Abstract
The global burden of dengue and its geographic distribution have increased over the past several decades. The introduction of dengue in new areas has often been accompanied by high case-fatality rates. Drawing on the experience in managing dengue cases at the Queen Sirikit National Institute of Child Health in Bangkok, Thailand, this article provides the authors’ perspectives on key clinical lessons to improve dengue-related outcomes. Parallels between this clinical experience and outcomes reported in randomized controlled trials, results of efforts to disseminate practice recommendations, and suggestions for areas for further research are also discussed.
Keywords: dengue, plasma leakage, hemorrhage, case management, clinical care, case-fatality rate.
Notwithstanding the striking advances in the science of medicine, we continue to be reminded that medical progress is often driven by the observations of astute clinicians, even without the benefit of advanced technology. The recognition of massive gastrointestinal fluid loss associated with Ebola virus infection [1] and microcephaly due to congenital infection with Zika virus [2] are just 2 recent examples of major practice-changing connections made by clinicians.
In the late 1950s, outbreaks in Southeast Asia of severe illness presented a challenge to clinicians [3–5]. The constellation of fever, shock, and hemorrhage was distinct and unfamiliar. Mortality was unacceptably high as clinicians struggled to better recognize and manage cases of this new illness. Despite the substantial limitations at that time in the laboratory diagnosis of viral diseases and confounding outbreaks of other mosquito-borne illnesses such as chikungunya, the essential features of dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) were identified, and the major principles for appropriate management were defined [6]. Despite many years of effort to develop more specific therapeutics for dengue and DHF/DSS, the observations made by the astute physicians of the time remain very relevant today.
EXPERIENCE AT BANGKOK CHILDREN’S HOSPITAL/QUEEN SIRIKIT NATIONAL INSTITUTE OF CHILD HEALTH
Bangkok Children’s Hospital, which was designated the Queen Sirikit National Institute of Child Health (QSNICH) by Her Majesty the Queen in 1997, has been at the forefront of clinical experience in dengue case management since the first outbreak of DHF in Thailand in 1958. Professor Suchitra Nimmannitya from QSNICH proposed the original criteria for diagnosis of DHF/DSS and dengue management guidelines adopted by WHO in 1975, updated by WHO in 1986 [7] and 1997 [8], and adopted by WHO SouthEast Asia Regional Office in 2011 [9]. QSNICH has provided care to a large number of dengue patients; outpatient visits range 3000–10000 cases per year and in-patient admissions range 600–2500 cases per year. The number of cases of severe/complicated dengue referred to QSNICH ranges 100–250 per year.
The outcomes of care for dengue patients at QSNICH have served as a standard for comparison. The overall case-fatality rate (CFR) for dengue patients at QSNICH, including referrals, is 1%–5%, and the CFR for patients who present to the outpatient department (walk-in patients) is <0.1% (Table 1). QSNICH staff have been invited by the WHO or national governments on numerous occasions since the 1970s to assist local medical and public health personnel in responding to outbreaks of dengue. This assistance has included the review of dengue case records and the development and dissemination of clinical practice guidelines and has resulted in dramatic and sustained reductions in CFRs. QSNICH has been designated a WHO Collaborating Centre for Case Management of Dengue/DHF/DSS since 1997, with renewal of the designation in 2002, 2007, 2012, and 2016. QSNICH is also designated by the Department of Medical Services, Thailand Ministry of Public Health, as a Dengue Centre of Excellence for its breadth of clinical, research, and outreach activities (Table 2).
Table 1.
Number of Dengue Cases Admitted to the Dengue Ward and Numbers of Deaths, Queen Sirikit National Institute of Child Health, Bangkok, Thailand, 2008–2015
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | Total | |
|---|---|---|---|---|---|---|---|---|---|
| All | |||||||||
| Cases | 1297 | 790 | 789 | 713 | 393 | 881 | 419 | 508 | 5790 |
| Deaths | 8 | 5 | 2 | 7 | 2 | 3 | 0 | 5 | 32 |
| CFR | 0.62 | 0.63 | 0.25 | 0.98 | 0.51 | 0.34 | 0.00 | 0.98 | 0.55 |
| Walk-in | |||||||||
| Cases | 1117 | 686 | 721 | 637 | 335 | 776 | 380 | 408 | 5060 |
| Deaths | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 1 | 5 |
| CFR | 0.00 | 0.00 | 0.14 | 0.31 | 0.00 | 0.13 | 0.00 | 0.25 | 0.10 |
| Referred | |||||||||
| Cases | 180 | 104 | 68 | 76 | 58 | 105 | 35 | 100 | 726 |
| Deaths | 8 | 5 | 1 | 5 | 2 | 2 | 0 | 4 | 27 |
| CFR | 4.44 | 4.81 | 1.47 | 6.58 | 3.45 | 1.90 | 0.00 | 4.00 | 3.72 |
Abbreviation: CFR, case-fatality rate per hundred (deaths/cases × 100).
Table 2.
Activities of the Dengue Center of Excellence at the Queen Sirikit National Institute of Child Health
| Component | Activities |
|---|---|
| Tertiary care and referral center | - Regularly receive referred cases of severe/complicated dengue - Provide 24-hour hotline telephone consultations for all of Thailand (separate lines for doctors and for nurses/general population) |
| Training center | - Organize Dengue Case Management courses for new doctors, nurses, other healthcare personnel and the community (4–13 times/year, 50–100 participants) - Organize 1–2 week International Dengue Case Management training courses (at least 1/year, 10–25 participants) - Organize 1–5 day training in outbreak/endemic countries as requested |
| Research center | - Independent research by staff, pediatric residents, and ID fellows - Collaborative research- nationally and internationally - Active in publication of research findings on dengue |
| Policy advocacy | - Participate in policy advocacy at the Thai Ministry of Public Health - Propose annual goals for reducing the case-fatality rate of dengue |
| Reference center | - Invited by World Health Organization or governments to help with dengue case management in outbreak situations with immediate and sustained reductions in case-fatality rate - Led the development of the national dengue clinical practice guidelines in Thailand since 1999 - Assisted outbreak/endemic countries in formulating national guidelines - Invited to participate in audits of interesting and/or fatal cases of dengue from many countries (12–20 times/year) |
| Networking | - Lead a dengue case management network of 5 tertiary hospitals in different regions of Thailand |
LESSONS LEARNED FROM CLINICAL EXPERIENCE
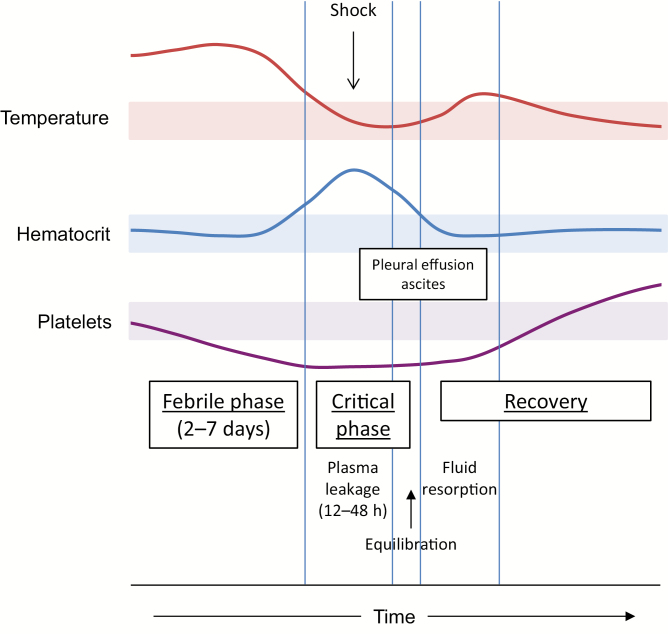
This extensive clinical experience in management of dengue cases at QSNICH and consultations with clinicians who are encountering unfamiliar outbreaks of severe dengue have led to the identification of several common themes for improvement of dengue case management to achieve sustained reductions in the CFR (Table 3). A major point of emphasis for training of clinical staff is awareness of the typical course of illness for each of the clinical categories: dengue fever (DF), dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS), and atypical manifestations/expanded dengue syndrome (EDS). As an example, the typical course of DHF/DSS is illustrated in Figure 1. Both the progression of the underlying disease process and the response to intervention are remarkably consistent; however, frequent and discerning clinical observation and appropriate laboratory support are required to recognize this course and any deviations from it so as to minimize complications.
Table 3.
Clinical Management to reduce Case-Fatality Rate in Dengue
| Problem | Solutions |
|---|---|
| Improper diagnosis and triage of dengue—delayed diagnosis of dengue and/or severe dengue | Earlier recognition and clinical/laboratory diagnosis - Suspect dengue in febrile cases, obtain CBC, and perform tourniquet test; positive tourniquet test and leukopenia increase the suspicion for dengue - Education of outpatients regarding warning signs, instructions for follow up - Daily follow up with CBC in suspected dengue cases (from day 3 of illness onward) - Rapid diagnostic test (NS1 and/or IgM) where available |
| Improper recognition and management of plasma leakage—delayed fluid resuscitation, fluid overload | Optimal fluid management of plasma leakage during the febrile and critical phases - Avoid unnecessary intravenous fluid during the febrile phase - Limit intravenous fluid during the leakage phase to maintain urine output - Use of hypertonic colloid/plasma volume expander (dextran-40) when response to crystalloid infusion is insufficient Recognition and management of volume overload during the resorption phase - Furosemide (with/without dextran-40) for treatment of volume overload |
| Improper recognition and management of bleeding—delayed recognition of significant hemorrhage, delayed blood transfusion | Maintain a high level of suspicion for occult bleeding in severe cases - Shock without hemoconcentration <20% - Severe abdominal pain, abnormal vaginal bleeding, and/or hemoglobinuria (especially in patients with thalassemia or glucose-6-phosphate deficiency) Optimal transfusion practice - Transfuse RBC (or whole blood) for hematocrit <40%–45% in shock cases (where hemoconcentration is expected) - Limit the use of platelet transfusions |
| Complications of prolonged shock- multiple organ failure/expanded dengue syndrome | Earlier recognition and management of severe dengue to avoid prolonged shock - Education of outpatients regarding warning signs, instructions for follow up - Daily follow-up with CBC in suspected dengue cases - Investigate for evidence of plasma leakage- ultrasound, albumin level Recognition and management of complications and less common dengue syndromes - Consider and treat BBH (bacterial infections, bleeding, and hepatitis) |
Abbreviations: CBC, complete blood count; IgM, immunoglobulin M; NS1, non-structural protein 1; RBC, red blood cell.
Figure 1.
Typical clinical course of dengue hemorrhagic fever. The progression of clinical events and laboratory findings during the transition from the febrile phase through the critical phase and into the recovery phase is illustrated. Shaded areas represent the normal ranges for temperature, hematocrit, and platelet count.
Early Diagnosis of Dengue Patients and Referral to the Appropriate Level of Care
The majority of dengue cases are mild; severe cases are mostly related to 2 well-known pathophysiologic hallmarks: plasma leakage and bleeding. Management in the early stage of illness is therefore aimed both at the detection and the prevention of progression to the more severe manifestations. Failure to suspect dengue sufficiently early in illness and to identify cases at increased risk for severe disease for referral for admission are major contributors to poor outcome. Maintaining a high level of suspicion, using clinical and laboratory findings to identify those cases reaching a critical phase of the illness, and providing careful instructions to outpatients have been important components of the management strategy at QSNICH.
To maintain an appropriate level of concern, it is important to consider dengue in the differential diagnosis of all acute febrile illnesses (AFI) in endemic countries. Symptoms or signs that increase the suspicion of dengue include bleeding, headache, retro-orbital pain, myalgia, arthralgia/bone pain, and rash [10]. Diarrhea and respiratory symptoms are less common in dengue but should not exclude it from consideration. Clinical practice at QSNICH is to perform a tourniquet test and complete blood count (CBC) in all AFI patients without an obvious site of infection; the presence of a positive tourniquet test (at least 10 petechiae/in2) and leukopenia (≤5000 WBC/mm3) is highly suggestive of dengue, with positive predictive value of 70%–80% in several case series [10]. When testing is available, mild elevations of aspartate transaminase (2–5 times the upper limit of normal [ULN]) also have high positive predictive value for dengue.
Because the clinical findings and laboratory tests mentioned above are not specific to dengue, their predictive values are dependent on the pretest probability of dengue. This predictive value therefore may be lower in patient populations that differ from QSNICH patients in age, underlying diseases, and common nondengue febrile illnesses, especially during periods of low dengue virus transmission. Rapid diagnostic tests for dengue are becoming increasingly available and can be helpful if used judiciously. Assays for NS1 antigen have high sensitivity early in illness, but the sensitivity declines by day 5. The sensitivity of NS1 assays is also generally lower in secondary dengue virus (DENV) infections [11], where the concern for severe illness is greater. Assays for anti-DENV immunoglobulin M antibody are most useful after day 5 of illness, but they also have lower sensitivity in secondary DENV infections.
For the outpatient evaluation of suspected dengue cases, patients are followed daily from day 3 of illness onward to ensure careful evaluation during the critical phase of illness (Figure 1). A CBC is obtained daily; the evolution of the hematologic parameters is very useful to guide the clinician in detecting the onset of plasma leakage. Leukopenia with relative lymphocytosis is a harbinger of defervescence in the next 24–48 hours and the need for close observation. Thrombocytopenia (platelet count ≤100000 cell/mm3) signals the onset of the period of plasma leakage in DHF, although it is also common in DF [10, 12]. A rise in hematocrit ≥20% of the previous or baseline value indicates plasma leakage; smaller increases in hematocrit (≥10%) may reflect early plasma leakage and should be closely monitored and managed accordingly by the clinician.
The potential for complications of dengue to develop rapidly demands ongoing caution. In addition to recommending daily follow-up, patients who are being followed as outpatients must be counseled on appropriate home care and on attending to warning signs warranting an earlier return to the clinic for re-evaluation. Patients should maintain a high intake of fluids and a soft diet. Acetaminophen is recommended for relief of fever, in addition to tepid sponging. Patients at QSNICH (and their caregivers) are provided a handout advising immediate return to the hospital upon the occurrence of any of several warning signs of more severe dengue illness (Table 4).
Table 4.
Clinical Criteria Used in Dengue Management at the Queen Sirikit National Institute of Child Health, Bangkok
| Application | Criteria |
|---|---|
| Warning signs of severe illness (instructions to patients/caregivers for immediate return to hospital) | - Transition to afebrility with no improvement in general well-being - Persistent vomiting (>3 times in a day) - Severe abdominal pain - Lethargy, restlessness, or sudden behavioral changes - Bleeding (epistaxis, black-colored stools, hematemesis, excessive menstrual bleeding, dark-colored or bloody urine) - Giddiness - Pale, cold clammy hands and feet - Decreased or no urine output for 4–6 h |
| Indications for hospital admission | - Moderate to severe dehydration - High-risk conditions - Shock or impending shock- narrowing of pulse pressure (≤20 mm Hg); hypotension (systolic BP <80 in adults or children aged >5 y); orthostatic hypotension; delayed capillary refill (> 2 secs); rapid and weak pulse; cold clammy skin; irritability or restlessness; no urine output > 4 h - Leukopenia and/or thrombocytopenia with poor appetite - Clinical deterioration when fever subsides - Change of consciousness - Residence distant from hospital or inconvenient to return to the hospital (if warning signs occur) - Family anxiety/concern |
| Parameters to be monitored during the critical phase of illness (hospitalized patients) | - General conditions: appetite, vomiting, bleeding, capillary refill time (normal < 2 sec) - Vital signs: T, P, RR, BP every 2–3 h in nonshock patients, every hour in shock patients, and every 15 min in profound shock patients (until BP is restored) - Hematocrit every 4–6 h in uncomplicated cases and more frequent in unstable patients or those with suspected/ massive bleeding - Urine output every 8 h in uncomplicated cases and hourly in patients with profound/prolonged shock or those with fluid overload (expected urine output is 0.5 mL/kg/h, based on ideal body weight) |
Abbreviations: BP, blood pressure; P, pulse; RR, respiration rate; T, temperature.
The principal reason for hospital admission for patients with dengue is for management during the critical (leakage) phase of illness, usually not until after 4 days of fever. Suspected dengue patients who are in the febrile phase of illness may warrant hospitalization for moderate to severe dehydration, which would arise from vomiting and/or very poor appetite. Patients who fall into ≥1 of the high-risk groups (infants aged <1 year, obese, elderly, or pregnant patients, and patients with underlying diseases including G6PD deficiency, hemoglobinopathy, diabetes, hypertension, heart disease, chronic kidney disease, or cirrhosis) may also warrant early admission.
Early Detection of Plasma Leakage and Optimal Fluid Management
The clinical experience at QSNICH, reinforced by clinical studies at many other institutions, has emphasized plasma leakage as the pathophysiologic feature of greatest significance to the outcome of dengue. Delayed treatment of intravascular hypovolemia can result in prolonged shock, leading to organ failure and a high mortality rate. The tools for correct management of this complication, standard crystalloid solutions, are widely available, and most patients will respond quickly to treatment. However, errors in management occur when intravenous fluids are administered without sufficient care. Training at QSNICH stresses the dynamic nature of plasma leakage in dengue and the need to titrate intravenous fluids based on frequent assessments of intravascular blood volume, which are done through a combination of clinical and laboratory observations. The consequences of inadequate replacement of a significantly depleted intravascular fluid volume are widely understood by clinicians. However, insufficient attention has been given to the risks of excessive fluid administration, which can lead to the delayed complication of respiratory failure during the phase of resorption of extravascular fluid.
The critical (plasma leakage) period of DHF typically starts around the time of the transition from the febrile to the afebrile phase. The recommended parameters to be monitored routinely in dengue patients hospitalized at QSNICH are listed in Table 3. Laboratory support for frequent CBC determinations is essential because the CBC helps to detect the critical phase. Thrombocytopenia (≤100000 cells/mm3) is a sensitive marker of dengue cases with plasma leakage, but its specificity is lower because it may also be observed in patients with DF [14]. At QSNICH, defervescence or thrombocytopenia trigger more frequent hematocrit measurements (every 4–6 hours). A rise in hematocrit of 10%–15% above baseline is an early objective indicator of plasma leakage. In contrast, clinical fluid accumulation in the chest (pleural effusion) or abdomen (ascites) is often a late finding [13]. A right lateral decubitus chest X-ray or chest and abdominal ultrasound is useful to document the presence and degree of plasma leakage, especially when there is diagnostic uncertainty. However, these tests are not usually essential for acute management because the more readily available clinical parameters mentioned above are adequate to guide care.
Fluid management for plasma leakage in dengue patients is guided by the principle of just adequate replacement of fluid loss to achieve the optimal balance of resuscitation from hypovolemia and minimization of the potential for fluid overload once extravascular fluid is resorbed. Patients with dengue respond differently than patients with sepsis to fluid therapy; with close monitoring, as described above, the clinician can respond quickly to the development of hypovolemia, while the patient will respond quickly to intervention. The type, volume, and duration of intravenous fluids are all important considerations. Because the fluid loss is approximately equivalent to plasma, isotonic crystalloid solutions are recommended, except in very young infants (<6 months of age), in whom 0.45% sodium chloride may be used. In patients with massive plasma leakage and those who do not respond to the minimum volume of crystalloid, hyperoncotic colloid solutions such as 10% dextran-40 in saline are used. Iso-oncotic colloids or albumin are not recommended. Except in patients with shock, the volume of fluid administration in patients with plasma leakage is limited to maintenance fluid requirements plus 5% deficit (5% of body weight) to account for dehydration; if patients are taking oral fluids, the recommended volume reflects the total of oral and intravenous fluids. Recommended infusion rates are given in Table 5. However, frequent assessment of volume and hydration status is essential to ensure adequate fluid replacement because the rate of plasma leakage varies between patients.
Table 5.
Intravenous Fluid Infusion Rates Used at the Queen Sirikit National Institute of Child Health, Bangkok
| Patient status | Infusion rate(s) |
|---|---|
| Prolonged/profound shock (undetectable blood pressure) | Initial: 20 mL/kg normal saline over 15–20 min or until BP restored Correct ABCS If no improvement: Recheck hematocrit, colloid If hematocrit falling: transfuse whole blood (10 mL/kg) or packed RBC (5 mL/kg) over 1 h If organ failure, special management needed (see text) When blood pressure restored: Follow recommendations under “Shock” below |
| Shock | Initial: 10 mL/kg/h isotonic crystalloid for 1–2 h If no improvement: Check hematocrit and ABCS Hematocrit rising: colloid 10 mL/kg/h Hematocrit falling: transfusion (see above under “Profound shock”) When patient improves: Stepwise reduction in infusion rate: 7, 5, 3, 1.5 mL/kg/h over 24–48 h |
| Nonshock (if inadequate oral intake) | Initial: Hemoconcentration ≥20%: 3 mL/kg/h (80 mL/h for adults) Hemoconcentration <20%: 1–2 mL/kg/h (20–40 mL/h for adults) |
Abbreviations: ABCS; acidosis, bleeding, calcium, and sugar; BP, blood pressure; RBC, red blood cell.
Limiting the duration of intravenous fluid therapy to the minimum necessary is essential to reduce the occurrence of intravascular fluid overload during the resorption phase, which typically begins 12–24 hours after resolution of leakage. Patients with shock are already well into the critical (leakage) phase, and intravenous fluids are typically needed for no more than 24–36 hours after resuscitation. Once these patients respond to the initial fluid infusion, the infusion rate is reduced at programmed intervals over the next 24 hours to 5 mL/kg/h by 6 hours, 3 mL/kg/h by 12 hours, and <3 mL/kg/h by 18 hours. Frequent clinical evaluations are necessary to detect any recurrence of shock. Patients with plasma leakage who are not in shock may have been identified at a very early point of the critical period. A lower initial infusion rate is used in these cases, adjusted for the degree of hemoconcentration (see Table 5), and frequent clinical evaluations are used to determine the need to increase the infusion rate. These patients may need a slightly longer duration of fluid administration because plasma leakage may increase before it resolves; however, fluids should not be continued longer than 60–72 hours unless other complications occur.
When patients with dengue do not respond adequately to fluid administration, it usually indicates the occurrence of ≥1 complication. The acronym ABCS has been used to highlight 4 common complications: acidosis, bleeding, calcium (hypocalcemia), and sugar (hypoglycemia). Laboratory evaluations important in the management of DHF grade IV (profound shock) and patients who respond poorly to therapy include capillary or venous blood gas, serum lactate, electrolytes, calcium, liver function tests, BUN, creatinine, uric acid, coagulation profile, cardiac enzymes, and amylase. A right decubitus chest X-ray and/or abdominal ultrasound is helpful to document the degree of plasma leakage; the latter examination can also help to evaluate the etiology of abdominal pain. Low serum albumin (<3.5 gm% in most patients and <4 gm% in obese patients) is a useful, although less reliable, indicator of plasma leakage when these imaging modalities cannot be obtained. If laboratory testing is not available, empiric administration of calcium gluconate and vitamin K is appropriate. Empiric administration of sodium bicarbonate is only recommended in cases with persistent shock after 30 minutes or more of resuscitation efforts.
In the great majority of cases managed appropriately, plasma leakage ceases within 24–48 hours. Recovery is indicated by the findings of stable pulse, blood pressure, and respiratory rate, normal temperature, return of appetite without vomiting or abdominal pain, good urinary output, stable hematocrit at baseline level, and absence of evidence of external or internal bleeding. A convalescent rash, which occurs as pruritic erythematous macules intermingling with areas of whitish discoloration on the extremities [15], may be seen; this is an indication that patients have entered the recovery phase. Some patients will show sinus bradycardia; in most cases, no treatment is indicated, but rare cases have been reported of myocarditis and/or heart block requiring specific treatment [16]. Mild tachypnea is seen in some patients due to resorption of large volumes of extravasated fluid back into the circulation. If respiratory distress occurs along with signs of pulmonary edema/congestion, diuretic treatment is needed. Patients are discharged from the hospital when fever has been absent for 48 hours, there has been improvement in general clinical condition (well-being, appetite, hemodynamic status, and urine output), and the hematocrit and platelet count have both stabilized off intravenous fluid therapy.
Management of Major Bleeding Complications
Bleeding is common in dengue [14]. In contrast with expectations arising from use of the term hemorrhagic fever, however, bleeding is mild in most patients, even though both thrombocytopenia and capillary fragility (demonstrated by a positive tourniquet test) are quite common. Such bleeding typically involves the skin (petechiae, ecchymoses) and mucosae (epistaxis, gum bleeding). However, internal bleeding is an important contributing factor for severity in a minority of patients and may lead to shock and fatality if not promptly recognized and managed. The gastrointestinal tract is the most common site for internal bleeding. Use of nonsteroidal anti-inflammatory drugs (NSAIDs) may be associated with a gastric ulcer and an increased risk of bleeding, particularly in adults. Hematemesis and melena are the most common clinical findings but may be absent or delayed in their presentation. Abdominal pain can be a prominent symptom. Other manifestations of bleeding include menorrhagia (in women), hematuria, and hemoptysis. Hemoglobinopathies are common in dengue endemic areas and may predispose these individuals to nonautoimmune hemolytic anemia and hemoglobinuria. The risk of bleeding is greatly increased in patients with severe plasma leakage who develop hypovolemic shock. Clinicians should maintain a high level of suspicion for internal bleeding in cases that do not respond to usual fluid resuscitation, especially in the presence of normal or low hematocrits. A decrease in hematocrit may be absent despite significant acute blood loss due to the time required for fluid redistribution and hemoconcentration resulting from plasma leakage.
Transfusion is indicated in patients with significant bleeding. The primary objective of transfusion in this setting is to improve oxygenation. Fresh whole blood should be given at 10 mL/kg/dose or 1 unit in adults. If volume overload is a concern, packed red blood cells should be given at 5 mL/kg/dose or 1 unit in adults. The transfusion should be carried out over 1 hour, and hematocrit readings should be obtained before and after transfusion. In the absence of ongoing blood loss, the hematocrit should increase by 5 points after transfusion at this recommended amount.
Prophylactic platelet transfusions have not been shown to be beneficial in pediatric or adult dengue [17, 18] and are not recommended. Platelet transfusion may be considered if the platelet count is <10000 cells/mm3 in adults with underlying conditions that might predispose to bleeding, such as hypertension or the use of medications that interfere with coagulation (eg, aspirin or other anticoagulants).
Early Recognition and Management of Less-Common Dengue Syndromes (Unusual Manifestations or Expanded Dengue Syndrome)
Plasma leakage and bleeding are the most frequent findings in severe dengue cases and are the major focus for clinical management. However, other complications of dengue are increasingly being recognized. End-organ involvement, such as liver and kidney failure and encephalopathy, is mostly the consequence of shock from plasma leakage or bleeding. Treatment for these conditions is primarily supportive. Early recognition of these complications is important so treatment can be initiated or adjusted accordingly. For instance, in cases with fluid overload and oliguric renal failure, renal replacement therapy such as dialysis or plasmapheresis may be needed.
Patients with DSS are sometimes initially misdiagnosed as septic shock. Atypical presentations, such as cases with high fever and leukocytosis with a predominance of neutrophils, increase the difficulty in recognizing dengue as the cause. Plasma leakage may not be apparent initially despite profound shock. Pleural effusion is difficult to detect on supine chest X-rays obtained on critically ill patients, and concealed internal bleeding obfuscates the expected increase in hematocrit from plasma leakage. Empiric treatment for bacterial infection is warranted until culture results are available, but fluid resuscitation following the more conservative guidelines is important because the higher volume of intravenous fluids used in septic shock will increase the risk for volume overload during the resorption phase of DSS. Early use of blood transfusion is another important lesson from the management of DSS because this will mitigate tissue hypoxia that would further contribute to prolonged shock, additional bleeding risk, and complications of organ dysfunction.
Central nervous system manifestations such as altered consciousness and convulsion are frequently observed in severe dengue cases [19]. This may reflect primary viral infection of the central nervous system (dengue encephalitis [20]), although more commonly it is secondary to liver failure (hepatic encephalopathy). The principle for the treatment of hepatic encephalopathy is to maintain adequate oxygenation and proper fluid balance and avoid further liver injury from drugs. Judicious use of fluid is critical to avoid or alleviate brain edema due to an increase in intracranial pressure (ICP); fluid intake should not exceed 80% of maintenance fluid requirements in hemodynamically stable cases. Hyperventilation and elevated head position may be used to decrease ICP. Measures to decrease ammonia production should be initiated, including administration of lactulose to induce osmotic diarrhea and oral neomycin to reduce gut bacteria and ammonia production. Dexamethasone (0.15 mg/kg/dose) may be administered intravenously every 6–8 hours to reduce ICP. Extra care is required to avoid administration of drugs with liver side effects or toxicity. In severe cases with fluid overload and oliguric renal failure, renal replacement therapy such as dialysis or plasmapheresis may be needed.
EVIDENCE BASE FOR CLINICAL LESSONS IN DENGUE MANAGEMENT
Randomized controlled clinical trials (RCTs) represent the acknowledged gold standard of evidence for medical interventions, yet few aspects of management of dengue have been subjected to this level of scrutiny. As a result, most elements of current practice guidelines rely on expert opinion. Given the many areas of general consensus and low CFRs at experienced centers, these elements are unlikely to be subjected to RCTs in the near future.
Randomized Controlled Trials
A search of the PubMed database yields >20 published RCTs in acute dengue illness, of which approximately two-thirds have been published since 2000 [21–36]. These studies have mostly failed to show statistically significant differences in outcomes and therefore largely have reinforced expert opinion. We review these briefly to highlight insights they provide into the typical outcomes from standard treatment guidelines and the difficulties in evaluating the components of these guidelines.
One group of RCTs focused on management of severe dengue cases. These studies enrolled subjects with established DHF or DSS; several studies required subjects to have failed to respond to standard fluid therapy. This study design led to enrollment late in the course of illness. The modalities tested have been different intravenous fluids using the same algorithms [21, 23, 26, 37] or the supplementation of standard care with potential disease modulators such as corticosteroids [38], activated factor VII [24], or pentoxyfylline [29]. Most studies showed either no differences between groups or found small differences in the clinical response to treatment that were not considered clinically meaningful. In earlier studies, CFRs in the control groups were substantial, as high as 44% in 1 study [39], providing clear and clinically important outcomes for statistical analyses. More recent studies have demonstrated much lower CFRs; in several studies, no deaths were recorded, even among subjects presenting in profound shock [21, 26, 37]. These results highlight the success of optimized treatment protocols and demonstrate the challenges for the design of clinical trials to improve on current management strategies.
A second group of studies, including most of those published since 2010, focused on management of dengue from an early stage. These studies enrolled subjects within the first 48–72 hours of onset of illness. Consequently, the frequency of severe clinical outcomes has been low. The treatment modalities tested have included candidate antiviral agents (eg, chloroquine [27, 32], balapiravir [30], or celgosivir [33]) and immunomodulators (eg, corticosteroids [31], lovastatin [36]), and the principal measures of efficacy have been a reduction in viremia and/or illness. These studies show that the typical duration of illness and viremia after the initial presentation is 2–5 days and that most patients with dengue seen during the early stage experience mild disease without complications and do not require specific therapy.
Comparisons of Clinical Outcomes Before and After Training
Although evidence to support current management strategies is largely lacking from RCTs, historical data support the value of the clinical lessons described above in reducing morbidity and mortality from dengue. Since the first designation of QSNICH as a WHO CC, staff have been expatriated as WHO Short Termed Consultants or Temporary Advisors to help in dengue case management in the following countries: Bangladesh, Bhutan, Brazil, Cambodia, Cape Verde, India, Indonesia, Lao PDR, Malaysia, Maldives, Myanmar, Pakistan, Philippines, Sri Lanka, Sudan, Timor Leste, and Vietnam. Table 6 compares the dengue CFRs before and after training of local staff in 10 outbreak areas between 2001 and 2011; each region saw a reduction in the CFR, which was often substantial. Details on the results of training in Timor Leste in 2005 have been published [40]; the program led to an immediate reduction in CFR from 12% to 3.6%, with improvement sustained for at least 1 year. Follow-up data are not available to assess the effects of training in Lahore, Pakistan, in 2011. However, the CFR was 1.7% (n = 364/21597) with 10–15 deaths recorded each day for 1 month before the training, and this was quickly reduced such that only 1 death was recorded in the 10 days after training was conducted. Reductions in CFR have been sustained through follow-up training programs in Thailand (from 1998–2015, CFR reduced from 0.34% [n = 432/127179] to 0.10% [n = 141/142925]), Cambodia (from 2010–2015, CFR reduced from 0.7% to 0.3%), and other SouthEast Asia Regional Office countries (from 2010–2012, CFR reduced from 0.56% [n = 1982/355525] to 0.48% [n = 1229/257204]).
Table 6.
Outcomes of Dengue After On-site Training on Dengue Case Management According to the Queen Sirikit National Institute of Child Health Treatment Guidelines
| Year | Country | Outcomes reported before assignment | Outcomes reported one year after assignment | ||
|---|---|---|---|---|---|
| Cases/deaths | CFR | Cases/deaths | CFR | ||
| 2001 | Bangladesh | 2430/44 | 1.81 | 6140/58 | 0.95 |
| 2004 | Bhutan | 2579/0 | 0.00 | 11/0 | 0.00 |
| 2005 | Timor Leste | 1128/40 | 3.55 | 162/0 | 0.00 |
| 2006 | Maldives | 2768/10 | 0.36 | 1680/2 | 0.12 |
| 2007 | Cambodia | 39618/396 | 1.00 | 9546/66 | 0.70 |
| 2009 | Bhutan | 351/8 | 2.28 | 887/4 | 0.45 |
| 2009 | Sri Lanka | 35010/346 | 0.99 | 34105/246 | 0.72 |
| 2009 | Cape Verde | 21383/6 | 0.03 | 406/0 | 0.00 |
| 2009 | Pakistan | 1940/13 | 0.67 | 15901/40 | 0.25 |
| 2011 | Maldives | 2909/12 | 0.41 | 1083/1 | 0.09 |
RESEARCH NEEDS
Notwithstanding the success of current strategies for management of dengue cases, there are substantial challenges in achieving optimal outcomes. This is particularly the case in settings with limited frontline availability of trained staff, clinical laboratory support, and/or supplies for treatment. As illustrated by the experience at QSNICH and elsewhere, lapses in care during the early phase of severe dengue have a lasting negative impact on clinical outcomes, even after transfer to an experienced, well-equipped referral center. We propose at least 4 areas of research to further improve outcomes.
Early Phase Case Management/Triage: Hospital Admission Criteria
Decisions on hospital admission versus outpatient follow up of suspected dengue cases and the frequency of re-evaluation remain challenging. Early during illness, indications of more severe illness are absent or subtle. Medical personnel with less training or experience may miss these early signs, contributing to delays in initiation of optimal therapy and consequently to increased morbidity and mortality. Recent guidelines promulgated by the WHO [41] have proposed a revised case classification of dengue illness and case management algorithms tied to each of the categories of dengue, dengue with warning signs, and severe dengue. We and others have expressed concerns about these revised management guidelines [42]. The expanded indications for referral for hospital admission in the revised guidelines were intended to improve early recognition of more severe cases, but the low specificity of these criteria has been found to result in overuse of admission [43, 44], which strains resources further and can lead to iatrogenic complications. Several different algorithms to identify patients at high risk for severe dengue at early time points have been generated from prospective studies [45–47]. However, none of these algorithms has been independently validated. Further research on implementation of algorithms for initial management of suspected cases is needed. The ideal instrument would use data readily obtained in low-resource settings and require minimal training or technical expertise.
Goal-Directed Fluid Management
As described above, optimal fluid management in dengue cases with plasma leakage requires close monitoring, experienced staff, and laboratory support. However, although there is broad agreement on the need to balance the restoration of intravascular volume with avoidance of volume overload, there are significant differences of opinion on the selection of fluids and infusion rates. The few RCTs mentioned above that have addressed fluid therapy have focused on the choice of intravenous fluids, and there have not been RCTs to establish an optimal fluid dosing regimen. Trials that more specifically define the clinical parameters to be targeted with intravenous fluid administration would facilitate training, particularly if they incorporate measurements that can readily be performed at the bedside such as with low-cost noninvasive devices [48].
Proof-of-Concept Antiviral/Immunomodulatory Therapies
Although results of RCTs have been disappointing to date, the consistent observations that severe dengue illness involves high viremia titers early in illness [49, 50] and the excessive activation of immune responses [51, 52] provides a strong rationale for the use of antiviral and/or immunomodulatory therapies in dengue. Relatively poor in vitro activity against DENV may explain the past failures of candidate antiviral agents. Immunomodulatory therapy may need to be more specifically targeted than the agents tested to date (eg, against vascular endothelial growth factor [VEGF] activity). Because the most familiar drugs have already been tested, candidate agents will likely need to be studied initially in small, proof-of-concept trials. Candidates that are not feasible for broad use, either because of difficulty in delivery or costs, could still provide critical information about effective classes of agents that would help to direct further drug development.
Biomarkers
Recent studies have identified biomarkers and noninvasive measurements that appear to have promise for guiding the triage and management of dengue patients [48, 51, 53–55]. However, these observations are based on very small studies that have not been validated. In addition, many of these parameters change significantly over the course of dengue illness, as is seen for viremia and cytokine levels [49, 52]. Further studies are needed to establish the sensitivity and specificity of these parameters in a range of real-world clinical scenarios. These studies should identify patient populations where their application is likely to have the greatest impact on the processes and outcomes of care. The development of low-cost, point-of-care devices to measure these parameters for use by frontline clinicians [48] would represent a significant advance in dengue case management.
CONCLUSIONS
Inasmuch as efforts to control dengue virus transmission have not yielded progress to date, it is fortunate that morbidity and mortality from dengue have been successfully reduced, even in settings with modest healthcare resources. Clinical experience has identified several key principles for timely recognition and attentive management of plasma leakage and severe bleeding, the major contributors to poor outcomes in dengue. Training programs that disseminate this knowledge from referral centers to new outbreak areas have achieved significant reductions in CFRs, but opportunities still exist for well-conducted clinical trials to clarify and improve best clinical practices.
Notes
Financial support. This work was supported by grant P01 AI034533 from the National Institutes of Health.
This work was in part supported by the National Health and Medical Research Council of Australia and the Queensland State Government (Accelerate Fellowship to D.A.M.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Leligdowicz A, Fischer WA, 2nd, Uyeki TM, et al. Ebola virus disease and critical illness. Crit Care 2016; 20:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al. Possible association between Zika virus infection and microcephaly—Brazil, 2015. Morb Mortal Wkly Rep 2016; 65:59–62. [DOI] [PubMed] [Google Scholar]
- 3. Hammon WM. Observations on dengue fever, benign protector and killer: a Dr. Jekyll and Mr. Hyde. Am J Trop Med Hyg 1969; 18:159–65. [DOI] [PubMed] [Google Scholar]
- 4. Fresh JW, Reyes V, Clarke EJ, Uylangco CV. Philippine hemorrhagic fever: a clinical, laboratory, and necropsy study. J Lab Clin Med 1969; 73:451–8. [PubMed] [Google Scholar]
- 5. Bhamarapravati N, Tuchinda P, Boonyapaknavik V. Pathology of Thailand haemorrhagic fever: a study of 100 autopsy cases. Ann Trop Med Parasitol 1967; 61:500–10. [DOI] [PubMed] [Google Scholar]
- 6. Nimmannitya S, Halstead SB, Cohen SN, Margiotta MR. Dengue and chikungunya virus infection in man in Thailand, 1962–1964. I. Observations on hospitalized patients with hemorrhagic fever. Am J Trop Med Hyg 1969; 18:954–71. [DOI] [PubMed] [Google Scholar]
- 7. Anonymous. Dengue haemorrhagic fever: diagnosis, treatment and control. Geneva: World Health Organization; 1986. 1–58. [Google Scholar]
- 8. Anonymous. Dengue haemorrhagic fever: diagnosis, treatment and control. Geneva: World Health Organization; 1997. 1–58. [Google Scholar]
- 9. Anonymous. Comprehensive guidelines for prevention and control of dengue and dengue haemorrhagic fever. Revised and expanded version. New Delhi: World Health Organization, Regional Office for South-East Asia; 2011. [Google Scholar]
- 10. Potts JA, Rothman AL. Clinical and laboratory features that distinguish dengue from other febrile illnesses in endemic populations. Trop Med Int Health 2008; 13:1328–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hunsperger EA, Muñoz-Jordán J, Beltran M, et al. Performance of dengue diagnostic tests in a single-specimen diagnostic algorithm. J Infect Dis 2016; 214:836–44. [DOI] [PubMed] [Google Scholar]
- 12. Kalayanarooj S, Vaughn DW, Nimmannitya S, et al. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis 1997; 176:313–21. [DOI] [PubMed] [Google Scholar]
- 13. Srikiatkhachorn A, Krautrachue A, Ratanaprakarn W, et al. Natural history of plasma leakage in dengue hemorrhagic fever: a serial ultrasonographic study. Pediatr Infect Dis J 2007; 26:283–90. [DOI] [PubMed] [Google Scholar]
- 14. Srikiatkhachorn A, Gibbons RV, Green S, et al. Dengue hemorrhagic fever: the sensitivity and specificity of the World Health Organization definition for identification of severe cases of dengue in Thailand, 1994–2005. Clin Infect Dis 2010; 50:1135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nelwan EJ, Pohan HT. Dengue convalescent rash in adult Indonesian patients. Acta Med Indones 2014; 46:339–40. [PubMed] [Google Scholar]
- 16. Shivanthan MC, Navinan MR, Constantine GR, Rajapakse S. Cardiac involvement in dengue infection. J Infect Dev Ctries 2015; 9:338–46. [DOI] [PubMed] [Google Scholar]
- 17. Lye DC, Lee VJ, Sun Y, Leo YS. Lack of efficacy of prophylactic platelet transfusion for severe thrombocytopenia in adults with acute uncomplicated dengue infection. Clin Infect Dis 2009; 48:1262–5. [DOI] [PubMed] [Google Scholar]
- 18. Lum LC, Abdel-Latif Mel-A, Goh AY, Chan PW, Lam SK. Preventive transfusion in Dengue shock syndrome—is it necessary? J Pediatr 2003; 143:682–4. [DOI] [PubMed] [Google Scholar]
- 19. Sahu R, Verma R, Jain A, et al. Neurologic complications in dengue virus infection: a prospective cohort study. Neurology 2014; 83:1601–9. [DOI] [PubMed] [Google Scholar]
- 20. Ramos C, Sánchez G, Pando RH, et al. Dengue virus in the brain of a fatal case of hemorrhagic dengue fever. J Neurovirol 1998; 4:465–8. [DOI] [PubMed] [Google Scholar]
- 21. Ngo NT, Cao XT, Kneen R, et al. Acute management of dengue shock syndrome: a randomized double-blind comparison of 4 intravenous fluid regimens in the first hour. Clin Infect Dis 2001; 32:204–13. [DOI] [PubMed] [Google Scholar]
- 22. Cam BV, Tuan DT, Fonsmark L, et al. Randomized comparison of oxygen mask treatment vs. nasal continuous positive airway pressure in dengue shock syndrome with acute respiratory failure. J Trop Pediatr 2002; 48:335–9. [DOI] [PubMed] [Google Scholar]
- 23. Wills BA, Nguyen MD, Ha TL, et al. Comparison of three fluid solutions for resuscitation in dengue shock syndrome. N Engl J Med 2005; 353:877–89. [DOI] [PubMed] [Google Scholar]
- 24. Chuansumrit A, Wangruangsatid S, Lektrakul Y, Chua MN, Zeta Capeding MR, Bech OM; Dengue Study Group Control of bleeding in children with dengue hemorrhagic fever using recombinant activated factor VII: a randomized, double-blind, placebo-controlled study. Blood Coagul Fibrinolysis 2005; 16:549–55. [DOI] [PubMed] [Google Scholar]
- 25. Jacobs J, Fernandez EA, Merizalde B, Avila-Montes GA, Crothers D. The use of homeopathic combination remedy for dengue fever symptoms: a pilot RCT in Honduras. Homeopathy 2007; 96:22–6. [DOI] [PubMed] [Google Scholar]
- 26. Kalayanarooj S. Choice of colloidal solutions in dengue hemorrhagic fever patients. J Med Assoc Thai 2008; 91Suppl 3:S97–103. [PubMed] [Google Scholar]
- 27. Tricou V, Minh NN, Van TP, et al. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl Trop Dis 2010; 4:e785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Castro JE, Vado-Solis I, Perez-Osorio C, Fredeking TM. Modulation of cytokine and cytokine receptor/antagonist by treatment with doxycycline and tetracycline in patients with dengue fever. Clin Dev Immunol 2011; 2011:370872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salgado D, Zabaleta TE, Hatch S, Vega MR, Rodriguez J. Use of pentoxifylline in treatment of children with dengue hemorrhagic fever. Pediatr Infect Dis J 2012; 31:771–3. [DOI] [PubMed] [Google Scholar]
- 30. Nguyen NM, Tran CN, Phung LK, et al. A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in adult dengue patients. J Infect Dis 2013; 207:1442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tam DT, Ngoc TV, Tien NT, et al. Effects of short-course oral corticosteroid therapy in early dengue infection in Vietnamese patients: a randomized, placebo-controlled trial. Clin Infect Dis 2012; 55:1216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borges MC, Castro LA, Fonseca BA. Chloroquine use improves dengue-related symptoms. Mem Inst Oswaldo Cruz 2013; 108:596–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Low JG, Sung C, Wijaya L, et al. Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): a phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect Dis 2014; 14:706–15. [DOI] [PubMed] [Google Scholar]
- 34. Somasetia DH, Setiati TE, Sjahrodji AM, et al. Early resuscitation of dengue shock syndrome in children with hyperosmolar sodium-lactate: a randomized single-blind clinical trial of efficacy and safety. Crit Care 2014; 18:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fredeking TM, Zavala-Castro JE, González-Martínez P, et al. Dengue patients treated with doxycycline showed lower mortality associated to a reduction in IL-6 and TNF levels. Recent Pat Antiinfect Drug Discov 2015; 10:51–8. [DOI] [PubMed] [Google Scholar]
- 36. Whitehorn J, Nguyen CV, Khanh LP, et al. Lovastatin for the treatment of adult patients with dengue: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2016; 62:468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dung NM, Day NP, Tam DT, et al. Fluid replacement in dengue shock syndrome: a randomized, double-blind comparison of four intravenous-fluid regimens. Clin Infect Dis 1999; 29:787–94. [DOI] [PubMed] [Google Scholar]
- 38. Tassniyom S, Vasanawathana S, Chirawatkul A, Rojanasuphot S. Failure of high-dose methylprednisolone in established dengue shock syndrome: a placebo-controlled, double-blind study. Pediatrics 1993; 92:111–5. [PubMed] [Google Scholar]
- 39. Min M, U T, Aye M, Shwe TN, Swe T. Hydrocortisone in the management of dengue shock syndrome. Southeast Asian J Trop Med Public Health 1975; 6:573–9. [PubMed] [Google Scholar]
- 40. Kalayanarooj S, Rimal HS, Andjaparidze A, et al. Clinical intervention and molecular characteristics of a dengue hemorrhagic fever outbreak in Timor Leste, 2005. AmJTropMed 2007; 77:534–7. [PubMed] [Google Scholar]
- 41. Anonymous. Dengue: guidelines for diagnosis, treatment, prevention and control—new edition. Geneva: World Health Organization, 2009. [PubMed] [Google Scholar]
- 42. Srikiatkhachorn A, Rothman AL, Gibbons RV, et al. Dengue—how best to classify it. Clin Infect Dis 2011; 53:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kalayanarooj S. Dengue classification: current WHO vs. the newly suggested classification for better clinical application? J Med Assoc Thai 2011; 94Suppl 3:S74–84. [PubMed] [Google Scholar]
- 44. Leo YS, Gan VC, Ng EL, et al. Utility of warning signs in guiding admission and predicting severe disease in adult dengue. BMC Infect Dis 2013; 13:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tanner L, Schreiber M, Low JG, et al. Decision tree algorithms predict the diagnosis and outcome of dengue fever in the early phase of illness. PLoS Negl Trop Dis 2008; 2:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee VJ, Lye DC, Sun Y, Leo YS. Decision tree algorithm in deciding hospitalization for adult patients with dengue haemorrhagic fever in Singapore. Trop Med Int Health 2009; 14:1154–9. [DOI] [PubMed] [Google Scholar]
- 47. Potts JA, Gibbons RV, Rothman AL, et al. Prediction of dengue disease severity among pediatric Thai patients using early clinical laboratory indicators. PLoS Negl Trop Dis 2010; 4:e769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Soller B, Srikiatkachorn A, Zou F, et al. Preliminary evaluation of near infrared spectroscopy as a method to detect plasma leakage in children with dengue hemorrhagic fever. BMC Infect Dis 2014; 14:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vaughn DW, Green S, Kalayanarooj S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 2000; 181:2–9. [DOI] [PubMed] [Google Scholar]
- 50. Libraty DH, Endy TP, Houng HS, et al. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis 2002; 185:1213–21. [DOI] [PubMed] [Google Scholar]
- 51. Srikiatkhachorn A, Green S. Markers of dengue disease severity. Curr Top Microbiol Immunol 2010; 338:67–82. [DOI] [PubMed] [Google Scholar]
- 52. Green S, Vaughn DW, Kalayanarooj S, et al. Early immune activation in acute dengue is related to development of plasma leakage and disease severity. J Infect Dis 1999; 179:755–62. [DOI] [PubMed] [Google Scholar]
- 53. Pang J, Lindblom A, Tolfvenstam T, et al. Discovery and validation of prognostic biomarker models to guide triage among adult dengue patients at early infection. PLoS One 2016; 11:e0155993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nhi DM, Huy NT, Ohyama K, et al. A proteomic approach identifies candidate early biomarkers to predict severe dengue in children. PLoS Negl Trop Dis 2016; 10:e0004435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cui L, Lee YH, Thein TL, et al. Serum metabolomics reveals serotonin as a predictor of severe dengue in the early phase of dengue fever. PLoS Negl Trop Dis 2016; 10:e0004607. [DOI] [PMC free article] [PubMed] [Google Scholar]