal mucosa of female mice [51, 52]. In addition to sexual transmission, ZIKV is also spread by arthropod vectors (such as Aedes aegypti) akin to flaviviruses (such as DENV and YFV) [53–60].
What makes ZIKV a neurotropic, sexually transmitted arboviral teratogen? The viral and host determinants that drive each of these interconnected aspects are poorly understood and are being aggressively pursued. To a large extent, tropism of the virus governs the nature of the disease pathology. For successful infection of a cell type, (1) the virus should be able to gain access to the target cells, (2) the cells should harbor the appropriate attachment factors/receptors to enable viral entry, (3) host proteins/factors that are required for viral life cycle should be present, and (4) the host immune response must be effectively counteracted. The holy grail in Zika research is to identify the unique bottlenecks circumvented by ZIKV that distinguish it from other closely related flaviviruses. Understanding the success strategy of ZIKV through these bottlenecks will provide mechanistic insights into the disease as well as unleash novel opportunities for intervention.
Clues from related neurotropic flaviviruses suggest that the E surface glycoprotein is an important determinant for disease. Glycosylation at Asn154 has been implicated in the neuroinvasiveness of WNV [61, 62]. Furthermore, mutations in the E protein of the JEV vaccine SA 14-14-2 strain were shown to be sufficient and required for attenuation of neurovirulence [63]. The E surface glycoproteins serve as ligands for attachment and entry factors located on the surface of host cells as well as molecular springs for inducing fusion of the viral and host membranes. They also serve as targets for neutralizing antibodies. What aspect of their function is compromised during attenuation is not completely understood. It is also unclear whether the regions/residues of E protein that are important for neurovirulence are conserved across neurotropic flaviviruses.
Several host molecules have been identified that could serve as attachment factors and/or receptors for flavivirus entry (see references [64, 65] for an exhaustive list). Glycosaminoglycans such as heparan sulfate has been suggested to serve as attachment factors for DENV [66] and ZIKV [67]. Basic residues Lys291 and Lys295 on domain III of the DENV E protein have been implicated in this interaction [68]. One of the most promising receptor/attachment factor for flaviviruses is the dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin (DC-SIGN) or CD209. This C-type (calcium dependent) lectin is expressed on macrophages and dendritic cells, which are the primary targets during DENV infection in vivo [69, 70] as well as targets for ZIKV infection [71]. The interaction has been shown by cryo-EM to be mediated by the glycans on Asn67 residues on domain II of 2 neighboring DENV E proteins and the carbohydrate recognition domain of DC-SIGN [72]. A homolog of DC-SIGN on endothelial cells of the liver and lymph nodes, called DC-SIGNR, L-SIGN, or CD209L, can also be used by flaviviruses to enter cells [70]. In addition, mannose receptor or CD206, another member of the C-type lectin family, was shown to function as a putative entry factor for DENV [73]. Glycans on E protein clearly play an important role during the entry process and possibly influence viral tropism. Flaviviruses can differ drastically in their surface carbohydrate content. The difference at the genotypic level is due to the presence or absence of glycosylation motifs in the surface proteins of different flaviviruses as well as the number and location of these motifs (see Table 1). At the phenotypic level, the diversity is imposed by the host environment. The carbohydrate processing machinery differs between organisms and cell types, and, accordingly, different producer cells yield viruses with distinct glycan composition. For instance, mosquito cells produced virions that have high mannose content, whereas mammalian cells raised virions that have a complex carbohydrate structure [74, 75]. The accessibility of the glycans to modifying enzymes can also influence their composition. Therefore, the glycan diversity on the virion surface translates to differential usage of lectin receptors by different flaviviruses [76, 77]. Furthermore, the carbohydrate landscape on flaviviruses can be influenced by the maturation state of the virus particle. This is because the pr peptide is glycosylated; the location and the number of sites varies across flaviviruses (Table 1). Therefore, the maturation and glycosylation of flaviviruses are intertwined. In fact, WNV strains with no glycosylation on E protein but a single glycosylation on prM (Asn15) can still bind to DC-SIGNR [77]. The lectin usage by ZIKV has not been investigated in detail. However, it has been shown that despite lacking glycosylation at Asn67, ZIKV grown in mosquito cells can utilize DC-SIGN [71]. The role of lectins in Zika infection in vivo needs to be evaluated.
Table 1.
Comparison of the Glycosylation Sites on prM and E Proteins of ZIKV to Other Mosquito-Transmitted Flaviviruses
| Virus | Strain | N-Linked Glycan Sites on prM | N-Linked Glycan Sites on E | GenBank Accession Code |
|---|---|---|---|---|
| ZIKV | H/PF/2013 | N70 | N154 | KJ776791 |
| DENV1 | SG/07K3640DK1/2008 | N69 | N67, N153 | GQ398255 |
| DENV2 | 16681 | N69 | N67, N153 | NC_001474 |
| DENV3 | SG/05K863DK1/2005 | N69 | N67, N153 | EU081190 |
| DENV4 | SG/06K2270DK1/2005 | N69 | N67, N153 | GQ398256 |
| WNV | NY99 | N15 | N154 | DQ211652 |
| WNV | Kunjin (MRM61C) | N15 | - | D00246 |
| JEV | SA14 | N15 | N154 | D90194 |
| YFV | Asibi | N13, N29, N51 | -a | AY640589 |
Abbreviations: E, envelope; N, aspargine; DENV, dengue virus; JEV, Japanese encephalitis virus; prM, premembrane; WNV, West Nile virus; YFV, yellow fever virus; ZIKV, Zika virus.
aPredicted motif at N309.
Phosphatidylserine (PS) receptors of the TIM (T-cell immunoglobulin and mucin domain) and TAM (Tyro3, Axl, and Mer) families are by far the most well characterized entry/attachment molecules in the context of ZIKV infection. These receptors are involved in sensing PS or “eat me” signal on the membranes of apoptotic cells, which triggers phagocytosis of these dying cells by immune cells. The TIM and TAMs also play a role in immune signaling via their cytoplasmic tail [78–80]. Dengue virus, WNV [81], and many other viruses seem to exploit these receptors for entry into cells by a process termed “apoptotic mimicry” [82]. These receptors are unique in that they bind to phospholipids (PS) on the surface of the virions instead of surface glycoproteins. The members of the TIM family (human TIM-1, TIM-3, and TIM-4) bind to lipids directly, whereas the members of the TAM receptor family (Tyro3, Axl, and Mertk) bind indirectly to lipids via ligands such as growth-arrest-specific protein 6 (Gas6) and protein S (ProS) ([82] and references within).
Binding of flaviviruses to PS receptors was an unexpected finding. This is because the structural studies suggest that the envelope glycoproteins cover the surface of the virion, and thus lipids are not readily accessible for binding to these receptors. However, the binding seen can be reconciled with the observation that the mature viruses are dynamic and may expose lipids on the surface, much like they expose cryptic epitopes that are captured by antibodies during viral breathing (as discussed in Structure of mature Zika virus section). In addition, structural studies suggest that immature viruses expose lipid bilayer on their surface. Due to inefficient maturation process, immature and partially immature viruses are known to be released by cells [26]. These mosaic particles could be potential targets for the PS receptors, and maturation may thus have a bearing in this interaction.
Binding to PS receptors has been shown to be influenced by the producer cells used to grow the viruses. Mammalian cell-produced WNV and DENV are unable to use Gas6-AXL as an entry (co)factor in the context of human umbilical vein endothelial cells (HUVECs), but the same viruses produced in insect cells can utilize this receptor. On the other hand, binding of ZIKV to Gas-6-AXL in HUVECs was not contingent on the source of these viruses [83]. How binding to PS receptors for different flaviviruses is influenced by the type of the receptor and source of the virus requires detailed analysis.
The localization of Axl on target cells that ZIKV infects [84], coupled with the observation that the entry of ZIKV is enhanced by the expression of Axl [71, 85] and is reduced by its ablation or treatment with antagonist or antibody specific for Axl [83, 86, 87], suggests a possible role of this molecule in the tropism of ZIKV. However, knockout of Axl in neural progenitor cells and organoids did not curb ZIKV infectivity or cell death [88]. Likewise, knockout of Axl (in combinations with other TAMs) in mice failed to prevent ZIKV pathology [89]. This could suggest that either Axl is not a bona fide entry receptor for ZIKV in the cells tested or there is functional redundancy in receptors. Axl is a receptor tyrosine kinase, and its activation suppresses type 1 interferon signaling [85, 90]. Given that Axl is a signaling molecule, it is likely that Axl plays multiple roles in ZIKV biology, some in favor and some against ZIKV. Support for this argument stems from work with WNV in which Mer and Axl knockout lowered replication in dendritic cells in vitro [91] but enhanced disease in vivo in a mouse model [92]. The TAM signaling was shown to be important for blood-brain barrier integrity; disruption of this signaling probably increased the neuroinvasiveness of WNV [92].
There is perhaps significant functional redundancy in ZIKV and flavivirurs receptors, which makes the identification of bona fide receptors extremely challenging. The αvβ3 integrin is a promising receptor lead for WNV and JEV [93], but the dependence on this molecule for WNV entry is not absolute [94]. Likewise, heat-shock protein (HSP)70 [95, 96] and HSP90 [97] and a tight junction protein, Claudin-1 [98, 99], have been suggested to assist in flavivirus entry. Claudin-1 is interesting in that it binds to the prM protein, and it highlights the expansive functional profile of this protein. Poorly neutralizing antibodies (against either E or prM) can also be used as surrogate receptors for flavivirus entry in Fc-receptor-bearing cells [100, 101]. The role of these antibodies in transplacental migration of ZIKV warrants further investigation.
Receptors are not the sole determinants of viral tropism. A permissive cell requires receptors as well as host factors that support viral replication and life cycle. Both targeted studies and large-scale CRISPR/Cas9 and RNAi screens [86] have also been carried out to accelerate the identification of crucial host molecules and receptors required for ZIKV infection, and these have revealed novel insights. Musashi-1 (MSI1) protein is a promising lead in this direction. The protein is heavily expressed in the neural progenitor cells, retina, and testis; it is required for neurodevelopment, and its depletion or mutation is associated with microcephaly. The MSI1 was shown to aid in the replication of ZIKV by binding to its 3’-untranslated region. Binding of this protein to the viral genome perhaps distracts its from its usual responsibilities in the cell that could lead to downstream neurodevelopmental deficits [102].
CONCLUSIONS
The last 2 years have been an exciting time for flavivirus research. Zika virus has awakened the world and the virology community to the fascinating biology behind this human pathogen. Bridging the gap between structure, function, and pathology is required for an integrated understanding of the ZIKV biology and will enhance the impact of therapeutic interventions.
Notes
Acknowledgments. We thank Thomas Klose and Matt D. Therkelsen for help with the production of images using the University of California, San Francisco (UCSF) Chimera package from the Computer Graphics Laboratory (UCSF). We thank Thomas Klose and Matthew D. Therkelsen (Purdue University) for technical help with the UCSF Chimera program. We also acknowledge Michael G. Rossmann for insights and discussions on flavivirus structure.
Financial support. This work was funded by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) (R01AI073755; to R. J. K.). Chimera package was used for creating molecular graphics of ZIKV. Chimera development by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco is funded by the NIH (NIGMS P41-GM103311).
Supplement sponsorship. This work is part of a supplement sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH).
Potential conflict of interest. Both authors: No reported conflicts of interest. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sirohi D, Chen Z, Sun L et al. The 3.8 Å resolution cryo-EM structure of Zika virus. Science 2016; 352:467–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kostyuchenko VA, Lim EX, Zhang S et al. Structure of the thermally stable Zika virus. Nature 2016; 533:425–8. [DOI] [PubMed] [Google Scholar]
- 3. Kuhn RJ, Zhang W, Rossmann MG et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 2002; 108:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang X, Ge P, Yu X et al. Cryo-EM structure of the mature dengue virus at 3.5-Å resolution. Nat Struct Mol Biol 2013; 20:105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kostyuchenko VA, Chew PL, Ng TS, Lok SM. Near-atomic resolution cryo-electron microscopic structure of dengue serotype 4 virus. J Virol 2014; 88:477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kostyuchenko VA, Zhang Q, Tan JL, Ng TS, Lok SM. Immature and mature dengue serotype 1 virus structures provide insight into the maturation process. J Virol 2013; 87:7700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fibriansah G, Tan JL, Smith SA et al. A highly potent human antibody neutralizes dengue virus serotype 3 by binding across three surface proteins. Nat Commun 2015; 6:6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mukhopadhyay S, Kim BS, Chipman PR, Rossmann MG, Kuhn RJ. Structure of West Nile virus. Science 2003; 302:248. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, Li SH, Zhu L et al. Near-atomic structure of Japanese encephalitis virus reveals critical determinants of virulence and stability. Nat Commun 2017; 8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goo L, Dowd KA, Smith ARY, Pelc RS, DeMaso CR, Pierson TC. Zika virus is not uniquely stable at physiological temperatures compared to other flaviviruses. MBio 2016; 7:e01396-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xie X, Yang Y, Muruato AE et al. Understanding Zika virus stability and developing a chimeric vaccine through functional analysis. MBio 2017; 8:e02134-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuhn RJ, Dowd KA, Beth Post C, Pierson TC. Shake, rattle, and roll: impact of the dynamics of flavivirus particles on their interactions with the host. Virology 2015; 479-480:508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dowd KA, Pierson TC. Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology 2011; 411:306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pierson TC, Diamond MS. Molecular mechanisms of antibody-mediated neutralisation of flavivirus infection. Expert Rev Mol Med 2008; 10:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pierson TC, Fremont DH, Kuhn RJ, Diamond MS. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe 2008; 4:229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lok SM, Kostyuchenko V, Nybakken GE et al. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nat Struct Mol Biol 2008; 15:312–7. [DOI] [PubMed] [Google Scholar]
- 17. Fibriansah G, Ng TS, Kostyuchenko VA et al. Structural changes in dengue virus when exposed to a temperature of 37°C. J Virol 2013; 87:7585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang X, Sheng J, Plevka P, Kuhn RJ, Diamond MS, Rossmann MG. Dengue structure differs at the temperatures of its human and mosquito hosts. Proc Natl Acad Sci U S A 2013; 110:6795–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rossignol ED, Peters KN, Connor JH, Bullitt E. Zika virus induced cellular remodelling. Cell Microbiol 2017; 19:e12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cortese M, Goellner S, Acosta EG et al. Ultrastructural characterization of Zika virus replication factories. Cell Rep 2017; 18:2113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Corver J, Chipman PR et al. Structures of immature flavivirus particles. EMBO J 2003; 22:2604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li L, Lok SM, Yu IM et al. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science 2008; 319:1830–4. [DOI] [PubMed] [Google Scholar]
- 23. Prasad VM, Miller AS, Klose T et al. Structure of the immature Zika virus at 9 Å resolution. Nat Struct Mol Biol 2017; 24:184–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu IM, Zhang W, Holdaway HA et al. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science 2008; 319:1834–7. [DOI] [PubMed] [Google Scholar]
- 25. Yu IM, Holdaway HA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. Association of the pr peptides with dengue virus at acidic pH blocks membrane fusion. J Virol 2009; 83:12101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Plevka P, Battisti AJ, Junjhon J et al. Maturation of flaviviruses starts from one or more icosahedrally independent nucleation centres. EMBO Rep 2011; 12:602–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Junjhon J, Edwards TJ, Utaipat U et al. Influence of pr-M cleavage on the heterogeneity of extracellular dengue virus particles. J Virol 2010; 84:8353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Junjhon J, Lausumpao M, Supasa S et al. Differential modulation of prM cleavage, extracellular particle distribution, and virus infectivity by conserved residues at nonfurin consensus positions of the dengue virus pr-M junction. J Virol 2008; 82:10776–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keelapang P, Sriburi R, Supasa S et al. Alterations of pr-M cleavage and virus export in pr-M junction chimeric dengue viruses. J Virol 2004; 78:2367–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nelson S, Jost CA, Xu Q et al. Maturation of West Nile virus modulates sensitivity to antibody-mediated neutralization. PLoS Pathog 2008; 4:e1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dejnirattisai W, Jumnainsong A, Onsirisakul N et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010; 328:745–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pierson TC, Diamond MS. Degrees of maturity: the complex structure and biology of flaviviruses. Curr Opin Virol 2012; 2:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dejnirattisai W, Wongwiwat W, Supasa S et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol 2015; 16:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dai L, Song J, Lu X et al. Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe 2016; 19:696–704. [DOI] [PubMed] [Google Scholar]
- 35. Barba-Spaeth G, Dejnirattisai W, Rouvinski A et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 2016; 536:48–53. [DOI] [PubMed] [Google Scholar]
- 36. Zhang S, Kostyuchenko VA, Ng TS et al. Neutralization mechanism of a highly potent antibody against Zika virus. Nat Commun 2016; 7:13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sapparapu G, Fernandez E, Kose N et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 2016; 540:443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hasan SS, Miller A, Sapparapu G et al. A human antibody against Zika virus crosslinks the E protein to prevent infection. Nat Commun 2017; 8:14722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miner JJ, Diamond MS. Zika virus pathogenesis and tissue tropism. Cell Host Microbe 2017; 21:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sarno M, Sacramento GA, Khouri R et al. Zika virus infection and stillbirths: a case of hydrops fetalis, hydranencephaly and fetal demise. PLoS Negl Trop Dis 2016; 10:e0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Coyne CB, Lazear HM. Zika virus - reigniting the TORCH. Nat Rev Microbiol 2016; 14:707–15. [DOI] [PubMed] [Google Scholar]
- 42. Ma W, Li S, Ma S et al. Zika virus causes testis damage and leads to male infertility in mice. Cell 2016; 167:1511–24.e10. [DOI] [PubMed] [Google Scholar]
- 43. Govero J, Esakky P, Scheaffer SM et al. Zika virus infection damages the testes in mice. Nature 2016; 540:438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sheng ZY, Gao N, Wang ZY et al. Sertoli cells are susceptible to ZIKV infection in mouse testis. Front Cell Infect Microbiol 2017; 7:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Uraki R, Hwang J, Jurado KA et al. Zika virus causes testicular atrophy. Sci Adv 2017; 3:e1602899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Matheron S, d’Ortenzio E, Leparc-Goffart I, Hubert B, de Lamballerie X, Yazdanpanah Y. Long-lasting persistence of Zika virus in Semen. Clin Infect Dis 2016; 63:1264. [DOI] [PubMed] [Google Scholar]
- 47. Foy BD, Kobylinski KC, Chilson Foy JL et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011; 17:880–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deckard DT, Chung WM, Brooks JT et al. Male-to-male sexual transmission of Zika virus–Texas, January 2016. MMWR Morb Mortal Wkly Rep 2016; 65:372–4. [DOI] [PubMed] [Google Scholar]
- 49. Murray KO, Gorchakov R, Carlson AR et al. Prolonged detection of Zika virus in vaginal secretions and whole blood. Emerg Infect Dis 2017; 23:99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus - New York City, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:716–7. [DOI] [PubMed] [Google Scholar]
- 51. Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. A mouse model of Zika virus sexual transmission and vaginal viral replication. Cell Rep 2016; 17:3091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yockey LJ, Varela L, Rakib T et al. Vaginal exposure to Zika virus during pregnancy leads to fetal brain infection. Cell 2016; 166:1247–56.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ciota AT, Bialosuknia SM, Zink SD et al. Effects of Zika virus strain and Aedes mosquito species on vector competence. Emerg Infect Dis 2017; 23:1110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Amraoui F, Atyame-Nten C, Vega-Rua A, Lourenco-de-Oliveira R, Vazeille M, Failloux AB. Culex mosquitoes are experimentally unable to transmit Zika virus. Euro Surveill 2016; 21. pii:30333. doi:10.2807/1560-7917.ES.2016.21.35.30333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hall-Mendelin S, Pyke AT, Moore PR et al. Assessment of local mosquito species incriminates Aedes aegypti as the potential vector of Zika virus in Australia. PLoS Negl Trop Dis 2016; 10:e0004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Richard V, Paoaafaite T, Cao-Lormeau VM. Vector competence of French Polynesian Aedes aegypti and Aedes polynesiensis for Zika virus. PLoS Negl Trop Dis 2016; 10:e0005024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thangamani S, Huang J, Hart CE, Guzman H, Tesh RB. Vertical transmission of Zika virus in Aedes aegypti mosquitoes. Am J Trop Med Hyg 2016; 95:1169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weger-Lucarelli J, Rückert C, Chotiwan N et al. Vector competence of American mosquitoes for three strains of Zika virus. PLoS Negl Trop Dis 2016; 10:e0005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Azar SR, Roundy CM, Rossi SL et al. Differential vector competency of Aedes albopictus populations from the Americas for Zika virus. Am J Trop Med Hyg 2017; 97:330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ferreira-de-Brito A, Ribeiro IP, Miranda RM et al. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Mem Inst Oswaldo Cruz 2016; 111:655–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shirato K, Miyoshi H, Goto A et al. Viral envelope protein glycosylation is a molecular determinant of the neuroinvasiveness of the New York strain of West Nile virus. J Gen Virol 2004; 85:3637–45. [DOI] [PubMed] [Google Scholar]
- 62. Beasley DW, Whiteman MC, Zhang S et al. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J Virol 2005; 79:8339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gromowski GD, Firestone CY, Whitehead SS. Genetic determinants of Japanese encephalitis virus vaccine strain SA14-14-2 that govern attenuation of virulence in mice. J Virol 2015; 89:6328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Perera-Lecoin M, Meertens L, Carnec X, Amara A. Flavivirus entry receptors: an update. Viruses 2013; 6:69–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cruz-Oliveira C, Freire JM, Conceição TM, Higa LM, Castanho MA, Da Poian AT. Receptors and routes of dengue virus entry into the host cells. FEMS Microbiol Rev 2015; 39:155–70. [DOI] [PubMed] [Google Scholar]
- 66. Chen Y, Maguire T, Hileman RE et al. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med 1997; 3:866–71. [DOI] [PubMed] [Google Scholar]
- 67. Kim SY, Zhao J, Liu X et al. Interaction of Zika virus envelope protein with glycosaminoglycans. Biochemistry 2017; 56:1151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Watterson D, Kobe B, Young PR. Residues in domain III of the dengue virus envelope glycoprotein involved in cell-surface glycosaminoglycan binding. J Gen Virol 2012; 93:72–82. [DOI] [PubMed] [Google Scholar]
- 69. Navarro-Sanchez E, Altmeyer R, Amara A et al. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep 2003; 4:723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tassaneetrithep B, Burgess TH, Granelli-Piperno A et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med 2003; 197:823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hamel R, Dejarnac O, Wichit S et al. Biology of Zika virus infection in human skin cells. J Virol 2015; 89:8880–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pokidysheva E, Zhang Y, Battisti AJ et al. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell 2006; 124:485–93. [DOI] [PubMed] [Google Scholar]
- 73. Miller JL, de Wet BJ, deWet BJ et al. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog 2008; 4:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jarvis DL. Developing baculovirus-insect cell expression systems for humanized recombinant glycoprotein production. Virology 2003; 310:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hsieh P, Robbins PW. Regulation of asparagine-linked oligosaccharide processing. Oligosaccharide processing in Aedes albopictus mosquito cells. J Biol Chem 1984; 259:2375–82. [PubMed] [Google Scholar]
- 76. Davis CW, Mattei LM, Nguyen HY, Ansarah-Sobrinho C, Doms RW, Pierson TC. The location of asparagine-linked glycans on West Nile virions controls their interactions with CD209 (dendritic cell-specific ICAM-3 grabbing nonintegrin). J Biol Chem 2006; 281:37183–94. [DOI] [PubMed] [Google Scholar]
- 77. Davis CW, Nguyen HY, Hanna SL, Sánchez MD, Doms RW, Pierson TC. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J Virol 2006; 80:1290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev 2010; 235:172–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer 2014; 14:769–85. [DOI] [PubMed] [Google Scholar]
- 80. Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res 2008; 100:35–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Meertens L, Carnec X, Lecoin MP et al. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe 2012; 12:544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Amara A, Mercer J. Viral apoptotic mimicry. Nat Rev Microbiol 2015; 13:461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Richard AS, Shim BS, Kwon YC et al. AXL-dependent infection of human fetal endothelial cells distinguishes Zika virus from other pathogenic flaviviruses. Proc Natl Acad Sci U S A 2017; 114:2024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. Expression analysis highlights AXL as a candidate zika virus entry receptor in neural stem cells. Cell Stem Cell 2016; 18:591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Meertens L, Labeau A, Dejarnac O et al. Axl mediates ZIKA virus entry in human glial cells and modulates innate immune responses. Cell Rep 2017; 18:324–33. [DOI] [PubMed] [Google Scholar]
- 86. Savidis G, McDougall WM, Meraner P et al. Identification of Zika virus and dengue virus dependency factors using functional genomics. Cell Rep 2016; 16:232–46. [DOI] [PubMed] [Google Scholar]
- 87. Retallack H, Di Lullo E, Arias C et al. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci U S A 2016; 113:14408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wells MF, Salick MR, Wiskow O et al. Genetic ablation of AXL does not protect human neural progenitor cells and cerebral organoids from Zika Virus infection. Cell Stem Cell 2016; 19:703–8. [DOI] [PubMed] [Google Scholar]
- 89. Hastings AK, Yockey LJ, Jagger BW et al. TAM receptors are not required for Zika virus infection in mice. Cell Rep 2017; 19:558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lemke G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol 2013; 5:a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bhattacharyya S, Zagorska A, Lew ED et al. Enveloped viruses disable innate immune responses in dendritic cells by direct activation of TAM receptors. Cell Host Microbe 2013; 14:136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Miner JJ, Daniels BP, Shrestha B et al. The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nat Med 2015; 21:1464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chu JJ, Ng ML. Interaction of West Nile virus with alpha v beta 3 integrin mediates virus entry into cells. J Biol Chem 2004; 279:54533–41. [DOI] [PubMed] [Google Scholar]
- 94. Medigeshi GR, Hirsch AJ, Streblow DN, Nikolich-Zugich J, Nelson JA. West Nile virus entry requires cholesterol-rich membrane microdomains and is independent of alphavbeta3 integrin. J Virol 2008; 82:5212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Das S, Laxminarayana SV, Chandra N, Ravi V, Desai A. Heat shock protein 70 on Neuro2a cells is a putative receptor for Japanese encephalitis virus. Virology 2009; 385:47–57. [DOI] [PubMed] [Google Scholar]
- 96. Thongtan T, Wikan N, Wintachai P et al. Characterization of putative Japanese encephalitis virus receptor molecules on microglial cells. J Med Virol 2012; 84:615–23. [DOI] [PubMed] [Google Scholar]
- 97. Reyes-Del Valle J, Chávez-Salinas S, Medina F, Del Angel RM. Heat shock protein 90 and heat shock protein 70 are components of dengue virus receptor complex in human cells. J Virol 2005; 79:4557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Che P, Tang H, Li Q. The interaction between claudin-1 and dengue viral prM/M protein for its entry. Virology 2013; 446:303–13. [DOI] [PubMed] [Google Scholar]
- 99. Gao F, Duan X, Lu X et al. Novel binding between pre-membrane protein and claudin-1 is required for efficient dengue virus entry. Biochem Biophys Res Commun 2010; 391:952–7. [DOI] [PubMed] [Google Scholar]
- 100. Halstead SB, Porterfield JS, O’Rourke EJ. Enhancement of dengue virus infection in monocytes by flavivirus antisera. Am J Trop Med Hyg 1980; 29:638–42. [DOI] [PubMed] [Google Scholar]
- 101. Halstead SB, Marchette NJ, Sung Chow JS, Lolekha S. Dengue virus replication enhancement in peripheral blood leukocytes from immune human beings. Proc Soc Exp Biol Med 1976; 151:136–9. [DOI] [PubMed] [Google Scholar]
- 102. Chavali PL, Stojic L, Meredith LW et al. Neurodevelopmental protein Musashi-1 interacts with the Zika genome and promotes viral replication. Science 2017; 357:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Liu W, Xie Y, Ma J et al. IBS: an illustrator for the presentation and visualization of biological sequences. Bioinformatics 2015; 31:3359–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pettersen EF, Goddard TD, Huang CC et al. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 2004; 25:1605–12. [DOI] [PubMed] [Google Scholar]