Summary
Antibody (immunoglobulins A and G) responses to rotavirus are associated with protection against reinfection, but do not predict whether rotavirus infections manifest as gastroenteritis. Risk for rotavirus infections to manifest as gastroenteritis declines with older age, independent of naturally acquired immunity.
Keywords: rotavirus, gastroenteritis, immunity, natural infection, birth cohort
Abstract
Background
Observational studies in socioeconomically distinct populations have yielded conflicting conclusions about the strength of naturally acquired immunity against rotavirus gastroenteritis (RVGE), mirroring vaccine underperformance in low-income countries. We revisited birth cohort studies to understand naturally acquired protection against rotavirus infection and RVGE.
Methods
We reanalyzed data from 200 Mexican and 373 Indian children followed from birth to 2 and 3 years of age, respectively. We reassessed protection against RVGE, decomposing the incidence rate into the rate of rotavirus infection and the risk of RVGE given infection, and tested for serum antibody correlates of protection using regression models.
Results
Risk for primary, secondary, and subsequent infections to cause RVGE decreased per log-month of age by 28% (95% confidence interval [CI], 12%–41%), 69% (95% CI, 30%–86%), and 64% (95% CI, –186% to 95%), respectively, in Mexico City, and by 10% (95% CI, –1% to 19%), 51% (95% CI, 41%–59%) and 67% (95% CI, 57%–75%), respectively, in Vellore. Elevated serum immunoglobulin A and immunoglobulin G titers were associated with partial protection against rotavirus infection. Associations between older age and reduced risk for RVGE or moderate-to-severe RVGE given infection persisted after controlling for antibody levels.
Conclusions
Dissimilar estimates of protection against RVGE may be due in part to age-related, antibody-independent risk for rotavirus infections to cause RVGE.
Rotavirus gastroenteritis (RVGE) remains a leading cause of pediatric gastrointestinal disease burden globally [1], with approximately 10 million severe cases and 193000 deaths annually among children <5 years old [2]. Live oral vaccines are being integrated into national immunization plans of developing countries following a decade of use in higher-income settings. Because immunity against rotavirus infection is imperfect, understanding how naturally acquired and vaccine-derived protection influences rotavirus epidemiology is critical for planning interventions and assessing vaccine impact [3–6].
Birth cohort studies have sought to measure naturally acquired immune protection against rotavirus among young children [7–11]. Under comparable designs, studies have yielded near-identical estimates of naturally acquired protection against rotavirus infection. However, protection against RVGE, and against moderate-to-severe RVGE in particular, has appeared to differ considerably. In a cohort of Mexican children, 2 infections seemed to confer complete protection against moderate-to-severe RVGE [9], while some children in Vellore, India, experienced severe disease following ≥3 infections [8]. These apparent differences in protection mirror variation in vaccine performance. Despite delivering >90% protection against moderate-to-severe RVGE in the United States and Europe [12], estimated protection has ranged from 81% in middle-income Latin American countries, including Mexico [13], to 39%–48% in low-income African and Asian settings [14, 15].
Several hypotheses have been proposed to account for differences in the epidemiology of diarrheal pathogens across settings. Nutrition-related immune deficiencies, host-genetic factors, and physiological damage to the gut from previous infections or chronic conditions may contribute to susceptibility and weak immune protection in low-resource, high-burden settings such as Vellore. Recent multicenter cohort studies have been undertaken to determine how these risk factors relate to diarrhea susceptibility [16–18] and the performance of oral polio and rotavirus vaccines [19]. A question the earlier cohort studies can answer, however, is whether age-dependent host responses to rotavirus infection further confound direct comparison of estimates from studies undertaken in distinct settings. Children exposed to differing levels of rotavirus transmission are expected to acquire primary, secondary, and later infections at distinct ages, when they may experience differential risk for RVGE given infection [20].
We revisited 2 large-scale birth cohort studies undertaken in Mexico City, Mexico and Vellore, India, to understand why protection against RVGE appeared to differ between the settings despite similar levels of protection against rotavirus infection [8, 9]. In side-by-side analyses of the studies, we evaluated serum antibody concentrations and age as determinants of rates at which children acquired rotavirus infection, and as determinants of risk for rotavirus infections to cause RVGE.
METHODS
Birth Cohort Studies
The studies followed similar protocols [8, 9] aiming to identify all rotavirus infections among children during follow-up, and to characterize gastrointestinal symptoms associated with each infection. Children were enrolled at birth in slum communities and followed to ages 24 and 36 months in Mexico City and Vellore, respectively. Surveillance for rotavirus infection included scheduled testing of asymptomatic stool specimens for rotavirus (weekly in Mexico City and biweekly in Vellore). Sera were drawn every 4 and 6 months in Mexico City and Vellore, respectively, to detect rises in anti-rotavirus immunoglobulin G (IgG) and immunoglobulin A (IgA) titers (Table 1). Both studies defined IgG seroconversion by a 4-fold rise in titers; the definition of IgA seroconversion (4-fold rise in Mexico City vs 3-fold rise in Vellore) accommodated antibody waning over the longer period between tests in India.
Table 1.
Study Design, Enrollment, and Follow-Up
| Study Attributes | Mexico City Cohort | Vellore Cohort |
|---|---|---|
| Duration of follow-up | 24 mo | 36 mo |
| Frequency of asymptomatic stool testing | Weekly | Every 2 wk |
| Frequency of serological testing | Every 4 mo | At least every 6 mo |
| Definition of rotavirus shedding | ELISA positive | 2x ELISA positive or RT-PCR positive |
| Definition of seroconversion | 4-fold rise in IgG or IgA | 4-fold rise in IgG or 3-fold rise in IgA |
| Study population | 200 children | 373 childrena |
| Child-months of observation | 3699/4800 (77%)b | 13341/13428 (99%) |
| Asymptomatic stool samples tested | 15503 | 26902 |
| Of all scheduled tests | 15503/20800 (75%) | 26902/29094 (92%) |
| Of all scheduled tests while child was retained in follow-up | 15503/16029 (97%) | 26902/28906 (93%) |
| Diarrheal episodes tested, of all reported diarrheal episodes | 963/1133 (85%) | 1829/1856 (99%) |
| Serum samples tested, of all scheduled tests | 1037/1080 (96%) | 2565/2598 (99%) |
| Infections detected | 315 | 1103 |
| From diarrheal episodes | 89/315 (28%) | 282/1103 (26%) |
| From asymptomatic shedding | 88/315 (28%) | 237/1103 (21%) |
| From seroconversion only | 139/315 (44%) | 584/1103 (53%) |
| Mean (SD) age, d, asymptomatic infections detected by seroconversion only | 445 (167)c | 626 (308)d |
| Mean age, d, asymptomatic infections detected by shedding | 339 (187)c | 542 (342)d |
| Rotavirus-negative diarrhea samples | 874/963 (91%) | 1547/1829 (85%) |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; IgA, immunoglobulin A; IgG, immunoglobulin G; RT-PCR, reverse-transcription polymerase chain reaction; SD, standard deviation.
aSample restricted to children who completed 3 years of follow-up (83% of initial cohort of 452 children).
bNinety-eight of 200 enrolled children (49%) in Mexico City completed the scheduled 2 years of follow-up.
cIn the Mexico City cohort, the mean age of asymptomatic infections detected by shedding is significantly lower than the mean age of asymptomatic infections detected by seroconversion alone (P < .0001 by cluster bootstrap).
dIn the Vellore cohort, the mean age of asymptomatic infections detected by shedding is significantly lower than the mean age of asymptomatic infections detected by seroconversion alone (P < .001 by cluster bootstrap).
Field workers visited homes to collect diarrheal stool when mothers reported changes in children’s stool pattern; clinical specimens were collected if children presented for care. Diarrhea episodes were defined as ≥3 watery stools in 24 hours (or ≥2 more than considered “normal” by the mothers), and ended the first day bowel movements returned to the usual daily pattern. Rotavirus infections were considered asymptomatic if no diarrhea occurred within 5 (in Mexico City) or 7 (in Vellore) days before and after rotavirus detection in stool, or during the interval over which a serological response was detected. Physicians assessed diarrheal severity on the 20-point Vesikari scale in clinic or based on symptom data from field workers and mothers. For consistency, we defined moderate-to-severe diarrhea as a Vesikari score of ≥11. In total, 200 children were recruited in Mexico City and retained in follow-up for 77% of the planned child-months of observation. Our analysis of the Vellore cohort included the 373 children (of 452 newborns recruited) who completed all 36 months of follow-up, consistent with primary analyses of the studies; no differences in baseline nutritional and demographic covariates were observed between those who completed or dropped out of follow-up in Vellore [8].
Statistical Analyses
Descriptive Analyses
Within each cohort we calculated age-specific incidence rates of rotavirus infection, asymptomatic infection, and RVGE, as well as all-cause and rotavirus-negative diarrhea, by fitting Poisson regression models measuring the incidence rate as a continuous function of age (in months). Polynomial terms for age were added until the Bayesian information criterion indicated no improvement in model fit. We estimated models using generalized estimating equations (GEE) with robust standard errors to account for repeated sampling of children.
Age and Previous Infection as Determinants of Risk for Rotavirus Infection to Cause RVGE
Primary analyses of the datasets provided similar estimates of relative rates at which children were reinfected with rotavirus after 1, 2, or 3 previous infections. However, these studies suggested weaker protection against incidence of RVGE and moderate-to-severe RVGE in Vellore. Based on differences in age at which infections occurred between the 2 settings, we hypothesized that older age may affect risk for RVGE given rotavirus infection. We examined this first by testing for differences in the proportions of primary, secondary, and subsequent rotavirus infections causing RVGE within age-specific strata defined by intervals between serological tests. For consistency with our use of GEE with robust standard errors in regression analyses, we constructed confidence intervals around proportions using a cluster bootstrap of individuals [21]. We used the same approach to compare risk of moderate-to-severe RVGE, in this case computing credible intervals via the Jeffreys prior [22] due to sparse counts within strata.
To characterize the effect of age on risk (conditional probability) of RVGE or moderate-to-severe RVGE given infection, we calculated the relative risk for these symptomatic manifestations during primary, secondary, and later rotavirus infections as a function of age using the modified Poisson regression approach with robust standard errors [23]. We tested different transformations of the exposure variable (age), identifying via the Bayesian information criterion that a natural log transformation of age provided a superior fit over inclusion of first-, second-, or third-order polynomials for age.
Role of Serum Antibody and Age in Risk for Rotavirus Infection and RVGE
We next used data on serum IgG and IgA antibody titers to better understand the independent roles of acquired immunity and age in protection against rotavirus infection, and protection against RVGE or moderate-to-severe RVGE given infection. We used repeated-events Cox proportional hazards models to measure how time to reinfection related to age and serum IgA and IgG antibody levels. We used Poisson regression models to calculate relative risks for RVGE and moderate-to-severe RVGE given infection acquired at differing ages and serum antibody titers. As in the other analyses, model parameters were estimated via GEE with robust variance to account for repeated sampling of children. Because no threshold IgA and IgG concentration is known to confer complete protection against rotavirus infection or RVGE [24–27], we measured antibody titers as continuous variables, calculating hazard ratios and relative risks associated with each 2-fold rise in titers above previously established [9] minimum dilutions (≤1:50 IgA and ≤1:400 IgG). Consistent with the analysis described above, we included age as a log-transformed, continuous variable. To ensure reliable baseline titer measurements, we limited analyses to the first infection occurring in each serological interval if multiple infections were reported.
Association of Previous RVGE With Risk for Rotavirus Infection and RVGE
Observations that RVGE occurred frequently on successive infections in Vellore led us to hypothesize that certain children may be predisposed to repeat infections, or to experiencing diarrhea when infected, for instance, due to frequent exposure or immune factors. We tested for an association between risk of RVGE given infection and susceptibility to future infection and RVGE; differences between the studies prevented us from conducting further side-by-side analyses adjusting for other potential predictors of susceptibility. Specifically, we measured the association between the symptom status of a child’s previous infection (ie, whether or not previous infection caused RVGE) and time to reinfection, as well as risk for reinfection to cause RVGE. Consistent with the analyses described above, we used repeated-events Cox proportional hazards models to estimate hazard ratios for rotavirus reinfection, and used Poisson regression models to estimate the relative risk that secondary or later infections would cause RVGE as a function of whether the child’s preceding infection was symptomatic. In both cases, we used robust error variance to account for repeated sampling of children, and controlled for age (log-transformed) and serum IgA and IgG titers. To further rule out confounding due to differential immune responses after symptomatic and asymptomatic infections, we also tested whether antibody titer (IgA or IgG) was dependent upon whether the previous infection was symptomatic, controlling for previous titer and age (log-transformed). We did this analysis within the subset of serological measurements separated by 1 infection, estimating regression models via GEE with robust standard errors to account for repeated sampling of children.
RESULTS
Cohort Monitoring
The studies detected 315 and 1103 rotavirus infections in Mexico City and Vellore, respectively, with 89 (28%) and 282 (26%) involving RVGE (Table 1). Of all infections, 139 (44%) and 584 (53%) in Mexico City and Vellore, respectively, were detected solely from serological responses; their timing was imputed by the original investigators as the midpoint of the interval between serological tests.
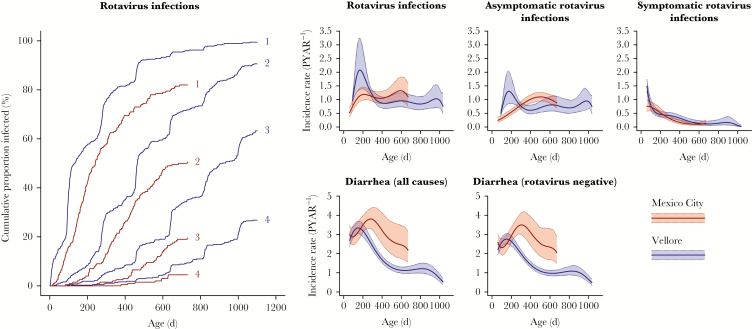
In Mexico City, rates of acquiring rotavirus infection peaked at 1.33 per year (95% confidence interval [CI], 0.95–1.83) at age 19.8 months, and increased with age, consistent with rates of asymptomatic infection. In Vellore, acquisition rates increased earlier in life and peaked at 2.08 per year (95% CI, 1.36–3.24) at age 5.5 months, again consistent with asymptomatic infection (Figure 1). RVGE incidence rates declined with age, and tended not to differ between the 2 settings.
Figure 1.
Cumulative and age-specific incidence of rotavirus infection and rotavirus gastroenteritis (RVGE), all-cause diarrhea, and rotavirus-negative diarrhea. We plot cumulative incidence of rotavirus infection (left panel) and age-specific incidence rates of rotavirus infection and gastroenteritis by age (right panels), fitted using polynomial terms for age with robust standard errors to account for repeated observations from children. Shaded areas denote 95% confidence intervals. Abbreviation: PYAR, person-years at risk.
There were 874 (91%) and 1547 (85%) rotavirus-negative diarrhea episodes in Mexico City and Vellore, respectively. Reported incidence of all-cause and rotavirus-negative diarrhea was equivalent between the cohorts or higher in Mexico City across ages (Figure 1).
Age-Related Reductions in Proportions of Rotavirus Infections Causing RVGE
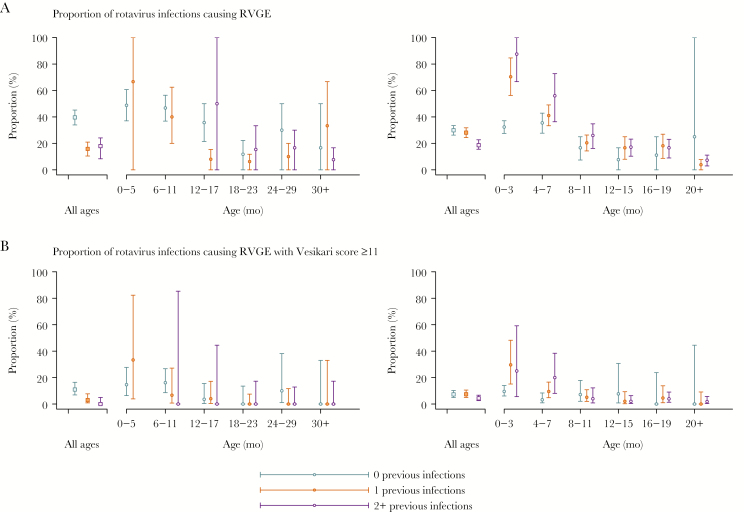
In both cohorts, the proportion of rotavirus infections causing RVGE and moderate-to-severe RVGE declined with the number of previous infections (Figure 2 and Supplementary Table 1). However, the association between the number of infections a child had experienced and the conditional probability for an infection to cause RVGE or moderate-to-severe RVGE differed between the 2 cohorts. In Mexico City, second, third, and fourth infections had 61% (95% CI, 37%–77%), 50% (95% CI, 9%–77%), and 79% (95% CI, 3%–99%) lower risk for causing RVGE, respectively, compared to first infections. We observed no reduction in risk by the second infection in Vellore, and only a 40% (95% CI, 9%–64%) reduction after 3 previous infections (Supplementary Table 1). No children in Mexico City experienced moderate-to-severe RVGE following ≥2 previous infections, but in Vellore, previous infection was not associated with reduced risk for infections to cause moderate-to-severe RVGE (Supplementary Table 1).
Figure 2.
Aggregate and age-specific proportions of primary, secondary, and subsequent rotavirus infections involving diarrhea. Vellore (left panels) and Mexico City (right panels) cohort data with point estimates and 95% confidence intervals obtained by cluster-bootstrap resampling (A) or the Jeffreys prior (B). Age strata are defined as the interval between serological measurements in each study. Error bars denote 95% confidence/credible intervals. Asterisks denote where at least 1 significant within-age group risk difference appears across infections: *P < .05; ** P < .01; ***P < .001. Abbreviation: RVGE, rotavirus gastroenteritis.
When we compared infections at similar ages, however, reductions in proportions of primary, secondary, and later rotavirus infections causing RVGE or moderate-to-severe RVGE were less apparent (Figure 2). On the contrary, RVGE and moderate-to-severe RVGE occurred in a higher proportion of secondary and later infections compared to primary infections among infants 0–3 and 4–7 months of age in Vellore.
In Poisson regression models measuring relative risk of RVGE given infection, we found that each log-month increase in the age at which primary, secondary, and later infections occurred was associated with 28% (95% CI, 12%–41%), 69% (95% CI, 30%–86%), and 64% (95% CI, –186% to 95%) lower risk for infections to cause RVGE, respectively, in Mexico City, and with 10% (95% CI, –1% to 19%), 51% (95% CI, 41%–59%), and 67% (95% CI, 57%–75%) lower risk in Vellore (Table 2). Similarly, risk for infections to cause moderate-to-severe RVGE declined with each log-month of age by 44% (95% CI, 11%–65%) and 93% (95% CI, 76%–98%) for primary and secondary infections in Mexico City, respectively. In Vellore, we calculated 14% (95% CI, –10% to 33%), 62% (95% CI, 45%–74%), and 74% (95% CI, 51%–86%) lower risk for primary, secondary, and later infections, respectively, to cause moderate-to-severe RVGE with each log-month of age (Table 2). These outcomes confirmed patterns that were visually evident in the proportion of infections causing symptoms within broader age groups (Figure 2). Patterns we identified in the main analyses persisted in sensitivity analyses of the Vellore dataset excluding G10P[11] infections (Supplementary Figure 1), which were not associated with protection in previous analyses [28].
Table 2.
Effect of Age on the Relative Risk of Rotavirus Gastroenteritis (RVGE) and Moderate-to-Severe RVGE Given Infection
| No. of Previous Infections | RR per Log-month (95% CI)a,b | |
|---|---|---|
| Mexico City | Vellore | |
| RVGE | ||
| 0 | 0.72 (.59–.88) | 0.90 (.81–1.01) |
| 1 | 0.31 (.14–.70) | 0.49 (.41–.59) |
| ≥2 | 0.36 (.05–2.86) | 0.33 (.25–.43) |
| RVGE with Vesikari score ≥11 | ||
| 0 | 0.56 (.35–.89) | 0.86 (.67–1.10) |
| 1 | 0.07 (.02–.24) | 0.38 (.26–.55) |
| ≥2 | … | 0.26 (.14–.49) |
Values in boldfaced text indicate statistically significant effect size estimates (defined as a 95% confidence interval excluding 1).
Abbreviations: CI, confidence interval; RR, relative risk; RVGE, rotavirus gastroenteritis.
aRelative risks are computed from Poisson regression models fitted with generalized estimating equations with a robust error variance to account for repeated sampling of individual children; models include an interaction between the number of previous infections and the log-transformed age of children at the time of infection.
bEffect sizes for the decline per log-month of age should be interpreted in the context of differences in the age range within the 2 studies (2 years in Mexico City; 3 years in Vellore).
Role of Age and Immunity in Rotavirus Infection and RVGE Given Infection
Serum IgA and IgG concentrations increased with age, but this association disappeared when controlling for the number of previous infections (Supplementary Table 2). We found that each 2-fold increase in serum IgA titer was associated with a 13% (95% CI, 4%–21%) lower rate of acquiring rotavirus in Mexico City, and with an 8% (95% CI, 2%–12%) lower rate of infection in Vellore when controlling for age (Table 3). However, we note that effect sizes may not be directly comparable due to the differential frequency of serum collection in the 2 settings, as antibody waning can occur. We also estimated 9% (95% CI, 0%–7%) and 17% (95% CI, 14%–20%) lower rates of rotavirus infection in Mexico City and Vellore, respectively, with each 2-fold increase in serum IgG concentration. In contrast to this evidence for antibody-dependent protection against infection, there were no significant associations between serum antibody titer and risk for rotavirus infections to cause RVGE or moderate-to-severe RVGE after controlling for age. Consistent with analyses described above (Table 2), we estimated lower risk for rotavirus infections acquired at older ages to cause RVGE or moderate-to-severe RVGE, independent of serum antibody levels.
Table 3.
Serum Antibody and Age as Determinants of Rotavirus Infection and Risk for Infection to Cause Rotavirus Gastroenteritis
| Predictors | Incidence of Infection of Any Symptom Status | Risk for Infection to Cause RVGE of Any Severity | Risk for Infection to Cause RVGE With Vesikari Score ≥11 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | aHR (95% CI) | RR (95% CI) | aRR (95% CI) | RR (95% CI) | aRR (95% CI) | |
| Mexico City | ||||||
| Serum IgA titer | ||||||
| Per 2-fold increasea | 0.81 (.76–.87) | 0.87 (.79–.96) | 0.78 (.64–.94) | 0.85 (.70–1.02) | 0.75 (.44–1.28) | 0.82 (.47–1.45) |
| Serum IgG titer | ||||||
| Per 2-fold increaseb | 0.82 (.77–.88) | 0.91 (.83–1.00) | 0.89 (.78–1.02) | 0.99 (.88–1.11) | 0.95 (.70–1.29) | 1.06 (.81–1.39) |
| Age | ||||||
| Per log-monthc | 0.81 (.69–.95) | 1.00 (.83–1.19) | 0.43 (.29–.63) | 0.51 (.35–.75) | 0.27 (.12–.62) | 0.33 (.11–.99) |
| Vellore | ||||||
| Serum IgA titer | ||||||
| Per 2-fold increasea | 0.80 (.76–.84) | 0.92 (.88–.98) | 1.01 (.92–1.12) | 1.07 (.98–1.17) | 0.91 (.71–1.17) | 1.01 (.80–1.28) |
| Serum IgG titer | ||||||
| Per 2-fold increaseb | 0.83 (.81–.86) | 0.83 (.80–.86) | 1.03 (.97–1.09) | 0.97 (.90–1.04) | 0.97 (.85–1.11) | 0.89 (.75–1.06) |
| Age | ||||||
| Per log-monthc | 0.90 (.87–.92) | 0.88 (.85–.91) | 0.76 (.70–.82) | 0.74 (.68–.80) | 0.70 (.60–.83) | 0.67 (.55–.81) |
Values in boldfaced text indicate statistically significant effect size estimates (defined as a 95% confidence interval excluding 1).
Abbreviations: aHR, adjusted hazard ratio; aRR, adjusted relative risk; CI, confidence interval; HR, hazard ratio; IgA, immunoglobulin A; IgG, immunoglobulin G; RR, relative risk; RVGE, rotavirus gastroenteritis.
aThe minimum dilution is <1:50; increases in serum IgA titers are measured on a scale of 2-fold increases over this level.
bThe minimum dilution is <1:400; increases in serum IgG titers are measured on a scale of 2-fold increases over this level.
cEffect sizes for the decline per log-month of age should be interpreted in the context of differences in the age range within the 2 studies (2 years in Mexico City; 3 years in Vellore).
Association Between Rotavirus Susceptibility and Previous Symptomatic Infection
Our observation in Vellore of elevated RVGE risk during secondary and later infections during the first 7 months of life prompted us to consider whether certain children may have especially high risk for acquiring rotavirus, or for experiencing RVGE when infected. We found that children in Vellore who experienced RVGE during a rotavirus infection had 74% (95% CI, 19%–156%) higher risk for their next infection to cause RVGE as well, independent of age and serum IgA and IgG titers (Table 4). This association was not apparent among children in Mexico City; we also observed no independent association between previous symptomatic infection and time to next infection. The association in RVGE risk across rotavirus infections was not likely to be attributable to differential immune responses after symptomatic and asymptomatic infections; in addition to controlling for age and serum antibody titers in the analysis, we identified no difference in the magnitude of serum IgA or IgG responses following symptomatic vs asymptomatic infections in either cohort (Table 4).
Table 4.
Antibody-Independent Association of Previous Symptomatic Infection With the Rate of Subsequent Rotavirus Infection and Risk for Infection to Cause Rotavirus Gastroenteritis
| Predictors | Incidence of Next Infectiona | Risk for Next Infection to Cause RVGEb | Fold Increase in Antibody Titerc | |||
|---|---|---|---|---|---|---|
| HR (95% CI) |
aHR (95% CI) |
RR (95% CI) |
aRR (95% CI) |
Relative Increase in Serum IgA, Adjusted (95% CI) | Relative Increase in Serum IgG, Adjusted (95% CI) | |
| Mexico City | ||||||
| Symptomatic previous infection | ||||||
| ref. asymptomatic previous infection | 1.44 (.98–2.14) | 1.08 (.72–1.61) | 0.58 (.21–1.57) | 0.69 (.25–1.93) | 0.90 (.49–1.71) | 1.06 (.52–2.16) |
| Vellore | ||||||
| Symptomatic previous infection | ||||||
| ref. asymptomatic previous infection | 1.22 (1.00–1.49) | 1.07 (.88–1.31) | 1.93 (1.32–2.81) | 1.74 (1.19–2.56) | 0.87 (.62–1.22) | 0.84 (.56–1.24) |
Values in boldfaced text indicate statistically significant effect size estimates (defined as a 95% confidence interval excluding 1).
Abbreviations: aHR, adjusted hazard ratio; aRR, adjusted relative risk; CI, confidence interval; HR, hazard ratio; IgA, immunoglobulin A; IgG, immunoglobulin G; RR, relative risk; RVGE, rotavirus gastroenteritis.
aWe calculated HRs and aHR with repeated-events Cox proportional hazards models of time to next infection using a robust error variance to account for repeated observations from children. As in the other analyses, we adjusted for age at the time of the preceding serum draw (log-transformed) and serum IgA and IgG concentrations.
bWe calculated RRs and aRR with Poisson regression models using generalized estimating equations with a robust error variance to account for repeated observations from children. As in the other analyses, we adjusted for age at time of infection (log-transformed) and serum IgA and IgG concentrations.
cWe calculated the association between the increase in antibody concentration resulting from an infection and whether this infection was symptomatic or asymptomatic using regression models of concentration, estimated with a robust error variance to account for repeated observations from children. Unadjusted estimates are not presented as the change in concentration can only be measured when controlling for previous serum IgA or IgG measurement; models further adjust for age (log-transformed), as in the other analyses.
DISCUSSION
Birth cohort studies have been foundational to our understanding of naturally acquired immunity against rotavirus. Evidence of naturally acquired protection against rotavirus infection and RVGE from studies in Mexico City and other settings provided important rationale behind vaccine development [9, 29, 30]. Lower estimates of protection against RVGE from natural infection in Vellore have aided efforts to address the underperformance of live oral vaccines in low-income settings [8, 31, 32]. Whereas naturally acquired protection against reinfection appeared equivalent in Mexico City and Vellore, uncertainty has persisted as to why immunity against RVGE would appear less protective among Indian children. Because rotavirus infections occur at earlier ages and with higher frequency in Vellore, we conducted paired analyses of the original datasets assessing the role of age and previous immunity in risk for rotavirus infection and RVGE.
In both settings, older age appears to be associated with lower risk for rotavirus infections to cause RVGE or moderate-to-severe RVGE, independent of acquired immunity. In contrast, serum IgA and IgG titers are associated with partial protection against infection. These outcomes suggest that acquired immune protection, by reducing the acquisition rate, defers reinfection to older ages when RVGE or moderate-to-severe RVGE is less likely. Between-cohort differences in the age distribution of primary, secondary, and subsequent infections may have confounded previous comparisons of protection calculated using RVGE incidence endpoints.
Stratifying the proportion of rotavirus infections by age and previous infection reveals a counterintuitive observation among young children in Vellore: At matched ages, secondary and later infections are more likely than primary infections to cause RVGE. Whereas this finding may reflect maternal antibodies preventing diarrhea during the earliest infections, it could also be an example of the Simpson paradox, if children susceptible to acquiring 2 or more infections in the first months of life are also more likely to experience RVGE when infected. Indeed, we find that having experienced RVGE during past infection is associated with future risk for RVGE given infection among children in Vellore, but not in Mexico City, independent of age and serum antibody concentrations.
However, our analysis cannot explain why individual variation in susceptibility to RVGE given infection emerges in Vellore but not Mexico City. As the studies were undertaken in distinct settings, socioeconomic risk factors are not directly comparable between studies; in previous analyses, household hygiene measures and involvement in making bidis (indigenous cigarettes) predicted rotavirus infection in Vellore, but do not have socioeconomic analogues in Mexico City. Although primary analyses did not point to a consistent set of nutritional factors predicting infection or RVGE, associations between these nutritional measures and susceptibility to rotavirus are difficult to interpret because diarrheal illnesses can cause poor growth, malnourishment, and micronutrient deficiencies. In addition, conflicting evidence about associations between nutrition and rotavirus susceptibility in observational and intervention studies underscores the need to interpret associations cautiously. For instance, vitamin A supplementation exacerbated susceptibility to rotavirus infection and RVGE in Guinea-Bissau [33], despite a protective association between vitamin A intake and diarrhea in observational studies [34]. Similarly, although longer breastfeeding was associated with lower RVGE incidence in Mexico City [9], such an association was not evident in Vellore [8]. Although few host-genetic correlates of rotavirus susceptibility and immune response have been investigated, associations with histo-blood group antigen phenotypes including Lewis positivity and FUT2 secretor status have been reported [35–38]. Recent cohort studies [16–19] investigating relationships among nutritional, genetic, and immunological risk factors and diarrhea endpoints may suggest specific covariates leading to differences such as those observed between the Mexico City and Vellore studies.
Our finding of decreasing RVGE risk with older age on primary, secondary, and subsequent rotavirus infections suggests age is one such contributing factor [4]. Infections tended to occur at younger ages and with higher frequency in Vellore compared to Mexico City, but age-specific RVGE incidence was similar in the 2 cohorts. In populations exposed to high rates of transmission, secondary and later infections are expected to occur in closer succession, and thus at younger ages, than in settings where exposure to rotavirus occurs with lower frequency. Although factors determining why age is associated with lower risk of RVGE given infection are not known, postnatal intestinal development, immune maturation, and establishment of gut microbial communities contribute to age-dependent risk for infection to cause RVGE in animal models [39–44].
Our analysis has several limitations. Low-replicating rotavirus infections that would otherwise be asymptomatic could be misclassified as RVGE episodes if they co-occur with other diarrhea-causing conditions (chronic or infectious), thus biasing estimates of protection. However, our finding of higher reported rotavirus-negative and all-cause diarrhea incidence in Mexico City, rather than Vellore (Figure 1), suggests misclassification of rotavirus-positive diarrhea as RVGE would not fully explain discrepancies in estimates of protection. Additional rotavirus infections could be misclassified due to imperfect diagnostic specificity. Whereas around half of infections were detected from seroincidence data, only 1 infection, at most, can be ascribed to the time between serological tests. This, too, is unlikely to undermine our inferences because the time between serological tests was well below the average time between infections; seroincidence data were also coupled with routine stool collection to identify asymptomatic rotavirus shedding. We cannot verify that mothers in the cohorts were equally likely to report diarrhea to field workers; however, similar age-specific proportions of rotavirus infections associated with diarrhea and moderate-to-severe diarrhea suggest consistent reporting between studies. Last, our analysis does not distinguish homotypic and heterotypic reinfections according to G-type or P-type, as not all infections were genotyped, and the studies were underpowered to detect significant differences in homotypic and heterotypic protection based on population-level estimates of this effect [45].
Findings from our reanalysis suggest the immunological mechanism of naturally acquired immunity against RVGE should be revisited. Age-associated reductions in the probability for rotavirus infections to cause RVGE may work in tandem with naturally acquired immunity in protecting older children. The cohort studies undertaken in Mexico City and Vellore demonstrate that previous infection provides moderate protection against reinfection. Our findings suggest variation in the efficacy and impact of live oral rotavirus vaccines against RVGE in low- and high-incidence settings may relate to the age at which rotavirus infections occur. Laboratory and epidemiological studies of factors underlying host- and age-related determinants of RVGE risk are important to improve rotavirus vaccine impact.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. The work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant number R01AI112970 to V. E. P.).
Potential conflicts of interest. J. A. L. has received funding from Pfizer to Harvard University. G. R.-P. has received funding to his institution from GlaxoSmithKline unrelated to the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Liu J, Platts-Mills JA, Juma J et al. . Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 2016; 388:1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walker CL, Rudan I, Liu L et al. . Global burden of childhood pneumonia and diarrhoea. Lancet 2013; 381:1405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pitzer VE, Viboud C, Simonsen L et al. . Demographic variability, vaccination, and the spatiotemporal dynamics of rotavirus epidemics. Science 2009; 325:290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pitzer VE, Atkins KE, de Blasio BF et al. . Direct and indirect effects of rotavirus vaccination: comparing predictions from transmission dynamic models. PLoS One 2012; 7:e42320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lopman BA, Curns AT, Yen C, Parashar UD. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis 2011; 204:980–6. [DOI] [PubMed] [Google Scholar]
- 6. Payne DC, Staat MA, Edwards KM et al. . Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US counties, 2006–2009. Clin Infect Dis 2011; 53:245–53. [DOI] [PubMed] [Google Scholar]
- 7. Reves RR, Hossain MM, Midthun K et al. . An observational study of naturally acquired immunity to rotaviral diarrhea in a cohort of 363 Egyptian children. Calculation of risk for second episodes using age-specific person-years of observation. Am J Epidemiol 1989; 130:981–8. [DOI] [PubMed] [Google Scholar]
- 8. Gladstone BP, Ramani S, Mukhopadhya I et al. . Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med 2011; 365:337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velázquez FR, Matson DO, Calva JJ et al. . Rotavirus infection in infants as protection against subsequent infections. N Engl J Med 1996; 335:1022–8. [DOI] [PubMed] [Google Scholar]
- 10. Mata L, Simhon A, Urrutia JJ, Kronmal RA, Fernández R, García B. Epidemiology of rotaviruses in a cohort of 45 Guatamalan Mayan Indian children observed from birth to the age of three years. J Infect Dis 1983; 148:452–61. [DOI] [PubMed] [Google Scholar]
- 11. Fischer TK, Valentiner-Branth P, Steinsland H et al. . Protective immunity after natural rotavirus infection: a community cohort study of newborn children in Guinea-Bissau, West Africa. J Infect Dis 2002; 186:593–7. [DOI] [PubMed] [Google Scholar]
- 12. Vesikari T, Matson DO, Dennehy P et al. ; Rotavirus Efficacy and Safety Trial (REST) Study Team Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23–33. [DOI] [PubMed] [Google Scholar]
- 13. Linhares AC, Velázquez FR, Pérez-Schael I et al. ; Human Rotavirus Vaccine Study Group Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet 2008; 371:1181–9. [DOI] [PubMed] [Google Scholar]
- 14. Zaman K, Dang DA, Victor JC et al. . Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615–23. [DOI] [PubMed] [Google Scholar]
- 15. Armah GE, Sow SO, Breiman RF et al. . Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606–14. [DOI] [PubMed] [Google Scholar]
- 16. Kotloff KL, Nataro JP, Blackwelder WC et al. . Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 17. Acosta AM, Chavez CB, Flores JT et al. . The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis 2014; 59:S193–206. [DOI] [PubMed] [Google Scholar]
- 18. Mohan VR, Ramanujam K, Babji S et al. . Rotavirus infection and disease in a multi-site birth cohort: results from the MAL-ED study [manuscript published online ahead of print 3 May 2017]. J Infect Dis 2017. doi:10.1093/infdis/jix199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirkpatrick BD, Colgate ER, Mychaleckyj JC et al. ; PROVIDE Study Teams The Performance of Rotavirus and Oral Polio Vaccines in Developing Countries (PROVIDE) study: description of methods of an interventional study designed to explore complex biologic problems. Am J Trop Med Hyg 2015; 92:744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grenfell BT, Anderson RM. The estimation of age-related rates of infection from case notifications and serological data. J Hyg (Lond) 1985; 95:419–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng G, Yu Z, Huang JZ. The cluster bootstrap consistency in generalized estimating equations. J Multivar Anal 2013; 115:33–47. [Google Scholar]
- 22. Jeffreys H. An invariant form for the prior probability in estimation problems. Proc R Soc Lond A Math Phys Sci 1946; 186:453–61. [DOI] [PubMed] [Google Scholar]
- 23. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–6. [DOI] [PubMed] [Google Scholar]
- 24. Patel M, Glass RI, Jiang B, Santosham M, Lopman B, Parashar U. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Dis 2013; 208:284–94. [DOI] [PubMed] [Google Scholar]
- 25. Clarke E, Desselberger U. Correlates of protection against human rotavirus disease and the factors influencing protection in low-income settings. Mucosal Immunol 2015; 8:1–17. [DOI] [PubMed] [Google Scholar]
- 26. Premkumar P, Lopman B, Ramani S et al. . Association of serum antibodies with protection against rotavirus infection and disease in South Indian children. Vaccine 2014; 32(suppl 1):A55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Velázquez FR, Matson DO, Guerrero ML et al. . Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis 2000; 182:1602–9. [DOI] [PubMed] [Google Scholar]
- 28. Banerjee I, Gladstone BP, Le Fevre AM et al. . Neonatal infection with G10P[11] rotavirus did not confer protection against subsequent rotavirus infection in a community cohort in Vellore, South India. J Infect Dis 2007; 195:625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lopman B, Kang G. In praise of birth cohorts: norovirus infection, disease, and immunity. Clin Infect Dis 2014; 58:492–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glass RI, Parashar UD, Bresee JS et al. . Rotavirus vaccines: current prospects and future challenges. Lancet 2006; 368:323–32. [DOI] [PubMed] [Google Scholar]
- 31. Nelson EA, Glass RI. Rotavirus: realising the potential of a promising vaccine. Lancet 2010; 376:568–70. [DOI] [PubMed] [Google Scholar]
- 32. Lopman BA, Pitzer VE, Sarkar R et al. . Understanding reduced rotavirus vaccine efficacy in low socio-economic settings. PLoS One 2012; 7:e41720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diness BR, Christoffersen D, Pedersen UB et al. . The effect of high-dose vitamin A supplementation given with bacille Calmette-Guérin vaccine at birth on infant rotavirus infection and diarrhea: a randomized prospective study from Guinea-Bissau. J Infect Dis 2010; 202(suppl):S243–51. [DOI] [PubMed] [Google Scholar]
- 34. Fawzi WW, Herrera MG, Willett WC, Nestel P, el Amin A, Mohamed KA. Dietary vitamin A intake and the incidence of diarrhea and respiratory infection among Sudanese children. J Nutr 1995; 125:1211–21. [DOI] [PubMed] [Google Scholar]
- 35. Payne DC, Currier RL, Staat MA et al. . Epidemiologic association between FUT2 secretor status and severe rotavirus gastroenteritis in children in the United States. JAMA Pediatr 2015; 169:1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nordgren J, Sharma S, Bucardo F et al. . Both Lewis and secretor status mediate susceptibility to rotavirus infections in a rotavirus genotype-dependent manner. Clin Infect Dis 2014; 59:1567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Günaydın G, Nordgren J, Sharma S, Hammarström L. Association of elevated rotavirus-specific antibody titers with HBGA secretor status in Swedish individuals: the FUT2 gene as a putative susceptibility determinant for infection. Virus Res 2016; 211:64–8. [DOI] [PubMed] [Google Scholar]
- 38. Van Trang N, Vu HT, Le NT, Huang P, Jiang X, Anh DD. Association between norovirus and rotavirus infection and histo-blood group antigen types in Vietnamese children. J Clin Microbiol 2014; 52:1366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ciarlet M, Conner ME, Finegold MJ, Estes MK. Group A rotavirus infection and age-dependent diarrheal disease in rats: a new animal model to study the pathophysiology of rotavirus infection. J Virol 2002; 76:41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ciarlet M, Gilger MA, Barone C, McArthur M, Estes MK, Conner ME. Rotavirus disease, but not infection and development of intestinal histopathological lesions, is age restricted in rabbits. Virology 1998; 251:343–60. [DOI] [PubMed] [Google Scholar]
- 41. Ramig RF. The effects of host age, virus dose, and virus strain on heterologous rotavirus infection of suckling mice. Microb Pathog 1988; 4:189–202. [DOI] [PubMed] [Google Scholar]
- 42. Saif LJ, Ward LA, Yuan L, Rosen BI, To TL. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Arch Virol Suppl 1996; 12:153–61. [DOI] [PubMed] [Google Scholar]
- 43. Pott J, Stockinger S, Torow N et al. . Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathog 2012; 8:e1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ball JM, Tian P, Zeng CQ, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 1996; 272:101–4. [DOI] [PubMed] [Google Scholar]
- 45. Pitzer VE, Bilcke J, Heylen E et al. . Did large-scale vaccination drive changes in the circulating rotavirus population in Belgium? Sci Rep 2015; 5:18585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.