Results from the Strategic Timing of Antiretroviral Treatment (START) study showed that serum albumin is associated with serious non-AIDS occurrence in HIV-infected persons with high CD4 cell counts.
Keywords: albumin, biomarker, HIV, non-communicable disease, non-AIDS comorbidity
Abstract
Background
Serum albumin may be used to stratify human immunodeficiency virus (HIV)–infected persons with high CD4 count according to their risk of serious non-AIDS endpoints.
Methods
Cox proportional hazards models were used to analyze the risk of serious non-AIDS events in the Strategic Timing of Antiretroviral Treatment (START) study (NCT00867048) with serum albumin as a fixed and time-updated predictor. Models with exclusion of events during initial follow-up years were built to assess the ability of serum albumin to predict beyond shorter periods of time. Secondarily, we considered hospitalizations and AIDS events.
Results
Among 4576 participants, 71 developed a serious non-AIDS event, 788 were hospitalized, and 63 experienced an AIDS event. After adjusting for a range of variables associated with hypoalbuminemia, higher baseline serum albumin (per 1 g/dL) was associated with a decreased risk of serious non-AIDS events (hazard ratio, 0.37 [95% confidence interval, .20–.71]; P = .002). Similar results were obtained in a time-updated model, after controlling for interleukin 6, and after excluding initial follow-up years. Serum albumin was independently associated with hospitalization but not with risk of AIDS.
Conclusions
A low serum albumin level is a predictor for short- and long-term serious non-AIDS events, and may be a useful marker of risk of noncommunicable diseases, particularly in resource-limited settings.
(See the editorial commentary by Siedner and Hunt, on pages 347–9.)
With the advent of modern antiretroviral therapy (ART), human immunodeficiency virus (HIV)–infected persons are living longer and noncommunicable diseases are becoming a global health issue in this population [1]. Identification of clinically available prognostic markers may help inform noncommunicable disease pathogenesis in HIV-infected persons as well as provide added value for a personalized approach to these conditions.
One disease marker that is regularly obtained in most settings, and has been well studied in acute and chronic conditions in the general population, is serum albumin. Serum albumin is involved in a number of biological processes including oncotic pressure maintenance [2], storage and transport properties [3], and antioxidant activity [4]. Low serum albumin levels may be associated with multiple conditions that are related to HIV (eg, poor nutritional status, inflammation, liver disease, and nephropathy) [5]. Thus, an association between serum albumin and health may be expected in this population.
A number of observational studies have examined the association between serum albumin and mortality in HIV-infected persons. As in the general population [6], these studies have shown a consistent and strong inverse association between serum albumin and incident health-related outcomes, including overall mortality [7–16] and AIDS-related morbidity [13–16]. Serum albumin was also found to be associated with non-AIDS morbidity in a US Veteran’s study assessing cardiovascular disease (CVD) specifically [8].
In people with HIV who have a high CD4 cell count, the role of serum albumin among predictors of serious non-AIDS events has not been extensively described. The Strategic Timing of Antiretroviral Treatment (START) study enrolled ART-naive HIV-infected individuals with CD4 counts >500 cells/μL and randomized them to either immediate ART initiation or deferred initiation when the CD4 count dropped below 350 cells/μL or when an AIDS-defining illness occurred. We assessed the ability of serum albumin to predict serious non-AIDS over varying follow-up intervals in the context of other prognostic and/or potentially causal factors.
METHODS
Study Population and Design
The multicontinental randomized START trial has been described elsewhere [17, 18]. The study was approved by the institutional review board or ethics committee at each participating site, and written informed consent was obtained from all participants. A total of 4684 people were enrolled in the trial between 2009 and 2013. After an average of 3.0 years of follow-up, the trial was unblinded on 26 May 2015. This report presents data accrued through the unblinding date. Locally measured baseline serum albumin was available from 4576 (98%) participants. Participants were followed up 1 month and 4 months after randomization and every 4 months thereafter for data collection and routine follow-up clinical evaluation.
Study Endpoints
The primary endpoint for this analysis was serious non-AIDS events consisting of the following conditions: CVD (myocardial infarction, stroke, or coronary revascularization) or death from CVD, end-stage renal disease (initiation of dialysis or renal transplantation) or death from renal disease, liver disease (decompensated liver disease) or death from liver disease, non-AIDS-defining cancer (except for basal cell or squamous cell skin cancer) or death from cancer, and any death not attributable to AIDS, accident, or violence. Secondary endpoints included (i) unscheduled hospitalizations that were not related to AIDS; (ii) AIDS events (ie, death from AIDS or any AIDS-defining event); (iii) CVD events; and (iv) non-AIDS cancer. All primary endpoints in START (serious non-AIDS, AIDS, and death) were reviewed by a committee, using preestablished International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) criteria [19]. The primary predictor serum albumin was measured at baseline and annually. Serum albumin was modeled as a continuous variable (fixed and time updated) and categorized in tertiles (defined by cutoffs of 4.2 g/dL and 4.5 g/dL) for Kaplan–Meier survival curves. Baseline categorical covariates investigated included a joint gender/risk group variable (heterosexual women, heterosexual men, men who have sex with men; intravenous drug use [any gender], other [any gender]), race/ethnicity (Asian, black, white, Latino, or Hispanic, other), region (Africa, Asia, Europe/Israel, North America, Oceania, South America), smoking status (current vs former/never), hepatitis B or C, and randomization arm. Baseline numeric covariates investigated were age, randomization date, body mass index (BMI), systolic blood pressure, hemoglobin, platelet count, neutrophil count, lymphocyte count, CD4 and CD8 counts, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), cholesterol, alanine aminotransferase, estimated glomerular filtration rate (eGFR; by Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] formula), urine dipstick protein (negative, trace, 1+, 2+, 3+, 4+, 5+), HIV type 1 (HIV-1) RNA (log10), and interleukin 6 (IL-6).
Statistical Methods
Summary statistics were calculated for the entire cohort and persons with hypoalbuminemia (<3.5 g/dL). Univariate and multivariate linear regression analyses were used to assess the association between baseline albumin levels and covariates. A longitudinal mixed model was fit using PROC MIXED in SAS software to assess the difference in the mean change in laboratory markers from study entry, between the treatment groups. Kaplan–Meier survival curves and log-rank tests were used to depict and compare cumulative incidence for serious non-AIDS events stratified by baseline serum albumin tertiles.
Multivariable Cox proportional hazards models were used to model the time to serious non-AIDS events. Univariable and multivariable hazard ratios (HRs) and 95% confidence intervals (CIs) were computed. Serum albumin was also fitted as a time-updated covariate. Follow-up was until the time of a serious non-AIDS event. For those not experiencing the event, follow-up was censored on the last day of study contact, or at study unblinding, whichever occurred earliest. To assess the ability of serum albumin to predict beyond shorter periods of time, and to address the possibility of bias by reverse causality, we considered the effect of left truncation of the survival time. Selection of potential confounders to be adjusted for in multivariable Cox models was based on clinical knowledge about the relation between serum albumin and the primary endpoint, with the thinking made explicit via the use of directed acyclic graphs. Besides unadjusted analyses (model 1), we considered a Cox model controlled for all covariates considered as potentially related to the outcome and listed above (model 2), as well as a Cox model controlling only for variables that were significantly (P < .05) associated with the outcome (model 3). The proportional hazards assumption was tested by fitting the interaction between baseline albumin and log (follow-up time). An interaction term for serum albumin and randomization arm was included, as well as gender and region, to test if the effect of serum albumin was different according to treatment status, gender, or region. Finally, receiver operating characteristic (ROC) analysis and area under the curve (AUC) was used as another means to assess the diagnostic ability of baseline serum albumin.
Missing covariates were infrequent and analyses were based on a complete case scenario. All P values reported were 2-sided. Analyses were conducted in SAS version 9.4 (SAS Institute, Cary, North Carolina) and graphs in R (version 3.3.0).
RESULTS
A total of 4576 HIV-infected START participants with nonmissing baseline albumin levels were included. One hundred eight individuals did not have a serum albumin level at baseline. This included 2 of the 63 participants experiencing a serious non-AIDS event and 18 of the 788 experiencing hospitalization. Over a total of 14312 person-years of follow-up, 71 participants experienced a serious non-AIDS event (immediate initiation group, n = 24; deferred initiation group, n = 47). The first such event was a non-AIDS-defining cancer in 27 people, a CVD event in 26, and a liver or renal event in 4; for 20 people, the event was fatal. For the secondary outcomes, there were 788 participants with unscheduled hospitalizations and 63 with AIDS events.
Demographic and baseline clinical characteristics of the study population and persons with hypoalbuminemia (<3.5 g/dL; n = 77) are depicted in Table 1. Overall, the centiles for albumin were 1%: 3.3 g/dL; 5%: 3.7 g/dL; 10%: 3.9 g/dL; 25%: 4.1 g/dL; 50%: 4.4 g/dL; 75%: 4.6 g/dL; 90%: 4.8 g/dL; 95%: 5.0 g/dL; 99%: 5.2 g/dL. Mean albumin level was 4.4 g/dL (standard deviation [SD], 0.4) in Europe/United States/Australia, 4.2 g/dL (SD, 0.4) in Africa, 4.5 g/dL (SD, 0.3) in Latin America, and 4.4 g/dL (SD, 0.4) in Asia (P < .001). Most participants in START were nonsmokers, and the geographic regions with the most participants enrolled were Europe and Israel (n = 1476), South America (n = 1173), and Africa (n = 957). Among persons with hypoalbuminemia at baseline, 4 (6% [95% CI, 2%–13%) experienced a serious non-AIDS event. Cross-sectional multivariate comparisons at baseline showed that lower serum albumin levels were associated with several factors including older age, being heterosexual male, black race, living in Australia, current smoking, higher BMI, hepatitis B and C infection, lower hemoglobin, higher HDL, LDL and cholesterol, higher eGFR, higher urinary protein, and higher HIV RNA (Table 2).
Table 1.
Baseline Characteristics of Strategic Timing of Antiretroviral Treatment Study (START) Participants (N = 4576)
| Characteristics | Hypoalbuminemia (<3.5 g/dL) (n = 77) |
All Participants (N = 4576) |
|---|---|---|
| Age, y, mean (SD) | 41.0 (10.1) | 36.8 (10.2) |
| Female sex | 39 (50.6) | 1218 (26.6) |
| Ethnicity | ||
| Black | 54 (70.1) | 1361 (29.8) |
| White | 19 (24.7) | 2031 (44.4) |
| Asian, Latino, and other | 4 (5.2) | 1184 (25.9) |
| Route of infection with HIV | ||
| Same sex | 15 (19.5) | 2544 (55.6) |
| Opposite sex | 51 (66.2) | 1748 (38.2) |
| Intravenous drug use | 5 (6.5) | 60 (1.3) |
| Other | 6 (7.8) | 224 (4.9) |
| Geographic region of residence | ||
| Africa | 41 (53.3) | 957 (20.9) |
| Asia | 3 (3.9) | 356 (7.8) |
| Europe/Israel | 15 (19.5) | 1476 (32.3) |
| North America | 14 (18.2) | 505 (11.0) |
| Oceania | 1 (1.3) | 109 (2.4) |
| South America | 3 (3.9) | 1173 (25.6) |
| Hepatitis B positive | 4 (5.4) | 128 (2.9) |
| Hepatitis C positive | 9 (12.0) | 167 (3.7) |
| Ever smoker | 20 (26.0) | 1466 (32.0) |
| BMI, kg/m2, mean (SD) | 28.0 (7.8) | 25.7 (5.4) |
| SBP, mm Hg, mean (SD) | 123.9 (20.4) | 121.6 (14.8) |
| CD4 count, cells/μL, mean (SD) | 695 (156) | 700 (170) |
| HDL, mg/dL, mean (SD) | 36.6 (13.0) | 43.3 (12.8) |
| LDL, mg/dL, mean (SD) | 95.5 (29.9) | 104.7 (31.8) |
| HIV RNA, log10 copies per mL, mean (SD) | 4.2 (0.9) | 4.0 (0.9) |
| Hemoglobin, g/dL, mean (SD) | 12.7 (1.9) | 14.3 (1.5) |
| ALT, IU/L, mean (SD) | 29.2 (19.2) | 30.1 (29.5) |
| eGFRa, mL/min/1.73m2, mean (SD) | 117 (20.1) | 110 (18.6) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; LDL, low-density lipoprotein; SBP, systolic blood pressure; SD, standard deviation.
aUsing the creatinine Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.
Table 2.
Cross-Sectional Multivariate Risk Factor Analyses for Baseline Serum Albumin Concentration (N = 4576)
| Characteristics | Univariate Analysis Coefficient (β) (95% CI)a |
Multivariate Analysis Coefficient (β) (95% CI)a |
P Valueb |
|---|---|---|---|
| Age per decade | –0.08 (–.09 to –.01) | –0.08 (–.09 to –.07) | <.0001 |
| Ethnicity | |||
| White | Ref | Ref | |
| Black | –0.20 (–.23 to –.18) | –0.06 (–.10 to –.02) | .002 |
| Latino | 0.04 (.01–.07) | 0.04 (.01–.08) | .027 |
| Asian | –0.05 (–.09 to –.01) | 0.01 (–0.11 to .12) | .935 |
| Other | 0.01 (–.05 to .07) | 0.01 (–.11 to .12) | .938 |
| Geographic region of residence | |||
| North America | Ref | Ref | |
| Africa | –0.07 (–.11 to –.02) | 0.03 (–.02 to .07) | .160 |
| South America | 0.17 (.13–.21) | 0.07 (.03–.11) | .001 |
| Europe/Israel | 0.16 (.12–.20) | 0.08 (.04–.12) | .001 |
| Oceania | –0.01 (–.09 to .07) | –0.09 (–.16 to –.02) | .016 |
| Asia | 0.09 (.04–.14) | 0.02 (–.10 to .14) | .706 |
| Joint risk group/ gender | |||
| Heterosexual female | Ref | Ref | |
| Heterosexual male | –0.18 (–.22 to –.14) | –0.05 (–.09 to –.01) | .010 |
| MSM | 0.08 (.04–.11) | –0.01 (–.05 to .02) | .462 |
| Intravenous drug use (any gender) | –0.20 (–.30 to –.10) | –0.09 (–.19 to .01) | .078 |
| Other (any gender) | –0.08 (–0.14 to –.03) | –0.07 (–.12 to –.01) | .015 |
| Hepatitis B positive | –0.11 (–.17 to –.04) | –0.09 (–.15 to –.03) | .002 |
| Hepatitis C positive | –0.12 (–.19 to –.06) | –0.04 (–.10 to .02) | .175 |
| Current smoker | 0.02 (–0.01 to .04) | –0.06 (–.08 to –.04) | <.0001 |
| BMI, kg/m2 | –0.01 (–.01 to –.01) | –0.01 (–.01 to –.00) | <.0001 |
| CD4 count per 100 cells/μL | –0.00 (–.01 to .00) | 0.00 (–.01 to .01) | .712 |
| HIV RNA, log10 copies per mL | –0.02 (–.03 to –.00) | –0.03 (–.04 to –.02) | <.0001 |
| SBP per 10 mm Hg | 0.00 (–.01 to .01) | 0.01 (.01–.02) | .001 |
| Hemoglobin, g/dL | 0.10 (.10–.11) | 0.08 (.07–.09) | <.0001 |
| ALT per 10 IU/L | 0.00 (.00–.00) | 0.00 (.00–.00) | .918 |
| eGFRc per 10 mL/ min/1.73m2 | –0.01 (–.02 to –.01) | –0.01 (–.02 to .01) | .001 |
| HDL per 10 mg/dL | 0.04 (.03–.05) | 0.04 (.03–.05) | <.0001 |
| LDL per 10 mg/dL | 0.01 (.01–.02) | 0.01 (.01–.02) | <.0001 |
| Urinary (per 1 category higher) | –0.08 (–.09 to –.06) | –0.05 (–0.7 to –.3) | <.0001 |
| D-dimer, µg/mL (log10) | –0.22 (–.23 to –.20) | –0.06 (–.07 to –.03) | <.0001 |
| IL-6 pg/mL, (log10) | –0.14 (–.16 to –.12) | –0.07 (–.03 to –.01) | <.0001 |
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; IL-6, interleukin 6; LDL, low-density lipoprotein; MSM, men who have sex with men; SBP, systolic blood pressure; SD, standard deviation; START, Strategic Timing of Antiretroviral Treatment study.
aβ-coefficient expresses the expected increase/decrease in serum albumin (mg/dL) for specified change in a predictor from regression models.
b P value for the multivariate model only.
cUsing the creatinine Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.
Immediate initiation of ART was significantly associated with higher average serum albumin concentrations over follow-up (0.086 [95% CI, .070–.102] g/dL; P < .0001) compared with deferral.
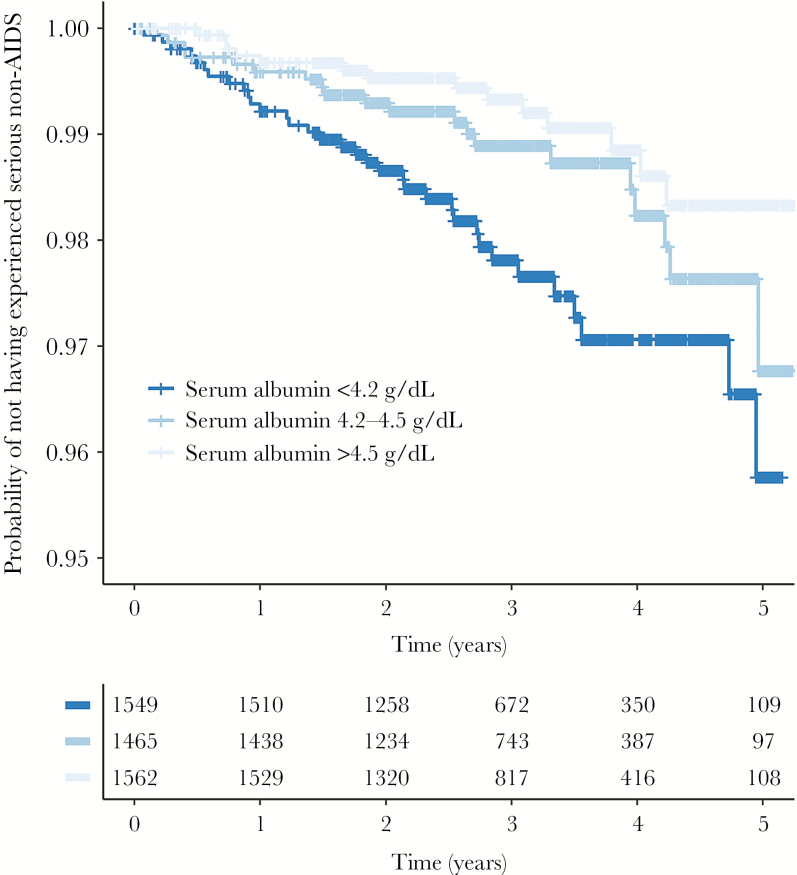
We then performed prospective analyses for the risk of the primary composite endpoint (serious non-AIDS event) by baseline serum albumin. In crude analysis (model 1), higher serum albumin was associated with a decreased risk of serious non-AIDS events (HR, 0.26 [95% CI, .15–.46] per 1 g/dL higher level; P < .0001; Table 3). We also utilized serial measurements of serum albumin and considered serum albumin as a time-updated variable that moved the effect estimate slightly toward 1 (HR, 0.34 [95% CI, .22–.54]; P < .0001). In analyses adjusted for all factors potentially related with the outcome (model 2), serum albumin persisted to be a predictor for serious non-AIDS events (HR, 0.39 [95% CI, .20–.79]; P = .009; Table 3). The interaction term between serum albumin and randomization arm was nonsignificant (P = .391). While all models were stratified by region, we did fit 1 unstratified model in which we divided regions into Europe/United States/Australia, Africa, Latin America, and Asia and assessed whether there was an interaction between this and the effect of albumin on serious non-AIDS, but we found no evidence for this (P > .1). There was also no evidence for an interaction between gender and the effect of albumin (P > .1). In model 3, factors that were univarately associated with the outcome (ie, age, total cholesterol, eGFR, hemoglobin, randomization arm, and systolic blood pressure), higher serum albumin was also associated with a decreased risk of serious non-AIDS events (HR, 0.37 [95% CI, .20–0.71]; P = .002; Table 3). IL-6 (log10) was added to model 3, which had little effect on the estimate (HR, 0.47 [95% CI, .25–.91]; P = .024). Left truncating events and follow-up occurring in the first, second, and third year of follow-up had little effect on the HRs, although the CIs widened due to reduced numbers of events (Table 4). Kaplan–Meier survival curves for serious non-AIDS events stratified by baseline serum albumin tertiles are depicted in Figure 1 (log-rank P < .01).
Table 3.
Hazard Ratios for the Risk of Serious Non-AIDS Events, Hospitalization, and AIDS per 1 g/dL Higher Baseline Serum Albumin
| Outcome (No. of Participants With Event) |
Crude HR (95% CI) | Adjusted HR Model 2a (95% CI) |
P Value | Adjusted HR Model 3b (95% CI) |
P Value | Adjusted HR Model 3 + IL-6c (95% CI,) |
P Value |
|---|---|---|---|---|---|---|---|
| Serious non-AIDS (71) | 0.26 (.15–.46) | 0.39 (.20–.79) | .009 | 0.37 (.20–.71) | .002 | 0.47 (.25–.91) | .024 |
| Hospitalization (788) | 0.61 (.51–.73) | 0.77 (.62– .96) | .019 | 0.78 (.64–.96) | .018 | 0.77 (.62–.95) | .013 |
| AIDS (63) | 1.07 (.57–2.03) | … | … | … | … | … | … |
| CVD (26) | 0.58 (.21– 1.57) | … | … | … | … | … | … |
| Non-AIDS cancer (27) | 0.29 (.12–.72) | 0.40 (.12– 1.31) | .131 | … | … | … | … |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; IL-6, interleukin 6.
aIn model 2, all covariates were included—that is, age, joint gender/risk group, ethnicity/race, randomization arm, smoking, hepatitis C virus, hepatitis B virus, body mass index (BMI), systolic blood pressure, hemoglobin, lymphocyte count, neutrophil count, platelets, CD4 and CD8 cell count, human immunodeficiency virus (HIV) RNA (log10), cholesterol, low-density lipoprotein (LDL), high-density lipoprotein, estimated glomerular filtration rate (eGFR), alanine aminotransferase, and urinary protein.
bIn model 3, covariates were included if they were univariately associated with the outcome (P < .05). For the primary outcome (serious non-AIDS events), this included age, randomization arm, hemoglobin, eGFR, and systolic blood pressure, and for hospitalization this included joint gender/risk group, current smoking, BMI, hemoglobin, CD4/CD8 ratio, HIV RNA, LDL, and urinary protein.
cFinally, IL-6 (log10) was added to model 3. Only crude analyses for AIDS and CVD are reported as serum albumin was not univariately associated with these endpoints.
Table 4.
Hazard Ratios for the Risk of Serious Non-AIDS Events, Hospitalization, and AIDS per 1 g/dL Higher Baseline Serum Albumin by Year of Follow-up
| Outcome (No. of Participants With Event) | Adjusted HR (95% CI) |
|---|---|
| Serious non-AIDS | |
| Restricted to follow-up beyond 1 y from baseline (50) | 0.46 (.23–.93) |
| Restricted to follow-up beyond 2 y from baseline (34) | 0.35 (.15–.82) |
| Restricted to follow-up beyond 3 y from baseline (20) | 0.47 (.16–1.37) |
| Hospitalization | |
| Restricted to follow-up beyond 1 y from baseline (587) | 0.61 (.49–.76) |
| Restricted to follow-up beyond 2 y from baseline (321) | 0.59 (.44–.79) |
| Restricted to follow-up beyond 3 y from baseline (138) | 0.61 (.39–.95) |
All models were age-adjusted.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Figure 1.
Kaplan–Meier survival curves for serious non-AIDS events according to baseline serum albumin levels. Kaplan–Meier survival curves with risk table for serious non-AIDS events (n = 71) stratified by serum albumin tertiles at baseline. Log-rank test of equality of strata (P = .001).
We also assessed the ability of baseline serum albumin to discriminate according to the future occurrence of a serious non-AIDS event. AUC was 0.64. A ROC curve for baseline serum albumin is depicted in Supplementary Material 1.
Secondary Analysis
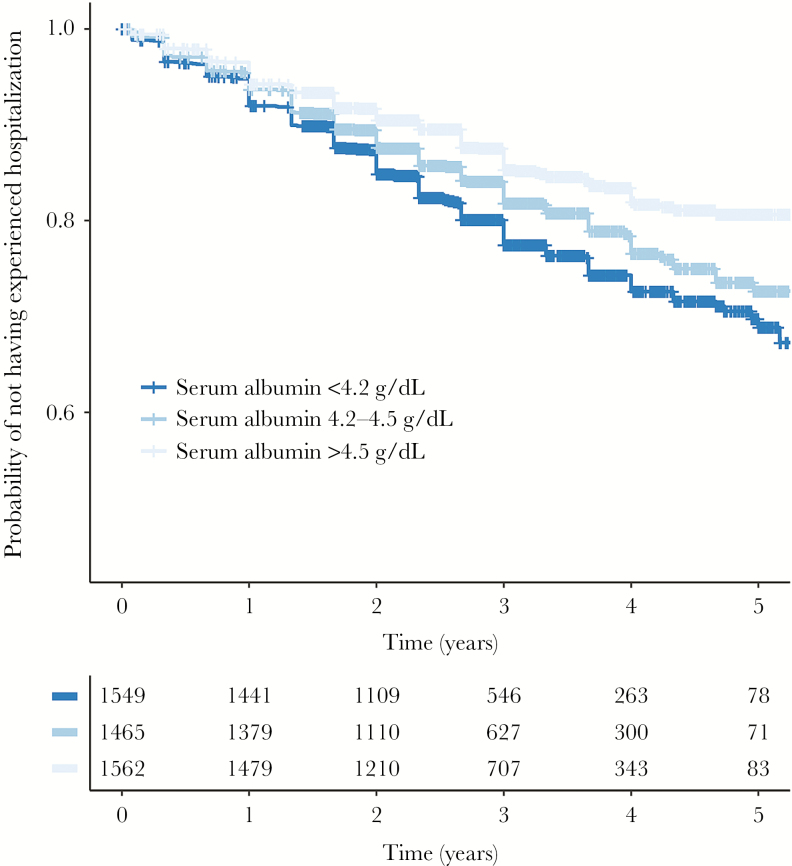
Serum albumin was not associated with AIDS but was found to be independently associated with hospitalization in all models, including when IL-6 was added (Table 3). Kaplan–Meier survival curves for hospitalization stratified by baseline serum albumin tertiles are depicted in Figure 2 (log-rank P < .001). As with the primary outcome, restriction of follow-up time to after years 1, 2, and 3 did not change the effect estimates on hospitalization, although the CIs widened (Table 3). Finally, components of the serious non-AIDS endpoints were assessed separately. In crude analysis, serum albumin was associated with the rate of non-AIDS cancers (HR, 0.29 [95% CI, .12–.72]; P < .01), but this association did not persist after controlling for additional factors (Table 3). Serum albumin was not found to be associated with CVD in crude analysis (HR, 0.58 [95% CI, .21–1.57]; P < .284). Interaction between treatment status and serum albumin was not significant for any of the above outcomes.
Figure 2.
Kaplan–Meier survival curves for hospitalization according to baseline serum albumin levels. Kaplan–Meier survival curves with risk table for hospitalization events (n = 788) stratified by serum albumin tertiles at baseline. Log-rank test of equality of strata (P < .0001).
DISCUSSION
We found that lower serum albumin was a strong predictor of serious non-AIDS events and hospitalization in seemingly healthy HIV-infected persons entering the START study with high CD4 counts (median, 652 cells/μL). These associations were independent of traditional risk factors and various laboratory measures. Serum albumin had an ability to predict serious non-AIDS events over both short and potentially long-term. Although early initiation of ART increased the serum albumin level, baseline albumin level remained a predictor of serious non-AIDS and hospitalization despite controlling for randomization arm.
The association between serum albumin and noncommunicable diseases was first reported in the British Regional Heart Study in 1989, where serum albumin was shown to be associated with all-cause mortality and CVD mortality [6]. Since then a number of studies have found a consistent and strong association between serum albumin and various disease endpoints in the general population [20–22]. To our knowledge, only 1 HIV cohort study has investigated serum albumin and a non-AIDS endpoint [8]. This study found a 12-fold and 3-fold increased hazard for heart failure and atherosclerotic events among HIV-infected US Veterans, respectively, for persons in the lowest albumin quartile (2.5–2.9 g/dL). As these results were comparable across pre and post-ART eras, the authors suggested that albumin is a strong predictor irrespective of ART status. Although the change in serum albumin since study entry was higher in the immediate-initiation group compared to the deferred-initiation arm in the START study, we confirm these data, and show that adjustment of early ART initiation did not change the predictive ability of serum albumin.
We also found evidence that albumin might provide information about the outlook for serious non-AIDS events over the longer term. The hazard ratio remained similar after excluding the initial years of follow-up for both serious non-AIDS and hospitalization, although the CIs widened. These results indicate that prediction for later years may be as strong as prediction in the initial years of follow-up. Although these results may contradict a previous analysis of overall mortality in HIV-infected persons, which showed that serum albumin primarily predicted all-cause mortality occurring within 1 year [8], early studies from the general population have also shown that serum albumin may predict cardiac and cancer-related deaths when the initial years of follow-up were excluded [6]. Finally, we also utilized serial serum albumin measurements and fitted albumin as a time-updated model for the primary outcome. The HR for this model moved slightly toward 1, and although serum albumin remained a strong predictor in this model, these findings suggest that serial measurements of albumin may not improve its predictive ability.
As the prognostic ability of HIV RNA and CD4 may be diminished in the ART era [23], and these markers may not always be available in resource-limited settings [24], serum albumin has also been investigated in the context of HIV progression. In an earlier study of 111 hemophilia patients coinfected with hepatitis C, serum albumin was associated with risk of developing AIDS (of which half of the population did) [15]. Low serum albumin was also independently associated with all-cause mortality, pulmonary tuberculosis, severe anemia, wasting, and weight loss in a study examining the effect of multivitamins in 2145 Tanzanian adults initiating ART, most of whom were in World Health Organization HIV stage III [16]. It has consequently been suggested that serum albumin may be used as a low-cost predictor of HIV disease progression. We were, however, not able to confirm these findings and found no association between serum albumin and AIDS. Individuals were screened for good health before entering the START study and had well preserved CD4 counts, and serum albumin may therefore only be capable of predicting HIV progression in a population with a poorer disease stage. Albumin appeared to be lowest in Africa and may thus be associated with other environmental (or host) factors that may affect its prognostic capacity in resource-limited settings. However, we did not find a significant interaction between albumin and region. We also found no interaction of albumin and gender.
It is not clear how hypoalbuminemia should alter clinical management of HIV-infected individuals, and the association found in this study does not necessarily mean that serum albumin is valuable for risk stratification at an individual level. Serum albumin has been associated with various diseases and is consequently nonspecific. However, serum albumin may help triage individuals, and hypoalbuminemia should probably prompt further clinical investigations to pinpoint the cause. Clarifying the cause of hypoalbuminemia is also important as manipulations to increase albumin production, such as nutritional support, would be indicated for reduced synthesis but not for increased albumin catabolism in which the underlying pathology would have to be treated.
To our knowledge, only 1 prognostic index has actually utilized serum albumin. The Child-Pugh score uses serum albumin tertiles to predict mortality and need for liver transplantation in patients with cirrhosis [25]. Other HIV-specific risk stratification indices, such as the Veterans Aging Cohort Study Index, have not utilized serum albumin [26]. We only assessed serum albumin as a stand-alone biomarker and included covariates in our models based on clinical assumptions. Serum albumin is also more easily obtained than other markers that have previously been associated with non-AIDS disease (ie, markers of chronic inflammation, hypercoagulation, microbial translocation, and immune activation) [27–30], of which none has been accepted into routine clinical practice. Thus, based on our data, and previous reports, serum albumin may be considered to be included in future HIV prognostic indices for non-AIDS morbidity.
Even though strong associations between serum albumin and disease outcomes have been found, such observations preclude us from drawing conclusions about causality. To our knowledge, there is no evidence from Mendelian randomization studies in the general population to support a causal relation between serum albumin and noncommunicable diseases. Thus, hypoalbuminemia could well be an epiphenomena to other disease condition (eg, poor nutritional status, liver disease, renal disease), which may confound the association explored in this study. However, serum albumin remained an independent predictor in multivariate analyses, and the association even persisted after controlling for IL-6, which may affect hepatic production of albumin [31]. Thus, it is likely that serum albumin may capture something broader than an inflammatory state in HIV-infected individuals. Low serum albumin could also be associated with structural abnormalities of the gut (leakage) that are also hallmarks of HIV [32] and which we were not able to control for. A direct protective effect of the molecule may include its ability to scavenge free radicals [33], its binding capacity of endogenous and exogenous compounds (eg, fatty acids and carcinogens) [3], or its potential anti-aggregatory effect on platelets [34], which could all be associated with serious non-AIDS pathogenesis.
Our study has limitations. First, although these results may suggest that low serum albumin levels may be present predisease, we cannot rule out that the long natural preclinical course of most serious non-AIDS events per se may have caused diminished levels of the molecule. In any case, serum albumin concentrations seemed to be low before disease manifestation, which is an interesting observation. Second, we were not powered to study different serious non-AIDS events separately, and it is possible that serum albumin may only be proxy for some events and not others. Third, we considered serious non-AIDS and AIDS separately and there may be an issue of competing risk, as these 2 endpoints may be related. However, when studying each outcome we did not censor at the other, and only 2 patients had both events. Fourth, we studied serum albumin alone and combining other biomarkers may have provided additional information.
The study also has strengths. The controlled nature of the START study with serial measurements of albumin at prespecified time points enabled us to study serum albumin as a time-updated variable. It also enabled us to rule out the confounding effect of blood sampling that registry-based studies are exposed to. Moreover, as opposed to previous studies, we were able to control for the effect of early ART initiation, and a review committee validated all endpoints.
In conclusion, serum albumin was found to be a predictor for short- and potentially long-term serious non-AIDS events. This effect was found to be independent of other measures of poor health, early ART initiation, and measures of systemic inflammation. Further studies are needed to ascertain whether serum albumin can be combined with other biomarkers in prognostic or frailty indexes, if serum albumin is associated with development of specific non-AIDS pathologies, and if it is possible to identify interventions, other than ART, that lower or raise serum albumin.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. All authors contributed to the formulation of the hypotheses and research questions and the analysis plan, and provided critical input into the draft manuscript. Data collection: S. S., J. V. B., R. M., T. D., L. C., N. N., J. D. L. Statistical analyses: A. R. (in part) and A. N. P. Interpretation of results: all authors. Drafted the manuscript: A. R. and A. N. P. Revised the work for important intellectual content: all authors. A complete list of members in the Strategic Timing of Antiretroviral Treatment (START) Study Group is available in a supplement to reference [17].
Acknowledgments. We thank all the patients who participated in this study.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases; National Institutes of Health Clinical Center; National Cancer Institute; National Heart, Lung, and Blood Institute; Eunice Kennedy Shriver National Institute of Child Health and Human Development; National Institute of Mental Health; National Institute of Neurological Disorders and Stroke; National Institute of Arthritis and Musculoskeletal and Skin Diseases; Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (France); National Health and Medical Research Council (Australia); National Research Foundation (Denmark); Bundesministerium für Bildung und Forschung (Germany); European AIDS Treatment Network; Medical Research Council, National Institute for Health Research, and National Health Service (United Kingdom); and University of Minnesota. National Institute of Health grant numbers for START: UM1-AI068641 and UM1-AI120197. Antiretroviral drugs were donated to the central drug repository by AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV Healthcare, Janssen Scientific Affairs, and Merck.
Potential conflicts of interest. A. R. reports travel grants from Gilead. A. N. P. reports speaker’s fees from Gilead Sciences. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Smit M, Brinkman K, Geerlings S et al. . ATHENA Observational Cohort Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol 1896; 19:312–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fasano M, Curry S, Terreno E et al. . The extraordinary ligand binding properties of human serum albumin. IUBMB Life 2005; 57:787–96. [DOI] [PubMed] [Google Scholar]
- 4. Taverna M, Marie AL, Mira JP, Guidet B. Specific antioxidant properties of human serum albumin. Ann Intensive Care 2013; 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial 2004; 17:432–7. [DOI] [PubMed] [Google Scholar]
- 6. Phillips A, Shaper AG, Whincup PH. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet 1989; 2:1434–6. [DOI] [PubMed] [Google Scholar]
- 7. Feldman JG, Burns DN, Gange SJ et al. . Serum albumin as a predictor of survival in HIV-infected women in the Women’s Interagency HIV study. AIDS 2000; 14:863–70. [DOI] [PubMed] [Google Scholar]
- 8. Lang J, Scherzer R, Weekley CC, Tien PC, Grunfeld C, Shlipak MG. Serum albumin and short-term risk for mortality and cardiovascular disease among HIV-infected veterans. AIDS 2013; 27:1339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodriguez RA, Mendelson M, O’Hare AM, Hsu LC, Schoenfeld P. Determinants of survival among HIV-infected chronic dialysis patients. J Am Soc Nephrol 2003; 14:1307–13. [DOI] [PubMed] [Google Scholar]
- 10. Scherzer R, Heymsfield SB, Rimland D et al. . Study of Fat Redistribution, Metabolic Change in HIV Infection (FRAM) Association of serum albumin and aspartate transaminase with 5-year all-cause mortality in HIV/hepatitis C virus coinfection and HIV monoinfection. AIDS 2017; 31:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Justice AC, Feinstein AR, Wells CK. A new prognostic staging system for the acquired immunodeficiency syndrome. N Engl J Med 1989; 320:1388–93. [DOI] [PubMed] [Google Scholar]
- 12. Dao CN, Peters PJ, Kiarie JN et al. . Hyponatremia, hypochloremia, and hypoalbuminemia predict an increased risk of mortality during the first year of antiretroviral therapy among HIV-infected Zambian and Kenyan women. AIDS Res Hum Retroviruses 2011; 27:1149–55. [DOI] [PubMed] [Google Scholar]
- 13. Feldman JG, Gange SJ, Bacchetti P et al. . Serum albumin is a powerful predictor of survival among HIV-1-infected women. J Acquir Immune Defic Syndr 2003; 33:66–73. [DOI] [PubMed] [Google Scholar]
- 14. Mehta SH, Astemborski J, Sterling TR, Thomas DL, Vlahov D. Serum albumin as a prognostic indicator for HIV disease progression. AIDS Res Hum Retroviruses 2006; 22:14–21. [DOI] [PubMed] [Google Scholar]
- 15. Sabin CA, Griffioen A, Yee TT et al. . Markers of HIV-1 disease progression in individuals with haemophilia coinfected with hepatitis C virus: a longitudinal study. Lancet 2002; 360:1546–51. [DOI] [PubMed] [Google Scholar]
- 16. Sudfeld CR, Isanaka S, Aboud S et al. . Association of serum albumin concentration with mortality, morbidity, CD4 T-cell reconstitution among Tanzanians initiating antiretroviral therapy. J Infect Dis 2013; 207:1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lundgren JD, Babiker AG, Gordin F et al. . Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Babiker AG, Emery S, Fätkenheuer G et al. . INSIGHT START Study Group Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials 2013; 10:S5–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. International Network for Strategic Initiatives in Global HIV Trials (INSIGHT). Criteria for event reporting in START http://insight.ccbr.umn.edu/start/index.php?tudy=start&page=&menu=eventreport. 2010. Accessed 1 May 2017.
- 20. Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol 1997; 50:693–703. [DOI] [PubMed] [Google Scholar]
- 21. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010; 9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Corti MC, Guralnik JM, Salive ME, Sorkin JD. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA 1994; 272:1036–42. [PubMed] [Google Scholar]
- 23. Gilbert PB, DeGruttola V, Hammer SM, Kuritzkes DR. Virologic and regimen termination surrogate end points in AIDS clinical trials. JAMA 2001; 285:777–84. [DOI] [PubMed] [Google Scholar]
- 24. Lecher S, Williams J, Fonjungo PN et al. . Progress with scale-up of HIV viral load monitoring—seven sub-Saharan African countries, January 2015–June 2016. MMWR Morb Mortal Wkly Rep 2016; 65:1332–5. [DOI] [PubMed] [Google Scholar]
- 25. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006; 44:217–31. [DOI] [PubMed] [Google Scholar]
- 26. Tate JP, Justice AC, Hughes MD et al. . An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS 2013; 27:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Borges ÁH, O’Connor JL, Phillips AN et al. . INSIGHT SMART Study and ESPRIT Groups Interleukin 6 is a stronger predictor of clinical events than high-sensitivity C-reactive protein or D-dimer during HIV infection. J Infect Dis 2016; 214:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mooney S, Tracy R, Osler T, Grace C. Elevated biomarkers of inflammation and coagulation in patients with HIV are associated with higher Framingham and VACS risk index scores. PLoS One 2015; 10:e0144312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burdo TH, Lo J, Abbara S et al. . Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duffau P, Wittkop L, Lazaro E et al. . ANRS CO3 Aquitaine Cohort Study Group Association of immune-activation and senescence markers with non-AIDS-defining comorbidities in HIV-suppressed patients. AIDS 2015; 29:2099–108. [DOI] [PubMed] [Google Scholar]
- 31. Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J 1990; 265:621–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mudd JC, Brenchley JM. Gut mucosal barrier dysfunction, microbial dysbiosis, and their role in HIV-1 disease progression. J Infect Dis 2016; 214(suppl 2):S58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med 2012; 33:209–90. [DOI] [PubMed] [Google Scholar]
- 34. Evans TW. Review article: albumin as a drug–biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther 2002; 16(suppl 5):6–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.