High transcript levels of the CMS-associated gene orf288 in the hau CMS line of Brassica juncea induce defects in mitochondrial ultrastructure during the development of archesporial cells and cause cytoplasmic male sterility.
Keywords: Anther development, Brassica, cytoplasmic male sterility, hau, CMS, orf288, plant mitochondria
Abstract
Cytoplasmic male sterility (CMS) is primarily caused by chimeric genes located in the mitochondrial genomes. In Brassica juncea, orf288 has been identified as a CMS-associated gene in the hau CMS line; however, neither the specific abortive stage nor the molecular function of the gene have been determined. We therefore characterized the hau CMS line, and found that defective mitochondria affect the development of archesporial cells during the L2 stage, leading to male sterility. The expression level of the orf288 transcript was higher in the male-sterility line than in the fertility-restorer line, although no significant differences were apparent at the protein level. The toxicity region of ORF288 was found to be located near the N-terminus and repressed growth of Escherichia coli. However, transgenic expression of different portions of ORF288 indicated that the region that causes male sterility resides between amino acids 73 and 288, the expression of which in E. coli did not result in growth inhibition. Transcriptome analysis revealed a wide range of genes involved in anther development and mitochondrial function that were differentially expressed in the hau CMS line. This study provides new insights into the hau CMS mechanism by which orf288 affects the fertility of Brassica juncea.
Introduction
Cytoplasmic male sterility (CMS) is primarily caused by chimeric genes located in the mitochondrial genome, and it can be suppressed by restoration-of-fertility (Rf) genes in the nuclear genome (Schnable, 1998; Chase, 2007). This system is not only a useful method for hybrid seed production, but also serves as a model for investigating nuclear–mitochondrial interactions (Hanson and Bentolila, 2004). Various CMS systems have been identified, and they are widely used in different crop plants, including maize, rice, wheat, petunia, pepper, and Brassica species (Li et al., 2007; Chen and Liu, 2014). Importantly, specific CMS mechanisms are often completely distinct in different plants. To date, four models have been proposed to explain CMS mechanisms, namely the cytotoxicity model, the energy deficiency model, the aberrant programmed cell death (PCD) model, and the retrograde regulation model (Chen and Liu, 2014). Although some CMS-associated genes can suppress the growth of prokaryotic cells (Korth et al., 1991; Nakai et al., 1995; Duroc et al., 2005; Wang et al., 2006; Jing et al., 2012), direct evidence for CMS proteins mediating cytotoxicity in plants is still lacking. In the energy deficiency model, CMS proteins cause male sterility by disrupting normal mitochondrial energy requirements during male reproductive development (Ducos et al., 2001; Ji et al., 2013; Wang et al., 2013). Premature tapetal PCD has been observed in the CMS-PET1 cytoplasm in sunflower (Balk and Leaver, 2001) and CMS-WA (wild-abortive-type) cytoplasm in rice (Luo et al., 2013), which supports the aberrant PCD model. Mitochondrial CMS genes can also regulate the expression of nuclear genes involved in anther development through retrograde signaling, consistent with the retrograde regulation model. In plants with Chinese wild rice-type CMS, overexpression of the RMS (RETROGRADE-REGULATED MALE STERILITY) gene in a fertility restorer line caused male sterility, whereas RNAi suppression of RMS restored fertility to CMS plants (Fujii and Toriyama, 2009). Although CMS has been extensively exploited in hybrid seed production in crop species, the molecular mechanisms underlying differential male sterility remain elusive.
Numerous different CMS systems have been reported in Brassicaceae, including among others nap CMS, pol CMS, ogu CMS, Tour CMS, Moricandia arvensis CMS, Nsa CMS, NCa CMS, hau CMS, and inap CMS (Grewe et al., 2014; Heng et al., 2015; Yang et al., 2016). However, only a few CMS-associated genes have been reported. In B. napus, the transcript expression level of the pol CMS-associated gene orf224 was found to be higher in the male-sterile line than in the male-fertile line (L’Homme and Brown, 1993; Menassa et al., 1999; Liu et al., 2016). Expression of the orf222/nad5c/orf139 region may be associated with nap CMS (L’Homme et al., 1997; Liu et al., 2017). ORF138 is a mitochondrial protein that acts at the inner mitochondrial membrane pore (Duroc et al., 2009) and is responsible for ogura CMS in Brassiceae (Grelon et al., 1994; Duroc et al., 2005). Transcription of the Kosena CMS-associated gene orf125, which is homologous to orf138, is strongly associated with the CMS phenotype in B. napus (Landgren et al., 1996). Studies on mitochondrial RNA and protein banding patterns revealed that orf263 is associated with CMS in B. tournefortii cytoplasm (Landgren et al., 1996). The orf108 gene is also associated with CMS in the cytoplasm of Moricandia arvensis (Ashutosh et al., 2008; Kumar et al., 2012). A chimeric orf220 gene from CMS B. juncea (stem mustard) causes male sterility (Yang et al., 2010) and MSH1-RNAi from spontaneous fertile-revertant lines was found to increase the orf220 copy number and induce male sterility (Zhao et al., 2016).
Flowers are one of the most complex plant structures, and anther development in particular is orchestrated by complex gene regulatory networks (Pearce et al., 2015). Molecular genetic studies have revealed many crucial genes involved in early anther development. For example, WUSCHEL (WUS) is critical for stem-cell fate determination in the shoot apical meristem of higher plants (Su et al., 2009). In Arabidopsis, SPL/NZZ, which belongs to the MADS-box transcription factor family (Liu et al., 2009), regulates anther patterns and sporocyte development. Moreover, spl/nzz mutants do not have microsporocytes and do not form an anther somatic wall layer (Schiefthaler et al., 1999; Yang et al., 1999). AP3 and PI, which belong to the floral homeotic B function MADS-box gene family, determine petal and stamen development and orchestration (Jack et al., 1992; Goto and Meyerowitz, 1994). EMS1 encodes a leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther (Zhao et al., 2002). DYT1, which encodes a putative bHLH transcription factor, controls anther development (Zhang et al., 2006). To date, many genes and regulation networks involved in anther development and cell fate specification have been reported (Gómez et al., 2015). Microarray and RNA-Seq have been widely used to analyse essential genes involved in B. juncea anther development. A number of mitochondrial genes and those involved early anther development (e.g. WUS, SPL, AP3, PI, DYT1, and AMS) were found to be down-regulated in male-sterility lines (Yang et al., 2010; Zhao et al., 2016).
Previous studies have identified hau CMS as being a novel CMS system in B. juncea, and the cytoplasm has been transferred to B. napus (Wan et al., 2008) and B. rapa (Heng et al., 2015). The orf288 gene is responsible for male sterility in hau CMS in B. juncea (Jing et al., 2012). After comparative analysis of the hau CMS mitochondrial genome and other sequenced mitochondrial genomes, the cytoplasm of hau CMS was identified as an alloplasmic cytoplasm in Brassica crops (Heng et al., 2014). However, the specific stage of anther development that is affected and how the CMS-associated gene orf288 triggers male sterility are poorly understood. Here, we describe the hau CMS line in B. juncea, which aborts at the archesporial cell stage of anther development. The hau CMS-associated gene orf288 was expressed at higher levels in anthers from the hau CMS line than in the fertility-restorer line. We also found that the genetic region of orf288 that inhibits the growth of E. coli is not associated with the hau CMS phenotype. Finally, expression of the orf288 transcript affects anther development in other Brassica species.
Materials and methods
Plant material
The hau CMS line (6-102A) and its iso-nuclear maintainer line (6-102B) in Brassica juncea (Wan et al., 2008) and the hau CMS line, its iso-nuclear maintainer line, and the fertility-restorer line in B. napus were grown at the rapeseed research field site at Huazhong Agricultural University (Wuhan, China) in September 2014. Wild-type (WT) Arabidopsis thaliana Columbia (Col-0) plants were grown under white fluorescent light (16 h light/8 h dark) at 22 °C during the day and 18 °C at night, with a relative humidity of 50%.
Histological analyses
Scanning electron microscopy (SEM) was used to examine the surfaces of 6-102A and 6-102B anthers. Fresh anthers <1 mm in length were fixed overnight in 2% glutaraldehyde. The dehydrated samples were sputter-coated with gold and examined using a LEO 435VP scanning electron microscope (LEO Electron Microscopy Ltd). Fresh anthers from 6-102A and 6-102B at different developmental stages were fixed in 2.5% (w/v) glutaraldehyde in 0.2 M phosphate buffer (pH 7.2) for transmission electron microscopy (TEM) analysis. The procedures were performed as previously described (Yi et al., 2010). Ultra-thin sections were obtained by using a Leica UC6 ultramicrotome and stained with uranyl acetate and lead citrate. A Hitachi H-7650 transmission electron microscope was used to record the images. Semi-thin sections were used to examine the hau CMS abortive stage, as described previously (Dun et al., 2011). Flower buds from 6-102A and 6-102B were fixed and vacuum-treated overnight in FAA solution (alcohol: acetic acid: formalin: water ,10:1:2:7). A graded ethanol series (70, 85, 95, and 100%) was used to dehydrate the fixed anthers. The anthers were then embedded in Technovit 7100 (Heraeus) resin for the semi-thin section analysis. The samples were cut into approximately 2-μm thick sections, stained with Toluidine Blue (Sigma-Aldrich), and photographed using bright-field microscopy.
Northern and western blotting
RNA was isolated from the top floral buds (<0.5 mm) from the hau CMS line in B. juncea and B. napus, its maintainer line in B. napus, and floral buds of different sizes (<0.5 mm; 0.5–2 mm; 2–4 mm) from the fertility-restorer line in B. napus in April 2015. Northern blotting analysis was performed as previously described (Jing et al., 2012) to detect the orf288 expression patterns in the hau CMS line and its fertility-restorer line. Total RNA from different samples was fractionated on a 1.2% denaturing agarose gel containing 2% formaldehyde and transferred to Hybond N+ membranes (Amersham, UK). The orf288 and atp6 expression levels were detected using a Promega labeling kit and HYB-101 Perfect Hyb (ToYoBo). Membranes were exposed on a phosphor storage screen for 2 h, and the signals were scanned using a Typhoon FLA 9000 imaging system (Fujifilm, Japan).
Total protein was also extracted from floral buds (<0.5 mm) from the hau CMS line in B. juncea and B. napus, its maintainer line, and the fertility-restorer line in B. napus in April 2015. Proteins were separated using 10% SDS–PAGE or Tricine gels and transferred to a PVDF membrane for western blotting (Millipore). A peptide antigen corresponding to 150 residues of ORF288 was synthesized using a chemical synthesis method (ABclonal) and then used to immunize rabbits for antibody production. An anti-β-actin antibody was used as a positive control.
Expression of different truncated orf288 transcripts in E. coli
Full-length and truncated orf288 fragments were amplified from flower bud cDNAs (primers are listed in Table S1 at the Dryad Digital Repository, https://doi.org/10.5061/dryad.9s68p; the BamHI and HindIII restriction sites in the primer sequences are underlined). Six different truncated orf288 fragments were amplified (see Results) and cloned into the PET32a bacterial expression vector (Novagen). When the optical density (OD) of the samples reached 0.6, isopropyl-β-D-thiogalactopyranoside (IPTG; 0.5 mM) was added to induce expression of the full-length and truncated ORF288 fragments in E. coli BL21 (DE3) plysS cells (Promega). The OD of each sample was measured every 30 min at 600 nm using a UV-1601 spectrophotometer (Shimadzu, Japan) five times with three replicates each.
Plant expression vector construction and transformation complementation test
A DNA fragment containing a mitochondrial targeting peptide (159 bp of the restorer gene Rfp) and the full-length or truncated ORF288 sequences were cloned into the pCAMBIA2300 binary vector under a double CaMV 35S promoter (Fig. S1 at Dryad). The Agrobacterium tumefaciens (GV3101)-mediated floral dip transformation method was performed in A. thaliana (Clough and Bent, 1998). The transgenic plants were analysed by PCR with primers specific to the CMS-associated genes (primers are listed in Table S1 at Dryad).
RNA sequencing and data processing
The RNAprep Pure Plant Kit (TIANGEN DP441) was used to extract total RNA from the different floral buds (<0.5 mm) from 6-102A and 6-102B, with three replications each. Sequencing was performed using the Illumina NextSeq 500 platform. Tophat2 was used to align the RNA-Seq reads against the B. juncea genome (http://brassicadb.org). HTSeq 0.6.1p2 was used to calculate the read counts for each gene. The reads per kilobase per million mapped reads (RPKM) value was used to estimate the expression levels of the different genes. Genes with a fold change >2 and a P-value <0.05 were identified as differentially expressed genes (DEGs) when using DESeq (v1.16) for the gene expression analysis.
First-strand cDNA was synthesized using the QuantiTect Reverse Transcription Kit according to the manufacturer’s instructions. Quantitative real time PCR (qRT-PCR) was performed using the SYBR Green Real-time PCR Master Mix (TOYOBO, Japan) on a Bio-Rad CFX96 instrument. PCRs had the following cycling conditions: (1) 95 °C for 5 min; (2) 40 cycles at 95 °C for 10 s, 60 °C for 15 s, and 72 °C for 30 s; and (3) a final extension step at 72 °C for 5 min. Results were analysed using the CFX Manager software program according to the 2−ΔΔCT method (Livak and Schmittgen, 2001). The primers used for qRT-PCR are listed in Table S1 at Dryad.
Results
Flower morphological defects in the hau CMS line in B. juncea
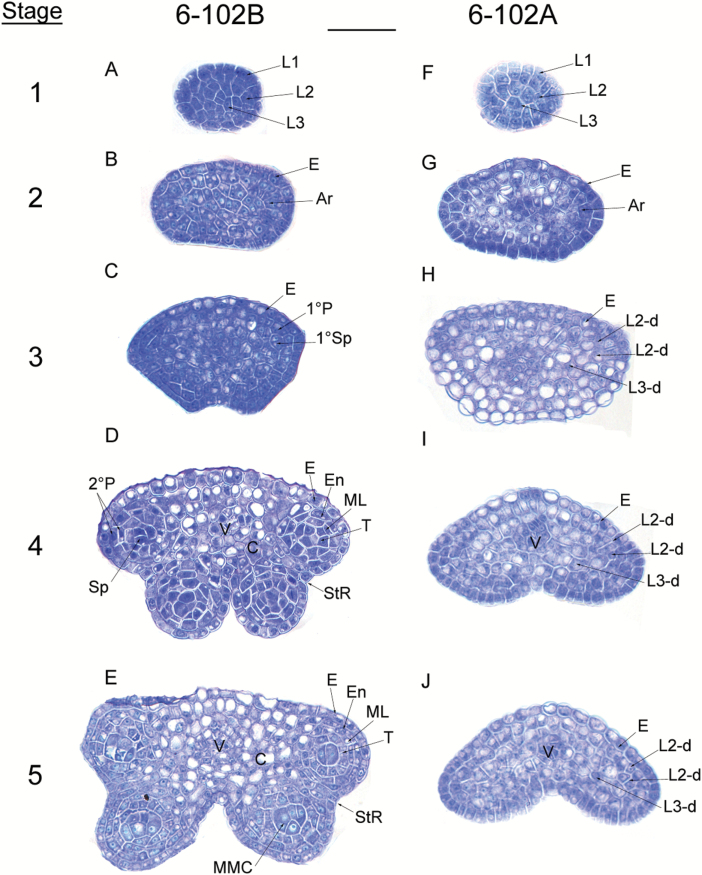
The morphological features of 6-102A (hau CMS) and 6-102B (hau CMS maintainer) were compared. Petals from 6-102A were reduced in size compared with those from 6-102B (Fig. 1D), And short filaments were also observed in flowers from the hau CMS line (Fig. 1E). The stamens of the hau CMS line were transformed into thickened petal-like structures (Fig. 1D, E). SEM results revealed that the petal-like stamens in the hau CMS line formed at an early stage of flower development (Fig. 1F). No differences in vegetative growth were observed between the plants. To better understand which cell types were affected in the stamens of the hau CMS line, transverse sections of 6-102A and 6-102B anthers at different stages of development were examined. Anther development can be divided into 14 stages in Arabidopsis (Sanders et al., 1999), and similar developmental stages have been described in B. juncea. Anther development was similar in the hau CMS line and its iso-nuclear maintainer line before the differentiation of archesporial cells (Fig. 2A, F, and B, G). Archesporial cells in 6-102B anthers underwent asymmetric and symmetrical cell divisions and differentiated into the endothecium, middle layer, tapetum, and microspore mother cells during stages 2–5. However, in an effect that was derived from the L2 stage of development, cell differentiation stopped and the divisions of each layer did not generate the secondary parietal layers and sporogenous cells in 6-102A (Fig. 2H). Anthers from the hau CMS line were completely aborted, with no pollen sacs (Fig. 2 H– J). Based on these cytological observations, we concluded that the archesporial cells arrest differentiation at stage 2, leading to abnormal stamens and male sterility in the 6-102A plants.
Fig. 1.
Anther phenotypes in the hau CMS lines and the maintainer line in B. juncea. (A, B) A flower from the hau CMS maintainer line (6-102B). (D, E) A flower from the hau CMS line (6-102A). Scale bars =20 mm. (C, F) A mature anther from the hau CMS maintainer line (C) and the hau CMS line (F). Scale bars =200 µm. (This figure is available in colour at JXB online.)
Fig. 2.
Anther development defects in the hau CMS line in B. juncea. The images are semi-thin sections from (A–E) the hau CMS maintainer line (6-102B) and (F–J) the hau CMS line (6-102A) showing anther development from stages 1–5. Abbreviations: Ar, archesporial cell; E, epidermis; En, endothecium; L1, L2, and L3, the three cell layers of the stamen primordia; L2-d and L3-d, the L2- and L3-derived cells; ML, middle layer; MMC, microspore mother cell; Sp, sporogenous cells; StR, stomium region; T, tapetum; V, vascular tissue; 1°P, primary parietal layer; 1°Sp, primary sporogenous layer; and 2°P, secondary parietal cell layers. Scale bar =25 µm. (This figure is available in colour at JXB online.)
Defective mitochondria in the anther cause male sterility in the hau CMS line
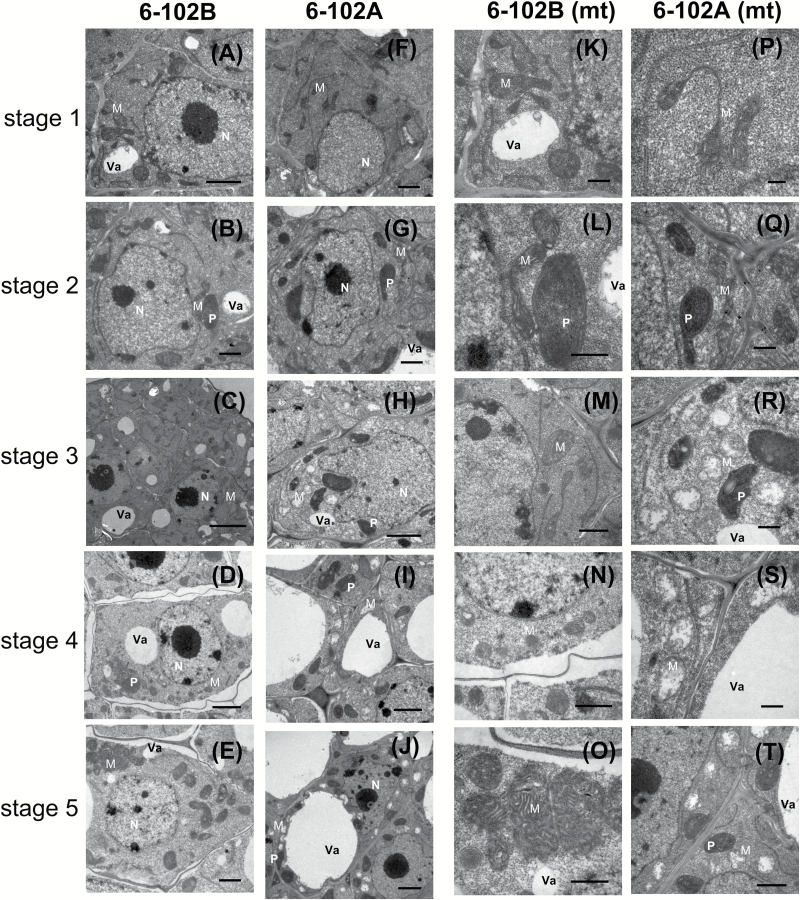
Notably, CMS systems in different plants are primarily caused by mitochondrial disturbances in the anther. TEM was used to compare the cell structures between 6-102A and 6-102B, and no significant differences were observed prior to stage 2 of anther development. The mitochondria were integrated with a double-membrane structure, and mitochondrial crests were clearly visible (Fig. 3F, G, P, Q). By contrast, numerous vacuolated mitochondria could be seen in sterile male anthers with swollen cells after stage 3 (Fig. 3R–T). More defective mitochondria were present in cells with sterile anthers. These observations suggest that orf288 affects mitochondrial function and interrupts tissue differentiation in anthers from the hau CMS line, leading to male sterility.
Fig. 3.
TEM analysis of anthers from the hau CMS maintainer line (6-102B) and the hau CMS line (6-102A) at different stages. Anther development from stages 1–5 in 6-102B (A–E) and in 6-102A (F–J). The mitochondrial structures of the cells from (A–E) and (F–J) are shown in (K–O) and (P–T), respectively. Abbreviations: M, mitochondria; P, plastid; N, nucleus; and Va, vacuole. Scale bars: (D) 0.2 µm, (B, F, H, L, P, R) 0.5 µm, (C, E, G, J, N, Q, T) 1 µm, (A, K, M, O, S) 2 µm, and (I) 5 µm.
Expression profile of the CMS-associated gene orf288
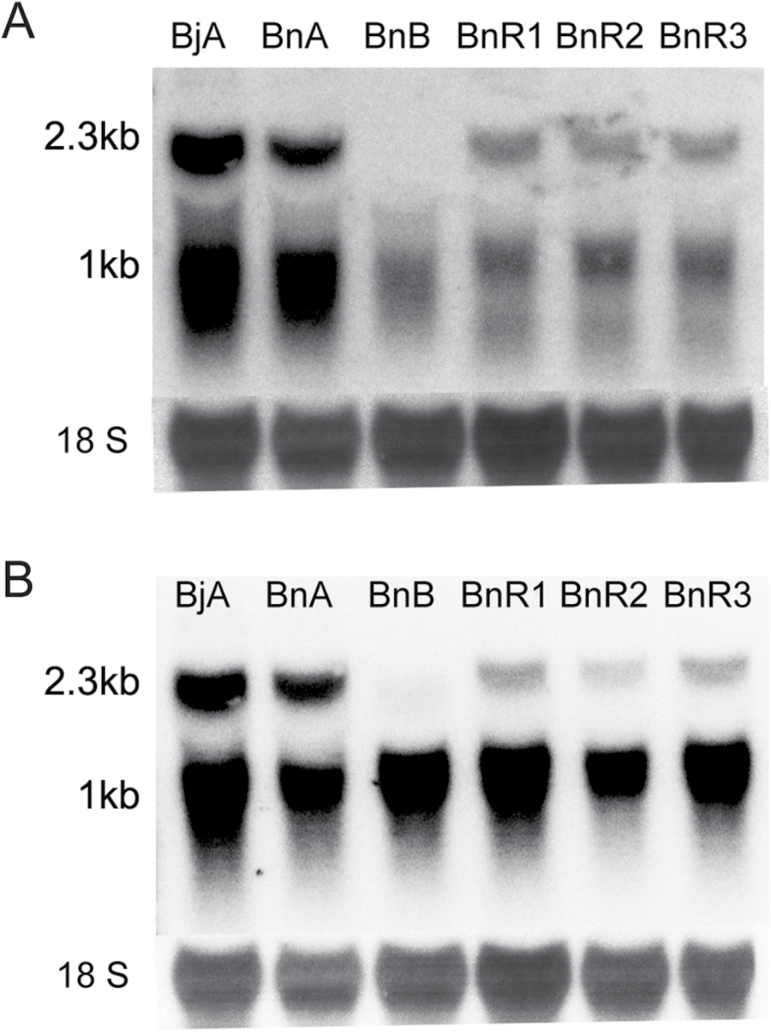
The CMS-associated gene orf288, which is located downstream of and co-transcribed with atp6, is only expressed in the hau CMS line (Jing et al., 2012). It was previously found to be constitutively expressed in all tested tissues of the hau CMS line, including flower buds, fresh leaves, roots, and hypocotyls (Heng et al., 2014). Northern blotting was used to determine the orf288 expression in different floral buds. Anthers from CMS B. juncea and B. napus plants and fertility-restorer B. napus plants showed both the 2.3-kb and 1-kb transcripts, but the intensity of both transcript bands was weak in fertility-restorer plants when the orf288 fragment was used as a probe (Fig. 4A). When the fragment from atp6 was used as a probe, the 2.3-kb and 1-kb transcripts were both present in the different lines, although the intensity of the 2.3-kb transcript band was weak in different floral buds of the B. napus fertility-restorer plants (Fig. 4B).
Fig. 4.
orf288 and atp6 expression patterns in the hau CMS lines. RNA gel-blotting analysis for (A) the orf288 and (B) the atp6 gene regions in the male-fertile, male-sterile, and nuclear-restorer floral buds: BjA, floral buds (<0.5 mm) from the hau CMS line (6-102A) in B. juncea; BjB, floral buds (<0.5 mm) from the hau CMS maintainer line (6-102B) in B. juncea; BnA, the male-sterile line in B. napus; and BnB, the maintainer line in B. napus. BnR1, BnR2 and BnR3 are different-sized floral buds (<0.5 mm; 0.5–2 mm; 2–4 mm) from the fertility-restorer line in B. napus.
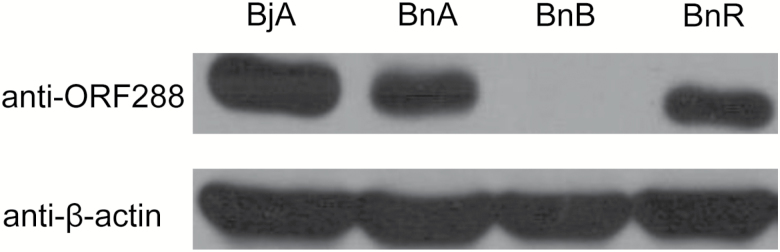
A western blotting assay was employed to further analyse ORF288 polypeptide expression levels in flower buds from the B. juncea and B. napus CMS lines and the B. napus fertility-restorer line. Intriguingly, equivalent amounts of ORF288 polypeptide were present in both the hau CMS line and the fertility-restorer line (Fig. 5).
Fig. 5.
Western blotting analysis of ORF288 between the hau CMS lines and the fertility-restorer lines. BjA, floral buds (<0.5 mm) from the hau CMS line in B. juncea; BnA, floral buds (<0.5 mm) from the male-sterile line in B. napus; BnB, floral buds (<0.5 mm) from the maintainer line in B. napus; and BnR, floral buds (<0.5 mm) from the fertility restorer line in B. napus.
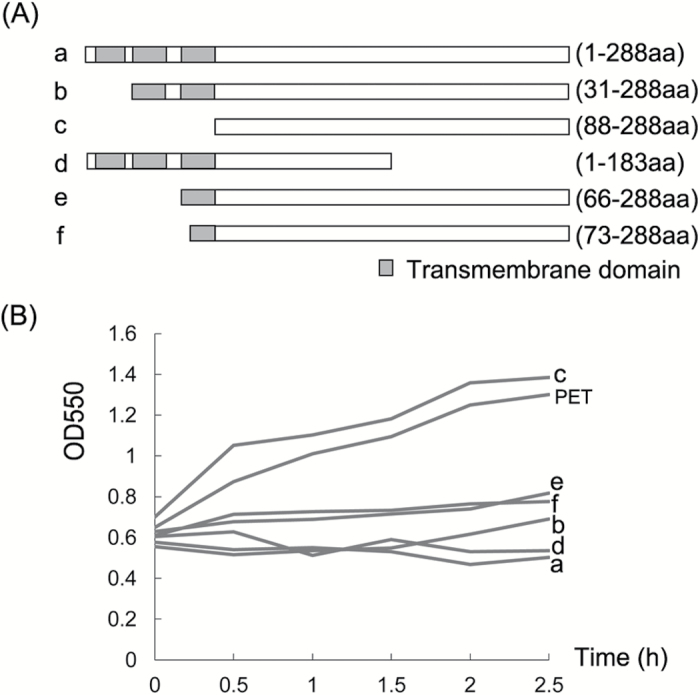
The cytotoxic region of the CMS-associated gene orf288
Previous results using the software TMHMM server showed that ORF288 contains three transmembrane regions in its N-terminus (1–88 aa), and the CMS-associated gene orf288 was found to significantly repress the growth of E. coli (Jing et al., 2012). To further analyse the cytotoxic region in this CMS-associated gene, the full-length (Fig. 6Aa) and five truncated (Fig. 6Ab-f) versions of ORF288 were cloned and expressed using the pET32a vector. The empty PET vector without gene fragments was used as a control. The growth of E. coli was normal only when the cloned gene fragments did not include the three transmembrane domains (1–88 aa); a part or all of the ORF288 transmembrane domains inhibited E. coli growth (Fig. 6B). Thus, the toxicity region is contained in the three ORF288 transmembrane domains.
Fig. 6.
The cytotoxic region of the CMS-associated gene orf288. (A) Truncated orf288 with different transmembrane domains: (a) full-length DNA sequence with three transmembrane domains; (b) truncated orf288 without the first transmembrane domain; (c) truncated orf288 without any transmembrane domains; (d) truncated orf288 that does not include 105-aa at the C-terminus; (e) truncated orf288 with only the third transmembrane domain; and (f) truncated orf288 with only part of the third transmembrane domain. (B) Effect of the overexpression of different truncated orf288 fragments on the growth of E. coli cells: (a–f) indicate the different truncated fragments shown in (A). IPTG was added when cell growth reached an OD550 of 0.6. PET indicates the control expression vector induced by IPTG.
A 215-aa region of the ORF288 C-terminus is sufficient to cause male sterility in Arabidopsis
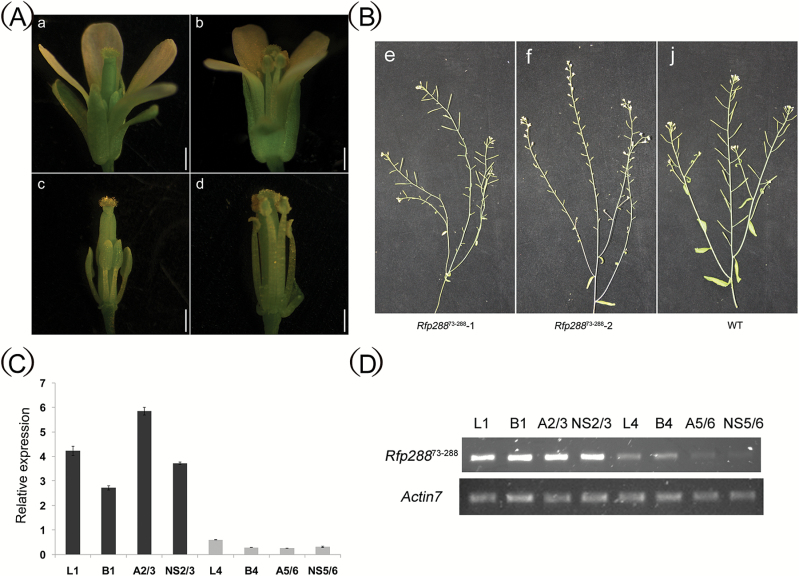
A previous study confirmed that orf288 expression in Arabidopsis can lead to male sterility (Jing et al., 2012). To further investigate which region of the CMS-associated orf288 gene is responsible for male sterility, we generated five different transgenic A. thaliana lines containing either full-length ORF288, amino acids 1–88, or amino acids 73–288, with or without a mitochondria-targeting peptide, under a constitutive double CaMV35S promoter. Fourteen 2 × 35S::Rfp288, eighteen 2 × 35S::Rfp28873–288, nine 2 × 35S::28873–288, twenty 2 × 35S::Rfp2881–88, and eleven 2 × 35S::2881–88 (Fig. S1 A–D at Dryad) T0 plants were grown in the greenhouse for phenotypic evaluation. Full-length ORF288 or amino acids 73–288 of ORF288, with or without a mitochondria-targeting peptide, caused male sterility, whereas amino acids 1–88 of ORF288, with or without the mitochondria-targeting peptide, did not (Fig. 7A; Table S2 at Dryad). Among the 13 2 × 35S::Rfp28873–288 transgenic lines, most displayed total male sterility (Fig. 7Bf), and the anthers from a few plants displayed semi-sterility (Fig. 7Be). The 2 × 35S::Rfp28873–288 male sterility line was further crossed with WT pollen, and the A. thaliana T3 progeny plants could co-segregate with the stably introduced DNA. The line contained a single copy of 2 × 35S::Rfp28873–288 and showed a 1:1 segregation rate for the male sterility and fertility phenotypes. At the same time, the semi-sterile plants with 35S::Rfp28873–288 were self-seeded by generation. Plants expressing ORF288 amino acids 1–88, with or without the mitochondria-targeting peptide, did not show male sterility.
Fig. 7.
Fertility associated with truncated ORF288 expression in Arabidopsis. (A) Anthers from transgenic truncated orf288 fragments (a, c) and the wild-type (b, d). (B) The siliques from transgenic truncated orf288 fragments (e, f) and the wild-type (j). (C, D) qPCR and RT-PCR analysis of the expression levels of the truncated orf288 fragments in different tissues from transgenic plants. L1 and B1, leaves and floral buds from a transgenic male sterility T3 plants; A2/3, anthers from two transgenic T3 male sterility plants; NS2/3, floral buds without anthers from two transgenic T3 male sterility plants; L4 and B4, leaves and floral buds from a transgenic semi-male sterility T3 plants; A5/6, anthers from two transgenic T3 semi-male sterility plants; and NS5/6, the floral buds without anthers from another two transgenic T3 semi-male sterility plants. (This figure is available in colour at JXB online.)
Quantitative PCR and real-time PCR with 2 × 35S:: Rfp28873–288-specific primers showed that Rfp28873–288 was relatively highly expressed in the leaves, floral buds, anthers, and floral buds without anthers in male-sterility T3 plants compared with semi-sterile T3 plants (Fig. 7C, D). Semi-thin sections were also used to compare anthers in the mature floral buds between WT and transgenic lines with 35S::Rfp28873–288. The anthers from the male-sterility flowers were completely abortive, and there were no pollen sacs or mature pollen (Fig. S2B at Dryad), distinct from the WT flowers (Fig. S2A at Dryad). However, the mature fertile anthers from the semi-sterile lines expressing 35S::Rfp28873–288 also showed abnormal development. Most anthers were affected to varying degrees, with some showing complete abortion and others showing only one or two pollen sacs with normal pollen grains (Fig. S2C, D at Dryad). Our transgenic analyses therefore confirmed that orf288 is a key gene that can cause male sterility, and that it is the C-terminal region of ORF288 and not the N-terminal transmembrane domain that causes the sterility.
Genes involved in early anther development are differentially expressed between the 6-102A and 6-102B lines
In this study, anther development was arrested during the archesporial cell differentiation stage in the hau CMS line. To better understand the processes associated with male anther sterility in this line, RNA was extracted from floral buds (<0.5 mm in length) of hau CMS plants and its iso-nuclear maintainer line and used to perform high-throughput transcriptome sequencing analysis (RNA-Seq). A total of 18 192 507 600 bp and 18 887 411 700 bp of clean data were separately generated with Q30 scores >92% from the hau CMS line and its iso-nuclear maintainer line, respectively (Table S3 at Dryad). The correlation coefficient (R2) between the different replicates calculated using RPKM values was greater than 0.95. In total, 5440 significant DEGs were identified between the hau CMS line and its iso-nuclear maintainer line (Table S4 at Dryad). Among these, 3256 unigenes were up-regulated and 2184 unigenes were down-regulated in the hau CMS line (Fig. S3 at Dryad).
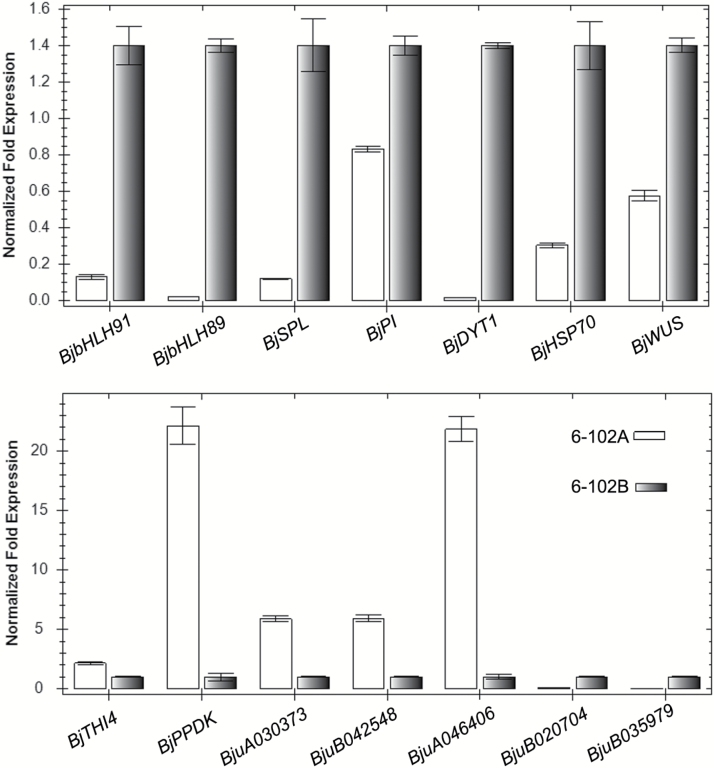
As the CMS phenotype is primarily caused by CMS genes located in the mitochondria, the expression profiles of DEGs in the mitochondria were further analysed. Genes involved in mitochondrial structure and ATP synthase were down-regulated in the hau CMS line (Table S5 at Dryad). Furthermore, genes involved in early anther development (e.g. BjWUS, BjSPL, BjPI, BjDYT1) were also down-regulated in anthers from the hau CMS line, whereas genes involved in autophagy, senescence, catalase, DNA repair, and mitochondrial DNA damage tolerance were up-regulated in this line (Table S5 at Dryad). In addition, a number of pentatricopeptide repeat (PPR) proteins were differentially expressed between the hau CMS and maintainer lines. qPCR results confirmed that these genes were differentially expressed between the hau CMS line and its maintainer line (Fig. 8). The hau CMS associated gene orf288 may influence these DEGs in a retrograde manner, contributing to the hau CMS phenotype.
Fig. 8.
qRT-PCR analysis of the expression profile of homologous genes involved in early anther development in B. juncea for the hau CMS line (6-102A) and its maintainer line (6-102B). Actin was used as an internal control to normalize the transcript levels.
Discussion
Mitochondrial ultrastructure damage in anthers causes male sterility in hau CMS plants
In Brassica, numerous CMS-associated genes are expressed in the mitochondria and induce male sterility, although the specific anther abortive stages are distinct in different species. In B. napus, the pol CMS anther produces little or no pollen and shows no differentiation of sporogenous cells (An et al., 2014; Liu et al., 2016). The abortive stage of ogu INRA CMS in rapeseed begins as early as the tetrad stage (González-Melendi et al., 2008; Yang et al., 2008), although petaloid stamens were observed in ogu CMS in B. juncea (Kirti et al., 1995; Meur et al., 2006). The abortive stages and detailed subcellular events in anther development in many different CMS plants have been analysed by light and electron microscopy (Chen and Liu, 2014). Mitochondrial structure in tapetal cells that developed within sterile anthers was found to be greatly affected compared with the WT in ogu INRA CMS (González-Melendi et al., 2008). Mitochondria from the tapetum cells in engineered male-sterile tobacco plants lost their cristae and appeared swollen (Hernould et al., 1998). Furthermore, tapetal cells became distorted when mitochondrial aberrations were present in Petunia hybrida (Bino, 1985). High amounts of hydrogen peroxide around the mitochondrial outer membranes were detected in the rice ZS97A tapetum at the microspore mother cell (MMC) stage, but not in ZS97B or in the later stage tapetum cells from ZS97A (Luo et al., 2013). As in other CMS plants, ultrastructural changes in mitochondria from hau CMS systems affected normal mitochondrial function, arrested archesporial cell development, and eventually caused male sterility. We speculate that male sterility in the hau CMS line is primarily due to mitochondrial dysfunction in the archesporial cells, which arrests cell development.
Expression levels of the CMS-associated gene orf288 affect the degree of male sterility
By comparing CMS lines and their fertility-restorer lines, we can better understand the expression profiles of CMS-associated genes. For example, the nap CMS-associated gene orf222 blocks pollen development at specific early stages. The nuclear Rf gene reduces orf222 transcript levels in a tissue-specific manner (Geddy et al., 2005). The pol CMS restorer-fertility gene Rfp acts to post-transcriptionally decrease orf224 transcript levels (Menassa et al., 1999; Liu et al., 2016). PPR-B, which is the ogu CMS Rf gene, does not affect the local accumulation of orf138 mRNA in young anthers, but it does inhibit ORF138 synthesis in the tapetum (Uyttewaal et al., 2008). Transcript levels of the CMS-associated gene orf108 were found to be reduced by the Rf gene in B. juncea containing Moricandia arvensis cytoplasm (Ashutosh et al., 2008; Kumar et al., 2012). Northern blotting analysis revealed that transcripts for the hau CMS-associated gene orf288 were present at higher levels in hau CMS flower buds than in the restorer line. However, the protein levels of ORF288 displayed no differences between the hau CMS line and its fertility-restorer line. Although maize Rf2 can restore fertility to T cytoplasm plants, it does not alter the accumulation of URF13 (Liu et al., 2001). In the I-12CMS(3) cytoplasm from wild beet, orf129 is responsible for male sterility, although the restorer genes do not affect ORF129 protein accumulation (Yamamoto et al., 2008). Our results suggest that Rf genes suppress atp6/orf288 through a post-translational mechanism to restore male fertility. In our transformation experiment, we placed different truncated orf288 fragments, with or without the mitochondria-targeting peptide, under the double CamV35S promoter and introduced them into Arabidopsis. Amino acids 73–288 from ORF288 induced male sterility, whereas amino acids 1–88 did not. In rice WA CMS, different truncated fragments of the WA CMS-associated gene WA352 were transformed into their maintainer line, and transgenic plants with MTS-WA352218–300 and MTS-WA352282–352 were male-sterile, whereas those with MTS-WA3521–227 were fertile (Luo et al., 2013); therefore, it was concluded that amino acids 218–352 were the core region that caused male sterility. The orf288 expression levels in different transgenic lines determined the anther abortive stage. The hau CMS cytoplasm was also transformed into B. napus: most of the plants were completely male-sterile, although some were not completely abortive in certain nuclear backgrounds. In this line, only one or two anthers had pollen grains; therefore, some minor restorative genes may influence the expression of orf288 in these plants. These results further indicate that orf288 transcript levels influence the degree of male fertility.
The cytotoxic region of ORF288 is not associated with cytoplasmic male sterility
The hau CMS-associated protein ORF288, which significantly represses E. coli growth, is toxic to the host cells (Jing et al., 2012); however, the identity of the regions that cause cytotoxicity and male sterility were unknown. In our study, various truncated fragments of orf288 with or without the transmembrane domains were cloned into an inducible vector and expressed using IPTG in E. coli. Interestingly, only the fragment with the transmembrane domain was cytotoxic to E. coli growth (Fig. 6B). This suggests that amino acids 1–88 of ORF288, which contain the three transmembrane domains, are the cytotoxic region. In rice, the WA CMS-associated protein WA352 has also been shown to be toxic to E. coli, although a truncated WA352 protein that did not contain the cytotoxic region still caused male sterility when expressed in transgenic plants (Chen and Liu, 2014). To better understand whether ORF288 cytotoxicity influences male sterility, we transformed amino acids 1–88 from ORF288, with or without a mitochondria-targeting peptide, into Arabidopsis, and found that they did not cause male sterility. But amino acids 73–288 from ORF288 with or without a mitochondria-targeting peptide did cause male sterility. The subcellular localization of ORF28873–288 was to mitochondria, as predicted by PredSL (http://aias.biol.uoa.gr/PredSL/;Petsalaki et al., 2006). These results may illustrate why the transformation with 2 × 35S::28873–288 lacking the mitochondrial targeting peptide could produce male sterility. A number of CMS-associated proteins in other crop plants have also been shown to be toxic to E. coli, such as URF13 in maize CMS-T (Korth et al., 1991), ORF522 in sunflower CMS-PET1 (Nakai et al., 1995), ORF138 in radish CMS-Ogu (Duroc et al., 2005), and ORF79 in rice CMS-BT (Wang et al., 2006). However, direct evidence of protein cytotoxicity causing male sterility is still lacking. Indeed, here we found that the cytotoxic regions in the hau CMS gene orf288 do not cause male sterility.
orf288 may control nuclear genes through retrograde regulation
High-throughput sequencing approaches have been used to reveal the genetic regulatory networks underlying early anther development by comparative gene expression analysis between mutant and WT plants. Different bioprocesses and genes involved in energy deficiency and early anther development are down-regulated in the pol CMS anther through nuclear–mitochondrial interactions (An et al., 2014). In B. juncea orf220-type CMS, some genes related to mitochondrial energy metabolism and pollen development were found to be down-regulated in transgenic plants (Yang et al., 2010). MSH1-RNAi lines with increased copy numbers of ORF220 caused male sterility, and numerous genes involved in anther development were up- or down-regulated in revertant and MSH1-RNAi lines (Zhao et al., 2016). In our study, genes involved in mitochondrial structure, ATP synthase, and early anther development were down-regulated in the hau CMS line, whilst genes involved in autophagy, senescence, catalase, DNA repair, and mitochondrial DNA damage tolerance were up-regulated in anthers from this line. These differentially expressed PPR genes may be responsible for many different post-transcriptional events, such as RNA editing and processing. These DEGs could affect archesporial cell differentiation and induce male sterility. The results suggest that CMS genes located in the mitochondria indirectly affect nuclear genes through retrograde regulation. Comparative analysis of these DEGs could provide a comprehensive understanding of the mechanism underlying hau CMS in B. juncea. Cytoplasmic male sterility promotes outcrosses and increases hybrid seed production in Brassica. However, exactly how the hau CMS-associated mitochondrial gene orf288 arrests anther development during the archesporial cell differentiation stage requires further study. Better characterization of the hau CMS phenotype should help to unravel the mechanism of male sterility in Brassica.
Data deposition
The following figures and tables are available at Dryad Data
Repository: https://doi.org/10.5061/dryad.9s68p.
Fig. S1. Schematic showing truncated orf288 gene construction.
Fig. S2. Semi-thin sections from transgenic Arabidopsis expressing truncated orf288.
Table S1. Primers used in this study.
Table S2. Fertility statistics for the mitochondria-targeted expression of the truncated orf288 fragment in Arabidopsis thaliana.
Table S3. Summary of RNA-Seq data in 6-102A and 6-102B.
Table S4. Detailed information on the differentially expressed genes between 6-102A and 6-102B.
Table S5. Selected differentially expressed genes.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (NSFC grant number 31271761), the National Key Research and Development Program of China (grant number 2016YFD0100804) and Nanhu Scholars Program for Young Scholars of XYNU and the Program for Modern Agricultural Industrial Technology System (nycytx-00501) and the earmarked fund for China Agriculture Research System (CARS-12).
References
- An H, Yang Z, Yi B, Wen J, Shen J, Tu J, Ma C, Fu T. 2014. Comparative transcript profiling of the fertile and sterile flower buds of pol CMS in B. napus. BMC Genomics 15, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashutosh, Kumar P, Dinesh Kumar V, Sharma PC, Prakash S, Bhat SR. 2008. A novel orf108 co-transcribed with the atpA gene is associated with cytoplasmic male sterility in Brassica juncea carrying Moricandia arvensis cytoplasm. Plant Cell Physiology 49, 284–289. [DOI] [PubMed] [Google Scholar]
- Balk J, Leaver CJ. 2001. The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. The Plant Cell 13, 1803–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bino RJ. 1985. Ultrastructural aspects of cytoplasmic male sterility in Petunia hybrida. Protoplasma 127, 230–240. [Google Scholar]
- Chase CD. 2007. Cytoplasmic male sterility: a window to the world of plant mitochondrial–nuclear interactions. Trends in Genetics 23, 81–90. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu YG. 2014. Male sterility and fertility restoration in crops. Annual Review of Plant Biology 65, 579–606. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Ducos E, Touzet P, Boutry M. 2001. The male sterile G cytoplasm of wild beet displays modified mitochondrial respiratory complexes. The Plant Journal 26, 171–180. [DOI] [PubMed] [Google Scholar]
- Dun X, Zhou Z, Xia S, Wen J, Yi B, Shen J, Ma C, Tu J, Fu T. 2011. BnaC.Tic40, a plastid inner membrane translocon originating from Brassica oleracea, is essential for tapetal function and microspore development in Brassica napus. The Plant Journal 68, 532–545. [DOI] [PubMed] [Google Scholar]
- Duroc Y, Gaillard C, Hiard S, Defrance MC, Pelletier G, Budar F. 2005. Biochemical and functional characterization of ORF138, a mitochondrial protein responsible for Ogura cytoplasmic male sterility in Brassiceae. Biochimie 87, 1089–1100. [DOI] [PubMed] [Google Scholar]
- Duroc Y, Hiard S, Vrielynck N, Ragu S, Budar F. 2009. The Ogura sterility-inducing protein forms a large complex without interfering with the oxidative phosphorylation components in rapeseed mitochondria. Plant Molecular Biology 70, 123–137. [DOI] [PubMed] [Google Scholar]
- Fujii S, Toriyama K. 2009. Suppressed expression of retrograde-regulated male sterility restores pollen fertility in cytoplasmic male sterile rice plants. Proceedings of the National Academy of Sciences, USA 106, 9513–9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddy R, Mahé L, Brown GG. 2005. Cell-specific regulation of a Brassica napus CMS-associated gene by a nuclear restorer with related effects on a floral homeotic gene promoter. The Plant Journal 41, 333–345. [DOI] [PubMed] [Google Scholar]
- Gómez JF, Talle B, Wilson ZA. 2015. Anther and pollen development: a conserved developmental pathway. Journal of Integrative Plant Biology 57, 876–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Melendi P, Uyttewaal M, Morcillo CN, Hernández Mora JR, Fajardo S, Budar F, Lucas MM. 2008. A light and electron microscopy analysis of the events leading to male sterility in Ogu-INRA CMS of rapeseed (Brassica napus). Journal of Experimental Botany 59, 827–838. [DOI] [PubMed] [Google Scholar]
- Goto K, Meyerowitz EM. 1994. Function and regulation of the Arabidopsis floral homeotic gene PISTILLATA. Genes & Development 8, 1548–1560. [DOI] [PubMed] [Google Scholar]
- Grelon M, Budar F, Bonhomme S, Pelletier G. 1994. Ogura cytoplasmic male-sterility (CMS)-associated orf138 is translated into a mitochondrial membrane polypeptide in male-sterile Brassica cybrids. Molecular & General Genetics 243, 540–547. [DOI] [PubMed] [Google Scholar]
- Grewe F, Edger PP, Keren I, Sultan L, Pires JC, Ostersetzer-Biran O, Mower JP. 2014. Comparative analysis of 11 Brassicales mitochondrial genomes and the mitochondrial transcriptome of Brassica oleracea. Mitochondrion 19, 135–143. [DOI] [PubMed] [Google Scholar]
- Hanson MR, Bentolila S. 2004. Interactions of mitochondrial and nuclear genes that affect male gametophyte development. The Plant Cell 16, S154–S169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng S, Gao J, Wei C, Chen F, Li X, Wen J, Yi B, Ma C, Tu J, Fu T, Shen J. 2017. Data from: Transcript levels of orf288 are associated with the hau cytoplasmic male sterility and altered nuclear gene expression in Brassica juncea. Dryad Digital Repository. https://doi.org/10.5061/dryad.9s68p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng S, Shi D, Hu Z, et al. 2015. Characterization and classification of one new cytoplasmic male sterility (CMS) line based on morphological, cytological and molecular markers in non-heading Chinese cabbage (Brassica rapa L.). Plant Cell Reports 34, 1529–1537. [DOI] [PubMed] [Google Scholar]
- Heng S, Wei C, Jing B, Wan Z, Wen J, Yi B, Ma C, Tu J, Fu T, Shen J. 2014. Comparative analysis of mitochondrial genomes between the hau cytoplasmic male sterility (CMS) line and its iso-nuclear maintainer line in Brassica juncea to reveal the origin of the CMS-associated gene orf288. BMC Genomics 15, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernould M, Suharsono, Zabaleta E, Carde JP, Litvak S, Araya A, Mouras A. 1998. Impairment of tapetum and mitochondria in engineered male-sterile tobacco plants. Plant Molecular Biology 36, 499–508. [DOI] [PubMed] [Google Scholar]
- Jack T, Brockman LL, Meyerowitz EM. 1992. The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell 68, 683–697. [DOI] [PubMed] [Google Scholar]
- Ji J, Huang W, Yin C, Gong Z. 2013. Mitochondrial cytochrome c oxidase and F1Fo-ATPase dysfunction in peppers (Capsicum annuum L.) with cytoplasmic male sterility and its association with orf507 and Ψatp6-2 genes. International Journal of Molecular Sciences 14, 1050–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing B, Heng S, Tong D, Wan Z, Fu T, Tu J, Ma C, Yi B, Wen J, Shen J. 2012. A male sterility-associated cytotoxic protein ORF288 in Brassica juncea causes aborted pollen development. Journal of Experimental Botany 63, 1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirti PB, Banga SS, Prakash S, Chopra VL. 1995. Transfer of Ogu cytoplasmic male sterility to Brassica juncea and improvement of the male sterile line through somatic cell fusion. Theoretical and Applied Genetics 91, 517–521. [DOI] [PubMed] [Google Scholar]
- Korth KL, Kaspi CI, Siedow JN. 1991. URF13, a maize mitochondrial pore-forming protein, is oligomeric and has a mixed orientation in Escherichia coli plasma membranes. Proceedings of the National Academy of Sciences, USA 88, 10865–10869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Vasupalli N, Srinivasan R, Bhat SR. 2012. An evolutionarily conserved mitochondrial orf108 is associated with cytoplasmic male sterility in different alloplasmic lines of Brassica juncea and induces male sterility in transgenic Arabidopsis thaliana. Journal of Experimental Botany 63, 2921–2932. [DOI] [PubMed] [Google Scholar]
- L’Homme Y, Brown GG. 1993. Organizational differences between cytoplasmic male sterile and male fertile Brassica mitochondrial genomes are confined to a single transposed locus. Nucleic Acids Research 21, 1903–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Homme Y, Stahl RJ, Li XQ, Hameed A, Brown GG. 1997. Brassica nap cytoplasmic male sterility is associated with expression of a mtDNA region containing a chimeric gene similar to the pol CMS-associated orf224 gene. Current Genetics 31, 325–335. [DOI] [PubMed] [Google Scholar]
- Landgren M, Zetterstrand M, Sundberg E, Glimelius K. 1996. Alloplasmic male-sterile Brassica lines containing B. tournefortii mitochondria express an ORF 3′ of the atp6 gene and a 32 kDa protein. Plant Molecular Biology 32, 879–890. [DOI] [PubMed] [Google Scholar]
- Li S, Yang D, Zhu Y. 2007. Characterization and use of male sterility in hybrid rice breeding. Journal of Integrative Plant Biology 49, 791–804. [Google Scholar]
- Liu F, Cui X, Horner HT, Weiner H, Schnable PS. 2001. Mitochondrial aldehyde dehydrogenase activity is required for male fertility in maize. The Plant Cell 13, 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Huang J, Parameswaran S, Ito T, Seubert B, Auer M, Rymaszewski A, Jia G, Owen HA, Zhao D. 2009. The SPOROCYTELESS/NOZZLE gene is involved in controlling stamen identity in Arabidopsis. Plant Physiology 151, 1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Dong F, Wang X, Wang T, Su R, Hong D, Yang G. 2017. A pentatricopeptide repeat protein restores nap cytoplasmic male sterility in Brassica napus. Journal of Experimental Botany 68, 4115–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yang Z, Wang X, Li K, An H, Liu J, Yang G, Fu T, Yi B, Hong D. 2016. A mitochondria-targeted PPR protein restores pol cytoplasmic male sterility by reducing orf224 transcript levels in oilseed rape. Molecular Plant 9, 1082–1084. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Luo D, Xu H, Liu Z, et al. 2013. A detrimental mitochondrial–nuclear interaction causes cytoplasmic male sterility in rice. Nature Genetics 45, 573–577. [DOI] [PubMed] [Google Scholar]
- Menassa R, L’Homme Y, Brown GG. 1999. Post-transcriptional and developmental regulation of a CMS-associated mitochondrial gene region by a nuclear restorer gene. The Plant Journal 17, 491–499. [DOI] [PubMed] [Google Scholar]
- Meur G, Gaikwad K, Bhat SR, Prakash S, Kirti PB. 2006. Homeotic-like modification of stamens to petals is associated with aberrant mitochondrial gene expression in cytoplasmic male sterile Ogura Brassica juncea. Journal of Genetics 85, 133–139. [DOI] [PubMed] [Google Scholar]
- Nakai S, Noda D, Kondo M, Terachi T. 1995. High-level expression of a mitochondrial orf522 gene from the male-sterile sunflower is lethal to E. coli. Japanese Journal of Breeding 45, 233–236. [Google Scholar]
- Pearce S, Ferguson A, King J, Wilson ZA. 2015. FlowerNet: a gene expression correlation network for anther and pollen development. Plant Physiology 167, 1717–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsalaki EI, Bagos PG, Litou ZI, Hamodrakas SJ. 2006. PredSL: a tool for the N-terminal sequence-based prediction of protein subcellular localization. Genomics, Proteomics & Bioinformatics 4, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders PM, Bui AQ, Weterings K, Mcintire KN, Hsu YC, Pei YL, Mai TT, Beals TP, Goldberg RB. 1999. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Plant Reproduction 11, 297–322. [Google Scholar]
- Schiefthaler U, Balasubramanian S, Sieber P, Chevalier D, Wisman E, Schneitz K. 1999. Molecular analysis of NOZZLE, a gene involved in pattern formation and early sporogenesis during sex organ development in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 96, 11664–11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnable P. 1998. The molecular basis of cytoplasmic male sterility and fertility restoration. Trends in Plant Science 3, 175–180. [Google Scholar]
- Su YH, Zhao XY, Liu YB, Zhang CL, O’Neill SD, Zhang XS. 2009. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. The Plant Journal 59, 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttewaal M, Arnal N, Quadrado M, Martin-Canadell A, Vrielynck N, Hiard S, Gherbi H, Bendahmane A, Budar F, Mireau H. 2008. Characterization of Raphanus sativus pentatricopeptide repeat proteins encoded by the fertility restorer locus for Ogura cytoplasmic male sterility. The Plant Cell 20, 3331–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Z, Jing B, Tu J, Ma C, Shen J, Yi B, Wen J, Huang T, Wang X, Fu T. 2008. Genetic characterization of a new cytoplasmic male sterility system (hau) in Brassica juncea and its transfer to B. napus. Theoretical and Applied Genetics 116, 355–362. [DOI] [PubMed] [Google Scholar]
- Wang K, Gao F, Ji Y, Liu Y, Dan Z, Yang P, Zhu Y, Li S. 2013. ORFH79 impairs mitochondrial function via interaction with a subunit of electron transport chain complex III in Honglian cytoplasmic male sterile rice. New Phytologist 198, 408–418. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zou Y, Li X, et al. 2006. Cytoplasmic male sterility of rice with boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. The Plant Cell 18, 676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto MP, Shinada H, Onodera Y, Komaki C, Mikami T, Kubo T. 2008. A male sterility-associated mitochondrial protein in wild beets causes pollen disruption in transgenic plants. The Plant Journal 54, 1027–1036. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu G, Zhao N, Chen S, Liu D, Ma W, Hu Z, Zhang M. 2016. Comparative mitochondrial genome analysis reveals the evolutionary rearrangement mechanism in Brassica. Plant Biology 18, 527–536. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Yang X, Zhang M. 2010. Mitochondrially-targeted expression of a cytoplasmic male sterility-associated orf220 gene causes male sterility in Brassica juncea. BMC Plant Biology 10, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Terachi T, Yamagishi H. 2008. Inhibition of chalcone synthase expression in anthers of Raphanus sativus with Ogura male sterile cytoplasm. Annals of Botany 102, 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WC, Ye D, Xu J, Sundaresan V. 1999. The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes & Development 13, 2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi B, Zeng F, Lei S, et al. 2010. Two duplicate CYP704B1-homologous genes BnMs1 and BnMs2 are required for pollen exine formation and tapetal development in Brassica napus. The Plant Journal 63, 925–938. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sun Y, Timofejeva L, Chen C, Grossniklaus U, Ma H. 2006. Regulation of Arabidopsis tapetum development and function by DYSFUNCTIONAL TAPETUM1 (DYT1) encoding a putative bHLH transcription factor. Development 133, 3085–3095. [DOI] [PubMed] [Google Scholar]
- Zhao DZ, Wang GF, Speal B, Ma H. 2002. The excess microsporocytes1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther. Genes & Development 16, 2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Xu X, Wamboldt Y, Mackenzie SA, Yang X, Hu Z, Yang J, Zhang M. 2016. MutS HOMOLOG1 silencing mediates ORF220 substoichiometric shifting and causes male sterility in Brassica juncea. Journal of Experimental Botany 67, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]