Biophysical and enzymatic analyses show that UDP- and ADP-glucose are more stable than claimed in a controversial study that questions the generally accepted pathway of starch synthesis in plants.
Keywords: ADP-glucose, NMR, plastid, starch, sucrose, UDP-glucose
Abstract
Nucleoside diphosphate sugars (NDP-sugars) are the substrates for biosynthesis of oligo- and polysaccharides, such as starch and cellulose, and are also required for biosynthesis of nucleotides, ascorbic acid, several cofactors, glycoproteins and many secondary metabolites. A controversial study that questions the generally accepted pathway of ADP-glucose and starch synthesis in plants is based, in part, on claims that NDP-sugars are unstable at alkaline pH in the presence of Mg2+ and that this instability can lead to unreliable results from in vitro assays of enzyme activities. If substantiated, this claim would have far-reaching implications for many published studies that report on the activities of NDP-sugar metabolizing enzymes. To resolve this controversy, we investigated the stability of UDP- and ADP-glucose using biophysical, namely nuclear magnetic resonance (NMR), and highly specific enzymatic methods. Results obtained with both techniques indicate that NDP-sugars are not as unstable as previously suggested. Moreover, their calculated in vitro half-lives are significantly higher than estimates of their in planta turnover times. This indicates that the physico-chemical stability of NDP-sugars has little impact on their concentrations in vivo and that NDP-sugar levels are determined primarily by the relative rates of enzymatic synthesis and consumption. Our results refute one of the main arguments for the controversial pathway of starch synthesis from imported ADP-glucose produced by sucrose synthase in the cytosol.
Introduction
Nucleoside diphosphate sugars (NDP-sugars) were discovered by Luis F Leloir and colleagues around the middle of the twentieth century (Leloir, 1951). This seminal discovery and the complementary work on enzyme characterization and elucidation of the many critical roles played by NDP-sugars in cells, led to Leloir being awarded the Nobel Prize in Chemistry in 1970 (Leloir, 1971; Leloir, 1983). Early studies determined that UDP-Glc is a key intermediate involved in the metabolism of galactose (Leloir, 1951) and is the glucosyl donor for trehalose synthesis in yeast (Leloir and Cabib, 1953), sucrose synthesis in plants (Cardini et al., 1955) and the elongation of α-1,4-polyglucan chains during glycogen synthesis in mammals (Leloir et al., 1959). UDP-Glc is also the substrate for cellulose synthesis and an intermediate in the production of other cell wall polymers (Carpita, 2011). Other NDP-sugars and NDP-sugar metabolizing enzymes were subsequently discovered, with the first report of an ADP-Glc synthesizing enzyme coming from soybean (Espada, 1962). Later studies showed that ADP-Glc, not UDP-Glc, is the glucosyl donor for glycogen synthesis in bacteria (Greenberg and Preiss, 1964) and starch synthesis in plants (Murata et al., 1963; Recondo et al., 1963; Ghosh and Preiss, 1966).
Starch is the most important carbon reserve in plants and the major source of calories in our staple crops. Transitory starch reserves are accumulated and degraded on a daily basis in the leaves of many plants to provide carbon and energy during the hours of darkness. Starch is also stored in many seeds, fruits, and tubers, as well as in the stems of woody perennials, providing longer term reserves for germination and regrowth after winter. In leaves, starch is synthesized in the chloroplasts from fructose 6-phosphate that is withdrawn from the Calvin-Benson cycle and converted to ADP-Glc via plastidial phosphoglucose isomerase (pPGI), plastidial phosphoglucomutase (pPGM), and ADP-Glc pyrophosphorylase (ADP-Glc PPase), which is restricted to the chloroplasts in leaves (Ballicora et al., 2004). In heterotrophic tissues, starch is made in specialized amyloplasts, in most cases via plastidial ADP-Glc PPase using imported hexose-phosphates. An exception is cereal endosperm, where additional cytosolic isoforms of ADP-Glc PPase are responsible for the majority of ADP-Glc production, with ADP-Glc being imported into the amyloplasts via a BRITTLE1-type nucleotide transporter for starch synthesis (Comparot-Moss and Denyer, 2009).
The classical pathway is supported by the demonstrated autonomy of illuminated chloroplasts to synthesize starch from CO2 (Gibbs and Cynkin, 1958; Heldt et al., 1977) and by the starch deficient phenotypes of pgi, pgm and adg mutants lacking pPGI, pPGM and ADP-Glc PPase, respectively (Caspar et al., 1985; Lin et al., 1988; Yu et al., 2000). Despite the considerable biochemical and genetic evidence supporting the ADP-Glc PPase-mediated pathway, its contribution to starch synthesis has been disputed by Pozueta-Romero and colleagues. They have proposed an alternative pathway in which ADP-Glc is produced by sucrose synthase (SUS) in the cytosol and then imported into plastids via a hypothetical ADP-Glc transporter (Baroja-Fernández et al., 2001). The initial version of the proposed SUS-mediated pathway was criticized because it did not explain the starch-deficient phenotypes of mutants lacking ADP-Glc PPase or pPGM (Neuhaus et al., 2005). To counter this criticism, Pozueta-Romero and colleagues postulated that starch synthesis is always accompanied by futile cycling of starch. They propose that starch is synthesized initially from imported ADP-Glc, but is constantly turned over via hydrolytic pathways that yield Glc and Glc-1P, and that plastidial hexokinase, pPGM and ADP-Glc PPase are needed to salvage these metabolites to resynthesize starch (Baroja-Fernández et al., 2001; Baroja-Fernández et al., 2004; Baroja-Fernández et al., 2005; Bahaji et al., 2011; Bahaji et al., 2014a,b; Baslam et al., 2017). More recently, these authors proposed that the starch deficient phenotype of pgi mutants is the result of reduced photosynthetic capacity, most likely as a consequence of reduced levels of plastidial cytokinins (Bahaji et al., 2015). In agreement with this hypothesis, Sánchez-López et al. (2016) showed that pgi mutants accumulate high levels of starch when exposed to volatile emissions from the fungus Alternaria alternata, which promote photosynthesis, growth, and accumulation of plastidic cytokinins.
The proposed SUS-mediated pathway and associated futile cycling of starch remains highly controversial (Neuhaus et al., 2005; Stitt and Zeeman, 2012; Streb and Zeeman, 2012) and several reverse genetic studies have been undertaken to test its validity. Arabidopsis thaliana mutants lacking key enzymes of starch degradation were crossed with a pgm mutant lacking pPGM activity (Streb et al., 2009). If the alternative SUS-mediated pathway and futile cycling of starch were operational, the double mutants would be expected to accumulate high levels of starch, but in fact all of them had a starch-deficient phenotype similar to the parental pgm plants (Streb et al., 2009). The epistatic effect of the pgm mutation is entirely consistent with the classical pathway. Arabidopsis has six SUS genes (Bieniawska et al., 2007), of which AtSUS1–AtSUS4 are expressed in leaf mesophyll cells where transitory starch is accumulated, while expression of AtSUS5 and AtSUS6 is restricted to the developing vascular tissue (Barratt et al., 2009). A quadruple sus1sus2sus3sus4 (sus1234) mutant that lacks SUS expression in mesophyll cells was found to accumulate wild-type levels of starch (Barratt et al., 2009). Although this observation was confirmed by Baroja-Fernández et al. (2012a), they claimed that Bieniawska et al. (2007) and Barratt et al. (2009) had underestimated SUS activity in the sus mutants and that the sus1234 mutant retained sufficient SUS activity to account for the observed wild-type rates of starch accumulation. This criticism was in part based on a claim that UDP-Glc would be unstable in the presence of Mg2+ under the mildly alkaline conditions of pH 9.4 of the assay used by Bieniawska et al. (2007) and Barratt et al. (2009), leading to loss of UDP-Glc during the assay and underestimation of SUS activity. The authors of the latter study responded that even if the residual SUS activity had been underestimated, this did not affect the main conclusion of Barratt et al. (2009) that knocking out all four of the SUS genes expressed in mesophyll cells had no discernible effect on starch accumulation (Smith et al., 2012). However, the question of whether the residual SUS activity in the sus1234 mutant was underestimated due to instability of UDP-Glc in the in vitro assay has not yet been addressed.
It has been known for many years that UDP-Glc spontaneously undergoes cleavage in mildly alkaline conditions to produce UMP and the cyclic monophosphoric ester Glc 1,2-phosphate (Paladini and Leloir, 1952). This process requires Mg2+ (Zervosen et al., 1998) and has also been described for ADP-Glc (Murata et al., 1964). These observations appear to support the claims of Baroja-Fernández et al. (2012a) that SUS activities were underestimated by Bieniawska et al. (2007) and Barratt et al. (2009). They also imply that the activities of UDP-Glc or ADP-Glc metabolizing enzymes could have been underestimated in many other previous studies, if inappropriate conditions had been used for in vitro assays. If substantiated, the proposed instability of NDP-sugars could also affect our understanding of their metabolism in vivo. Illumination of leaves leads to alkalinization of the chloroplast stroma and release of Mg2+ from the thylakoids, leading to a steady state pH of about 8.3 and a Mg2+ concentration of about 6 mM (Werdan et al., 1975; Portis and Heldt, 1976; Krause, 1977; Portis, 1981). It has been claimed that ADP-Glc would be highly unstable under such conditions, implying that the chemical environment of the stroma of illuminated chloroplasts is unfavourable for ADP-Glc synthesis (Baroja-Fernández et al., 2001).
The aim of the current study was to determine the pH sensitivity of UDP-Glc and ADP-Glc in the presence or absence of Mg2+. This will not only address the specific question of whether SUS activity could have been underestimated in the studies of Bieniawska et al. (2007) and Barratt et al. (2009), but also whether alkaline sensitivity of NDP-sugars could have compromised other studies of NDP-sugar metabolizing enzymes. Results presented in this work are critical for the proper study of all glycosyltransferases, not only in plants but also in other organisms. They are also relevant for biotechnological processes (De Bruyn et al., 2015), such as glycorandomization and in vitro production of glycoproteins, where the physico-chemical stability of NDP-sugar reactants could be a significant constraint.
We used biophysical, namely nuclear magnetic resonance (NMR), and enzymatic methods to investigate the stability of UDP-Glc and ADP-Glc over a range of pH and Mg2+ concentrations spanning the conditions used in the disputed in vitro assays of SUS activity. We determined the half-life of these UDP-sugars in conditions that simulate the in vivo environment of the stroma in illuminated chloroplasts and in other subcellular compartments.
Materials and methods
Chemicals and enzymes
UDP-Glc, ADP-Glc, UMP, AMP, sodium pyrophosphate, NADP+, Glc 1,6-bisphosphate, phosphoglucomutase from rabbit muscle, Glc-6-phosphate dehydrogenase from Baker’s yeast, BSA and MgCl2 were obtained from Sigma-Aldrich (St. Louis, MO, USA). Deuterium oxide was from Cambridge Isotope Laboratories Inc. (Andover, MA, USA). All other chemicals were of the highest quality available. Recombinant ADP-Glc and UDP-Glc pyrophosphorylases from Escherichia coli were expressed in E. coli and purified to near homogeneity as previously described (Ballicora et al., 2002; Ebrecht et al., 2015).
Sample preparation and NMR analysis
Samples 1 ml in volume containing 5 mM UDP-Glc, 10 mM MgCl2, 10% deuterium oxide, and 30 mM of either HEPES-NaOH at pH 7.0, BisTris propane-NaOH at pH 8.0, or CHES-NaOH at pH 8.5 and 9.0, were incubated in a 37°C water bath for 0, 12, 20, 45, or 90 min. All pH measurements were made at 37°C. Additionally, a 1 ml sample containing 5 mM ADP-Glc, 10 mM MgCl2, 10% deuterium oxide, and 30 mM CHES-NaOH at pH 9.0 was incubated in a 37°C water bath for 90 min. Once removed from the water bath, 250 μl of a solution containing 1 M HEPES-NaOH at pH 7.0 and 150 mM EDTA were added to the 1 ml samples. The neutral pH and chelation of Mg2+ by excess EDTA prevented further degradation of the NDP-Glc (Zervosen et al., 1998). Samples were immediately frozen with liquid nitrogen and kept at -80°C until use. After thawing, [31P]NMR was performed on these samples to quantify the extent of NDP-sugar degradation that occurred during the incubation period. Experiments determining the relative NDP-Glc, nucleoside monophosphate (NMP), and cyclic Glc 1,2-phosphate concentrations were performed using a Varian Inova 500 MHz NMR spectrometer. A dilute phosphoric acid sample served as the external standard, denoting 0 ppm. At least 6000 scans were performed on all samples using a 33° tip angle and a 3.2 s acquisition time, while at least 12 000 scans were performed on samples with more than 80% apparent NDP-Glc degradation. The relative integration values of the observed species were used to calculate the percentage of NDP-Glc degradation that occurred during the given time period. As NMPs are known to be stable in alkaline solutions containing Mg2+ (Nanninga, 1957), but the stability of cyclic Glc 1,2-phosphate is not known, calculations for the percentage of degradation were based on the integration values of the NMP and NDP-Glc using the following equation: (2×ANMP)/[(2×ANMP)+ANDP-Glc]×100, where ANMP is the integrated area for the NMP and ANDP-Glc is the area of NDP-Glc. Half-lives for UDP-Glc in each of the pH conditions were calculated using the formula: P=(1−2−t/z)×100, where P is the percentage of UDP-Glc degradation, t is time in minutes, and z is the calculated half-life.
The chemical shift peaks of NMP and NDP-Glc were determined from standard solutions. Each 5 mM solution was prepared with 30 mM HEPES-NaOH at pH 7.0, 10 mM MgCl2, and 10% deuterium oxide, to which 250 μl of a solution containing 1 M HEPES-NaOH at pH 7.0 and 150 mM EDTA were added. Peaks were found to be near 3 ppm for the NMP and near -11 and -13 ppm for the two phosphates of the NDP-Glc. Consistent with previously published results (Withers et al., 1981), we concluded that the peak observed near 11 ppm in our experimental samples belongs to cyclic Glc 1,2-phosphate.
To determine the spin-lattice relaxation times (T1), experiments of T1 inversion recovery were performed as previously described (Günther, 2013). A standard containing UDP-Glc, UMP, and cyclic Glc 1,2-phosphate was derived from a sample originally containing 10 mM UDP-Glc, 20 mM MgCl2, 10% deuterium oxide, and 30 mM CHES-NaOH at pH 9.0 which was incubated at 37°C for 2 h. After incubation, 250 μl of 1 M HEPES-NaOH at pH 7.0, with 150 mM EDTA, were added prior to NMR analysis to stop further NDP-sugar degradation. The longest T1 time of any analyte (UDP-Glc, UMP, or cyclic Glc 1,2-phosphate) was 3.3 s. As described elsewhere (Ernst and Anderson, 1966), the use of a 33° tip angle reduces this by a factor of 5.56, yielding an effective T1 time of 0.59 s. Therefore, when a 33° tip angle is used, an acquisition time of 3.2 s is greater than 5×T1 of any analyte being quantified. In addition, the T1 relaxation times for UDP-Glc that we observed matched well with previous research (Wehrli et al., 1992).
Enzymatic determination of NDP-sugars degradation
Four 1 ml solutions, each containing 0, 1.25, 2.5, or 5 mM NDP-Glc, 10 mM MgCl2 and 30 mM CHES-NaOH at pH 9.0, were incubated in a water bath at 37°C. The reaction was started by the addition of the NDP-sugar. After 90 min, 250 µl of 1 M HEPES-NaOH at pH 7.0 was added to achieve neutralization and to stop any further degradation of the NDP-sugar. Neutralization was confirmed by using pH indicator paper. Separately, four replicate ‘time zero’ samples were prepared in the same way, except that the 250 µl of 1 M HEPES-NaOH at pH 7.0 was added before the NDP-Glc and the reaction mixture was not incubated at 37°C. Following neutralization, the amount of intact NDP-Glc remaining in each of these solutions was enzymatically determined as previously described (Munch-Petersen, 1955; Sowokinos, 1976), with the following modifications: a 10-µl aliquot of neutralized sample was added to 160 µl of a solution containing 200 mM HEPES-NaOH at pH 7.3, 5 mM MgCl2, 1.5 mM sodium pyrophosphate, 10 mM sodium fluoride, 1.25 mM NADP+, 2 mM dithiothreitol, 0.01 mM Glc 1,6-bisphosphate, 3 U/ml phosphoglucomutase, 3 U/ml Glc-6-phosphate dehydrogenase, 0.2 mg/ml BSA, and 1 U of either UDP-Glc pyrophosphorylase or ADP-Glc PPase. The conversion of NADP+ to NADPH was monitored using a BioTek EL808 microplate reader (Winooski, VT, USA) measuring the absorbance at 340 nm every 15 s. The absorbance was measured for at least 4 min after there was no further change to ensure that the reaction had reached completion, resulting in measurements spanning approximately 8 min in total. The absorbance value of the blank samples without NDP-Glc was subtracted from all readings.
Results and discussion
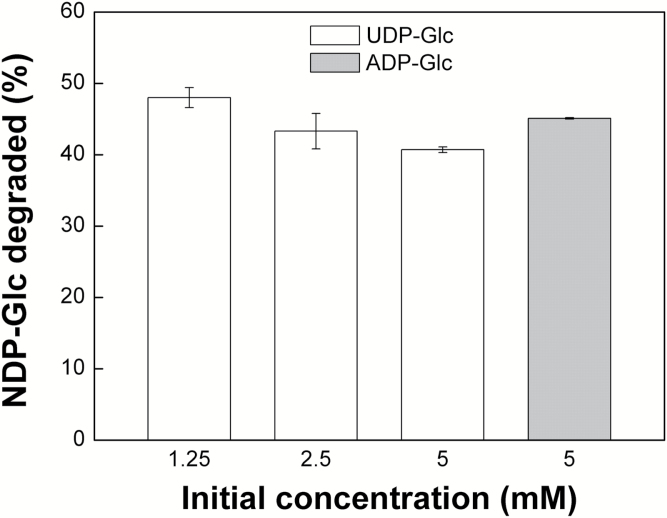
The stability of UDP-Glc and ADP-Glc was initially assessed by incubation of known amounts at pH 9.0 and 37°C, in the presence of 10 mM MgCl2, and measuring the amount remaining after 90 min by enzymatic analysis with UDP-Glc pyrophosphorylase and ADP-Glc PPase, respectively. These conditions were chosen as a physiologically extreme scenario, combining high pH and high Mg2+ concentration with high temperature. Under these extreme conditions, 40–48% of the UDP-Glc was degraded within 90 min (Fig. 1), with only a weak dependence on the initial concentration (Fig. 1). There was a similar loss of 45% of ADP-Glc under these conditions (Fig. 1).
Fig. 1.
Extent of NDP-Glc degradation determined using the enzymatic method. The results match well with those obtained from the NMR analysis and also show that the rate of degradation is marginally dependent on the initial concentration of the NDP-sugar.
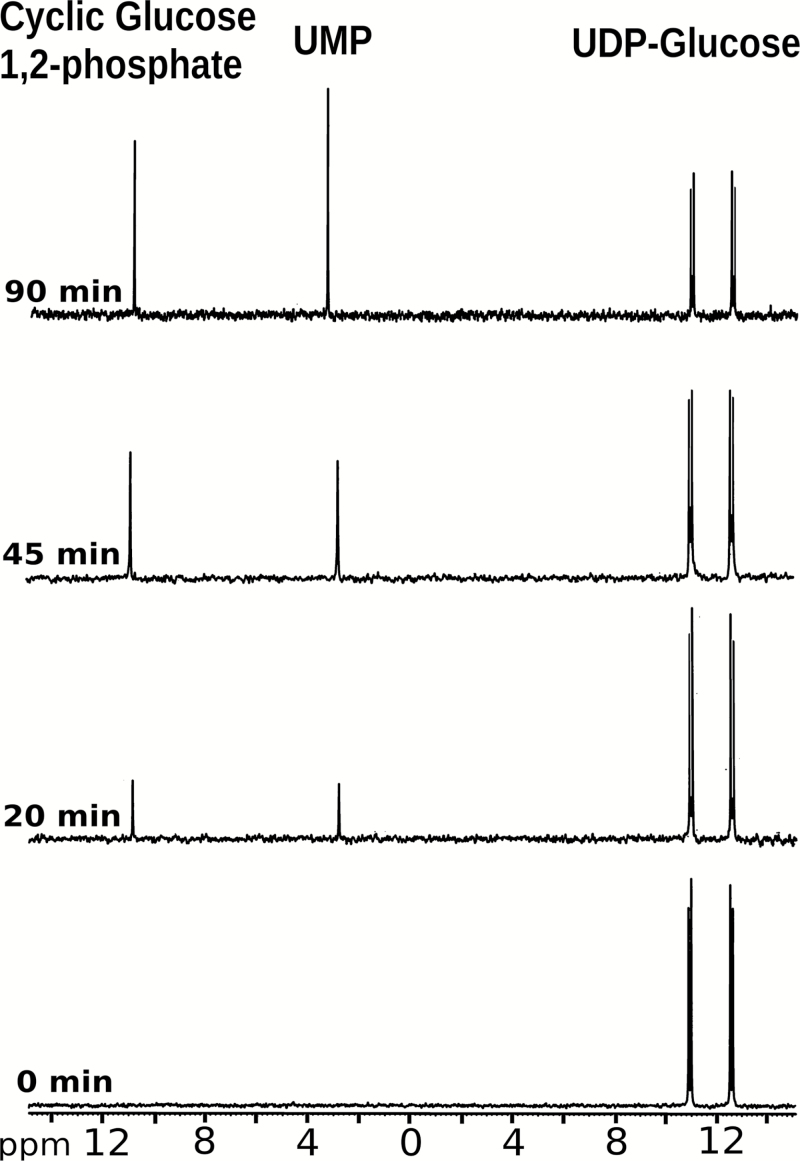
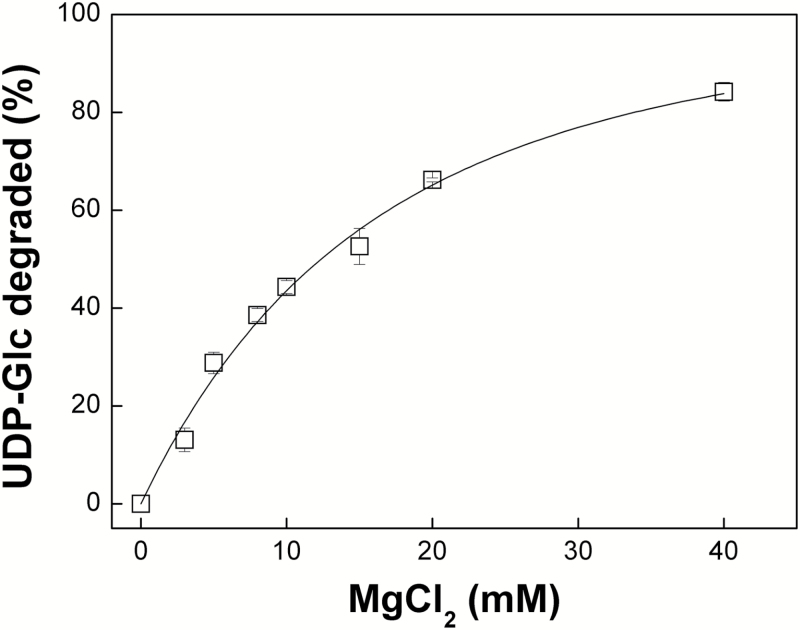
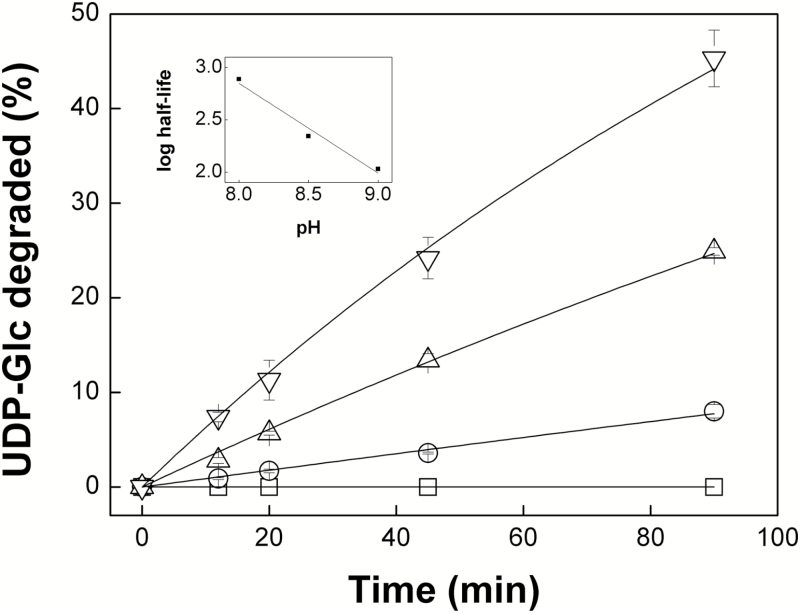
We used [31P]NMR to investigate the kinetics and dependence of the degradation on pH and Mg2+ concentration in more detail. When incubated at pH 9.0 in the presence of 10 mM MgCl2, the amplitude of the UDP-Glc chemical shift peaks decreased over time and was accompanied by the appearance of additional peaks corresponding to the chemical shifts of UMP and cyclic Glc 1,2-phosphate (Fig. 2), the characteristic degradation products from alkaline cleavage of UDP-Glc (Paladini and Leloir, 1952). The cleavage process required the presence of Mg2+, with the amount of UDP-Glc degraded showing a first order exponential response to increasing Mg2+ concentration, resulting in 84% degradation at 40 mM Mg2+ (Fig. 3). No detectable loss of UDP-Glc was observed at pH 7.0 but degradation was detectable at pH 8.0 and increased at higher pH values up to pH 9.0 (Fig. 4). Calculated half-lives for UDP-Glc were 773 min at pH 8.0, 220 min at pH 8.5, and 107 min at pH 9.0. There was a linear correlation between the log10 of the half-lives for UDP-Glc and the pH (Pearson’s r=-0.988), suggesting that the reaction depends on the concentration of hydroxyl ions (Fig. 4, inset). Degradation of ADP-Glc reached 47% after 90 min incubation at pH 9.0 with 10 mM Mg2+, a similar value to that observed for UDP-Glc (Fig. 4).
Fig. 2.
NMR spectra of UDP-Glc samples incubated for 0, 20, 45, and 90 min at pH 9.0 with 10 mM Mg2+. The time-dependent cleavage of UDP-Glc gives rise to UMP and cyclic Glc 1,2-phosphate.
Fig. 3.
Degradation of UDP-Glc at pH 9.0 and varying concentrations of Mg2+. Degradation of the NDP-sugar depends on the concentration of the divalent cation.
Fig. 4.
Degradation of UDP-Glc is higher at alkaline pH values. The main plot shows data derived from the NMR analysis of UDP-Glc samples that were incubated for 0, 12, 20, 45, and 90 min with 10 mM Mg2+ at pH 7.0 (square), 8.0 (circle), 8.5 (triangle) and 9.0 (inverted triangle). These curves were used to calculate the half-lives of UDP-Glc at different pH values. The inset shows the linear correlation between the log10 of the UDP-Glc half-lives and the pH.
These results confirm the previous reports from Paladini and Leloir (1952) and Murata et al. (1964) that UDP-Glc and ADP-Glc decompose under alkaline conditions. However, their susceptibility to degradation is strongly dependent on pH and Mg2+ concentration (Figs 3 and 4), and even under the most extreme conditions tested, namely pH 9.0, 10 mM MgCl2 and 37°C, over 50% of the initial UDP-Glc and ADP-Glc survived incubation for 90 min (Figs 1 and 4). In plant cells, UDP-Glc is located predominantly, or even exclusively, in the cytosol (Szecowka et al., 2013; Arrivault et al., 2014), which typically has a pH of 7.3–7.7 (Ishizawa, 2014) and a concentration of free Mg2+ of 0.2–0.4 mM (Yazaki et al., 1988; Gout et al., 2014). From our results, we can conclude that UDP-Glc is stable under such conditions. Its concentration in vivo will therefore be determined by the relative rates of UDP-Glc synthesis and consumption by UDP-Glc metabolizing enzymes, with its chemical instability at alkaline pH having little or no significant impact on in vivo levels.
During photosynthesis, ion movements across the thylakoid membranes result in alkalinization of the stroma to around pH 8.3 and a rise in Mg2+ concentration to around 6 mM (Werdan et al., 1975; Portis and Heldt, 1976; Krause, 1977; Portis, 1981). Although these conditions appear to be more favourable to chemical cleavage of NDP-Glc metabolites, in reality the impact of this instability is likely to be very limited. The calculated half-lives for UDP-Glc at alkaline pH values ranged from 107–773 min, which is over two orders of magnitude higher than the estimated turnover time for UDP-Glc of 36 s in planta (Arrivault et al., 2009). ADP-Glc displayed similar chemical stability to UDP-Glc (Figs 1 and 4); therefore, we can reasonably expect half-life values for ADP-Glc to be in the same range as UDP-Glc. Such values would be over four orders of magnitude greater than the estimated turnover time for ADP-Glc of 0.5 s in planta (Arrivault et al., 2009). Thus, our data do not support the idea that the chemical instability of ADP-Glc favors its accumulation in the cytosol rather than the chloroplast stroma, which was a theoretical argument put forward to support the alternative pathway of starch biosynthesis (Baroja-Fernández et al., 2001; Bahaji et al., 2011). We conclude that the chemical instability of UDP-Glc and ADP-Glc under mildly alkaline conditions has no significant impact on their concentrations in vivo.
Our results also demonstrate that even under the most extreme conditions tested, namely pH 9.0, 10 mM Mg2+ and 37°C, losses of UDP-Glc and ADP-Glc were less than 50% after 90 min incubation. The disputed SUS activity data reported by Bieniawska et al. (2007) and Barratt et al. (2009) came from in vitro assays with reaction mixtures containing 2.2 mM MgCl2, which were incubated at 20°C for only 20 min. Based on the data (Figs 3 and 4), we can estimate that the difference in Mg2+ concentration alone would reduce the loss of UDP-Glc by a factor of three compared with the most extreme conditions tested, and that the shorter incubation time of 20 min versus 90 min would reduce losses by a further factor of 4.5. Thus, the maximal loss of UDP-Glc to be expected under the assay conditions used by Bieniawska et al. (2007) and Barratt et al. (2009) would lead to underestimation of SUS activity by less than 10%. In reality, losses of UDP-Glc would be even lower because of the lower incubation temperature of 20°C versus 37°C. From these calculations, the instability of UDP-Glc would have had little impact on the SUS activities measured by Bieniawska et al. (2007) and Barratt et al. (2009), with the reported values underestimating the true activity by around 10% at most and very likely even less. The discrepancies between the various reports of SUS activities in sus mutants (Bieniawska et al. 2007; Barratt et al., 2009; Baroja-Fernández et al., 2012a) remain to be resolved. However, the criticism by Baroja-Fernández et al. (2012a) of the SUS activity data in the other two studies, based on the assumed instability of UDP-Glc under the in vitro assay conditions, is not supported by our results. It is also worth reiterating that even if residual SUS activity in the quadruple sus1234 mutant was underestimated by Barratt et al. (2009), none of this activity would be in the mesophyll cells where starch synthesis occurs (Barratt et al. 2009; Smith et al., 2012). Wild-type levels of starch in the quadruple loss-of-function mutant therefore indicate that SUS makes no significant contribution to starch synthesis in Arabidopsis leaves.
In conclusion, the chemical instability of UDP-Glc and ADP-Glc at alkaline pH in the presence of Mg2+ is unlikely to have any significant impact on in vivo concentrations of these metabolites in plants. Arguments in favor of the alternative pathway of starch synthesis based on NDP-sugar instability therefore have little merit.
Author contributions
AAI and MAB conceived the project; BLH, CMF and MDAD performed experiments; all authors analyzed data and wrote the manuscript.
Acknowledgements
This work was supported by NSF (MCB 1616851 to MAB) and ANPCyT (PICT-2015-1767 to AAI, PICT-2014-1305 to CMF and PICT-2014–3362 to MDAD). The authors acknowledge the NMR technical support of the late Dr David French. CMF, MDAD and AAI are Researchers from CONICET.
Glossary
Abbreviations:
- ADP-Glc PPase
ADP-glucose pyrophosphorylase
- NDP
nucleoside diphosphate
- NMP
nucleoside monophosphate
- pPGI
plastidial phosphoglucose isomerase
- pPGM
plastidial phosphoglucomutase.
References
- Arrivault S, Guenther M, Florian A et al. . 2014. Dissecting the subcellular compartmentation of proteins and metabolites in arabidopsis leaves using non-aqueous fractionation. Molecular & Cellular Proteomics 13, 2246–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrivault S, Guenther M, Ivakov A, Feil R, Vosloh D, van Dongen JT, Sulpice R, Stitt M. 2009. Use of reverse-phase liquid chromatography, linked to tandem mass spectrometry, to profile the Calvin cycle and other metabolic intermediates in Arabidopsis rosettes at different carbon dioxide concentrations. The Plant Journal 59, 826–839. [DOI] [PubMed] [Google Scholar]
- Bahaji A, Baroja-Fernández E, Sánchez-López AM et al. . 2014a. HPLC-MS/MS analyses show that the near-Starchless aps1 and pgm leaves accumulate wild type levels of ADPglucose: further evidence for the occurrence of important ADPglucose biosynthetic pathway(s) alternative to the pPGI-pPGM-AGP pathway. PLoS ONE 9, e104997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahaji A, Li J, Ovecka M et al. . 2011. Arabidopsis thaliana mutants lacking ADP-glucose pyrophosphorylase accumulate starch and wild-type ADP-glucose content: further evidence for the occurrence of important sources, other than ADP-glucose pyrophosphorylase, of ADP-glucose linked to leaf starch biosynthesis. Plant & Cell Physiology 52, 1162–1176. [DOI] [PubMed] [Google Scholar]
- Bahaji A, Li J, Sánchez-López ÁM et al. . 2014b. Starch biosynthesis, its regulation and biotechnological approaches to improve crop yields. Biotechnology Advances 32, 87–106. [DOI] [PubMed] [Google Scholar]
- Bahaji A, Sánchez-López AM, De Diego N et al. . 2015. Plastidic phosphoglucose isomerase is an important determinant of starch accumulation in mesophyll cells, growth, photosynthetic capacity, and biosynthesis of plastidic cytokinins in Arabidopsis. PLoS ONE 10, e0119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballicora MA, Iglesias AA, Preiss J. 2004. ADP-glucose pyrophosphorylase: a regulatory enzyme for plant starch synthesis. Photosynthesis Research 79, 1–24. [DOI] [PubMed] [Google Scholar]
- Ballicora MA, Sesma JI, Iglesias AA, Preiss J. 2002. Characterization of chimeric ADPglucose pyrophosphorylases of Escherichia coli and Agrobacterium tumefaciens. Importance of the C-terminus on the selectivity for allosteric regulators. Biochemistry 41, 9431–9437. [DOI] [PubMed] [Google Scholar]
- Baroja-Fernández E, Muñoz FJ, Akazawa T, Pozueta-Romero J. 2001. Reappraisal of the currently prevailing model of starch biosynthesis in photosynthetic tissues: a proposal involving the cytosolic production of ADP-glucose by sucrose synthase and occurrence of cyclic turnover of starch in the chloroplast. Plant & Cell Physiology 42, 1311–1320. [DOI] [PubMed] [Google Scholar]
- Baroja-Fernández E, Muñoz FJ, Akazawa T, Pozueta-Romero J. 2005. Response to Neuhaus et al.: No need to shift the paradigm on the metabolic pathway to transitory starch in leaves. Trends in Plant Science 10, 156–158. [DOI] [PubMed] [Google Scholar]
- Baroja-Fernández E, Muñoz FJ, Bahaji A, Almagro G, Pozueta-Romero J. 2012a Reply to Smith et al.: No evidence to challenge the current paradigm on starch and cellulose biosynthesis involving sucrose synthase activity. Proceedings of the National Academy of Sciences, USA 109, E777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroja-Fernández E, Muñoz FJ, Li J, Bahaji A, Almagro G, Montero M, Etxeberria E, Hidalgo M, Sesma MT, Pozueta-Romero J. 2012b Sucrose synthase activity in the sus1/sus2/sus3/sus4 Arabidopsis mutant is sufficient to support normal cellulose and starch production. Proceedings of the National Academy of Sciences, USA 109, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroja-Fernández E, Muñoz FJ, Zandueta-Criado A, Morán-Zorzano MT, Viale AM, Alonso-Casajús N, Pozueta-Romero J. 2004. Most of ADP-glucose linked to starch biosynthesis occurs outside the chloroplast in source leaves. Proceedings of the National Academy of Sciences, USA 101, 13080–13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt DH, Derbyshire P, Findlay K, Pike M, Wellner N, Lunn J, Feil R, Simpson C, Maule AJ, Smith AM. 2009. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proceedings of the National Academy of Sciences, USA 106, 13124–13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baslam M, Baroja-Fernández E, Ricarte-Bermejo A et al. . 2017. Genetic and isotope ratio mass spectrometric evidence for the occurrence of starch degradation and cycling in illuminated Arabidopsis leaves. PLoS ONE 12, e0171245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieniawska Z, Paul Barratt DH, Garlick AP, Thole V, Kruger NJ, Martin C, Zrenner R, Smith AM. 2007. Analysis of the sucrose synthase gene family in Arabidopsis. The Plant Journal 49, 810–828. [DOI] [PubMed] [Google Scholar]
- Cardini CE, Leloir LF, Chiriboga J. 1955. The biosynthesis of sucrose. The Journal of Biological Chemistry 214, 149–155. [PubMed] [Google Scholar]
- Carpita NC. 2011. Update on mechanisms of plant cell wall biosynthesis: how plants make cellulose and other (1->4)-β-D-glycans. Plant Physiology 155, 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T, Huber SC, Somerville C. 1985. Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiology 79, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comparot-Moss S, Denyer K. 2009. The evolution of the starch biosynthetic pathway in cereals and other grasses. Journal of Experimental Botany 60, 2481–2492. [DOI] [PubMed] [Google Scholar]
- De Bruyn F, Maertens J, Beauprez J, Soetaert W, De Mey M. 2015. Biotechnological advances in UDP-sugar based glycosylation of small molecules. Biotechnology Advances 33, 288–302. [DOI] [PubMed] [Google Scholar]
- Ebrecht AC, Orlof AM, Sasoni N, Figueroa CM, Iglesias AA, Ballicora MA. 2015. On the ancestral UDP-glucose pyrophosphorylase activity of GalF from Escherichia coli. Frontiers in Microbiology 6, 1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst RR, Anderson WA. 1966. Application of Fourier transform spectroscopy to magnetic resonance. Review of Scientific Instruments 37, 93–102. [Google Scholar]
- Espada J. 1962. Enzymic synthesis of adenosine diphosphate glucose from glucose 1-phosphate and adenosine triphosphate. The Journal of Biological Chemistry 237, 3577–3581. [Google Scholar]
- Ghosh HP, Preiss J. 1966. Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. The Journal of Biological Chemistry 241, 4491–4504. [PubMed] [Google Scholar]
- Gibbs M, Cynkin MA. 1958. Conversion of carbon-14 dioxide to starch glucose during photosynthesis by spinach chloroplasts. Nature 182, 1241–1242. [DOI] [PubMed] [Google Scholar]
- Gout E, Rébeillé F, Douce R, Bligny R. 2014. Interplay of Mg2+, ADP, and ATP in the cytosol and mitochondria: Unravelling the role of Mg2+ in cell respiration. Proceedings of the National Academy of Sciences, USA 111, E4560–E4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg E, Preiss J. 1964. The occurrence of adenosine diphosphate glucose: glycogen transglucosylase in bacteria. The Journal of Biological Chemistry 239, 4314–4315. [PubMed] [Google Scholar]
- Günther H. 2013. The physical basis of the nuclear magnetic resonance experiment. Part II: pulse and Fourier-transform NMR. In: Günther H, ed. NMR Spectroscopy: Basic Principles, Concepts, and Applications in Chemistry. Weinheim: Wiley-VCH, 233–280. [Google Scholar]
- Heldt HW, Chon CJ, Maronde D. 1977. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiology 59, 1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa K. 2014. Intracellular pH regulation of plant cells under anaerobic conditions. In: van Dongen JT, Licausi F, eds. Low-Oxygen Stress in Plants. Wein: Springer-Verlag, 59–74. [Google Scholar]
- Krause GH. 1977. Light-induced movement of magnesium ions in intact chloroplasts. Spectroscopic determination with Eriochrome Blue SE. Biochimica et Biophysica Acta 460, 500–510. [DOI] [PubMed] [Google Scholar]
- Leloir LF, Cabib E. 1953. The enzymatic synthesis of trehalose phosphate. Journal of the American Chemical Society 75, 5445–5446. [Google Scholar]
- Leloir LF, Olavarria JM, Goldemberg SH, Carminatti H. 1959. Biosynthesis of glycogen from uridine diphosphate glucose. Archives of Biochemistry and Biophysics 81, 508–520. [DOI] [PubMed] [Google Scholar]
- Leloir LF. 1951. The enzymatic transformation of uridine diphosphate glucose into a galactose derivative. Archives of Biochemistry and Biophysics 33, 186–190. [DOI] [PubMed] [Google Scholar]
- Leloir LF. 1971. Two decades of research on the biosynthesis of saccharides. Science 172, 1299–1303. [DOI] [PubMed] [Google Scholar]
- Leloir LF. 1983. Far away and long ago. Annual Review of Biochemistry 52, 1–15. [DOI] [PubMed] [Google Scholar]
- Lin TP, Caspar T, Somerville C, Preiss J. 1988. Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) heynh lacking ADPglucose pyrophosphorylase activity. Plant Physiology 86, 1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch-Petersen A. 1955. Investigations of the properties and mechanism of the uridine diphosphate glucose pyrophosphorylase reaction. Acta Chemica Scandinavica 9, 1523–1536. [Google Scholar]
- Murata T, Minamikawa T, Akazawa T, Sugiyama T. 1964. Isolation of adenosine diphosphate glucose from ripening rice grains and its enzymic synthesis. Archives of Biochemistry and Biophysics 106, 371–378. [DOI] [PubMed] [Google Scholar]
- Murata T, Minamikawa T, Akazawa T. 1963. Adenosine diphosphate glucose in rice and its role in starch synthesis. Biochemical and Biophysical Research Communications 13, 439–443. [Google Scholar]
- Nanninga LB. 1957. Formation constants and heat stability of calcium and magnesium complexes of adenosinetri-, di- and mono-phosphate. The Journal of Physical Chemistry 61, 1144–1149. [Google Scholar]
- Neuhaus HE, Häusler RE, Sonnewald U. 2005. No need to shift the paradigm on the metabolic pathway to transitory starch in leaves. Trends in Plant Science 10, 154–156; author reply 156. [DOI] [PubMed] [Google Scholar]
- Paladini AC, Leloir LF. 1952. Studies on uridine-diphosphate-glucose. The Biochemical Journal 51, 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis AR. 1981. Evidence of a low stromal Mg concentration in intact chloroplasts in the dark: I. studies with the ionophore A23187. Plant Physiology 67, 985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis AR Jr, Heldt HW. 1976. Light-dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochimica et Biophysica Acta 449, 434–436. [DOI] [PubMed] [Google Scholar]
- Recondo E, Dankert M, Leloir LF. 1963. Isolation of adenosine diphosphate D-glucose from corn grains. Biochemical and Biophysical Research Communications 12, 204–207. [DOI] [PubMed] [Google Scholar]
- Sánchez-López ÁM, Bahaji A, De Diego N et al. . 2016. Arabidopsis responds to Alternaria alternata volatiles by triggering plastid phosphoglucose isomerase-independent mechanisms. Plant Physiology 172, 1989–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Kruger NJ, Lunn JE. 2012. Source of sugar nucleotides for starch and cellulose synthesis. Proceedings of the National Academy of Sciences, USA 109, E776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowokinos JR. 1976. Pyrophosphorylases in Solanum tuberosum: I. Changes in ADP-glucose and UDP-glucose pyrophosphorylase activities associated with starch biosynthesis during tuberization, maturation, and storage of potatoes. Plant Physiology 57, 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Zeeman SC. 2012. Starch turnover: pathways, regulation and role in growth. Current Opinion in Plant Biology 15, 282–292. [DOI] [PubMed] [Google Scholar]
- Streb S, Egli B, Eicke S, Zeeman SC. 2009. The debate on the pathway of starch synthesis: a closer look at low-starch mutants lacking plastidial phosphoglucomutase supports the chloroplast-localized pathway. Plant Physiology 151, 1769–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb S, Zeeman SC. 2012. Starch metabolism in Arabidopsis. The Arabidopsis Book 9, e0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szecowka M, Heise R, Tohge T et al. . 2013. Metabolic fluxes in an illuminated Arabidopsis rosette. The Plant Cell 25, 694–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli SL, Palmieri MJ, Berry GT, Kirkman HN, Segal S. 1992. 31P NMR analysis of red blood cell UDPGlucose and UDPGalactose: comparison with HPLC and enzymatic methods. Analytical Biochemistry 202, 105–110. [DOI] [PubMed] [Google Scholar]
- Werdan K, Heldt HW, Milovancev M. 1975. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochimica et Biophysica Acta 396, 276–292. [DOI] [PubMed] [Google Scholar]
- Withers SG, Madsen NB, Sykes BD. 1981. Active form of pyridoxal phosphate in glycogen phosphorylase. Phosphorus-31 nuclear magentic resonance investigation. Biochemistry 20, 1748–1756. [DOI] [PubMed] [Google Scholar]
- Yazaki Y, Asukagawa N, Ishikawa Y, Ohta E, Sakata M. 1988. Estimation of cytoplasmic free Mg2+ levels and phosphorylation potentials in mung bean root tips by in vivo 31P NMR spectroscopy. Plant and Cell Physiology 29, 919–924. [Google Scholar]
- Yu TS, Lue WL, Wang SM, Chen J. 2000. Mutation of Arabidopsis plastid phosphoglucose isomerase affects leaf starch synthesis and floral initiation. Plant Physiology 123, 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC. 2015. Carbohydrate metabolism. In: Buchanan BB, Gruissem W, Jones RL, eds. Biochemistry & Molecular Biology of Plants, Second Edition. Chichester: John Wiley & Sons, Ltd, 567–609. [Google Scholar]
- Zervosen A, Römer U, Elling L. 1998. Application of recombinant sucrose synthase-large scale synthesis of ADP-glucose. Journal of Molecular Catalysis B: Enzymatic 5, 25–28. [Google Scholar]