HSV-2 ΔgD-2, but not adjuvanted, recombinant glycoprotein D, completely protects male mice from challenge with clinical isolates of HSV and prevents the establishment of latency in peripheral nerves. Protection correlates with FcγRIV-activating antibodies that confer passive protection to naive mice.
Keywords: ADCC, HSV, vaccine
Abstract
Herpes simplex virus (HSV) infections manifest as recurrent oral or genital mucosal lesions, meningoencephalitis, corneal blindness, and perinatal disease. Subunit vaccines have advanced into the clinic without success. None were tested preclinically in male mice. We compared a single-cycle candidate vaccine deleted in HSV-2 glycoprotein D (ΔgD-2) and subunit gD-2 or gD-1 protein vaccines in a male murine skin model. The ΔgD-2 provided complete protection against 10 times the lethal dose of HSV-1 or HSV-2 clinical isolates, and no latent virus was detected, whereas gD-1- and gD-2-adjuvanted proteins provided little or no protection. Protection correlated with Fc receptor activating but not neutralizing antibody titers.
Herpes simplex virus (HSV)-2 infects over 400 million people worldwide, and HSV-1 causes approximately 30% of new genital infections in the developed world [1, 2]. Herpes simplex virus replicates at mucosal sites and migrates to the dorsal root ganglia (DRG) where it establishes latency characterized by subclinical or clinical recurrences [3]. Herpes simplex virus-2 infection significantly increases the risk of human immunodeficiency virus (HIV) acquisition and transmission, which highlights the potential for HSV vaccines to have additional benefit against HIV [4].
Several vaccine candidates have been developed, predominantly subunit vaccines designed to elicit (seronegative participants) or boost (seropositive participants) neutralizing antibodies targeting glycoprotein D (gD) [5]. Three Phase 3 clinical trials were conducted with recombinant gD-2 combined with monophosphoryl lipid A (MPL) and alum as adjuvants (gD/AS04; GlaxoSmithKline). The gD-2/AS04 vaccine provided no protection against HSV-2 in seronegative or seropositive men or in seropositive women in studies of serodiscordant couples [6]. In the subsequent field trial in seronegative women, no protection against HSV-2 was observed [7]. More importantly, no preclinical animal studies were conducted in males.
We adopted a different strategy and developed a single-cycle candidate HSV-2 vaccine strain deleted in gD (ΔgD-2). This vaccine completely protects against lethal vaginal and skin challenge in female mice challenged with clinical isolates of HSV-1 and HSV-2 [8, 9]. Passive transfer studies demonstrate that protection is mediated by antibodies that activate the murine Fc receptor, FcγRIV, to mediate antibody-dependent cell-mediated cytotoxicity (ADCC) and phagocytosis. The current studies were designed to test the efficacy and immunogenicity of HSV-2 ΔgD compared with gD protein vaccination in a male mouse skin scarification model.
METHODS
Mice and Ethics Statement
Age-matched male C57BL/6 mice were purchased from the Jackson Laboratory (JAX, Bar Harbor, ME). The use of animals was approved by the Institutional Animal Care and Use Committee at the Albert Einstein College of Medicine (Protocol 2015-0805).
Cell Lines and Viruses
Vero (Green Monkey Kidney cells line; American Type Culture Collection) and VD60 [10] cells were grown in Dulbecco’s modified Eagle’s medium ([DMEM] Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and 1% penicillin-streptomycin (Invitrogen). The ΔgD-2 was propagated in complementing VD60 cells expressing HSV-1 gD and was titered on VD60 and Vero cells. The HSV-2 SD90 (clinical isolate) was obtained from David Knipe (Harvard Medical School). The HSV-1 B3x1.1 and HSV-2 4674 (clinical isolates) were obtained from the Montefiore Clinical Virology Laboratory. Clinical isolates were propagated on Vero cells [9].
Preparation of Recombinant Glycoprotein D-1 and Glycoprotein D-2
Sequences encoding HSV-1 (accession Q05059, residues 25 to 341) and HSV-2 (accession AAA45841, residues 26 to 340) gD were synthesized (Gen9 and Integrated DNA Technologies, respectively) and cloned into a modified version of pIRES-acGFP with the human erythropoietin signal peptide and a C-terminal deca-His tag. HEK293 cells (Life Technologies) were transfected with the plasmids using linear polyethylenimine; valproic acid was added 24 hours posttransfection, and culture supernatants were harvested 6 days posttransfection. Protein was affinity purified with nickel resin (HIS60; Clontech) and through gel filtration (Superdex S200 26/60; GE) in phosphate-buffered saline. Endotoxin was quantified by limulus amebocyte lysate kinetic-QCL (Lonza) and was <1 EU/mg.
Immunizations and Herpes Simplex Virus Infections
Mice received 2 doses of the following vaccines subcutaneously at 3-week intervals: 5 × 106 plaque-forming units (pfu) ΔgD-2 infected VD60 cell lysate; control VD60 lysate; 5 µg of gD-1 or gD-2 combined with 150 µg of alum (Imject Alum; Pierce Biotechnology, Rockland, IL) and 12.5 µg of MPL (Invivogen, San Diego, CA). Three weeks after the second dose, mice were challenged on the skin with HSV-1 or HSV-2 [8]. The flanks were depilated to remove the fur 24 hours before abrasion with an emery board and administration of the virus. Mice were monitored daily for epithelial and neurological disease and scored as follows: (1) erythema at inoculation site; (2) spread to distant site, zosteriform lesions, edema; (3) ulceration, epidermal spread, limb paresis; (4) hind limb paralysis; and (5) death. Mice were euthanized at a score of 4 and assigned a score of 5 the following day. For passive transfer studies, mice received serum containing 750 µg of total immunoglobulin (Ig)G from mice prime-boost vaccinated with ΔgD-2 or rgD-2-alum/MPL 24 hours before challenge with an LD90 of HSV-2.
Quantitative Polymearse Chain Reaction Analysis
At the time of euthanasia (when mice succumbed or day 14 postchallenge), sacral nerves and DRG were collected. Deoxyribonucleic acid (DNA) was isolated using the Qiagen Blood and Tissue DNA isolation kit (QIAGEN), and HSV DNA was quantified by quantitative polymerase chain reaction (qPCR) [9]. In brief, 10 ng of DNA per sample was loaded, and primers and probes targeting HSV polymerase were used to quantify HSV DNA (forward primer sequence, 5′-GGCCAGGCGCTTGTTGGTGTA-3′; reverse primer sequence, 5′-ATCACCGACCCGGAGAGGGA-3′; probe sequence, 5′-CCGCCGAACTGAGCAGACACCCGC-3′). Mouse β actin was used as a loading control (Applied Biosystems, Foster City, CA), and qPCR was run in an Applied Biosystems QuantStudio 7 Flex. Samples with fewer than 4 copies detected were considered negative [9].
Antibody Detection by Enzyme-Linked Immunosorbent Assay
Total HSV-binding IgG and IgG isotypes were determined by enzyme-linked immunosorbent assay (ELISA) [8, 9]. The ELISA plates were coated with lysates of Vero cells infected with HSV-1 B3x1.1 or HSV-2 SD90 at a multiplicity of infection (MOI) of 0.1 for 24 hours or uninfected Vero cell lysates as control. Serum dilutions were incubated with coated plates overnight at 4°C, and bound IgG or IgG1, IgG2, and IgG3 were quantified using specific biotin-labeled secondary antibodies (BD Pharmingen, San Jose, CA).
Neutralization Assay
Neutralizing titers were determined as described [8, 9]. Serial 2-fold dilutions of heat-inactivated serum were incubated with virus (50 pfu/well) for 1 hour at 37°C and then applied to Vero cell monolayers for 1 hour at 37°C. Cells were fixed with methanol and stained with Giemsa stain after a 48-hour incubation. Plaques were counted, and the neutralization titer was defined as the highest dilution to result in a 50% reduction in plaque numbers.
FcγRIV Activation Assay
FcγRIV activation was determined using the mFcγRIV ADCC Reporter Bioassay (Promega, Madison, WI) [9]. In brief, target Vero cells were infected with HSV-1 B3x1.1 or HSV-2 SD90 at an MOI of 0.1 for 12 hours. Infected or uninfected control cells were transferred to white, flat-bottomed 96-well plates and incubated with heat-inactivated serum from immunized mice (1:5 dilution in DMEM) for 15 minutes at room temperature. FcγRIV reporter cells were added for 6 hours at 37°C 5% CO2. FcγRIV activation was detected by the addition of luciferin substrate. Plates were read in a SpectraMax M5e (Molecular Devices). Fold induction was calculated relative to luciferase activity in the absence of serum.
Statistical Analysis
Analyses were performed using GraphPad Prism version 7.0 software (GraphPad Software Inc., San Diego, CA). A P value of 0.05 was considered statistically significant. Survival curves were compared using the Gehan-Breslow-Wilcoxon test; other results were compared using analysis of variance with multiple testing as indicated.
RESULTS
Glycoprotein D-2 Is Immunogenic in Male Mice
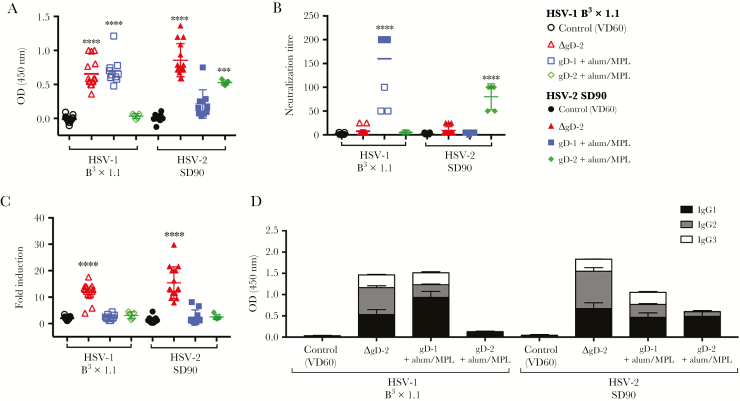
To evaluate immunogenicity, blood was obtained 1 week after the second dose of vaccine and assayed for HSV-1 (B3x1.1) or HSV-2 (SD90) binding IgG by ELISA (Figure 1A). Mice vaccinated with ΔgD-2 (n = 10/group) generated significant IgG responses to HSV-1 and HSV-2. The response to adjuvanted gD-1 (n = 10/group) or gD-2 (n = 5/group) was similar in magnitude but serotype specific. The ΔgD-2 induced little or no neutralizing antibodies (Figure 1B) (neutralization titer mean of 7.6 and 9 for HSV-1 and HSV-2, respectively), but the serum strongly activated the FcγRIV (11.9-fold and 15.3-fold induction of activation relative to control-vaccinated mouse serum [n = 15/group]; P < .0001) (Figure 1C). In contrast, antibodies elicited by gD-alum/MPL vaccination elicited serotype-specific neutralizing responses. Immunization with gD-1 neutralized HSV-1 Bx31.1-infected cell lysates (mean neutralization titer 160), but not HSV-2 SD90 lysates, and elicited only a 2.3-fold increase in FcγRIV activation. Likewise, gD-2-alum/MPL neutralized HSV-2 (but not HSV-1) infected cell lysates (mean neutralization titer 80) but elicited only a 2.2-fold increase in FcγRIV activation. Consistent with these functional differences, HSV-2 ΔgD induced a higher relative proportion of IgG2:IgG1+IgG3 compared with the gD protein vaccines (Figure 1D). IgG2 is the isotype most strongly associated with activation of FcγRIV [11].
Figure 1.
Herpes simplex virus (HSV)-2ΔgD is immunogenic in male mice and generates a nonneutralizing, Fc receptor-activating antibody response. Male C57BL/6 mice were prime-boost vaccinated with 5 × 106 plaque-forming units of ΔgD-2, 5 µg of rgD-1 or rgD-2 with alum/monophosphoryl lipid A adjuvant or a VD60 lysate control. One week after boost vaccination, serum samples were collected and assessed for HSV-1 (B3x1.1) or HSV-2 (SD90) specific immunoglobulin (Ig)G titer by enzyme-linked immunosorbent assay ([ELISA] 1:90 000 dilution) (A) and HSV-1- and HSV-2-specific neutralization titer by a standard in vitro neutralization assay (B). (C) FcγRIV activation was measured using a Promega mFcγRIV ADCC Reporter Bioassay, with cells infected with HSV-1 or HSV-2 as target cells and Jurkat effector cells expressing murine FcγRIV with an NFAT-luciferase reporter. Serum was diluted 1:5. (D) IgG isotypes were quantified by HSV-1- and HSV-2-specific ELISA. Data shown are as mean with standard error of the mean (1:1000 serum dilution). n = 10/group; 2 independent experiments, except for VD60 n = 15/group and rgD-2 n = 5/group. ***, P < .01 and ****, P < .0001 by analysis of variance with Tukey’s multiple comparison test. Significance is shown relative to control VD60 lysate.
Differences in Immunogenicity Translate to Protection Against Disease
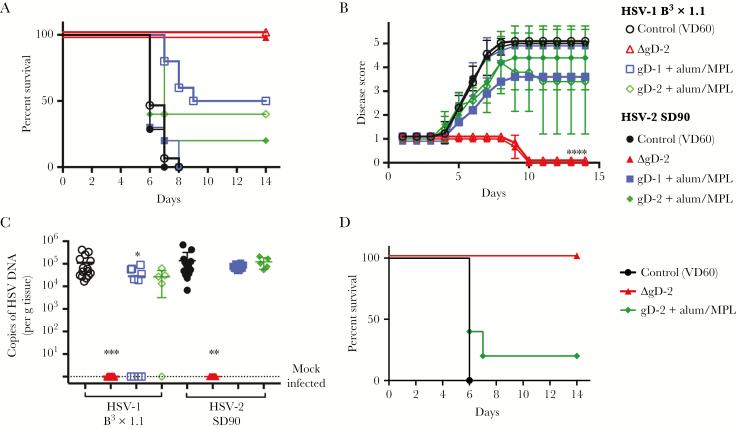
Mice were challenged on the skin 3 weeks after the second vaccine dose with 10 times LD90 doses of HSV-1 B3x1.1 or HSV-2 SD90. One hundred percent of ΔgD-2-immunized mice were protected from disease (peak score 1), and no virus was detected in the nervous tissue after challenge with either HSV-1 or HSV-2. In contrast, there was no significant difference in disease scores compared with control-immunized (VD60 lysate) in mice vaccinated with gD-1 or gD-2 protein, although there was a modest, but nonsignificant increase in survival for mice challenged with HSV-1 (5 of 10 and 2 of 5 of mice immunized with gD-1 and gD-2, respectively). Herpes simplex virus DNA was recovered from neuronal tissue in all gD-protein immunized mice challenged with SD90, and the majority of mice challenged with B3x1.1 (6 of 10 and 4 of 5 mice immunized with gD-1 or gD-2, respectively), at levels similar to control-immunized mice. In addditon, all male mice that received 750 µg of total IgG from ΔgD-2-immunized mice (5 of 5) survived subsequent skin challenge with HSV-2, whereas 4 of 5 mice that received immune serum from gD-2-alum/MPL and all control-recipient mice succumbed to infection (Figure 2D).
Figure 2.
Herpes simplex virus (HSV)-2ΔgD vaccination protects male mice from lethal HSV challenge and latency. Male C57BL/6 mice were prime-boost vaccinated with 5 × 106 plaque-forming units (pfu) of ΔgD-2, 5 µg of rgD-1 or rgD-2 with alum/monophosphoryl lipid A (MPL) adjuvant or a VD60 lysate control. (A) Percentage survival after challenge on the skin with a 10 times lethal dose (LD90) of HSV-1 B3x1.1 or HSV-2 SD90. (B) Mean disease scores after challenge with HSV-1 (open symbols) or HSV-2 (closed symbols), with standard deviation. Mice were monitored daily and scored for clinical disease as follows: (1) erythema at inoculation site; (2) spread to distant site, zosteriform lesions, edema; (3) ulceration, epidermal spread, limb paresis; (4) hind limb paralysis; and (5) death. Mice were euthanized at a score of 4 and assigned a score of 5 the following day. (C) Copies of HSV deoxyribonucleic acid (DNA) detected in the dorsal root ganglia (DRG) of mice at time of sacrifice (VD60, gD-1, gD-2) or at day 14 postchallenge (survivors). Data are displayed as copies per gram of tissue; n = 10/group; 2 independent experiments, except for VD60 n = 15/group, rgD-2 n = 5/group. (D) Male mice received 750 µg of total immunoglobulin G from mice vaccinated with ΔgD-2, gD-2-alum/MPL, or VD60 lysate 24 hours before challenge with an LD90 of HSV-2 (4674). Percentage survival is shown; n = 5/group. *, P < .05, **, P < .01, ***, P < .001, and ****, P < .0001 by Gehan-Breslow-Wilcoxon test (A and D) or by analysis of variance with Tukey’s (B) or Dunnett’s (C) multiple comparison test. Significance is shown relative to VD60 lysate control.
Discussion
Herpes simplex virus ΔgD-2 completely protects male mice from challenge with virulent clinical isolates of HSV-1 and HSV-2 and prevents the establishment of latency. In contrast, despite eliciting a similar total HSV-specific anibody response and significantly higher neutralizing anibody titers, gD proteins provided little or no protection against HSV-2. These findings recapitulate the clinical trial outcomes with gD/AS04, where no protection against HSV-2 was observed in men, who were excluded from the subsequent Phase 3 field trial [6, 7].
In addition to the observed sex differences in efficacy of gD-2 subunit vaccination in human subjects [6, 7], there are data suggesting sex differences in responses to SIV vaccinations in rhesus macaques [12, 13]. A delay in simian immunodeficiency virus acquisition associated with mucosal antibody as well as B and plasma cell responses in mucosal tissue was observed in vaccinated female but not male macaques [12]. The results from this study suggest that there are no substantial sex-based differences in the immune response to ΔgD-2. We previously reported that ΔgD-2 provides “sterilizing” immunity (defined as preventing establishment of latency) against several different clinical isolates of HSV-1 and HSV-2 (including Bx31.1 and SD90) after skin or vaginal challenge in female mice by inducing antibodies that activate the Fc receptor to elicit ADCC responses [8, 9]. Consistent with those findings, vaccination of male mice with ΔgD-2 induced antibodies that exhibit little neutralizing activity but were able to activate the murine FcγRIV, which is strongly associated with ADCC activity [11] and resulted in protective immunity. These antibodies are also sufficient to passively protect naive male mice from lethal challenge with HSV-2 [8].
The immune response to the adjuvanted gD-2 protein in male mice was similar to the responses previously reported in female mice [14]. In both sexes, gD-2 induced a potent neutralizing Ab response, but this response failed to protect male mice from challenge with the clinical isolate, SD90, and failed to protect against latency. The inability of gD-2-alum/MPL to protect against latency is consistent with published results using other challenge strains in female mice [14]. We observed similar levels of viral DNA in gD-alum/MPL and control-vaccinated mice.
It is notable that ΔgD-2 elicited similar total and FcγRIV-activating antibody responses to HSV-2 and HSV-1, whereas the gD proteins elicited serotype-specific neutralizing antibody responses. This suggests that despite only a 21% amino acid divergence between gD-1 and gD-2, the neutralizing antibodies may recognize serotype-specific epitopes [15]. These results also suggest that neutralizing antibodies alone are not sufficient to protect mice against clinical isolates of either serotype or to prevent virus from reaching neuronal tissue.
Conclusions
The protection elicited against challenge with virulent clinical isolates of HSV-1 and HSV-2 after ΔgD-2 vaccination in male and female mice suggests that this vaccine is a promising candidate for future investigation. We have protected more than 400 female mice (98%) from lethal challenge with clinical isolates of HSV by multiple routes of infection and now show similar protection in male mice. Taken together, our findings suggest that challenge with clinical isolates in male and female murine models may prove more predictive of clinical outcomes. Moreover, these results support the contention that antibodies that activate the FcγR to induce ADCC may provide a better correlate of protection against HSV infection than neutralizing antibodies.
Notes
Acknowledgments. We thank John Kim for technical assistance. We are also grateful to Mei Cong, Aileen Paguio, and Vanessa Ott at Promega for providing the murine FcγRIV activation assays.
Financial support. This work was funded by the National Institutes of Health (Grant RO1 AI117321-01; to W. R. J. J. and B. C. H.) and X-VAX Technology, Inc. C. B. is supported by an HHMI International Student Research Fellowship.
Potential conflicts of interest. W. R. J. J. and B. C. H. are inventors on a pending patent on the HSV-2 ΔgD-2 vaccine. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 2015; 10:e114989–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis 2003; 30:797–800. [DOI] [PubMed] [Google Scholar]
- 3. Corey L, Schiffer JT. Rapid host immune response and viral dynamics in herpes simplex virus-2 infection. Nat Med 2013; 19:280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Looker KJ, Elmes JAR, Gottlieb SL et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis 2017; 17:1303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Awasthi S, Friedman HM. Status of prophylactic and therapeutic genital herpes vaccines. Curr Opin Virol 2014; 6:6–12. [DOI] [PubMed] [Google Scholar]
- 6. Stanberry LR, Spruance SL, Cunningham AL et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 2002; 347:1652–61. [DOI] [PubMed] [Google Scholar]
- 7. Belshe RB, Leone PA, Bernstein DI et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 2012; 366:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petro C, González PA, Cheshenko N, Jandl T. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. Elife 2015; 4. doi: 10.7554/eLife.06054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petro CD, Weinrick B, Khajoueinejad N et al. HSV-2 ΔgD elicits FcγR-effector antibodies that protect against clinical isolates. JCI Insight. 2016; 1:e88529. doi:10.1172/jci.insight.88529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ligas MW, Johnson DC. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol 1988; 62:1486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity 2005; 23:41–51. [DOI] [PubMed] [Google Scholar]
- 12. Tuero I, Mohanram V, Musich T et al. Mucosal B cells are associated with delayed SIV acquisition in vaccinated female but not male rhesus macaques following SIVmac251 rectal challenge. PLoS Pathog 2015; 11:e1005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mohanram V, Demberg T, Musich T et al. B cell responses associated with vaccine-induced delayed SIVmac251 acquisition in female rhesus macaques. J Immunol 2016; 197:2316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delagrave S, Hernandez H, Zhou C et al. Immunogenicity and efficacy of intramuscular replication-defective and subunit vaccines against herpes simplex virus type 2 in the mouse genital model. PLoS ONE 2012; 7:e46714 https://doi.org/10.1371/journal.pone.0046714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lamers SL, Newman RM, Laeyendecker O et al. Global diversity within and between human herpesvirus 1 and 2 glycoproteins. J Virol 2015; 89:8206–18. [DOI] [PMC free article] [PubMed] [Google Scholar]