Summary
Mucosal inflammation is a hallmark of amebic colitis. Understanding the mechanisms by which Entamoeba histolytica drives inflammation is crucial for improving preventive and therapeutic strategies. Here, we identified a specific parasite-encoded cytokine, E. histolytica migration inhibitory factor (EhMIF), as a mediator of host mucosal inflammation.
Keywords: Chemokines, colitis, diarrhea, Entamoeba histolytica, host–parasite interaction, macrophage migration inhibitory factor, mucosal inflammation, neutrophils.
Abstract
Understanding the mechanisms by which Entamoeba histolytica drives gut inflammation is critical for the development of improved preventive and therapeutic strategies. E. histolytica encodes a homolog of the human cytokine macrophage migration inhibitory factor (MIF). Here, we investigated the role of E. histolytica MIF (EhMIF) during infection. We found that the concentration of fecal EhMIF correlated with the level of intestinal inflammation in persons with intestinal amebiasis. Mice treated with antibodies that specifically block EhMIF had reduced chemokine expression and neutrophil infiltration in the mucosa. In addition to antibody-mediated neutralization, we used a genetic approach to test the effect of EhMIF on mucosal inflammation. Mice infected with parasites overexpressing EhMIF had increased chemokine expression, neutrophil influx, and mucosal damage. Together, these results uncover a specific parasite protein that increases mucosal inflammation, expands our knowledge of host–parasite interaction during amebic colitis, and highlights a potential immunomodulatory target.
The mucosal surfaces of the nasal, intestinal, respiratory, and genitourinary tracts are the points of first contact for many protozoan parasites. Mucosal inflammation triggered by the interaction with these parasites plays a key role in human disease. However, the mechanisms by which parasites induce mucosal inflammation are incompletely understood.
Globally, diarrheal disease is second only to pneumonia as a leading cause of death in children under 5 years of age [1]. Entamoeba histolytica is a protozoan parasite that causes colitis, a leading cause of severe diarrhea in low-income countries. [2, 3]. E. histolytica infection is also a concern among returning travelers with infectious gastrointestinal disease: E. histolytica infection occurs at an estimated rate of 14 per 1000 returned unwell travelers [4]. Fulminant amebic colitis is an uncommon but life-threatening complication and is associated with high mortality and morbidity despite antimicrobial therapy, with case fatality rates ranging from 40% to 89% [5]. There is neither an effective vaccine nor have there been advancements in therapies for amebic colitis for over half a century, following the introduction of the nitroimidazole agents [6].
Mucosal inflammation is a hallmark of amebic colitis, explaining why it is often misdiagnosed as inflammatory bowel disease [5]. While inflammatory cells represent a line of defense [7], there is significant evidence that the inflammatory response contributes to the tissue damage seen in amebic colitis [8]. During amebic colitis, neutrophils infiltrate the intestinal tract [9]. It has been known for decades that neutrophilic enzymes such as myeloperoxidase (MPO) generate oxygen-free radicals, which kill invading pathogens. Oxygen-free radicals are also responsible for collateral tissue damage during the inflammatory period [10]. There is a direct positive correlation between MPO activity in the colon and the extent of intestinal tract epithelial damage [10].
Neutrophil migration depends on chemokines produced by epithelial cells. Interleukin-8 (IL-8) is a potent neutrophil chemoattractant that contributes to mucosal inflammation in various infectious and inflammatory diseases. Persons with severe forms of amebic colitis have higher colonic tissue levels of IL-8 and neutrophils [11, 12]. Both IL-8 inhibition and neutrophil depletion resulted in less mucosal damage during E. histolytica infection in a mouse-human intestinal xenograft model [13, 14]. Neutralization of a crucial parasite mediator of host immunopathology may prevent or attenuate disease. However, key parasite mediators of mucosal neutrophil influx during amebic infection remain incompletely understood.
Macrophage migration inhibitory factor (MIF) is a proinflammatory cytokine that is a critical upstream mediator of the innate immune response. MIF enhances the secretion of inflammatory mediators, and there is a strong association between MIF and colitis [15–19]. E. histolytica encodes a homolog of the cytokine MIF. However, the effect of the E. histolytica–encoded MIF homolog on mucosal inflammation during infection is unknown. In the present study, we examined the role of E. histolytica MIF (EhMIF) during infection using in vitro approaches, mouse model, and in persons with intestinal amebiasis. Taken together, the results of our study suggest that EhMIF is a key contributor of parasite-induced mucosal inflammation.
METHODS
Study Approval
All animal studies were performed in compliance with the federal regulations set forth in the Animal Welfare Act, the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and the guidelines of the University of Virginia Institutional Animal Care and Use Committee. All protocols for animal use were approved by the University of Virginia Institutional Animal Care and Use Committee. Use of serum and stool samples from human participants were approved by the Institutional Review Board at the University of Virginia, the Research and Ethical Research Review Committees of the International Center for Diarrheal Disease Research, Bangladesh, and the Research and Ethics Committee of the University of Venda, South Africa.
Parasites and Cell Culture
Entamoeba histolytica strain HM1:IMSS trophozoites were grown at 37°C in TYI-S-33 medium. The human intestinal epithelial cell line Caco-2 (American Type Culture Collection) was grown in Dulbecco’s Modified Eagle medium (Gibco). Cell lines tested negative for Mycoplasma (Lonza). Coculturing of epithelial cells with ameba were done at a ratio of 10:1 host cells to parasite in M199 medium [20]. IL-8 in cell culture supernatant was measured by enzyme-linked immunosorbent assay (ELISA; eBioscience). For overexpression of EhMIF in E. histolytica trophozoites, the EhMIF gene with no tag was cloned into the pKT3M expression vector [21] and confirmed by sequencing. Parasites were transfected by a previously described technique [22]. Transfectants were selected with 12 µg/ml G-418 (Gibco). EhMIF protein overexpression was confirmed by immunoblot analysis using specific anti-EhMIF antibodies [23]. Parasites transfected only with pKT3M expression vector were used as empty vector controls. Parasite growth was measured using CyQUANT Direct Cell Proliferation Assay kit (Invitrogen) according to the manufacturer’s instructions. For amebic cytotoxicity assays, E. histolytica trophozoites were added to intestinal epithelial cell line monolayers in M199 as previously described [20]. Lactate dehydrogenase (LDH) levels in the supernatant were measured using Cyto Tox-ONE Homogeneous Membrane Integrity Assay (Promega) as directed. The maximum amount of LDH released was determined by the addition of Triton-X to intestinal epithelial cells alone. Percent cytotoxicity was calculated as: [(LDH release+E. histolytica)−(LDH – E. histolytica)] / [maximum LDH release]. Conditions were tested in triplicates. Each experiment was repeated at least 3 times, and representative experiments are shown.
Measurement of EhMIF and Stool Myeloperoxidase
We developed an ELISA to measure EhMIF levels, similar to a recently described method [24]. Corning 96-well high-protein-binding polystyrene plates were coated with 5 μg/ml rabbit polyclonal anti-EhMIF [23] in phosphate-buffered saline (PBS) overnight and blocked for 1 hour with PBS containing 1% bovine serum albumin. Recombinant EhMIF was used as a protein standard. Stool samples were incubated overnight at 4oC, then washed before the addition of biotinylated anti-EhMIF at 0.25 μg/ml. After incubation and washing, avidin-conjugated horseradish peroxidase (eBioscience) was added, and detection was performed with 3,3′,5,5′-tetramethylbenzidine ELISA detection reagent (eBioscience). The sensitivity of the ELISA was 15.6 pg. There was no cross-reactivity to human MIF. EhMIF concentrations were measured in deidentified diarrheal stool samples from 35 South African patients with intestinal amebiasis. Stool Myeloperoxidase (MPO) levels were measured by ELISA (ALPCO) according to the manufacturer’s instructions [25].
Mice
Wild-type CBA/J mice were obtained from the Jackson Laboratory. Male mice were used at 10 weeks of age.
Parasite Infection
Infections were carried out via intracecal inoculation of mice with E. histolytica trophozoites [26]. A total of 5 × 105 trophozoites in 100 μl of TYI media were injected intracecally after laparotomy for antibody-mediated neutralization and EhMIF overexpression studies. For antibody neutralization studies, 0.5 mg mouse anti-EhMIF blocking antibodies were given by intraperitoneal injection 24 hours before and intracecally at the time of infection. Isotype antibodies given at the same dose, route, and timing were used as controls. Intracecal injection with media only was used as uninfected controls. No differences in the inflammatory markers and cytokine levels were observed among mice that did not received intracecal injection and those injected with media only and PBS. Mice were sacrificed 24 hours postinfection. TechLab E. histolytica–II kit was used to determine amebic antigen burden in cecal contents [26]. Cecal tissue lysates were prepared as in [27]. C-X-C motif (CXC) chemokine ligand 1 (CXCL1), CXC chemokine ligand 2 (CXCL2), and matrix metalloproteinase-3 (MMP3) levels in cecal lysates were measured by ELISA (R&D Systems). Myeloperoxidase activity in cecal tissue was determined using the same standard protocol as used in [28].
Human Samples
Serum sample concentrations of anti-EhMIF antibodies were measured in 79 children (2–5 years old) from a well-characterized cohort in the endemic area of Mirpur within Dhaka, Bangladesh [23]. After serum sample collection, monthly stool samples were routinely obtained from all children and tested for E. histolytica using real-time polymerase chain reaction as previously published [29].
Secretion Assay
Preparation of E. histolytica–secreted fractions were modified as described in [30]. Briefly, 1 × 107 trophozoites per mL were suspended in M199 media (Gibco) and incubated at 37°C for 2 hours. Cell-free supernatant representing the secreted fraction was collected for further analysis. Proteins in the secreted fraction was not due to cell stress or cell death, as only a minor portion of cells stained positive for Trypan blue (less than 5%), and no cellular actin was found by immunoblot analysis. For inhibition assays, trophozoites were incubated with pharmacological agents brefeldin A and probenicid [31].
Immunohistochemical Staining and Histopathological Examination
Mouse immunohistochemical staining was performed by the University of Virginia Biorepository and Tissue Research Facility. Staining was performed using the DAKO Autostainer Universal Staining System with specific antibody directed against EhMIF at a dilution of 1:600. Mouse cecal tissue was fixed in Bouin’s solution (Sigma) and stored in 70% ethanol. Tissue was stained with hematoxylin and eosin by the University of Virginia Research Histology Core. Histological scoring for inflammatory infiltration and epithelial cell damage was performed by 2 independent blinded scorers as previously described [32].
Mass Spectrometry
Proteins from the E. histolytica–secreted fraction were separated by gel electrophoresis. The section spanning 10–20 kilodaltons was then excised from the gel. The gel sample was submitted to the W. M. Keck Biomedical Mass Spectrometry Laboratory for mass spectrometry analysis.
Statistics
Statistical differences between 2 groups were determined using the Mann–Whitney U test or Student t test. Pearson’s correlation was used for correlation analysis. Survival differences were analyzed by the log-rank test. A P value less than .05 was considered statistically significant.
RESULTS
Association of EhMIF With Intestinal Inflammation in Persons With Intestinal Amebiasis
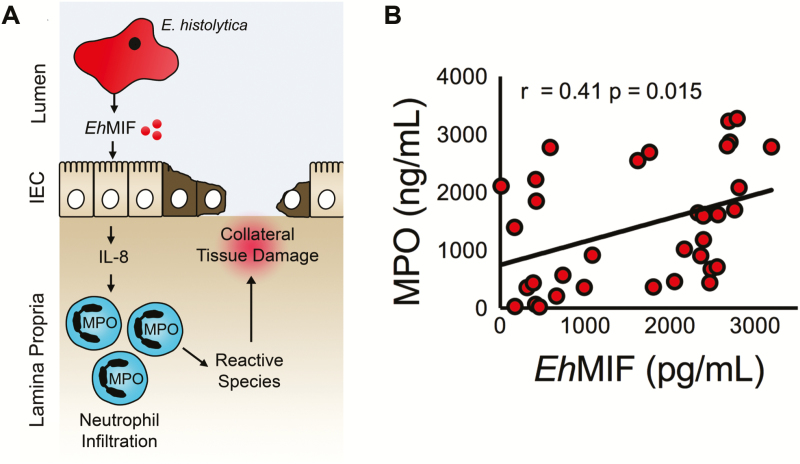
Given that inflammation is a characteristic feature of amebic colitis and EhMIF is expressed during human infection, we tested in humans for a proinflammatory effect of EhMIF: MPO is a major component of neutrophils, and the concentration of MPO in stool samples is a widely used marker of intestinal inflammation [25, 33]. We measured the concentrations of EhMIF and MPO in the stools samples of persons with intestinal amebiasis and found a positive association between EhMIF and intestinal inflammation by Pearson’s correlation (n = 35, r = 0.41; P = .015 (Figure 1A and 1B). We concluded that the correlation of stool EhMIF with MPO was consistent with a potential role for EhMIF in colonic inflammation in humans with amebiasis.
Figure 1.
Association between EhMIF and intestinal inflammation. A, Schematic of the hypothesis of how secreted EhMIF promotes mucosal inflammation. B, Significant positive correlation between fecal EhMIF levels and the MPO marker of intestinal inflammation in persons with amebiasis (n = 35). A P value < .05 was considered statistically significant.
Abbreviations: EhMIF, Entamoeba histolytica macrophage migration inhibitory factor; MPO, myeloperoxidase.
EhMIF Induces IL-8 Secretion From Human Intestinal Epithelial Cells
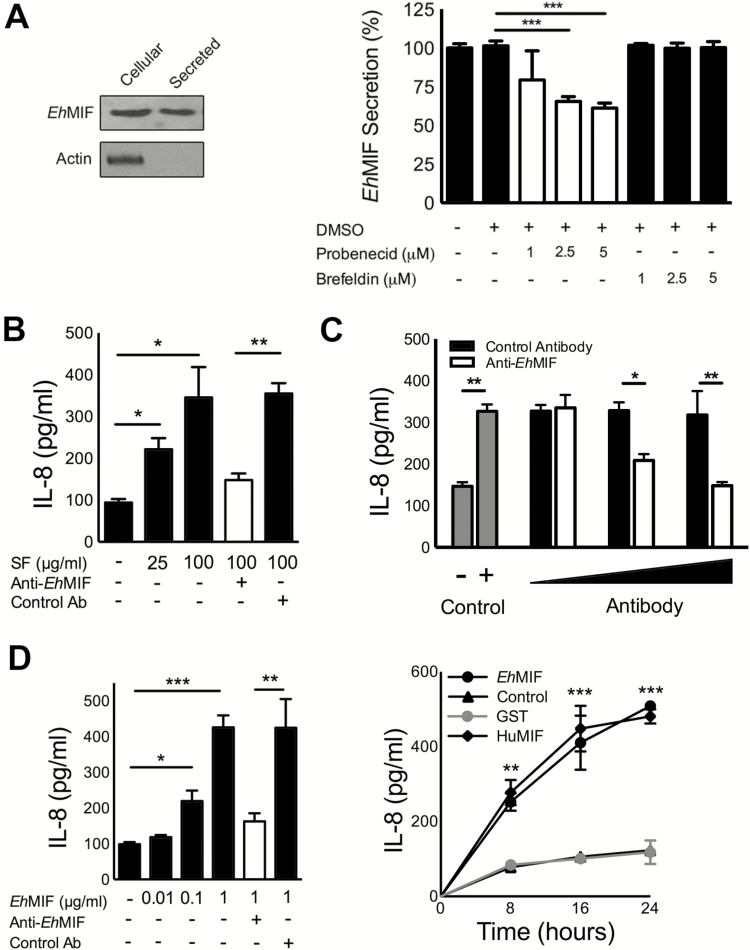
Cytokines such as IL-1β and MIF are secretory proteins that lack a signal peptide and therefore do not follow the classical endoplasmic reticulum–to-Golgi pathway of secretion. Human MIF is constitutively expressed, accumulated in the cytoplasm, and secreted by a nonclassical pathway involving an ATP-binding cassette (ABC) transporter [31, 34]. Similar to human MIF, EhMIF lacks a signal peptide and ABC transporters can be found in E. histolytica [35]. We used mass spectrometry to further confirm the protein expression of EhMIF (Supplemental Figure 1). We investigated whether EhMIF is secreted and found it present in secreted fractions by ELISA and immunoblot (Figure 2A). We further investigated the effect of transport inhibitors on EhMIF secretion. We found that EhMIF secretion was not inhibited by brefeldin A, an inhibitor of the classical secretory pathway. Probenicid, an inhibitor of nonclassical protein export, blocked EhMIF secretion (Figure 2A). These findings suggest that EhMIF shared with other MIF homologs secretion by a nonclassical pathway. In our study, the maximum inhibition achieved was approximately 50%. This raises the possibility of other secretion pathways for EhMIF. EhMIF was tested for its ability to induce IL-8 using a cellular approach, given that epithelial cells are the first host cells to encounter secretory products released by enteric parasites. It has long been hypothesized that E. histolytica, by means of soluble mediators, stimulates chemokine production from host cells [36]. The E. histolytica secretory protein fraction was previously shown to induce IL-8 production by Caco-2 human colonic epithelial cells [37]. We were able to reproduce this finding and found that IL-8 production was inhibited by antibodies that blocked EhMIF (Figure 2B). The rabbit anti-EhMIF antibodies used for these experiments did not cross-react with human MIF (Supplemental Figure 2A). In addition, anti-EhMIF antibodies inhibited the IL-8 secretion induced by coculturing intestinal epithelial cells (IECs) with E. histolytica parasites (Figure 2C). We also determined the effect of endotoxin-free recombinant EhMIF (<1 pg LPS/μg protein) on IECs. Recombinant glutathione S-transferase, an irrelevant protein, was used as a negative control and human MIF as a positive control. EhMIF induced IL-8 production in a time- and dose-dependent manner, and treatment with anti-EhMIF inhibited EhMIF-induced IL-8 production (Figure 2D). These data indicate that EhMIF was a cause of IL-8 secretion by E. histolytica.
Figure 2.
EhMIF induces IL-8 production from human intestinal epithelial cells. A, Secretion of EhMIF by amebic trophozoites. Immunoblot analyses of the cell lysate and secreted fractions of E. histolytica using anti-EhMIF antibodies. Actin detection serves as negative controls for cell lysis. EhMIF ELISA of E. histolytica secreted fractions. EhMIF secretion is not inhibited by the classical pathway inhibitor brefeldin A. Probenicid, an inhibitor of nonclassical protein export, blocked EhMIF secretion. B, Anti-EhMIF antibodies blocked E. histolytica secretory fraction-induced IL-8 production by colonic epithelial cells (Caco-2 cells). C, E. histolytica parasites cocultured with IECs in the presence of antibodies. D, EhMIF stimulates IL-8 production in a dose- and time-dependent manner. Data represent mean and SD of triplicates from 1 experiment and are representative of 3 independent experiments. *P < .05; **P < .01; ***P < .001. Abbreviations: EhMIF, Entamoeba histolytica macrophage migration inhibitory factor; ELISA, enzyme-linked immunosorbent assay; IECs, intestinal epithelial cells; IL-8, interleukin-8; SF, secretory fraction.
Anti-EhMIF Antibody Treatment Reduces Mucosal Inflammation
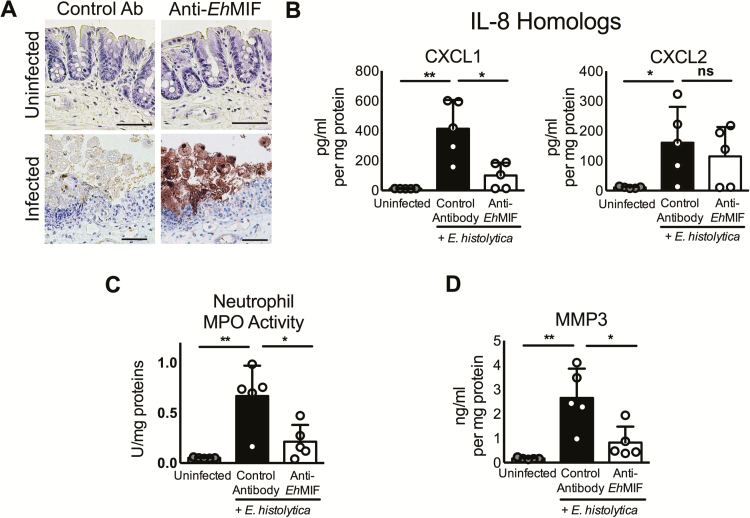
We further investigated in an amebic mouse model the role of EhMIF on chemokine secretion and mucosal inflammation. CXCL1/KC and CXCL2/MIP-2 are mouse homologs of human IL-8 and are key chemokines in neutrophil recruitment and inflammation. Host MIF was shown to induce mouse IL-8 homolog production from alveolar epithelial cells [38, 39]. Mice infected with E. histolytica had elevated levels of CXCL1 and CXCL2 (Figure 3B), in keeping with previous studies [28]. We found that mice treated with mouse anti-EhMIF-blocking antibodies had reduced CXCL1 (Figure 3B). Neutrophil MPO activity, an indicator of neutrophil infiltration [6, 32, 40], was significantly lower in anti-EhMIF-treated mice compared with controls (Figure 3C). The reduction of neutrophil infiltration by anti-EhMIF antibodies was consistent with its effect on chemokine production. These anti-EhMIF antibodies did not cross-react with mouse MIF (Supplemental Figure 2B). We concluded that anti-EhMIF blocked neutrophil recruitment to the gut in the mouse model of amebic colitis. In a previous study, anti-EhMIF antibodies were detected in the serum samples of children living in an endemic area [23]. We tested whether anti-EhMIF was associated with protection from amebiasis. Children in the top 50th percentile for anti‐EhMIF serum immunoglobulin G had a significantly higher probability of survival free of E. histolytica infection, compared with children in the lower 50th percentile (Supplemental Figure 3). This finding supports the hypothesis that anti-EhMIF antibodies have a protective role.
Figure 3.
Anti-EhMIF antibody treatment reduces E. histolytica–induced inflammation. A, Immunohistochemical stain showing EhMIF protein expression (brown) and interaction with host during infection. Scale bars, 50μm. B-D, Mice treated with anti-EhMIF antibodies had reduced intestinal tissue levels of CXCL1 chemokine, neutrophil infiltration, and MMP-3. Data represent mean and SD (n = 5 mice per group).
*P < .05; **P < .01.
Abbreviations: CXCL1, C-X-C motif chemokine ligand 1; EhMIF, Entamoeba histolytica macrophage migration inhibitory factor; MMP-3, matrix metalloproteinase 3; ns, not significant.
Overexpression of EhMIF Enhances Mucosal Inflammation
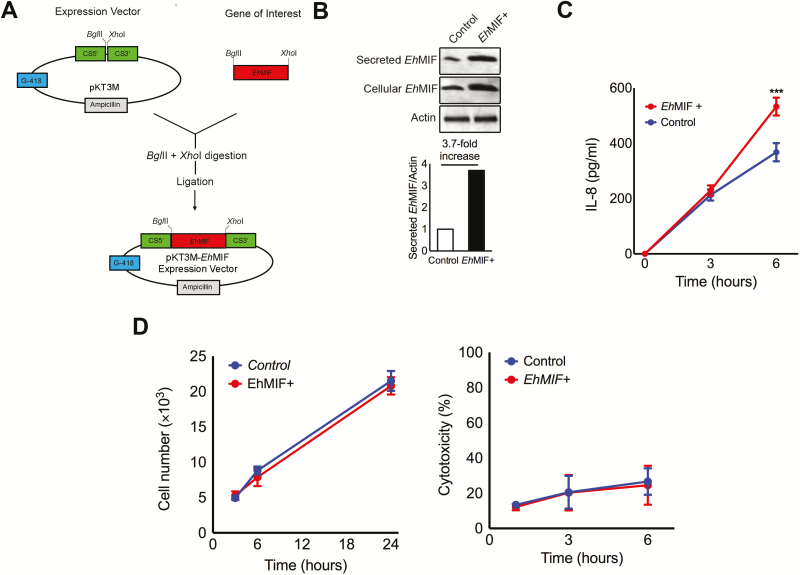
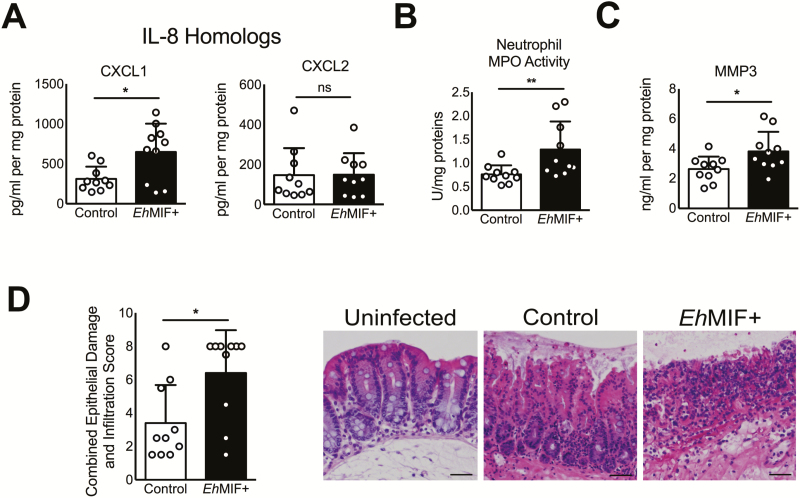
In addition to antibody-mediated neutralization, we used a genetic approach to test the effect of EhMIF on mucosal inflammation. We generated E. histolytica trophozoites that overexpress EhMIF (Figure 4A and 4B), given that EhMIF is a soluble secreted nontoxic protein, and gene overexpression can be technically accomplished in amebic strains that are adapted for virulence in the mouse model [22, 41, 42]. Mice infected with parasites overexpressing EhMIF showed increased chemokine production, mucosal inflammation, and pathology compared with parasites transfected with the empty vector (Figure 4C, 5A–D). No significant differences in parasite antigen load were observed between mice infected with parasites overexpressing EhMIF and controls postchallenge. This was also true for groups given isotype antibody control or anti-EhMIF antibody, indicating that the 2 groups were exposed to the same levels of E. histolytica antigens. In addition, parasites overexpressing EhMIF did not exhibit any growth or cytotoxicity difference compared with controls (Figure 4D). These data indicate that overexpression of EhMIF increased intestinal inflammation and damage.
Figure 4.
EhMIF overexpression by E. histolytica parasites. A, Schematic for the preparation of the pKT3M-EhMIF expression vector. EhMIF gene (EHI7A_051880). CS5': CS promoter (EHI_024230). CS3': CS UTR (EHI_024230). G-418 and ampicillin resistance genes. B, EhMIF expression assessed by immunoblot analysis. Actin was used as a loading control. Quantification of secreted EhMIF bands relative to actin by densitometry. C, IECs cocultured with E. histolytica parasites overexpressing EhMIF (EhMIF+) compared to empty vector controls. D, No difference in parasite growth or parasite-induced cytotoxicity between EhMIF+ parasites and WT parasite controls with empty vector. Data represent mean and SD of triplicates from 1 experiment and are representative of 3 independent experiments. A P value < .05 was considered statistically significant.
***P < .001. Abbreviations: EhMIF, Entamoeba histolytica macrophage migration inhibitory factor; IECs, intestinal epithelial cells; WT, wild-type.
Figure 5.
EhMIF overexpression increases inflammation. A–C, Increased CXCL1, neutrophil influx, and MMP-3 tissue levels in mice infected with EhMIF+ parasites compared with controls. D, Representative H&E-stained images and combined epithelial damage and infiltration scores. Scale bars, 100μm. Data represent mean and SD (n = 10 mice per group).
*P < .05; **P < .01; ***P < .001.
Abbreviations: CXCL1, C-X-C motif chemokine ligand 1; EhMIF, Entamoeba histolytica macrophage migration inhibitory factor; H&E, hematoxylin and eosin; MMP-3, matrix metalloproteinase 3; ns, not significant
EhMIF and Matrix Metalloproteinases Expression
Matrix metalloproteinases (MMPs) are enzymes capable of degrading extracellular matrix proteins. MMPs are expressed in all infections with protozoan parasites [43]. Matrix metalloproteinase 1 (MMP-1) and matrix metalloproteinase 3 (MMP-3) genes were among the most overexpressed genes in persons suffering from intestinal amebiasis [9]. In the human colon explant model, MMP-3 was shown to play a central role in amebic colitis, and inhibition of MMP activity blocked colonic mucosa invasion by E. histolytica [44]. We found that mice given anti-EhMIF antibodies had reduced MMP-3 mucosal levels, and parasites overexpressing EhMIF generated higher MMP-3 production during infection compared with controls (Figure 4D and Figure 5C). Proinflammatory cytokines, including human MIF have shown to stimulate the expression of MMPs [45]. However, in our hands, recombinant EhMIF failed to directly induce MMP-3 production from intestinal epithelial cells and fibroblasts in vitro. These findings suggest that MMP3 elevation might be due to EhMIF-induced mucosal inflammation and not a direct effect of EhMIF on host cell MMP-3 secretion.
DISCUSSION
Mucosal inflammation resulting from infection with E. histolytica is a hallmark of amebic colitis. In this study, we examined the role of the cytokine MIF homolog of E. histolytica in mucosal inflammation. We found a positive correlation between EhMIF levels and intestinal inflammation in infected persons. Using cellular and mouse models, we demonstrated that EhMIF induces chemokine secretion from intestinal epithelial cells, resulting in neutrophil influx. These findings implicate EhMIF as a causal factor of mucosal inflammation during infection.
Severe forms of amebic colitis are associated with both high mortality and morbidity. Antibiotics alone are often not enough to treat disease, and surgical removal of the inflamed colon may not prevent death [5]. Metronidazole is the antibiotic of choice for treating amebic colitis. In preclinical mouse models, metronidazole was shown to be very effective at killing ameba but had little effect on E. histolytica–induced mucosal inflammation [6, 46]. Adjunctive anti-inflammatory strategies may be needed to improve the clinical outcome of amebic colitis. Neutralization of a parasite mediator of host inflammation such as EhMIF may attenuate disease. However, further studies are needed to determine whether the combination of metronidazole and anti-EhMIF antibodies is superior to metronidazole alone for treatment of severe amebic colitis.
Mucosal inflammation also plays a key role in other human protozoan infections. Mucosal leishmaniasis is a destructive disease caused by the protozoan parasite Leishmania. Neutrophil recruitment and an exaggerated inflammatory response perpetuates the disease in mucosal leishmaniasis [47].Trichomonas vaginalis causes the most prevalent nonviral sexually transmitted infection worldwide. Vaginitis is characterized by infiltration of the vaginal mucosa with neutrophils, which contributes to the symptoms of vaginal discharge [48]. Toxoplasmosis is a parasitic disease, caused by Toxoplasma gondii, which can infect the brain, eye, and the developing fetus. The parasite first enters through the intestine and induces recruitment of neutrophils to the site of intestinal infection that was recently shown to facilitate the spread of infection [49]. Similar to E. histolytica, these parasites encode their own MIF homolog. It is possible that these parasite MIF homologs are contributing to the mucosal influx of neutrophils during their respective infections.
A recent study found that the Plasmodium-encoded MIF, through its proinflammatory properties, interfered with the development of immunological memory by inducing the development of short-lived effector cells rather than memory cells. This rendered the host susceptible to reinfection by the parasite [50]. This finding could help explain why antibodies against EhMIF were associated with protection from reinfection. Additional studies, however, are needed to validate our preliminary findings.
In conclusion, we identified EhMIF as a specific amebic mediator of host chemokine expression, neutrophil infiltration, and mucosal immunopathology during infection. Intestinal amebiasis remains a major global health problem, especially in children living in low-income countries. With no vaccine and only a single drug class to treat this devastating disease, EhMIF may represent a promising immunotherapeutic target to prevent or attenuate amebic disease.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank William A. Petri, Jr for helpful advice; Carol Gilchrist and the laboratory of Kris Chadee for technical advice; and the University of Virginia Research Histology, Pat Pramoonjago, and the Biorepository and Tissue Research Facilities.
Financial support. This work was supported by the National Institutes of Health (NIH) D43TW006578 (to R. N.): T35AI060528, Infectious Diseases Society of America (IDSA) Medical Scholars Program, and Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship (to N. M. J.): R01AI026649, K08AI119181, and the Robert Wood Johnson Foundation–Harold Amos Medical Faculty Development Program Award (to S. N. M.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010; 375:1969–87. [DOI] [PubMed] [Google Scholar]
- 2. Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 3. Taniuchi M, Sobuz SU, Begum S, et al. Etiology of diarrhea in Bangladeshi infants in the first year of life analyzed using molecular methods. J Infect Dis 2013; 208:1794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Swaminathan A, Torresi J, Schlagenhauf P, et al. ; GeoSentinel Network. A global study of pathogens and host risk factors associated with infectious gastrointestinal disease in returned international travellers. J Infect 2009; 59:19–27. [DOI] [PubMed] [Google Scholar]
- 5. Shirley DA, Moonah S. Fulminant amebic colitis after corticosteroid therapy: a systematic review. PLOS Negl Trop Dis 2016; 10:e0004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Debnath A, Parsonage D, Andrade RM, et al. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat Med 2012; 18:956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Naylor C, Burgess S, Madan R, et al. Leptin receptor mutation results in defective neutrophil recruitment to the colon during Entamoeba histolytica infection. mBio 2014; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moonah SN, Jiang NM, Petri WA., Jr Host immune response to intestinal amebiasis. PLOS Pathog 2013; 9:e1003489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peterson KM, Guo X, Elkahloun AG, et al. The expression of REG 1A and REG 1B is increased during acute amebic colitis. Parasitol Int 2011; 60:296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hao XP, Lucero CM, Turkbey B, et al. Experimental colitis in SIV-uninfected rhesus macaques recapitulates important features of pathogenic SIV infection. Nat Commun 2015; 6:8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sierra-Puente RE, Campos-Rodríguez R, Jarillo-Luna RA, et al. Expression of immune modulator cytokines in human fulminant amoebic colitis. Parasite Immunol 2009; 31:384–91. [DOI] [PubMed] [Google Scholar]
- 12. Ventura-Juárez J, Barba-Gallardo LF, Muñoz-Fernández L, et al. Immunohistochemical characterization of human fulminant amoebic colitis. Parasite Immunol 2007; 29:201–9. [DOI] [PubMed] [Google Scholar]
- 13. Seydel KB, Li E, Zhang Z, Stanley SL., Jr Epithelial cell–initiated inflammation plays a crucial role in early tissue damage in amebic infection of human intestine. Gastroenterology 1998; 115:1446–53. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Z, Duchêne M, Stanley SL. A monoclonal antibody to the amebic lipophosphoglycan-proteophosphoglycan antigens can prevent disease in human intestinal xenografts infected with Entamoeba histolytica. Infect Immun 2002; 70:5873–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Jong YP, Abadia-Molina AC, Satoskar AR, et al. Development of chronic colitis is dependent on the cytokine MIF. Nat Immunol 2001; 2:1061–6. [DOI] [PubMed] [Google Scholar]
- 16. Yao J, Leng L, Sauler M, et al. Transcription factor ICBP90 regulates the MIF promoter and immune susceptibility locus. J Clin Invest 2016; 126:732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang J, Li Y, Zhang X. Meta-analysis of macrophage migration inhibitory factor (MIF) gene -173G/C polymorphism and inflammatory bowel disease (IBD) risk. Int J Clin Exp Med 2015; 8:9570–4. [PMC free article] [PubMed] [Google Scholar]
- 18. Roger T, Schneider A, Weier M, et al. High expression levels of macrophage migration inhibitory factor sustain the innate immune responses of neonates. Proc Natl Acad Sci USA 2016; 113:E997–E1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishihira J. Molecular function of macrophage migration inhibitory factor and a novel therapy for inflammatory bowel disease. Ann NY Acad Sci 2012; 1271:53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marie C, Verkerke HP, Theodorescu D, Petri WA. A whole-genome RNAi screen uncovers a novel role for human potassium channels in cell killing by the parasite Entamoeba histolytica. Sci Rep 2015; 5:13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saito-Nakano Y, Yasuda T, Nakada-Tsukui K, Leippe M, Nozaki T. Rab5-associated vacuoles play a unique role in phagocytosis of the enteric protozoan parasite Entamoeba histolytica. J Biol Chem 2004; 279:49497–507. [DOI] [PubMed] [Google Scholar]
- 22. Abhyankar MM, Haviland SM, Gilchrist CA, Petri WA., Jr Development of a negative selectable marker for Entamoeba histolytica. J Vis Exp 2010; 12:2410 doi: 10.3791/2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moonah SN, Abhyankar MM, Haque R, Petri WA., Jr The macrophage migration inhibitory factor homolog of Entamoeba histolytica binds to and immunomodulates host macrophages. Infect Immun 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holowka T, Castilho TM, Garcia AB, Sun T, McMahon-Pratt D, Bucala R. Leishmania-encoded orthologs of macrophage migration inhibitory factor regulate host immunity to promote parasite persistence. FASEB J 2016; 30:2249–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naylor C, Lu M, Haque R, et al. ; PROVIDE study teams. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine 2015; 2:1759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barroso L, Abhyankar M, Noor Z, et al. Expression, purification, and evaluation of recombinant LecA as a candidate for an amebic colitis vaccine. Vaccine 2014; 32:1218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cowardin CA, Buonomo EL, Saleh MM, et al. The binary toxin CDT enhances Clostridium difficile virulence by suppressing protective colonic eosinophilia. Nat Microbiol 2016; 1:16108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kissoon-Singh V, Moreau F, Trusevych E, Chadee K. Entamoeba histolytica exacerbates epithelial tight junction permeability and proinflammatory responses in Muc2(-/-) mice. Am J Pathol 2013; 182:852–65. [DOI] [PubMed] [Google Scholar]
- 29. Korpe PS, Liu Y, Siddique A, et al. Breast milk parasite-specific antibodies and protection from amebiasis and cryptosporidiosis in Bangladeshi infants: a prospective cohort study. Clin Infect Dis 2013; 56:988–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cornick S, Moreau F, Chadee K. Entamoeba histolytica cysteine proteinase 5 evokes mucin exocytosis from colonic goblet cells via αvβ3 integrin. PLOS Pathog 2016; 12:e1005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flieger O, Engling A, Bucala R, Lue H, Nickel W, Bernhagen J. Regulated secretion of macrophage migration inhibitory factor is mediated by a non-classical pathway involving an ABC transporter. FEBS Lett 2003; 551(1-3):78–86. [DOI] [PubMed] [Google Scholar]
- 32. Spalinger MR, Kasper S, Gottier C, et al. NLRP3 tyrosine phosphorylation is controlled by protein tyrosine phosphatase PTPN22. J Clin Invest 2016; 126:1783–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kosek M, Haque R, Lima A, et al. ; MAL-ED network. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg 2013; 88:390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Regis EG, Barreto-de-Souza V, Morgado MG, et al. Elevated levels of macrophage migration inhibitory factor (MIF) in the plasma of HIV-1-infected patients and in HIV-1-infected cell cultures: a relevant role on viral replication. Virology 2010; 399:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sauvage V, Aubert D, Escotte-Binet S, Villena I. The role of ATP-binding cassette (ABC) proteins in protozoan parasites. Mol Biochem Parasitol 2009; 167:81–94. [DOI] [PubMed] [Google Scholar]
- 36. Huldt G, Davies P, Allison AC, Schorlemmer HU. Interactions between Entamoeba histolytica and complement. Nature 1979; 277:214–6. [DOI] [PubMed] [Google Scholar]
- 37. Yu Y, Chadee K. Entamoeba histolytica stimulates interleukin 8 from human colonic epithelial cells without parasite-enterocyte contact. Gastroenterology 1997; 112:1536–47. [DOI] [PubMed] [Google Scholar]
- 38. Takahashi K, Koga K, Linge HM, et al. Macrophage CD74 contributes to MIF-induced pulmonary inflammation. Respiratory Research 2009; 10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weiser JN, Roche AM, Hergott CB, et al. Macrophage migration inhibitory factor is detrimental in pneumococcal pneumonia and a target for therapeutic immunomodulation. J Infect Dis 2015; 212:1677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Banerjee A, Kim BJ, Carmona EM, et al. Bacterial Pili exploit integrin machinery to promote immune activation and efficient blood-brain barrier penetration. Nat Commun 2011; 2:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morf L, Pearson RJ, Wang AS, Singh U. Robust gene silencing mediated by antisense small RNAs in the pathogenic protist Entamoeba histolytica. Nucleic Acids Res 2013; 41:9424–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matthiesen J, Bar AK, Bartels AK, et al. Overexpression of specific cysteine peptidases confers pathogenicity to a nonpathogenic Entamoeba histolytica clone. mBio 2013; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Geurts N, Opdenakker G, Van den Steen PE. Matrix metalloproteinases as therapeutic targets in protozoan parasitic infections. Pharmacol Ther 2012; 133:257–79. [DOI] [PubMed] [Google Scholar]
- 44. Thibeaux R, Avé P, Bernier M, et al. The parasite Entamoeba histolytica exploits the activities of human matrix metalloproteinases to invade colonic tissue. Nat Commun 2014; 5:5142. [DOI] [PubMed] [Google Scholar]
- 45. Bai F, Asojo OA, Cirillo P, et al. A novel allosteric inhibitor of macrophage migration inhibitory factor (MIF). J Biol Chem 2012; 287:30653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Becker S, Hoffman P, Houpt ER. Efficacy of antiamebic drugs in a mouse model. Am J Trop Med Hyg 2011; 84:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boaventura VS, Santos CS, Cardoso CR, et al. Human mucosal leishmaniasis: neutrophils infiltrate areas of tissue damage that express high levels of Th17-related cytokines. Eur J Immunol 2010; 40:2830–6. [DOI] [PubMed] [Google Scholar]
- 48. Twu O, de Miguel N, Lustig G, et al. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host∶parasite interactions. PLOS Pathog 2013; 9:e1003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Coombes JL, Charsar BA, Han S-J, et al. Motile invaded neutrophils in the small intestine of Toxoplasma gondii–infected mice reveal a potential mechanism for parasite spread. Proc Natl Acad Sci USA 2013; 110:E1913–E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sun T, Holowka T, Song Y, et al. A Plasmodium-encoded cytokine suppresses T-cell immunity during malaria. Proc Natl Acad Sci USA 2012; 109: E2117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.