Summary
Persistent herpesviruses, including cytomegalovirus, herpes simplex virus type 1, and human herpesvirus type 6 were associated with greater telomere attrition over time in a sample of healthy adults aged 53–76 years.
Keywords: telomeres, herpes simplex virus, cytomegalovirus, Epstein-Barr virus, Whitehall II
Abstract
The determinants of telomere attrition, a potential marker of cellular aging, are not well understood. Persistent herpesvirus infections including cytomegalovirus (CMV) infection may be particularly important for telomere dynamics via mechanisms such as inflammation, oxidative stress, and their impact on peripheral blood lymphocyte composition. This study examined the association of 4 human herpesviruses (CMV, herpes simplex virus type 1, human herpesvirus type 6, and Epstein-Barr virus) with change in leukocyte telomere length (LTL) over 3 years in 400 healthy individuals (aged 53–76 years) from the Whitehall II cohort. CMV, herpes simplex virus type 1, and human herpesvirus 6 infection were independently associated with greater 3-year LTL attrition, with no association found for Epstein-Barr virus. The magnitudes of these associations were large, for example, the equivalent of almost 12 years of chronological age for those CMV seropositive. Seropositivity to more herpesviruses was additively associated with greater LTL attrition (3 herpesviruses vs none, β = −0.07 and P = .02; 4 infections vs none, β = −0.14 and P < .001). Higher immunoglobulin G antibody levels among those seropositive to CMV were also associated with shorter LTL at follow-up. These associations were robust to adjustment for age, sex, employment grade, body mass index, and smoking status. These results suggest that exposure to infectious agents should be an important consideration in future studies of telomere dynamics.
(See the editorial commentary by Griffiths, on pages 511–3.)
The length of telomeres, the DNA-protein structures that cap and stabilize the physical ends of chromosomes, has been proposed as a marker of cellular aging [1, 2]. In most cells these telomeres shorten with each round of cell division, with critically short telomeres leading to cellular senescence and genomic instability [1, 3]. Shortened leukocyte telomere length (LTL) is associated with all-cause mortality and progression of age-related diseases—in particular, cardiovascular disease, cancer, diabetes, and dementia [4, 5]. Shorter telomere length may also play a role in clinically important age-related declines in immune function, as signified by the reduced lymphocyte proliferative capacity and associated impaired response to vaccines and acute infections [6, 7].
Although overall telomere shortening with age is well established, there is large variation in LTL among individuals of the same chronological age, with age accounting for only 10% or less of interindividual LTL variation [8]. Although environmental factors such as diet, obesity, and smoking have been associated with shorter LTL, these findings have been inconsistent across studies [9, 10]. Additional exposures underlying interindividual variation in LTL are not well characterized but may include infection history, particularly chronic viral infections [11–13]. Herpesviruses, such as cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus (HSV) type 1, and human herpesvirus (HHV) 6, are commonly acquired in childhood or early adulthood [14].
In most cases, the host is generally unable to eradicate these infections and the virus remains in a quiescent (ie, latent) state in the host’s cells, with intermittent phases of reactivation. The containment of herpesvirus replication takes up considerable immune resources, with CMV in particular seeming to be immunodominant, becoming the primary target of 10%–30% of all circulating CD4 T lymphocytes and up to 50% of all circulating CD8 T lymphocytes in CMV infected elderly individuals [15, 16]. These CMV-specific T cells contribute to large shifts in the cellular composition of peripheral blood, including a large increase in the number of so-called “effector-memory” T lymphocytes that are characterized by shorter telomeres [12, 17].
Such dramatic expansion of T cells that exhibit short telomeres is not seen for EBV and has not been well investigated for other herpesviruses [18–20]. However, there is some evidence that EBV-CMV coinfection may influence the human T-cell repertoire beyond the impact of each individual infection [19, 21]. The potential impact of other herpesviruses on peripheral blood LTL is less well explored, though it is known that HHV-6 achieves latency through integration with human telomeres and may lead to dysfunction of the enzyme telomerase, which is responsible for elongating telomeres, with clinical implications for stem cell transplants [22, 23]. Herpesviruses infections may contribute to telomere attrition through pathways other than repeated proliferation and subset differentiation, such as by stimulating inflammation and oxidative stress processes [24, 25].
To our knowledge, no prospective studies have examined the association between CMV or other herpesviruses and changes in LTL. Previous cross-sectional analyses in the current study population found an association between CMV infection and lower telomerase activity among women [26], but no association between CMV infection and cross-sectional LTL. This cross-sectional association with telomerase activity suggested potential longer-term impacts of CMV on telomere dynamics that could be explored only with data on telomere change over time [26]. Longitudinal studies of telomere change are also important owing to the large genetic component of LTL [27], which implies that cross-sectional associations with shorter LTL could reflect predispositions to risk factors and disease rather than causal associations [28].
The current study evaluated the association of seropositivity to 4 herpesviruses (CMV, HSV-1, HHV-6, and EBV) at baseline and change in LTL over 3 years of follow-up. The analyses considered the contribution of serological evidence of infections with the individual herpesviruses as well as their combination in relation to LTL change. Associations between LTL and immunoglobulin (Ig) G antibody levels to each herpesvirus were also examined, given evidence of positive associations between higher proportions of (short-telomere) peripheral blood lymphocytes [29] and previous cross-sectional associations between higher CMV IgG and lower telomerase activity in this sample [26].
MATERIALS AND METHODS
Study Sample
Participants were from the Heart Scan subsample of the Whitehall II epidemiological cohort, recruited during 2006–2008, to investigate the psychosocial, demographic and biological risk factors for coronary heart disease [30]. Participants were aged 53–76 years at baseline and were screened to ensure that they had no history or objective signs of coronary heart disease and no previous diagnosis or treatment for hypertension, diabetes, inflammatory diseases or allergies. Volunteers were of white European origin, and 56.5% were in full-time employment. Socioeconomic status (SES) was defined by current (or most recent) grade of employment within the British civil service, and selection into the study was stratified to include representation of higher-, intermediate-, and lower-grade employment groups. Participants were invited for reassessment after 3 years (mean interval, 1087 days). Measurement of LTL did not commence at the beginning of the study; therefore, only 434 participants had LTL data at baseline, and a total of 400 had complete LTL data at both time points and infection data at baseline. Ethical approval was obtained from the University College London Hospital Committee on the Ethics of Human Research, and all participants gave signed informed consent.
Isolation of Peripheral Blood Mononuclear Cells
To assess LTL at baseline and at follow-up, we used an adaptation of the method first described by Cawthon [31]. Genomic DNA was extracted from peripheral blood mononuclear cells in a QIAcube workstation (baseline) or manually (follow-up) with the QIAamp DNA blood mini kit (Qiagen), according to the manufacturer’s instructions, and stored in 10 mmol/L Tris-hydrochloride and 0.5 mmol/L ethylenediamine tetraacetate, (pH 9.0) at −20°C (baseline) or −80oC (follow-up). Relative mean LTL was measured with a monochrome multiplex quantitative real-time polymerase chain reaction (PCR) assay (Bio-Rad CFX96 Real-Time PCR Detection System; Bio-Rad) for samples obtained at baseline, and with a Roche Lightcycler 480 real-time PCR machine (Roche Diagnostics) at follow-up. Reactions containing serial dilutions of a reference DNA standard were included in each PCR plate to generate the telomere (T) and single copy gene (S) standard curves required for quantitation, and the relative mean LTL, expressed as a T/S ratio, was derived. The coefficient of variation of these assays was 2.3%. Absolute measures of LTL can vary across laboratories, but rankings of relative length are highly correlated [32]. We therefore computed standardized LTL z scores for the baseline and follow-up samples as a robustness check; statistical results were identical to those for absolute values, so the latter are presented.
CMV, HSV-1, EBV (EBV-EBNA), and HHV-6 IgG antibody titers were measured from thawed serum samples using a solid-phase enzyme immunoassay system, as described elsewhere [33]. Briefly, diluted aliquots of serum were reacted with the specific antigen bound to a solid-phase surface. After the addition of enzyme-linked anti-human IgG and enzyme substrate, IgG antibody titers were quantified based on the amount of color generated from the reaction of antigen-bound enzyme and soluble substrate, measured as optical densities read by a spectrophotometer. In each assay run, a standard sample was used, and the results were expressed as a ratio between the amount of color generated by the test sample and the amount generated by the standard. A sample was categorized as seropositive if the optical density ratio was >1.2.
Additional Covariates
We adjusted for several potential confounders, factors potentially associated with both infections and LTL. Employment grade was used as an indicator of SES; participants were classified according to their current or most recent civil service grade at baseline into lower (administrative assistant, administrative officer, or executive officer), intermediate (higher or senior executive officer) and higher (grades 7 to 1) SES. Smoking status at baseline was assessed by questionnaire and categorized as current, former, or never. Body mass index (BMI) at baseline, based on measured height and weight and expressed in kilograms per square meter, was categorized as normal weight (18.5–24.9), overweight (25.0–29.9), or obese (≥30).
Statistical Analysis
Associations between serostatus for each individual infection at baseline, the total number of infections (0–4), and LTL measured prospectively were analyzed using separate multivariable regression models. LTL at follow-up was the dependent variable, with age, sex, grade of employment, smoking status, BMI, and LTL at baseline included as controls. Additional models using change in LTL between periods (time 2 [follow-up] minus time 1 [baseline]) were also analyzed, with very similar results (available on request).
RESULTS
Herpesvirus Seropositivity and Leukocyte Telomere Length
The mean T/S ratio was 0.992 (standard deviations [SDs], 0.07) at baseline, and 0.897 (0.15) at follow-up. This indicates a significant decrease in T/S ratio among the study population over the 3-year follow-up period (P < .001). LTL at follow-up was inversely associated with age (P < .001) and was greater in women than in men (P < .001). At baseline, 47.25% of the sample were seropositive for CMV, 21% for HSV-1, 59% for HHV-6, and 63.25% for EBV (Table 1).
Table 1.
Descriptive Statistics in 400 Study Participants
| Variable | Participants, %a |
|---|---|
| LTL (T/S ratio), mean (SD) | |
| Time 2 | 0.897 (0.15) |
| Time 1 | 0.992 (0.07) |
| Age (baseline) | 63.413 (5.63) |
| Female sex | 53.25 |
| Pathogen seroprevalence | |
| CMV | 47.25 |
| HSV-1 | 21.00 |
| HHV-6 | 59.00 |
| EBV | 63.25 |
| Total pathogen burden | |
| 0 | 7.75 |
| 1 | 28.25 |
| 2 | 36.25 |
| 3 | 21.25 |
| 4 | 6.50 |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV, human herpesvirus; HSV, herpes simplex virus; SD, standard deviation; LTL, leukocyte telomere length; T/S ratio, telomere to single copy gene ratio.
aData represent percentage of participants unless otherwise specified.
Serological evidence of CMV infection was associated with shorter LTL at follow-up (β = −0.061 [standard error (SE), 0.014]; P < .001), which was also true for HSV-1 (β = −0.049 [0.016]; P = .002), and HHV-6 (β = −0.033 [0.015]; P = .02). adjusting for baseline age, sex, smoking, employment grade, BMI, and LTL (Table 2, model 1). Because associations for individual infections may be confounded by exposure to other herpesviruses, model 2 mutually adjusted for all infections. The coefficient estimates were virtually unchanged, but the association with HHV-6 lost statistical significance at conventional levels (P = .06). The coefficient for EBV infection was close to 0 in both models. The other independent predictors of shorter telomeres at follow-up were older age, male sex, and shorter LTL at baseline. Compared with these factors, the magnitude of the coefficient for CMV seropositivity was substantial, roughly equivalent to the coefficient for female versus male sex (β = −0.062 [SE, 0.014]; P < .001), or the equivalent of 11.8 years of additional age (βCMV/βAge). Smoking status, BMI, and employment grade were not significant predictors of LTL at follow-up in any model.
Table 2.
Herpesvirus Infections and LTL in Whitehall Heart Scan Study (N = 400)
| Whitehall Heart Scan Study | LTL (T/S Ratio (Follow-up) | |||
|---|---|---|---|---|
| Model 1a | Model 2a | |||
| β (SE) | P Value | β (SE) | P Value | |
| Pathogen present | ||||
| CMV | −0.061 (0.014) | <.001 | −0.059 (0.014) | <.001 |
| HSV-1 | −0.049 (0.016) | .002 | −0.047 (0.017) | <.006 |
| HHV-6 | −0.033 (0.015) | .02 | −0.027 (0.014) | .06 |
| EBV | 0.001 (0.015) | .95 | 0.009 (0.015) | .52 |
| Covariate | ||||
| Age | … | … | −0.005 (0.001) | <.001 |
| Female sex | … | … | 0.062 (0.014) | <.001 |
| LTL at baseline | … | … | 0.523 (0.093) | <.001 |
| Current vs never smoking | … | … | −0.007 (0.031) | .83 |
| Obese vs normal weight | … | … | −0.021 (0.021) | .31 |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV, human herpesvirus; HSV, herpes simplex virus; SE, standard error; LTL, leukocyte telomere length; T/S ratio, telomere to single copy gene ratio.
aAll models were adjusted for age, sex, smoking, employment grade, categorical body mass index, and baseline LTL. Model 1 shows coefficients for separate regressions with individual infections. In model 2, coefficients are mutually adjusted for the other infections.
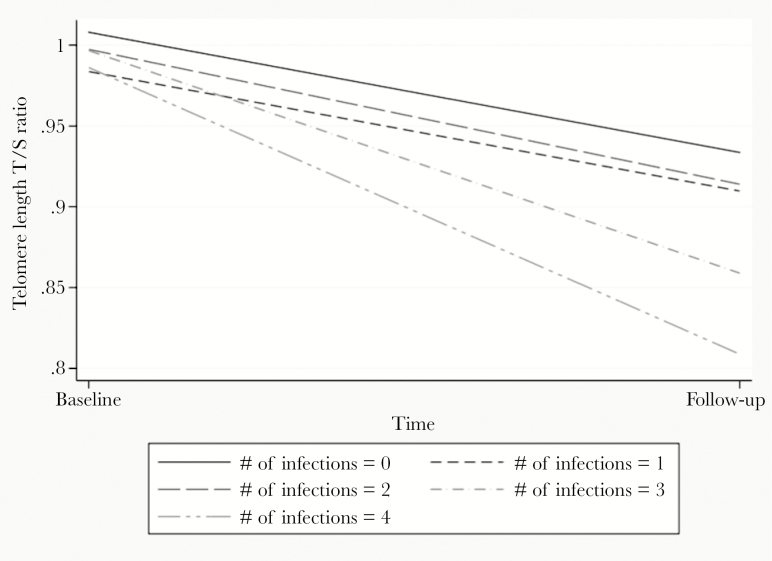
Serological evidence of exposure to multiple herpesviruses was also significantly associated with shorter LTL at follow-up (Table 3). Seropositivity for 3 herpesviruses versus none was associated with a decrease in LTL (β = −0.069 [SE, 0.029]; P = .02), with a doubling of this effect size for 4 infections compared with none (β = −0.139 [0.037]; P < .001). Figure 1 illustrates the pattern of mean change in LTL across the 2 time periods by the total number of infections for which an individual was seropositive.
Table 3.
Pathogen Burden, Coinfection, and LTL
| Whitehall Heart Scan Study | LTL (T/S Ratio (Follow-up) | |
|---|---|---|
| β (SE)a | P Valueb | |
| Total pathogen burden (0–4 pathogens) | ||
| 0 (n = 31) | Reference | … |
| 1 (n = 113) | −0.014 (0.028) | .62 |
| 2 (n = 145) | −0.025 (0.027) | .36 |
| 3 (n = 85) | −0.069 (0.029) | .02 |
| 4 (n = 26) | −0.139 (0.037) | <.001 |
| Pathogen combination | ||
| HSV−/CMV− (43.00%) | Reference | … |
| HSV+/CMV− (9.75%) | −0.030 (0.024) | .68 |
| HSV−/CMV+ (36.00%) | −0.050 (0.015) | .01 |
| HSV+/CMV+ (12.25%) | −0.118 (0.023) | <.001 |
| HHV-6−/CMV− (22.50%) | Reference | … |
| HHV-6+/CMV− (30.25%) | −0.008 (0.018) | .68 |
| HHV-6−/CMV+ (18.50%) | −0.041 (0.021) | .58 |
| HHV-6+/CMV+ (28.75%) | −0.082 (0.019) | <.001 |
| EBV−/CMV− (21.50%) | Reference | … |
| EBV+/CMV− (31.25%) | 0.009 (0.018) | .68 |
| EBV−/CMV+ (15.25%) | −0.066 (0.022) | .04 |
| EBV+/CMV+ (32.00%) | −0.051 (0.018) | .06 |
| EBV−/HSV− (30.00%) | Reference | … |
| EBV+/HSV− (49.00%) | 0.012 (0.016) | .68 |
| EBV−/HSV+ (6.75%) | −0.027 (0.029) | .68 |
| EBV+/HSV+ (14.25%) | −0.048 (0.021) | .24 |
| HHV-6−/EBV− (15.50%) | Reference | … |
| HHV-6+/EBV− (21.25%) | −0.010 (0.023) | .68 |
| HHV-6−/EBV+ (25.50%) | 0.019 (0.022) | .68 |
| HHV-6+/EBV+ (37.75%) | −0.020 (0.021) | .68 |
| HHV-6−/HSV− (35.00%) | Reference | … |
| HHV-6+/HSV− (44.00%) | −0.015 (0.016) | .68 |
| HHV-6−/HSV+ (6.00%) | −0.026 (0.031) | .68 |
| HHV-6+/HSV+ (15.00%) | −0.069 (0.020) | .02 |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV, human herpesvirus; HSV, herpes simplex virus; SE, standard error; LTL, leukocyte telomere length; T/S ratio, telomere to single copy gene ratio.
aAll models are adjusted for age, sex, smoking, employment grade, and LTL at baseline.
bFor pathogen combinations, P values are adjusted for Benjamini-Hochberg false discovery rate.
Figure 1.
Number of herpesvirus infections at baseline and mean leukocyte telomere length (LTL) change (telomere [T]/single copy gene [S] ratio) over 3 years.
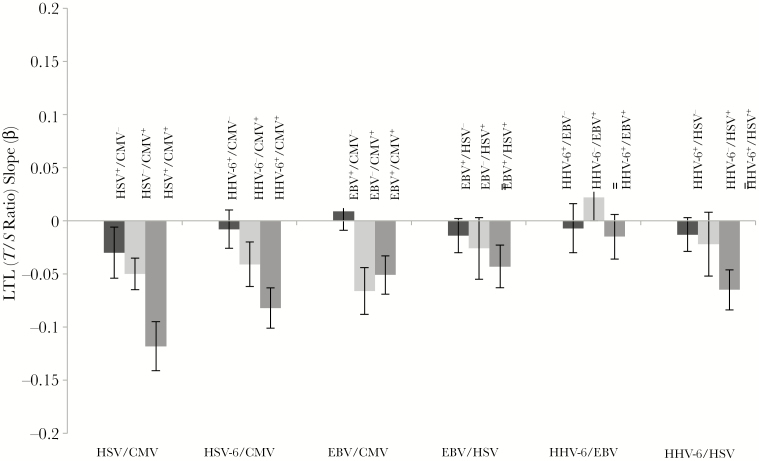
To explore whether one particular herpesvirus, such as CMV, was primarily driving these results, we explored all combinations of infection pairs and their association with LTL, illustrated in Table 3 and Figure 2. The Benjamini-Hochberg procedure was used to adjust for multiple comparisons/false discovery rate, with adjusted P values shown in Table 3 [34]. Overall, there was evidence of an important role for CMV infection and some interaction effects with other herpesviruses. Coinfection with both HSV-1 and CMV showed the strongest association with shorter LTL, suggesting an interactive effect of seropositivity for both infections compared with only one. Coinfection with HHV-6 and CMV also showed a stronger association than either infection by itself. In both of these cases, exposure to HHV-6 and HSV-1 among those who were CMV seronegative was not independently associated with shorter telomeres, but their presence amplified the association with LTL among those who were CMV seropositive. This interaction was not seen for CMV and EBV. Table 3 also shows the remainder of coinfection pair-wise combinations, with some evidence for the importance of HHV-6 coinfection for the association between HSV-1 and LTL.
Figure 2.
Association of herpesvirus coinfections at baseline and leukocyte telomere length (LTL; telomere [T]/single copy gene [S] ratio) at follow-up (β coefficient; linear regression model adjusted for age, sex, and LTL at baseline). Error bars indicate 95% confidence intervals. Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV-6, human herpesvirus type 6; HSV, herpes simplex virus.
IgG Antibody Response and LTL
Because higher CMV IgG antibody has previously been associated with reduced telomerase activity, we also examined LTL with respect to continuous IgG immune response among those who were CMV seropositive (n = 189). Higher CMV IgG antibody levels (mean, 3.01 OD; SD, 0.77) were significantly associated with greater LTL attrition (β = −0.029 [SE, 0.013]; P = .03). Higher EBV IgG antibody levels among those seropositive was also associated with shorter LTL (β = −0.023 [0.009]; P = .02). No significant associations were found for HSV-1 (β = 0.085 [0.075]; P = .26) or HHV-6 (β = −0.019 [0.013]; P = .16) IgG levels.
Sensitivity Analysis
Because of previous findings of associations between CMV and telomerase activity in women only, we tested for interactions between sex and each infection, as well as burden of infection. No significant sex-infection interactions were found. Because inflammation is a hypothesized mechanism through which infections may affect telomere shortening, we also tested models adjusting for available markers of inflammation (serum C-reactive protein, interleukin 6, and fibrinogen, measured at baseline). None of these inflammatory markers were associated with LTL shortening, and thus they did not mediate the associations between infections and LTL.
DISCUSSION
Although LTL is correlated with many important aging-related outcomes, its determinants are not well understood. Persistent viral infections may be an important determinant of individual variation in LTL, but have not been extensively studied in vivo [11, 12]. This study was the first to test the association of multiple highly prevalent persistent herpesvirus infections with prospective LTL in humans. We found decreases in LTL associated with infections of a substantively important magnitude—the equivalent of almost half a SD in LTL for those CMV positive versus CMV negative, and a full SD in LTL for those infected with all 4 herpesviruses versus none. These findings suggest an important role for these infections for understanding LTL dynamics.
There are several reasons why CMV, more than other pathogens, may contribute to telomere shortening. CMV infection is thought to be associated with a continuous low-grade reactivation, causing a highly characteristic differentiation among T cells that yields a robust expansion of T cells that down-regulate telomerase and exhibit short telomeres [35]. A study of 159 healthy volunteers aged 20–95 years found that CMV seropositivity amplified the association of increased age with shorter telomeres in T-cell populations, particularly in the lymphocytes of those aged ≥60 years. The causality of this association was confirmed in the same study, showing that primary CMV infection among 19 renal transplant recipients coincided with a steep drop in lymphocyte LTL. This drop was related to the change in the T-cell subset distribution toward a differentiated low LTL phenotype of T cells in both CD4 and CD8 T cells [11]. Thus, the LTL decreases associated with CMV infection may driven in part be by a change in the cellular composition of the peripheral blood, the main cellular source of LTL determination, and not a direct cellular effect of telomere attrition.
The association between CMV and LTL shortening may also involve endocrine pathways. Recent results from this sample found that greater cortisol responsivity to acute stressors predicted more rapid telomere attrition [36]. CMV can infect and replicate in human adrenocortical cells, thereby triggering steroidogenesis [37], which in turn has been shown to inhibit telomerase activity in CD4 and CD8 T cells [38]. Steptoe et al [39] previously reported an altered diurnal cortisol slope in healthy CMV-positive individuals, in which those who are infected exhibited a flatter decline over the day than those who are not infected. Such a flattened secretion pattern of cortisol has repeatedly been associated with impaired immunity and enhanced inflammatory activity [40, 41], which, in turn, may further promote elevated viral reactivation. Indeed, studies have suggested that cells exposed to glucocorticoids increased replication of HSV and CMV in vitro [42].
The mechanisms underlying the observed associations of HSV-1 and HHV-6 and telomere shortening are not known and should be replicated and explored in future work. Likewise, the synergistic effects seen for CMV coinfection with HSV-1 and HHV-6 merit additional study. The lack of an association of EBV with shorter LTL, in contrast to the other herpesvirus infections, is consistent with fundamental differences in how these viruses modulate the host immune system, with dramatic expansion of T cells with short telomeres not seen in EBV as in CMV [18–20].
A major strength of this study is that our findings come from a well-characterized longitudinal population cohort, in contrast to the previous small clinical sample used to explore CMV and lymphocyte LTL [11]. The prospective assessment of LTL over 3 years and data on the serostatus to multiple herpesviruses at baseline is also novel. Our findings identify for the first time independent associations between LTL and both HSV-1 and HHV-6. Previous cross-sectional work showed that CMV seropositivity was associated with lower levels of telomerase in women [26], but this did not seem to generalize to shorter LTL in that sample. The present study indicated that despite the lack of association of CMV with baseline LTL, both CMV seropositivity and CMV IgG antibody response were associated with prospective change in LTL. Future work should test whether CMV plays a role, at least in part, in the association between LTL and the onset of chronic disease, especially vascular disease. Results from a small clinical study found that, among patients with coronary heart disease, LTL in CD8 cytotoxic T cells was shorter in CMV-positive than in CMV-negative patients, and this LTL shortening was correlated with a decrease in left ventricular function, suggesting a role for CMV in the coevolution of coronary heart disease and immunosenescence [43].
Our findings of an association between higher CMV IgG and shorter telomeres warrants further investigation, particularly given epidemiological evidence that higher CMV IgG is associated with increased risk of mortality [44], weaker vaccine response [29], and lower levels of psychological well-being [45]. Strong and consistent associations have also been observed between stress and increased herpesvirus IgG [46]. Given the evidence that chronic stress contributes to shorter telomeres [47], our results could have implications for understanding the pathways through which psychosocial factors affect cellular aging. The role of stress-related reactivation of herpesviruses as a mediator of stress and telomere dynamics should be further explored.
Our study has several limitations. LTL was measured in peripheral blood mononuclear cells, and values may differ in lymphocyte subpopulations. Future flow cytometric analysis of cell subpopulations would allow better mechanistic understanding of how infections decrease LTL. Measures were also made with 2 different PCR machines at baseline and follow-up, but though this may affect comparisons of absolute values, it did not affect the relative results shown here, as indicated by identical findings with standardized measures of LTL. Furthermore, our results are virtually identical when we use only follow-up LTL data, providing further assurance that changes in LTL measurement methods are not driving our results. Because IgG antibodies reflect any past exposure, it is not known when these herpesviruses were acquired, though it is likely that many were acquired early in life, given the concentration of risk factors for early exposure to these infections [48]. Furthermore, although shorter LTL has been shown to increase the risk of experimentally induced upper respiratory tract infections in younger adults [7], the we infections analyzed were not associated with LTL at baseline, reducing the risk that reverse causality from shorter LTL to increased risk of infection was driving the observed associations.
In conclusion, our results suggest large and significant associations between highly prevalent persistent herpesvirus infections and telomere shortening in humans. The magnitude of the association for CMV seropositivity was equal to almost half an SD in LTL, or the equivalent of 12 years of chronological age as estimated in this sample. In contrast, previously identified risk factors for cross-sectional LTL, including smoking, obesity, SES, and inflammatory markers, were not associated with LTL attrition in this sample, suggesting that infection history may be a more robust predictor of LTL than these prior commonly identified risk factors. The strong associations seen for CMV and LTL may be clinically important and are consistent with evidence that CMV is associated with increased mortality risk in the overall population [49, 50]. We encourage additional research to replicate these findings and continue to advance our understanding of the infectious determinants of cellular aging. Such knowledge could improve primary prevention and potentially reduce telomere-related chronic disease and accelerated aging.
Notes
Acknowledgments. We gratefully acknowledge Jorge Erusalimsky and Lee Butcher from Cardiff Metropolitan University for analyzing baseline LTL and the Stanley Neurovirology Laboratory, Johns Hopkins University School of Medicine, for performing the herpesvirus infection assays.
Funding Statement. This work was supported by the National Institutes of Health (grant 1R01AG040115 to J. B. D. and A. E. A.). The Heart Scan study was supported by the British Heart Foundation and Medical Research Council, United Kingdom.
Potential conflict of interest. J. L. is cofounder of Telome Health, a diagnostic company measuring telomere biology. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med 2006; 12:1133–8. [DOI] [PubMed] [Google Scholar]
- 2. Gabriele S, Thomas Z. Replicative aging, telomeres, and oxidative stress. Ann N Y Acad Sci 2002; 959:24–9. [DOI] [PubMed] [Google Scholar]
- 3. Donate LE, Blasco MA. Telomeres in cancer and ageing. Philos Trans R Soc Lond B Biol Sci 2011; 366:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bojesen SE. Telomeres and human health. J Intern Med 2013; 274:399–413. [DOI] [PubMed] [Google Scholar]
- 5. Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003; 361:393–5. [DOI] [PubMed] [Google Scholar]
- 6. Najarro K, Nguyen H, Chen G et al. Telomere length as an indicator of the robustness of B- and T-cell response to influenza in older adults. J Infect Dis 2015; 212:1261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen S, Janicki-Deverts D, Turner RB et al. Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. JAMA 2013; 309:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iwama H, Ohyashiki K, Ohyashiki JH et al. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Hum Genet 1998; 102:397–402. [DOI] [PubMed] [Google Scholar]
- 9. Cassidy A, De Vivo I, Liu Y et al. Associations between diet, lifestyle factors, and telomere length in women. Am J Clin Nutr 2010; 91:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin J, Epel E, Blackburn E. Telomeres and lifestyle factors: roles in cellular aging. Mutat Res 2012; 730:85–9. [DOI] [PubMed] [Google Scholar]
- 11. van de Berg PJEJ, Griffiths SJ, Yong SL et al. Cytomegalovirus infection reduces telomere length of the circulating T cell pool. J Immunol 2010; 184:3417–23. [DOI] [PubMed] [Google Scholar]
- 12. Effros RB. Telomere/telomerase dynamics within the human immune system: effect of chronic infection and stress. Exp Gerontol 2011; 46:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ilmonen P, Kotrschal A, Penn DJ. Telomere attrition due to infection. PLoS One 2008; 3:e2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delaney AS, Thomas W, Balfour HH Jr. Coprevalence of Epstein-Barr virus, cytomegalovirus, and herpes simplex virus type-1 antibodies among United States children and factors associated with their acquisition. J Pediatric Infect Dis Soc 2015; 4:323–9. [DOI] [PubMed] [Google Scholar]
- 15. Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, Moss PA. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol 2007; 81:7759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sylwester AW, Mitchell BL, Edgar JB et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 2005; 202:673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol 2004; 25:406–10. [DOI] [PubMed] [Google Scholar]
- 18. Luz Correa B, Ornaghi AP, Cerutti Muller G et al. The inverted CD4:CD8 ratio is associated with cytomegalovirus, poor cognitive and functional states in older adults. Neuroimmunomodulation 2014; 21:206–12. [DOI] [PubMed] [Google Scholar]
- 19. Wikby A, Ferguson F, Forsey R et al. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci 2005; 60:556–65. [DOI] [PubMed] [Google Scholar]
- 20. Khan N, Hislop A, Gudgeon N et al. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol 2004; 173:7481–9. [DOI] [PubMed] [Google Scholar]
- 21. van den Heuvel D, Jansen MA, Dik WA et al. Cytomegalovirus- and Epstein-Barr virus-induced T-cell expansions in young children do not impair naive T-cell populations or vaccination responses: the Generation R Study. J Infect Dis 2016; 213:233–42. [DOI] [PubMed] [Google Scholar]
- 22. Arbuckle JH, Medveczky MM, Luka J et al. The latent human herpesvirus-6A genome specifically integrates in telomeres of human chromosomes in vivo and in vitro. Proc Natl Acad Sci U S A 2010; 107:5563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clark DA, Nacheva EP, Leong HN et al. Transmission of integrated human herpesvirus 6 through stem cell transplantation: implications for laboratory diagnosis. J Infect Dis 2006; 193:912–6. [DOI] [PubMed] [Google Scholar]
- 24. O’Donovan A, Pantell MS, Puterman E et al. ; Health Aging and Body Composition Study Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS One 2011; 6:e19687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wong JY, De Vivo I, Lin X, Fang SC, Christiani DC. The relationship between inflammatory biomarkers and telomere length in an occupational prospective cohort study. PLoS One 2014; 9:e87348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dowd JB, Bosch JA, Steptoe A et al. Cytomegalovirus is associated with reduced telomerase activity in the Whitehall II cohort. Exp Gerontol 2013; 48:385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Broer L, Codd V, Nyholt DR et al. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet 2013; 21:1163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holohan B, De Meyer T, Batten K et al. Decreasing initial telomere length in humans intergenerationally understates age-associated telomere shortening. Aging Cell 2015; 14:669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turner JE, Campbell JP, Edwards KM et al. Rudimentary signs of immunosenescence in cytomegalovirus-seropositive healthy young adults. Age (Dordr) 2014; 36:287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steptoe A, Hamer M, O’Donnell K, Venuraju S, Marmot MG, Lahiri A. Socioeconomic status and subclinical coronary disease in the Whitehall II epidemiological study. PLoS One 2010; 5:e8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 2009; 37:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martin-Ruiz CM, Baird D, Roger L et al. Reproducibility of telomere length assessment: an international collaborative study. Int J Epidemiol 2015; 44:1673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry 2001; 58:1032–7. [DOI] [PubMed] [Google Scholar]
- 34. Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol 2014; 67:850–7. [DOI] [PubMed] [Google Scholar]
- 35. Dock JN, Effros RB. Role of CD8 T cell replicative senescence in human aging and in HIV-mediated immunosenescence. Aging Dis 2011; 2:382–97. [PMC free article] [PubMed] [Google Scholar]
- 36. Steptoe A, Hamer M, Lin J, Blackburn EH, Erusalimsky JD. The longitudinal relationship between cortisol responses to mental stress and leukocyte telomere attrition. J Clin Endocrinol Metab 2017;102:962–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trevisan M, Matkovic U, Cusinato R, Toppo S, Palù G, Barzon L. Human cytomegalovirus productively infects adrenocortical cells and induces an early cortisol response. J Cell Physiol 2009; 221:629–41. [DOI] [PubMed] [Google Scholar]
- 38. Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun 2008; 22:600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steptoe A, Gylfe A, Shamaei-Tousi A, Bergstrom S, Henderson B. Pathogen burden and cortisol profiles over the day. Epidemiol Infect 2009; 137:1816–24. [DOI] [PubMed] [Google Scholar]
- 40. Edwards KM, Bosch JA, Engeland CG, Cacioppo JT, Marucha PT. Elevated macrophage migration inhibitory factor (MIF) is associated with depressive symptoms, blunted cortisol reactivity to acute stress, and lowered morning cortisol. Brain Behav Immun 2010; 24:1202–8. [DOI] [PubMed] [Google Scholar]
- 41. Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med 2006; 68:657–61. [DOI] [PubMed] [Google Scholar]
- 42. Inoue-Toyoda M, Kato K, Nagata K, Yoshikawa H. Glucocorticoids facilitate the transcription from the human cytomegalovirus major immediate early promoter in glucocorticoid receptor- and nuclear factor-I-like protein-dependent manner. Biochem Biophys Res Commun 2015; 458:180–5. [DOI] [PubMed] [Google Scholar]
- 43. Spyridopoulos I, Hoffmann J, Aicher A et al. Accelerated telomere shortening in leukocyte subpopulations of patients with coronary heart disease: role of cytomegalovirus seropositivity. Circulation 2009; 120:1364–72. [DOI] [PubMed] [Google Scholar]
- 44. Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol 2010; 172:363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rector JL, Dowd JB, Loerbroks A et al. Consistent associations between measures of psychological stress and CMV antibody levels in a large occupational sample. Brain Behav Immun 2014; 38:133–41. [DOI] [PubMed] [Google Scholar]
- 46. Gouin JP, Hantsoo L, Kiecolt-Glaser JK. Immune dysregulation and chronic stress among older adults: a review. Neuroimmunomodulation 2008; 15:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Epel ES, Blackburn EH, Lin J et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A 2004; 101:17312–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010; 20:202–13. [DOI] [PubMed] [Google Scholar]
- 49. Gkrania-Klotsas E, Langenberg C, Sharp SJ, Luben R, Khaw KT, Wareham NJ. Seropositivity and higher immunoglobulin G antibody levels against cytomegalovirus are associated with mortality in the population-based European prospective investigation of Cancer-Norfolk cohort. Clin Infect Dis 2013; 56:1421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One 2011; 6:e16103. [DOI] [PMC free article] [PubMed] [Google Scholar]