In a phase 1 clinical study, coadministration of the adsorbent-based DAV132 product together with the fluoroquinolone antibiotic moxifloxacin, while not affecting the plasma concentrations of the antibiotic, reduced by >99% exposure of the intestinal microbiota to the moxifloxacin.
Keywords: microbiome, antibiotics, fluoroquinolones, Clostridium difficile
Abstract
Background
Antibiotics are life-saving drugs but severely affect the gut microbiome with short-term consequences including diarrhea and selection of antibiotic-resistant bacteria. Long-term links to allergy and obesity are also suggested. We devised a product, DAV132, and previously showed its ability to deliver a powerful adsorbent, activated charcoal, in the late ileum of human volunteers.
Methods
We performed a randomized controlled trial in 28 human volunteers treated with a 5-day clinical regimen of the fluoroquinolone antibiotic moxifloxacin in 2 parallel groups, with or without DAV132 coadministration. Two control goups of 8 volunteers each receiving DAV132 alone, or a nonactive substitute, were added.
Results
The coadministration of DAV132 decreased free moxifloxacin fecal concentrations by 99%, while plasmatic levels were unaffected. Shotgun quantitative metagenomics showed that the richness and composition of the intestinal microbiota were largely preserved in subjects co-treated with DAV132 in addition to moxifloxacin. No adverse effect was observed. In addition, DAV132 efficiently adsorbed a wide range of clinically relevant antibiotics ex vivo.
Conclusions
DAV132 was highly effective to protect the gut microbiome of moxifloxacin-treated healthy volunteers and may constitute a clinical breakthrough by preventing adverse health consequences of a wide range of antibiotic treatments.
Clinical Trials Registration
Antibiotics constitute one of the most medically important and effective class of drugs. However, during systemic antibiotic treatments, the nonabsorbed part of orally administered drugs, as well as the possible fraction excreted into the upper intestine via bile for both oral and parenteral antibiotics, reaches the cecum and colon where it can exert devastating effects on the gut microbiome, with short- and long-term consequences [1–5]. Short-term effects include diarrhea, Clostridium difficile infection (CDI), and selection of antibiotic-resistant microorganisms [6–8]. CDI currently constitutes a major clinical challenge, and antibiotic treatments are the key factor for their occurrence in hospitalized and community patients [9–11]. Long-term links to allergy [4] and obesity [5] have also been suggested. Indeed, burgeoning research in recent years has shown that the intestinal microbiome plays an important role in many aspects of human physiology and health [12]. In particular, it is involved in the production of metabolites that may affect insulin sensitivity [13] and diet-related obesity [14]; this state has been shown to correlate with a gut microbiome of lower bacterial richness than in healthy individuals [15].
Strategies that would preserve the intestinal microbiome from deleterious consequences of dysbiosis during antibiotic treatments would be highly welcome for immediate protection of patients from CDI, and also for long-term public health consequences such as dissemination of resistant bacteria and the occurrence of metabolic disorders. Oral administration of a β-lactamase [16–20] prevented the impact of parenteral β-lactams on the microbiome, which is promising but limited to this class of antibiotics. Delivering a nonspecific adsorbent to the colon partially decreased fecal concentrations of orally administered ciprofloxacin without significantly affecting its plasma pharmacokinetics in rats [21]. We devised a product, DAV132, which delivers a powerful nonspecific adsorbent, a carefully chosen activated charcoal, to the late ileum in humans, and have shown in healthy volunteers that its administration did not affect the plasma pharmacokinetics of amoxicillin, given as a single dose [22]. Here, we performed a randomized clinical trial in volunteers receiving a full oral clinical course of the fluoroquinolone antibiotic moxifloxacin (MXF), and assessed DAV132 safety, as well as its resulting effects on MXF plasma concentrations, MXF free fecal concentrations, and intestinal dysbiosis. We also evaluated ex vivo the capacity of DAV132 to adsorb a wide range of clinically relevant antibiotics.
METHODS
Investigational Products
DAV132 was manufactured according to Da Volterra’s specifications [22] under Good Manufacturing Practice conditions at NextPharma (Bielefeld, Germany). The dosage form, consisting of 7.5 g DAV132, contained 5.11 g activated charcoal as active adsorbing ingredient. To facilitate oral intake, DAV132 pellets were suspended in an extemporaneously prepared gel suspension (batch number C1311007). A negative control (CTL) was made of a product similar to DAV132, in which the adsorbent was replaced by microcrystalline cellulose (batch number C1311006). MXF was from Bayer (Avalox 400 mg Filmtabletten, batch number BXGFBN1).
Subjects and Clinical Trial Design
Male and female healthy volunteers >18 years old having given written informed consent were included (body mass index <30 kg/m2, normal digestive transit and healthy (by medical history, physical examination, vital signs, electrocardiography, and blood laboratory results) at a screening visit 8–21 days prior to treatment beginning, defined as day 1. Subjects carrying C. difficile at screening or with a history of hospitalization or antibiotic exposure (both past 3 months) or vaccination (past 28 days) were not included.
Volunteers were included as outpatients (March–October 2014) in a prospective, randomized, controlled, repeated doses, open-label trial, blinded to analytical and microbiological evaluations, at the Clinical Investigation Centre of the Bichat Hospital, Paris (France) in respect with Good Clinical Practice and the Declaration of Helsinki as last amended. Volunteers were randomized (Supplementary Materials) to receive either MXF alone (n = 14), MXF + DAV132 (n = 14), DAV132 alone (n = 8), or CTL (n = 8) (Figure 1). The study was carried out after authorizations from French Health Authorities and the Independent Ethics Committee (“Comité de Protection des Personnes Ile-de-France IV,” Paris, France) had been obtained (January and February 2014, respectively). It was declared in ClinicalTrials.gov (identifier NCT02176005) and the French (ID-RCB number 2013-A01504-41) registers of clinical trials. A study-specific scientific committee (A. A., V. A., A. Duc., A. Duf., X. D., C. F., J. G., F. M., and M. V.) was set up to ensure scientific integrity.
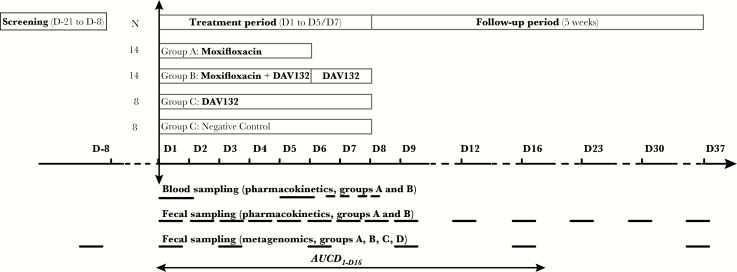
Figure 1.
Study design. The various periods of the study (screening, treatment, follow-up) are shown in boxes at the top. The times of blood and fecal sampling for moxifloxacin pharmacokinetics and metagenomics analysis are shown by horizontal bars in the bottom section of the graph. Abbreviations: AUC, area under the time curve; MXF, moxifloxacin.
Treatments
Moxifloxacin 400 mg was administered orally, once a day (after breakfast) from day 1 to day 5 under direct observed therapy. DAV132 or CTL, 7.5 g, was administered orally, thrice daily (before meals) from day 1 to day 7; on day 1, the first DAV132 dose was given 2 hours before MXF. Morning administrations of DAV132 and of CTL were performed under direct observed therapy, while noon and evening intake were reported by the subjects. Compliance was assessed by counting empty bottles each following day. Follow-up was until day 37. See Supplementary Materials for details on collection and storage of fecal and plasma samples.
MXF Assay in Plasma and Fecal Samples
Moxifloxacin assays were performed by Amatsi Group (Fontenilles, France) using specifically developed and validated bioanalytical methods (Supplementary Materials).
Statistical Methods
The primary objective was to evaluate the influence of DAV132 on free fecal MXF concentrations between day 1 and day 16 by comparing individuals in the MXF- and MXF + DAV132–treated groups. The primary endpoint was the area under the time curve from D1 to D16 (AUCD1-D16) of free fecal MXF concentrations.
The study sample size was calculated at the time of study design. Assuming an AUCD1-D16 variability similar to that of the AUCD1-D14 previously measured from individual data [23], a sample size of 11 subjects in each MXF-treated group (MXF and MXF + DAV132) would allow to detect a 2-fold change between these groups (90% power, 2-sided test, type I error 0.05). For security, we included 14 subjects in each of these groups. Additionally, we randomized 2 groups of 8 volunteers without MXF, but with DAV132 or CTL to study secondary objectives (DAV132 safety and intestinal microbiota composition).
As preplanned for the primary objective, comparison of log(AUCD1-D16) of free MXF fecal concentrations, in groups treated with MXF + DAV132 and MXF alone, was performed using a general linear model. AUCD1-D16 were calculated by the trapezoidal method using the actual time of stool emission and the results were expressed as geometric means of AUCD1-D16 and coefficient of variation. For MXF plasma concentrations, comparisons of log(AUC0-24h) and log(Cmax), in groups treated with MXF + DAV132 and MXF alone, were performed using a general linear model. AUC0-24h was calculated by the trapezoidal method. Statistical analysis of clinical and pharmacokinetic data was performed using SAS version 9.4 software (SAS Institute, Cary, North Carolina).
Metagenomic Methods and Analysis
Analysis of metagenomic data was exploratory and not prespe cified. Essentially, total fecal DNA was extracted as described previously [24, 25] and sequenced using SOLiD 5500 Wildfire (Life Technologies) resulting in 67.2 ± 19.8 M (mean ± standard deviation) sequences of 35-base-long single-end reads. High-quality reads were generated with quality score cutoff >20. Reads with a positive match with human, plant, cow, or SOLiD adapter sequences were removed. Filtered high-quality reads were mapped to the MetaHIT 3.9M gene catalog [26] using METEOR software [27]. The read alignments were performed in colorspace with Bowtie software (version 1.1.0) [28] with options: -v 3 (maximum number of mismatches) and -k 10000 (maximum number of alignments per reads). The raw SOLiD read data were deposited in the European Bioinformatics Institute European Nucleotide Archive under accession number PRJEB12391. Details of read mapping, data treatment, and statistical methods to analyze microbiome data are provided in the Supplementary Materials.
Adsorption of Antibiotics by Activated Charcoal Ex Vivo
To mimic at best the adsorption of antibiotics onto activated charcoal in the gut, we used cecal medium obtained from extemporaneously euthanized pigs, stored at –80°C. Antibiotics (400 µg/mL), and activated charcoal (4 mg/mL) obtained from DAV132 deformulated by incubation for 30 minutes at 37°C in 50 mM sodium phosphate buffer pH 7.5 containing 80 nM sodium chloride, were independently preincubated with cecal medium (1:1 v:v) for 2 hours at 37°C. Then, the 2 preincubation reactions were mixed and further incubated for 3 hours at 37°C with gentle agitation. For antibiotics sensitive to β-lactamases, endogenous enzymes were inactivated by heating at 70°C for 1 hour. Samples were centrifuged 3 minutes at 19890g, and nonadsorbed antibiotics in the supernatant were quantified in triplicate using a microbiological assay [29].
RESULTS
Subjects
Overall, 71 subjects were included in the DAV132-CL-1002 study between 20 March 2014 and 1 September 2014. Twenty-one subjects did not meet the inclusion/exclusion criteria and 5 subjects withdrew consent before randomization. Of 45 subjects randomized, 1 refused to take treatment and withdrew from the study; 44 were treated and completed the study: n = 14 in groups MXF and MXF + DAV132, n = 8 in groups DAV132 and CTL. All subjects meeting the inclusion/exclusion criteria, having taken at least 20 doses of DAV132 (95% of expected doses) and 5 doses of moxifloxacin (100% of expected doses) were evaluable and included in the per protocol population. There were no deviations from protocol during the treatment and follow-up periods. Therefore, the 44 subjects were included in both per protocol and safety analysis sets. The number of subjects analyzed in groups MFX and MFX + DAV132 ensured a study statistical power >90%; the characteristics of volunteers were similar in both groups (Supplementary Table 2).
MXF Pharmacokinetics in Feces and Plasma
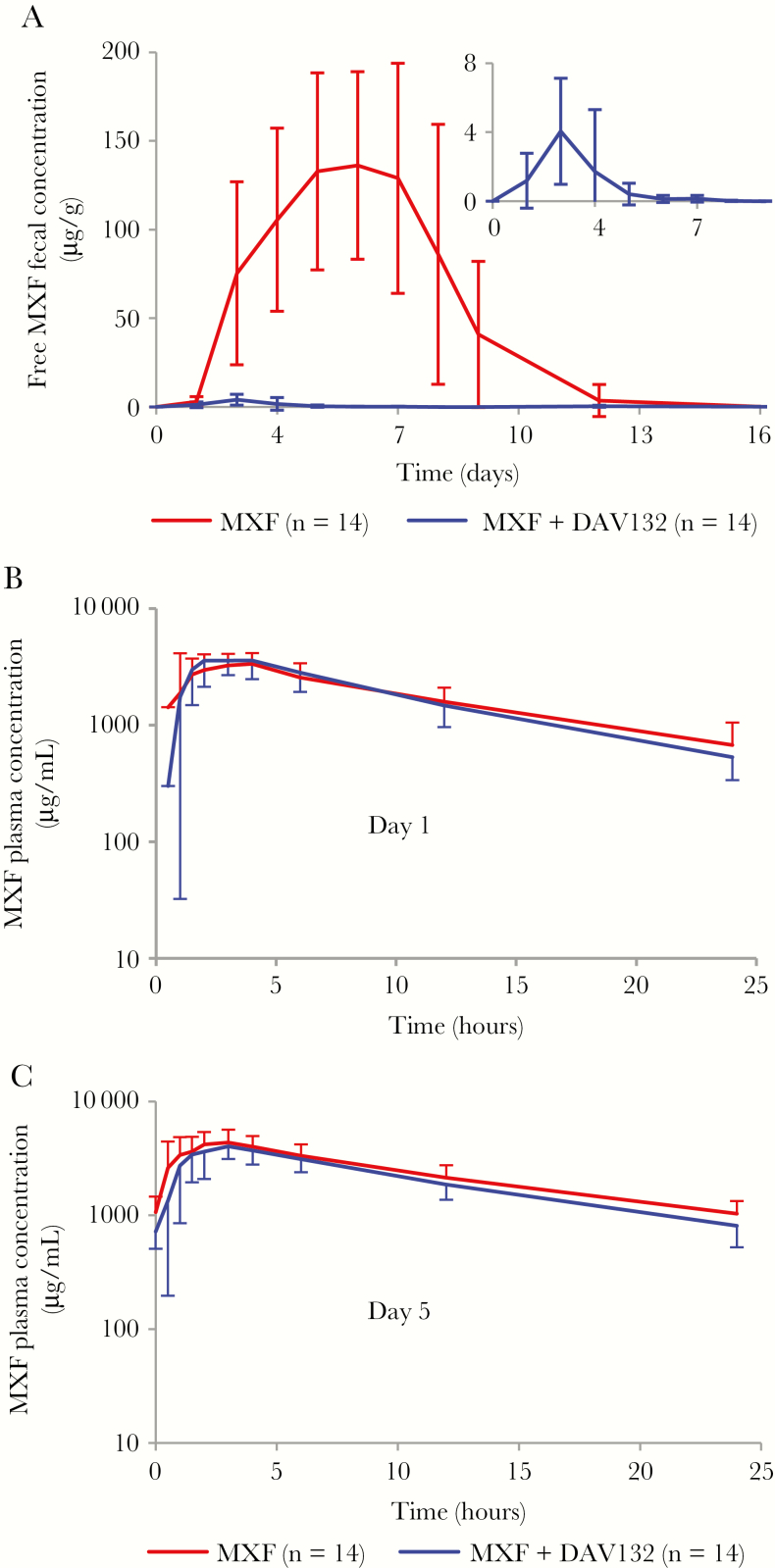
In volunteers with MXF alone, average fecal concentrations of free MXF peaked at 136.2 µg/g (with 39% intersubject coefficient of variation [CV]) at day 6 and returned to undetectable levels by day 16 (Figure 2A); they were markedly reduced in volunteers that received MXF together with DAV132, with free fecal MXF concentrations ranging from 1 to 14 µg/g feces between day 1 and day 6. Indeed, coadministration of DAV132 reduced the AUCD1-D16 of fecal free MXF by >99%, with geometric means of 699.2 µg/g/day (CV 41%) in the MXF group vs 6.4 µg/g/day (CV 69%) in the MXF + DAV132 group (p = 3.10–18). Despite the low concentrations of free fecal MXF in volunteers that were coadministered DAV132, no selection for resistance in coliforms was seen; some quinolone- and fluoroquinolone-resistant strains emerged, but no difference was observed between the treatment groups (Supplementary Table 1). When adjusting for each main individual characteristic of the volunteers (Supplementary Table 2) in a multivariate analysis, the effect of DAV132 on reducing logAUCD1-D16 of free fecal MXF concentration remained significant (analysis not shown).
Figure 2.
Effect of DAV132 on moxifloxacin (MXF) concentrations in feces and plasma of human volunteers. A, Free fecal MXF concentrations between day (D) 1 and D16 (P = 10–17 for the comparison of logAUCD1-D16). Inset: magnified scale for healthy volunteers (HVs) treated with MXF + DAV132. Plasma MXF concentrations on D1 (P = .8 for the comparison of logAUC0-24h) (B) and D5 (P = .1 for the comparison of logAUC0-24h) (C). HVs, 14 in each of these groups, were administered orally MXF 400 mg once daily from D1 to D5 (MXF), or MXF 400 mg once daily plus DAV132 7.5 g thrice daily from D1 to D5 and then DAV132 alone on D6–D7 (MXF + DAV132). Mean values ± standard deviation are shown.
By contrast, plasma concentrations of MXF at day 1 and day 5 were not significantly different in volunteers who received DAV132 or not, in addition to MXF, as shown by analysis of the geometric means of the AUC0-24h and Cmax (Figure 2B and 2C and Table 1).
Table 1.
Plasma Pharmacokinetic Parameters of Moxifloxacin in Healthy Volunteers Receiving or Not Receiving DAV132
| Parameter | MXF | MXF + DAV132 | P Value |
|---|---|---|---|
| D1 | |||
| AUC0-24h, µg/mL.h | 42.1 (17%) | 41.9 (23%) | .81 |
| Cmax, µg/mL | 4.02 (33%) | 4.63 (21%) | .10 |
| D5 | |||
| AUC0-24h, µg/mL.h | 57.6 (24%) | 50.5 (22%) | .14 |
| Cmax, µg/mL | 4.89 (25%) | 4.60 (27%) | .52 |
Geometric means (coefficient of variation %) are shown. Statistical comparison of groups was performed on log values.
Abbreviations: AUC0–24h, Area under the time curve between 0 and 24 h; Cmax, maximal concentration; MXF, moxifloxacin.
The safety analysis showed that repeated oral administration of DAV132 during 7 days was safe and well tolerated. Only 1 adverse effect, a per-treatment vulvovaginal mycotic infection, possibly related to MXF, was considered as related to a study product by the investigator. No adverse effect considered as related to DAV132 was reported. No clinically relevant abnormality in vital signs, 12-lead electrocardiographic parameters and laboratory results occurred in any subject during the study.
Prevention of Intestinal Dysbiosis by DAV132
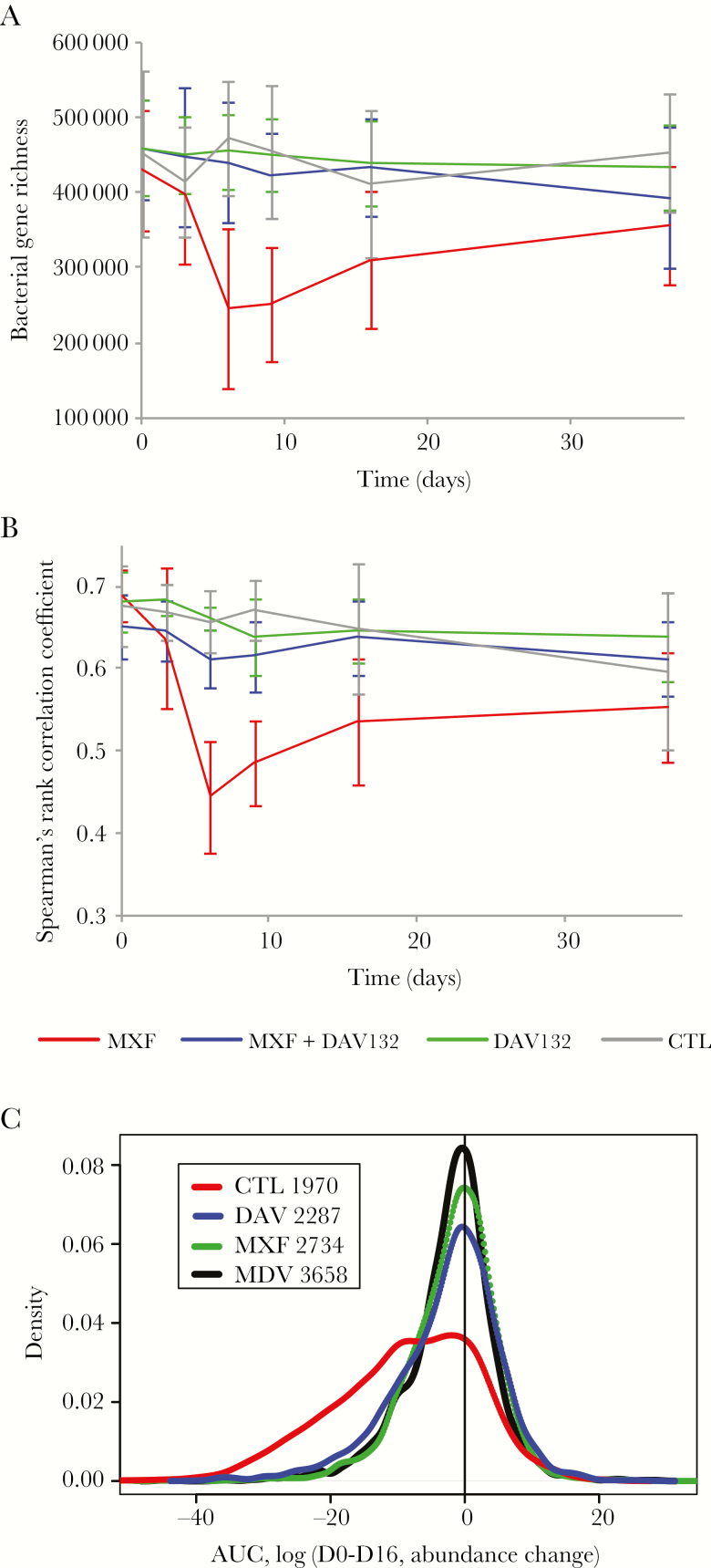
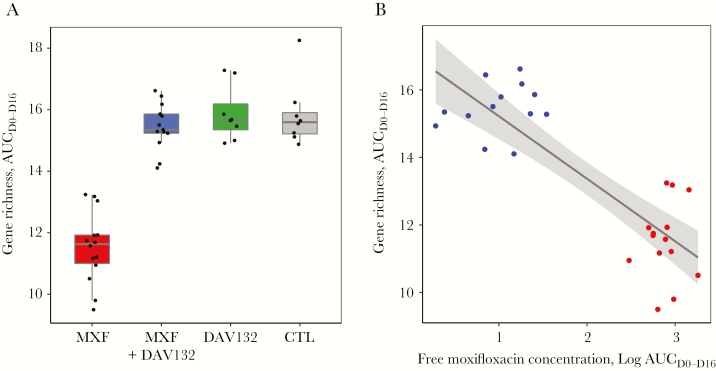
The global effect of DAV132 administration on the gut microbiome was explored in 2 ways, by assessing microbiome bacterial gene richness and overall composition. Richness was strongly decreased to 54.6% of baseline value at day 6 in volunteers who received MXF alone, and failed to return to the initial value even at day 37 (Figure 3A); this decrease was greatly attenuated by coadministration of DAV132 (97.8% of baseline value at day 6, close to what was observed for the CTL group). We also assessed the impact of treatments on bacterial gene richness over the length of the trial by computing the AUC, between day 0 and day 16, of its relative change from day 0 for each individual (Figure 4A). This interval was chosen because most of richness evolution took place within it, and no residual antibiotic was present at D16 (Figure 2A). The AUCD0-D16 of gene richness change was significantly different among the 4 groups of volunteers (p = 4.10–6) (Figure 4A). It was significantly lower in volunteers receiving MXF alone than in those in the CTL group (q = 1.10–5); it was significantly higher in those treated with MXF + DAV132 than in those receiving MXF alone (q = 4.10–7), and not significantly different from those in the CTL group (q = 0.8), thereby showing the protective effect of DAV132 (Figure 4A). Finally, the AUCD0-D16 of gene richness change was highly correlated with the AUCD1-D16 of free MXF fecal concentrations (Figure 4B), further illustrating the impact of MXF on richness.
Figure 3.
Effect of DAV132 on moxifloxacin (MXF)–induced alterations of the human gut microbiome of human volunteers. A, Gene richness: bacterial gene counts for each study group are displayed. B, Microbiome composition. Spearman rank correlation coefficients (ρ) computed from the abundance of all genes of the 3.9 M gene catalog carried by each individual between Dscreening and the indicated days are shown for each study group. C, Metagenomic species (MGSs). Distribution of areas under the time curve (AUC) values computed between D0 and D16 using log10 of abundance change from D0 of each MGS present in each individual is shown. The group sizes were: MXF, n = 14; MXF + DAV132, n = 13; DAV132, n = 8; negative control (CTL), n = 8. The number of available individual measures over all MGSs for each group is indicated in the inset. Red, blue, green, and black correspond to MXF, MXF + DAV132 (MDV), DAV132 (DAV), and CTL groups, respectively.
Figure 4.
Impact of moxifloxacin (MXF) and DAV132 on gene richness. A, AUCD0-D16 of gene richness change from D0; see Methods for details. Medians [min, max] were 11.63 [9.50, 13.24] for MFX, 15.34 [14.10, 16.62] for MXF + DAV132, 15.66 [14.91, 17.28] for DAV132, and 15.59 [14.88, 18.25] for negative control (CTL). Of note, in absence of any change from D0 the value of AUCD0-D16 would be 16. Median values, quartiles, and 1.5 interquartile range are shown. The distribution of the AUCD0-D16 of gene richness was significantly different between the 4 groups (Kruskal–Wallis test p = 4.10–6). In the pairwise comparisons, it was significantly lower in healthy volunteers (HVs) receiving MXF alone than in those receiving MXF + DAV132 (q = 4.10–7) or negative control (q = 1.10–5), whereas the difference between HVs receiving MXF + DAV132 and negative control was not significant (q = 0.8) as assessed by the Wilcoxon rank-sum test with Benjamini–Hochberg correction for the 4 pairwise comparisons. No difference was observed between the group receiving DAV132 alone and CTL (q = 0.8). The number of individuals in different groups was MXF, n = 14; MXF + DAV132, n = 13; DAV, n = 8; CTL, n = 8. B, Relationship between AUCD0-D16 of gene richness change from D0 and AUCD1-D16 MXF fecal concentration (r2 = 0.71, p = 4.10–8). Red and blue dots correspond to groups exposed to MXF or MXF + DAV132, respectively.
Overall changes of microbiome composition with time were assessed by computing, for each individual, the Spearman rank correlation coefficient of the relative abundance of bacterial genes between each time point and the screening pretreatment day (Figure 3B). Microbiome composition changed little over time in the CTL and DAV132 groups of volunteers that did not receive MXF (Figure 3B). By contrast, exposure to MXF resulted in marked microbiome changes, detected from D3, maximal at D6, and still partially present 30 days after the treatment ended; these changes were greatly attenuated by DAV132 (Figure 3B). The comparison of Spearman rank correlation coefficient values at D6 across the 4 groups was significant (p = 2.10–6). Those values were significantly lower in the MXF-treated group (median [min, max] 0.44 [0.34, 0.59]) than in the CTL group (0.65 [0.60, 0.70], q = 2.10–4) and in the MXF + DAV132 group (0.62 [0.53, 0.66], q = 1.10–5). DAV132 exerted an important, but not total protection of the microbiome from the effect of MXF, as the median in the MXF + DAV132 group was slightly lower than in the CTL group (q = 0.03).
Intestinal Microbiota Analysis at the Species Level
Six hundred twenty-nine of 741 (85%) metagenomic species (MGSs; see Supplementary Figure 1 and Supplementary Table 3) found in the MetaHit gut microbial catalogue of 3.9 M genes were present in at least 1 sample. The mode of their AUCD0-D16 distribution was 0 in the CTL group as well as in volunteers treated with DAV132 alone, indicating that the abundance of most MGSs did not change (Figure 3C). The distribution of AUC values for the MXF group was strikingly different, with a broad shoulder toward negative values, indicating a decrease in the abundance of numerous MGSs. This shoulder was largely absent in the MXF + DAV132 group, suggesting that many MGSs were protected by DAV132.
A detailed analysis of the MGSs that differed significantly between treatment groups (Supplementary Figure 1 and Supplementary Table 3) showed that of the 252 MGSs present at baseline in at least 4 volunteers per group, 99 were differentially abundant. Only 3 were affected by DAV132 given alone, whereas 86 were affected by the MXF treatment; among them, 81% were fully protected, and a further 12% partially protected from the effect of MXF by coadministration of DAV132.
Taxonomical Analysis
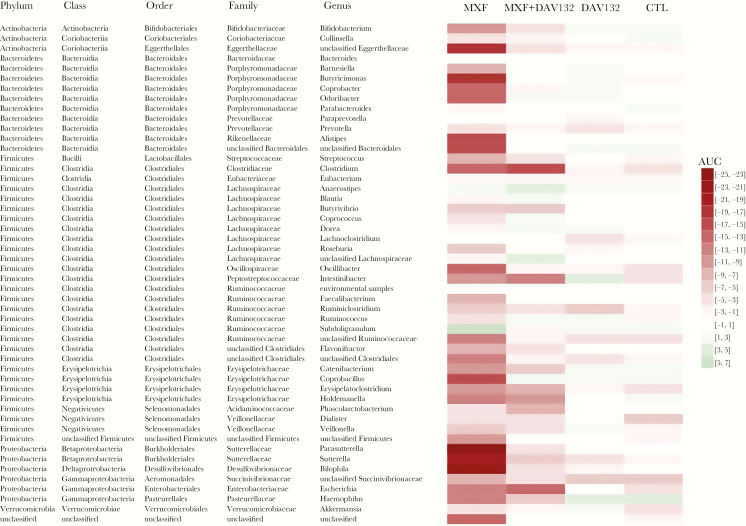
Taxonomical characterization at the genus level (Figure 5) showed that Alistipes, Bilophila, Butyciromonas, Coprobacillus, Fecalibacterium, Odoribacter, Oscillibacter, Parasutterella, Roseburia, and Sutterella genera were decreased in MXF-treated volunteers, and partially (Bilophila) or fully (all others) protected by DAV132. In contrast, Bacteroides, Paraprevotella, and Lachnoclostridium were unaffected by MXF as well as MXF + DAV132 treatments.
Figure 5.
Heatmap depicting the changes induced by the various treatments at the genus level. The heatmap represents, for each treatment group and for each bacterial genus, the median AUC of log10 change from D0 of the metagenomic species that constitute this genus. Green and red colors, respectively, indicate genera that are decreased or increased (the intensity of the color represents the extent of the change), while white indicates very limited changes. Abbreviations: AUC, area under the time curve; CTL, negative control; MXF, moxifloxacin.
We used a set of 34 MGS characteristic of the high-richness microbiome of healthy individuals present among the 252 examined above (Supplementary Table 4) to assess whether MXF may induce not only an overall loss of gut microbiome richness but also a shift to a composition expected in low-richness microbiomes. The AUCD0-D16 of log10 of relative abundance change from D0 of these 34 MGS was significantly different among the 4 groups of volunteers (p < 10–4; Supplementary Figure 2), being significantly lower under MXF treatment alone than under CTL (q = 1.10–4) or MXF + DAV132 (q = 1.10–4); just as for overall gene richness, this measure also showed no significant difference between the MXF + DAV132 and CTL groups (q = 0.6), further attesting to the protective effect of DAV132.
Ex Vivo Adsorption of Other Antibiotics
To assess whether DAV132 could also protect against antibiotics other than MXF routinely used in the clinic, we examined the capacity of the activated charcoal released from DAV132 to adsorb them under ex vivo conditions, that is, in pig cecal medium (Table 2). Among the 14 antibiotics routinely used in clinic tested, 13 were adsorbed to an extent of at least 95% by the charcoal after 3–5 hours of contact with deformulated DAV132. Only amoxicillin was a little less adsorbed, to the extent of 92%.
Table 2.
Adsorption of Antibiotics by Deformulated DAV132 Ex Vivo
| Antibiotic Class | Antibiotic |
Adsorption by Deformulated DAV132 at 3 Hours and 100:1 DAV132: Antibiotic Ratio,
% |
|---|---|---|
| Penicillins | Amoxicillin | 92.4a |
| Piperacillin | 95.4a | |
| Cephalosporins, first generation | Cefalexin | 97.6 |
| Cephalosporins, third generation | Cefotaxime | 96.2a |
| Ceftriaxone | 99.4 | |
| Carbapenems | Ertapenem | 98.0 |
| Imipenem | 99.7 | |
| Meropenem | 98.1b | |
| Fluoroquinolones | Ciprofloxacin | >99.9 |
| Levofloxacin | >99.9 | |
| Lomefloxacin | >99.4 | |
| Marbofloxacin | >99.9 | |
| Moxifloxacin | >99.7 | |
| Lincosamides | Clindamycin | >99.4 |
aAdsorption at 5 hours.
bAdsorption at 2 hours.
DISCUSSION
Our most important result was that in human volunteers treated with a clinical 5-day course of oral MXF, DAV132 spared the intestinal microbiome from exposure to free MXF by >99%, without affecting the plasma pharmacokinetics of the antibiotic or causing any serious adverse effects. This is a major advance over what we showed in the first DAV132 phase 1 trial that was limited to a small number of volunteers treated with DAV132 for 24 hours and receiving only a single dose of amoxicillin [22]. Here, DAV132 was associated with a full 5-day clinical course of a widely used fluoroquinolone antibiotic. The facts that all randomized volunteers completed the study and that only a small amount of data were missing ensure the validity of the results. We conclude that the coadministration of DAV132 with MXF is safe, and should not affect the systemic therapeutic effects of oral as well as parenteral antibiotic treatments. The fact that DAV132 is able to markedly reduce fecal MXF concentrations without significantly affecting systemic exposure to the antibiotic, contrarily to the use of nonformulated activated charcoal [30], is due to the targeted delivery of the adsorbent component to the ileocecal region [22].
Our second most important result was that coadministration of DAV132 largely protected richness and composition of the intestinal microbiota of MXF-treated volunteers. The changes observed with MXF alone that were maximal after 6 days of antibiotic, and persisted a month after the treatment ended, were reminiscent of those previously observed with ciprofloxacin [2]. Under coadministration of DAV132 with MXF, they were largely reduced and return to baseline was observed 11 days after treatment ended, suggesting that long-term consequences of antibiotics might be spared. Indeed, a third of the 252 MGSs identified in the gut microbiota of the volunteers were affected by MXF, but the coadministration of DAV132 fully protected 81% of the affected MGSs, and a further 12% partially. Of particular interest in that respect was that the 34 MGSs that had previously been shown to be associated with the high-richness microbiome in healthy individuals [15] were well protected.
A third important result from the study was that the adsorbent released from DAV132 could efficiently adsorb, under ex vivo conditions mimicking the cecum, antibiotics from several distinct and therapeutically important classes such as β-lactams of all categories (penicillins, cephalosporins, and carbapenems), fluoroquinolones, and lincosamides. This indeed suggests that the coadministration of DAV132 could protect the human gut microbiome against the deleterious effects of many antibiotics, including those administered orally, without affecting their plasma pharmacokinetics, as we previously showed with the β-lactam amoxicillin [22], and here with the fluoroquinolone MXF. The nonspecific nature of the adsorbent used in DAV132 might indeed be advantageous over the use of the recently proposed β-lactamase for prevention of intestinal dysbiosis and C. difficile infections, which is limited to association with parenteral treatments by penicillins and cephalosporins [20].
Another set of results in this trial was strongly favorable for the possibility to further use DAV132 in the clinic. First, concerning safety, despite the relatively important dose of charcoal that was given (7.5 g thrice daily), the treatment was associated with no significant side effects, in particular intestinal ones except for the black darkening of feces. DAV132 had no impact on blood electrolytes or coagulation parameters, suggesting that it did not interfere with electrolyte exchanges or vitamin K production, both of which take place in the colon. Compliance to treatment was not an issue for the volunteers. Second, we did not observe any remarkable differential modification in the emergence of quinolone/fluoroquinolone-resistant coliforms between groups of volunteers, even when the free fecal antibiotic concentrations were low as in those who received MXF+DAV132. Notwithstanding the small number of subjects, this is reassuring because some have suggested that low concentrations of fluoroquinolones might be prone to increase the selection of resistant bacteria [31]. The small number of subjects and the fact that they were naive volunteers might also have prevented the observation of a significant difference of emergence of fluoroquinolone-resistant enterobacteria in those who received MXF vs CTL, as was observed after ciprofloxacin treatment, in 10-fold larger groups of hospitalized patients [32].
Despite these positive results, our work has limitations. First, in this phase 1 trial we did not address directly the efficacy of DAV132 to protect patients against immediate consequences of antibiotic treatments such as C. difficile colitis. However, we recently published preclinical results in hamsters that suggest that such might well be the case [33]. Second we did not address the possibility that DAV132 might interfere with other drugs that could be taken concomitantly for therapeutic purposes by patients treated with antibiotics. This was far beyond the purpose of the current study but will have to be determined before testing the product in actual patients.
Whatever these limitations, the results of this phase 1 trial appear promising: DAV132 may constitute a breakthrough product to prevent short- and long-term detrimental effects of antibiotic treatments. Further studies are under way to validate the potential of DAV132 in a clinical setting.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments. We thank M. Ghidi and M. N. Bouverne (Da Volterra), as well as C. Féger (Emibiothech) for coordination and management of the clinical study; E. Arcaraz, E. Desmartin, A. Toutin, T. Mezzasalma, and C. Toutin (Amatsi Group) for the development and validation of bioanalytical methods, and for performing the fecal and plasma pharmacokinetic analyses; I. Wieder and C. Bourseau for performing the phenotypic microbiological analyses; Prof A. Dufour for biopharmacological analysis; P. Clerson (Orgamétrie) for statistical analysis of the clinical data; N. Galleron and B. Quinquis (Metagenopolis) for generating sequencing data; and P. Leonard (Metagenopolis) for informatics support.
Financial support. The randomized clinical trial was sponsored by Da Volterra (Paris) and funded in part by the European Union Seventh Framework Programme (FP7-HEALTH-2011-single-stage) under grant agreement number 282004, EvoTAR. Additional funding was from the Metagenopolis grant ANR-11-DPBS-0001, and from BPIFrance under the NOSOBIO collaborative program.
Potential conflicts of interest. A. D., F. S.-G., V. A., and M. V. are employees of Da Volterra. J. G., E. R., C. B., E. C., F. M., and A. A. are consultants for Da Volterra. J. G., A. D., F. S.-G., and V. A. are shareholders of Da Volterra. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: ID Week, San Diego, California, October 2015 (abstract 760).
References
- 1. Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010; 156:3216–23. [DOI] [PubMed] [Google Scholar]
- 2. Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A 2011; 108(Suppl 1):4554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pérez-Cobas AE, Gosalbes MJ, Friedrichs A et al. . Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut 2013; 62:1591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell 2014; 157:121–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cox LM, Blaser MJ. Antibiotics in early life and obesity. Nat Rev Endocrinol 2015; 11:182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Lastours V, Fantin B. Resistance to fluoroquinolones in 2010: what are the consequences for prescriptions in intensive care units?Réanimation 2010; 19:347–53. [Google Scholar]
- 7. Johanesen PA, Mackin KE, Hutton ML et al. . Disruption of the gut microbiome: Clostridium difficile infection and the threat of antibiotic resistance. Genes (Basel) 2015; 6:1347–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015; 372:1539–48. [DOI] [PubMed] [Google Scholar]
- 9. Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother 2013; 57:2326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deshpande A, Pasupuleti V, Thota P et al. . Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother 2013; 68:1951–61. [DOI] [PubMed] [Google Scholar]
- 11. Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother 2014; 69:881–91. [DOI] [PubMed] [Google Scholar]
- 12. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016; 375:2369–79. [DOI] [PubMed] [Google Scholar]
- 13. Pedersen HK, Gudmundsdottir V, Nielsen HB et al. ; MetaHIT Consortium. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016; 535:376–81. [DOI] [PubMed] [Google Scholar]
- 14. Cox LM, Yamanishi S, Sohn J et al. . Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158:705–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le Chatelier E, Nielsen T, Qin J et al. ; MetaHIT Consortium. Richness of human gut microbiome correlates with metabolic markers. Nature 2013; 500:541–6. [DOI] [PubMed] [Google Scholar]
- 16. Léonard F, Andremont A, Leclerq B, Labia R, Tancrède C. Use of beta-lactamase-producing anaerobes to prevent ceftriaxone from degrading intestinal resistance to colonization. J Infect Dis 1989; 160:274–80. [DOI] [PubMed] [Google Scholar]
- 17. Stiefel U, Pultz NJ, Harmoinen J et al. . Oral administration of beta-lactamase preserves colonization resistance of piperacillin-treated mice. J Infect Dis 2003; 188:1605–9. [DOI] [PubMed] [Google Scholar]
- 18. Stiefel U, Nerandzic MM, Koski P, Donskey CJ. Orally administered beta-lactamase enzymes represent a novel strategy to prevent colonization by Clostridium difficile. J Antimicrob Chemother 2008; 62:1105–8. [DOI] [PubMed] [Google Scholar]
- 19. Pitout JDD. IPSAT P1A, a class A beta-lactamase therapy for the prevention of penicillin-induced disruption to the intestinal microflora. Curr Opin Investig Drugs Lond Engl 2000 2009; 10:838–44. [PubMed] [Google Scholar]
- 20. Connelly S, Bristol JA, Hubert S et al. . SYN-004 (ribaxamase), an oral beta-lactamase, mitigates antibiotic-mediated dysbiosis in a porcine gut microbiome model. J Appl Microbiol 2017. http://www.pubmed.org/28245091. Accessed 28 February 2017. [DOI] [PubMed]
- 21. Khoder M, Tsapis N, Domergue-Dupont V, Gueutin C, Fattal E. Removal of residual colonic ciprofloxacin in the rat by activated charcoal entrapped within zinc-pectinate beads. Eur J Pharm Sci 2010; 41:281–8. [DOI] [PubMed] [Google Scholar]
- 22. de Gunzburg J, Ducher A, Modess C et al. . Targeted adsorption of molecules in the colon with the novel adsorbent-based medicinal product, DAV132: a proof of concept study in healthy subjects. J Clin Pharmacol 2015; 55:10–6. [DOI] [PubMed] [Google Scholar]
- 23. Edlund C, Beyer G, Hiemer-Bau M, Ziege S, Lode H, Nord CE. Comparative effects of moxifloxacin and clarithromycin on the normal intestinal microflora. Scand J Infect Dis 2000; 32:81–5. [DOI] [PubMed] [Google Scholar]
- 24. Godon JJ, Zumstein E, Dabert P, Habouzit F, Moletta R. Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl Environ Microbiol 1997; 63:2802–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suau A, Bonnet R, Sutren M et al. . Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol 1999; 65:4799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nielsen HB, Almeida M, Juncker AS et al. ; MetaHIT Consortium; MetaHIT Consortium. Identification and assembly of genomes and genetic elements in complex metagenomic samples without using reference genomes. Nat Biotechnol 2014; 32:822–8. [DOI] [PubMed] [Google Scholar]
- 27. Cotillard A, Kennedy SP, Kong LC et al. ; ANR MicroObes consortium. Dietary intervention impact on gut microbial gene richness. Nature 2013; 500:585–8. [DOI] [PubMed] [Google Scholar]
- 28. Langmead B. Aligning short sequencing reads with Bowtie. Curr Protoc Bioinforma 2010. doi:10.1002/0471250953.bi1107s32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kitzis MD. Antibiotic assay. Antibiogram. Courvalin P, Leclerc R, Rice LB Portland, OR: ESKA Publishing, ASM Press, 2010. [Google Scholar]
- 30. Stass H, Kubitza D, Möller JG, Delesen H. Influence of activated charcoal on the pharmacokinetics of moxifloxacin following intravenous and oral administration of a 400 mg single dose to healthy males. Br J Clin Pharmacol 2005; 59:536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andersson DI, Hughes D. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 2014; 12:465–78. [DOI] [PubMed] [Google Scholar]
- 32. Fantin B, Duval X, Massias L et al. . Ciprofloxacin dosage and emergence of resistance in human commensal bacteria. J Infect Dis 2009; 200:390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burdet C, Sayah-Jeanne S, Nguyen TT et al. . Protection of hamsters from mortality by reducing fecal moxifloxacin concentration with DAV131A in a model of moxifloxacin-induced Clostridium difficile colitis. Antimicrob Agents Chemother 2017; 61:e00543–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.