Continuous malaria control in Papua New Guinea has resulted in a marked decline of Plasmodium falciparum and P. vivax prevalence. Yet, an increasing proportion of submicroscopic infections, many of them carrying gametocytes, demands for novel strategies to target residual transmission.
Keywords: Malaria control, temporal trend, submicroscopic, asymptomatic, gametocyte
Abstract
Background
The scale-up of effective malaria control in the last decade has resulted in a substantial decline in the incidence of clinical malaria in many countries. The effects on the proportions of asymptomatic and submicroscopic infections and on transmission potential are yet poorly understood.
Methods
In Papua New Guinea, vector control has been intensified since 2008, and improved diagnosis and treatment was introduced in 2012. Cross-sectional surveys were conducted in Madang Province in 2006 (with 1280 survey participants), 2010 (with 2117 participants), and 2014 (with 2516 participants). Infections were quantified by highly sensitive quantitative polymerase chain reaction (PCR) analysis, and gametocytes were quantified by reverse-transcription qPCR analysis.
Results
Plasmodium falciparum prevalence determined by qPCR decreased from 42% in 2006 to 9% in 2014. The P. vivax prevalence decreased from 42% in 2006 to 13% in 2010 but then increased to 20% in 2014. Parasite densities decreased 5-fold from 2006 to 2010; 72% of P. falciparum and 87% of P. vivax infections were submicroscopic in 2014. Gametocyte density and positivity correlated closely with parasitemia, and population gametocyte prevalence decreased 3-fold for P. falciparum and 29% for P. vivax from 2010 to 2014.
Conclusions
Sustained control has resulted in reduced malaria transmission potential, but an increasing proportion of gametocyte carriers are asymptomatic and submicroscopic and represent a challenge to malaria control.
While increased malaria control has led to declining transmission in many countries [1, 2], an increasing proportion of asymptomatic and submicroscopic infections represent a major challenge to further progress toward elimination [3–5]. Clinical malaria episodes that are light microscopy (LM) and/or rapid diagnostic test positive can be diagnosed with tools that are now available in the field, but asymptomatic infections are not targeted by programs relying on passive case detection [6]. Asymptomatic and submicroscopic infections have been shown to carry gametocytes and to be infective to mosquitos [7–10]. Data on their frequency is crucial for the design and evaluation of strategies to interrupt malaria transmission.
After roll out of malaria control interventions, such as the distribution of bed nets, naturally acquired immunity in the population may remain high for a number of years [11]. Thus, parasite densities are likely to remain low, and few people will present with clinical malaria. After an extended period of lower transmission, however, immunity is expected to wane, resulting in more high-density and clinical infections. In parallel, malaria-naive individuals experiencing less frequent exposure will acquire immunity more slowly. In combination, these effects are expected to result in changes in treatment frequency, parasite distribution and gametocyte density, and infection duration. Yet, little is known about the extent and time frame of such changes.
Owing to differences in the biology of P. falciparum and P. vivax, the effects of control are often remarkably different for the 2 species, and in many countries P. vivax has proven more resilient to control [5, 12, 13]. P. vivax densities determined by microscopy are generally 5–10 times lower than P. falciparum densities [14–16], making diagnosis more difficult. Latent liver-stage parasites (hypnozoites) escape diagnosis, and standard treatment against blood-stage parasites does not affect them [17]. In regions where malaria is highly endemic, up to 80% of all blood-stage P. vivax infections in children are due to relapses [18, 19]. If the transmission level declines, individuals who have experienced high levels of transmission may harbor a large reservoir of hypnozoites, which will result in relapses for an extended period. Thus, the proportion of all blood-stage parasite infections in the population that are caused by relapsed P. vivax relapses as compared to primary infections might temporarily increase.
Few in-depth studies have assessed the effect of intensified control on parasite prevalence, clinical malaria, the proportion of asymptomatic and submicroscopic P. falciparum and P. vivax infections, and gametocyte carriage over several years in the same population. In the Madang area on the north coast of Papua New Guinea (PNG), P. falciparum and P. vivax prevalence determined by polymerase chain reaction (PCR) analysis had reached 30%–60% in the general population during 2001–2006 [14, 16, 20–22]. As a consequence of the corresponding high transmission intensity, children in PNG acquired natural immunity against clinical malaria during early childhood, and 78%–97% of infections in the general population were asymptomatic [14, 16]. In 2008/2009 and again in 2011/2012, long-lasting insecticidal nets were distributed in PNG. Rapid diagnostic tests to test all febrile cases in health centers, as well as artemisinin-based combination therapy with artemether-lumefantrine as first-line treatment, were implemented in 2012. Surveys conducted after the first round of long-lasting insecticidal net distribution found considerable decreases in entomological inoculation rate [23] and parasite prevalence detected by LM [24], suggesting these interventions had a pronounced effect on transmission.
To understand the full impact of intensified control, repeated cross-sectional surveys were conducted in Madang Province in 2006, 2010, and 2014 (Figure 1). Blood samples were collected from 5913 individuals, and highly sensitive molecular assays were used to diagnose malarial infections and gametocytes in the same population during distinct phases of malaria control.
Figure 1.
Map of study sites. Green dots represent study villages in the Malala, Mugil, and Utu catchments surveyed in 2014. As a reference, Madang Town is shown (purple dot).
METHODS
Ethics Statement
Informed written consent was obtained from participants, or, if participants were <18 years, from their parents or legal guardians. This study was approved by the PNG Institute of Medical Research Institutional Review Board (IMR IRB) (1116/1204), the PNG Medical Research Advisory Committee (MRAC) (11.21/1206), the Walter and Eliza Hall Institute Human Research Ethics Committee (WEHI HREC) (12/09), and the Case Western Reserve University University Hospitals of Cleveland Medical Center(CWRU UHCMC) (05-11-11).
Study Site and Sample Collection
Blood samples were collected in Madang Province (Figure 1), in coastal catchments for 2 health centers (Mugil and Malala), and 1 inland catchment (Utu). The climate is tropical, with a rainy season from December to April. Samples were collected during March–April in 2006 and between mid-May and early July during 2010 and 2014. A convenience sampling strategy including individuals aged >6 months was used. In 2014, among villages, 8.3%–45.1% of residents were sampled (Supplementary Table 1).
From each participant, a 250-μL blood sample obtained by finger prick was collected into ethylenediaminetetraacetic acid–lined tubes. For gametocyte detection, 50 μL of blood was transferred into tubes containing 250 μL of RNAprotect (Qiagen; performed during 2010 and 2014 only). In the field, samples were stored at 4°C and transferred every night to the laboratory at −20°C (for DNA extraction) or −80°C (for RNA extraction).
Parasite Quantification By qPCR and LM
Laboratory methods described elsewhere were used [25]. In brief, DNA was extracted from 200 μL of pelleted blood, using the Favorgen 96-well genomic DNA extraction kit and eluted in 200 μL of buffer. P. falciparum and P.vivax, as well as Plasmodium malariae and Plasmodium ovale (during the 2010 and 2014 surveys only), were quantified by highly sensitive qPCR assays, using 4 µL of DNA, corresponding to 4 µL of blood [26]. A dilution of plasmids containing the target sequence of the PCR was run as an external standard for absolute quantification. P. falciparum–positive samples were genotyped by msp2 [22, 27], and P. vivax–positive samples were genotyped by msp1F3 and MS2 [28, 29].
For gametocyte detection, RNA was extracted using the Qiagen RNeasy 96-kit, with additional DNAse treatment to remove residual DNA (Qiagen RNase-Free DNase Set). pfs25 and pvs25 transcripts were detected using published reverse-transcription qPCR protocols [30] and were quantified using plasmids to generate an external standard curve. A genus-specific qPCR assay [30] was run to ensure absence of DNA.
Data Analysis
Data were analyzed using Stata 12.1. Unless otherwise stated, results are based on qPCR analysis. Densities (determined by LM or qPCR analysis) are given as geometric means. Fever or history of fever was defined as measured fever >37.5°C or reported febrile illness in the past 2 days. Clinical malaria was defined as fever or history of fever and detection of parasites by microscopy. Logistic regression was used to assess risk factors of infection, and χ2 tests were used to compare rates of infection between age groups and catchments. Only in rare cases was an individual included in >1 survey, because the 3 surveys did not necessarily involve the same villages. These cases were treated as independent observations.
Model for Age-Prevalence Curves
The nonlinear association between parasite prevalence and age was first assessed using generalized additive models with thin-plate smoothing splines, for each survey. The shift in age-prevalence peaks across surveys was then investigated using a likelihood-based model inspired by the work of Smith et al [31]. The host population was described using a compartmental model similar to that of a classical SIRS model, using a set of 3 ordinary differential equations (ODEs). Instead of modeling the proportions of susceptible (sk), infected (ik), and retired (rk) individuals in survey k according to time, these were modeled according to age a:
Hence, this ODE model did not represent actual transmission events but rather provided an estimate of age-prevalence curves. A binomial likelihood function was used to fit the model to survey data: , where pa denoted the expected fraction of infectious individuals aged a. Constraining the same model by keeping values of λ, γ, and ν fixed across surveys yielded the null model where age-prevalence remained constant between 2006, 2010, and 2014. A likelihood ratio test was used to assess the statistical significance between the null and alternate models.
RESULTS
Parasite Prevalence and Density
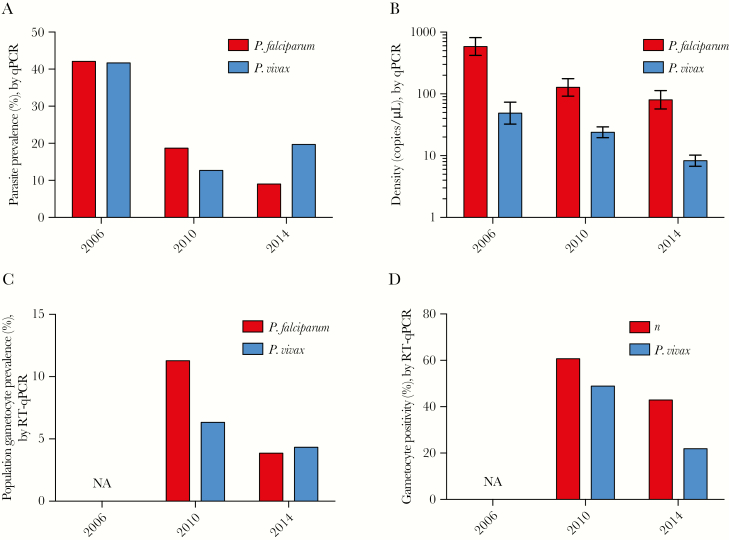
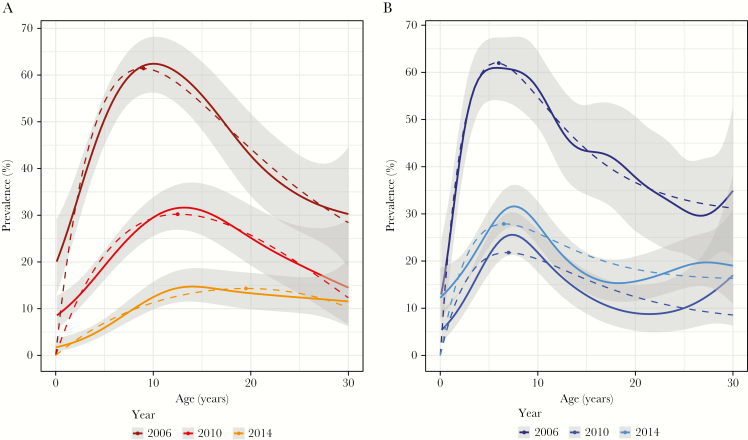
A total of 5913 individuals were surveyed over the study period, with 1280 participating in 2006, 2117 participating in 2010, and 2516 participating in 2014 (Supplementary Table 2). By LM, P. falciparum prevalence decreased from 34.0% in 2006 to 7.3% in 2010 and 2.8% in 2014 (P < .001). By qPCR analysis, P. falciparum prevalence decreased from 42.1% in 2006 to 18.7% in 2010 and 9.0% in 2014 (P < .001; Table 1 and Figure 2A). Prevalence peaked at 9 years in 2006, at 12.5 years in 2010, and at 19.5 years in 2014 (ODE model, P < .001; Figure 3A).
Table 1.
Plasmodium falciparum and Plasmodium vivax Prevalence, Submicroscopic Infection Percentage, Gametocyte Carriage Percentage, and Parasite Density, by Survey Year
| Parasite, Year | Parasite Prevalence | LM Positive/qPCR Negative | Submicroscopic Infection | Gametocyte Carriage by RT-qPCR | Parasite Densitya | ||
| By qPCR | By LM | By qPCR | By LM | ||||
| P. falciparum | |||||||
| 2006 | 42.1 (539/1280) | 34.0 (435/1280) | 11.2 (59/435) | 36.2 (195/539) | NA | 584.0 (419.4–813.2) | 378.3 (315.7–453.3) |
| 2010 | 18.7 (396/2117) | 7.3 (156/1094) | 7.1 (11/156) | 62.7 (224/389) | 60.6 (235/387) | 127.4 (91.9–176.7) | 808.0 (579.0–1127.0) |
| 2014 | 9.0 (226/2517) | 2.8 (69/2513) | 8.7 (6/69) | 72.1 (163/226) | 43.3 (97/224) | 80.3 (56.9–113.3) | 346.6 (217.6–552.2) |
| P. vivax | |||||||
| 2006 | 41.7 (534/1280) | 17.4 (223/1280) | 8.5 (19/223) | 62.0 (331/534) | NA | 48.9 (32.5–73.7) | 260.6 (212.6–319.2) |
| 2010 | 12.7 (271/2117) | 6.9 (147/2094) | 4.8 (7/147) | 48.2 (130/270) | 48.9 (132/270) | 23.9 (19.6–29.2) | 118.3 (97.9–142.8) |
| 2014 | 19.7 (496/2517) | 2.7 (68/2513) | 2.9 (2/68) | 86.7 (430/496) | 22.6 (111/492) | 8.3 (6.8–10.2) | 168.3 (118.2–240.0) |
Data are percentage (proportion) of samples, unless otherwise indicated.
Abbreviations: CI, confidence interval; LM, light microscopy; NA, not available; qPCR, quantitative polymerase chain reaction; RT, reverse transcription.
aData are the number of samples positive by qPCR and by LM (95% CI).
Figure 2.
Plasmodium falciparum and Plasmodium vivax prevalence (A) and density (with 95% confidence intervals; B), by quantitative polymerase chain reaction (qPCR) analysis, in 2006, 2010, and 2014; and population gametocyte prevalence (C) and proportion of all individuals with infection detected by qPCR who were positive for gametocytes (D), by reverse-transcription qPCR. NA, no data available.
Figure 3.
Age trends in Plasmodium falciparum (A) and Plasmodium vivax (B) prevalence by quantitative polymerase chain reaction analysis. Solid lines denote general additive model predictions (with 95% confidence intervals), and the dotted lines denote the ordinary differential equations model. P. falciparum prevalence peaks in older individuals in 2010 and 2014 as compared to 2006, while no change for P. vivax peak prevalence was observed.
P. vivax prevalence by LM similarly decreased from 17.4% in 2006 to 6.9% in 2010 and 2.7% in 2014 (P < .001). By qPCR analysis, P. vivax prevalence decreased from 41.7% in 2006 to 12.7% in 2010 but increased to 19.7% in 2014 (P < .001; Table 1 and Figure 2A). In all surveys, it peaked in children aged approximately 6 years (Figure 3B).
In 2006, 20.7% of individuals (265 of 1280) carried P. falciparum/P. vivax coinfection, 3.9% (82 of 2117) carried both parasites in 2010, and 1.6% (41 of 2517) carried both parasites in 2014 (P < .001). P. malariae prevalence was 1.3% (28 of 2117 individuals) in 2010 and 1.4% (36 of 2117 individuals) in 2014 (P = .758). P. ovale prevalence was 0.01% (2 of 2117 individuals) in 2010, and in 2014 no P. ovale was detected (P = .123). Thirty-five out of 64 P. ovale carriers, and both P. ovale carriers were coinfected with other species.
Mean P. falciparum and P. vivax gene copy numbers decreased 10-fold (P < .001) and 5-fold (P < .001), respectively, from 2006 to 2014 (Table 1 and Figure 2B). In all surveys, the mean P. falciparum density was 5–10-fold higher than the mean P. vivax density. As a result of lower parasite densities, the proportion of submicroscopic infections increased between 2006 and 2014, from 37.2% to 72.1% for P. falciparum (P < .001) and from 62.0% to 86.7% for P. vivax (P < .001; Table 1). A generalized additive model indicated that in response to increased training, LM-based diagnosis had become more sensitive over time (Supplementary Figure 1). Assuming identical LM sensitivity in all 3 surveys, the increase in the proportion of submicroscopic infections would have been even more pronounced.
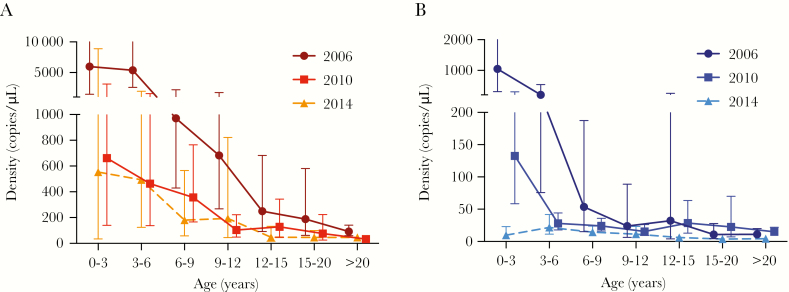
Densities of both species decreased with age. The decrease in P. falciparum densities was slower in 2010 and 2014 as compared to 2006 (interaction of log10 age with log10 density: P = .042; Figure 4A). This effect was even more pronounced for P. vivax, with little change of densities with age in 2014 (P < .001; Figure 4B).
Figure 4.
Geometric mean copy numbers across age groups for Plasmodium falciparum (A) and Plasmodium vivax (B), by quantitative polymerase chain reaction analysis. Error bars show 95% confidence intervals.
By genotyping, a pronounced increase in the proportion of single-clone infections was observed. The proportion of P. falciparum single-clone infections was 57.0% in 2006, 80.1% in 2010, and 82.3% in 2014 (P < .001). For P. vivax, the proportions were 50.9%, 61.3%, and 78.7% in 2006, 2010, and 2014, respectively (P < .001).
In multivariable analysis, age was highly associated with the risk of infection in all surveys and for both species (Supplementary Table 3). The P. falciparum prevalence differed between catchments in all surveys, but not the P. vivax prevalence. Treatment with antimalarials in the past 2 months resulted in an approximately 50% reduction of the odds of P. falciparum or P. vivax infection in 2006 (P < .001). No such association was observed in 2010 and 2014 (P ≥ .079).
Clinical Symptoms
Improvements in morbidity indicators were observed over the 8-year period (Table 2). The proportion of individuals who reported having experienced a malaria episode in the past 2 weeks or who had received antimalarials in the previous 2 months decreased 12-fold and 6-fold, respectively (P < .001 for both comparisons; Table 2). The proportion of qPCR-positive infections defined as clinical malaria decreased 2–3-fold (P ≤ .039; Table 2), and the population attributable fraction of fever or history of fever caused by LM-positive infections decreased substantially (P < .001; Table 2). In contrast, measured fever did not change significantly (P = .843).
Table 2.
Clinical Characteristics of Study Participants
| Variable | 2006 | 2010 | 2014 | P |
|---|---|---|---|---|
| Self-reported malaria episode in past 2 wk | 17.0 (217/1278) | 8.0 (168/2107) | 1.4 (34/2477) | <.001 |
| Self-reported antimalarial use in past 2 mo | 17.7 (225/1280) | 1.8 (39/2117) | 2.8 (71/2498) | <.001 |
| Measured fever | 1.3 (17/1280) | 1.4 (30/2092) | 1.2 (30/2430) | .843 |
| Clinical infectiona | ||||
| P. falciparum | 6.9 (38/539) | 7.6 (26/396) | 2.7 (6/226) | .039 |
| P. vivax | 3.2 (17/534) | 4.8 (13/271) | 1.0 (5/496) | .005 |
| Fever, PAF, %b | ||||
| P. falciparum | 24.1 | 8.2 | 3.7 | <.001 |
| P. vivax | 15.6 | 0 | 0.8 | <.001 |
| Anemiac | 7.2 (92/1274) | 6.1 (113/1844) | 3.5 (88/2514) | <.001 |
| Hemoglobin level, g/dL (95% CI) | 10.55 (10.46–10.66) | 10.64 (10.56–10.72) | 10.86 (10.79–10.93) | <.001d |
| Enlarged spleen | 30.2 (368/1279) | 3.2 (1928/2112) | 1.3 (62/2516) | <.001 |
Data are percentage (proportion) of samples, unless otherwise indicated.
Abbreviations: CI, confidence interval; LM, light microscopy; P. falciparum, Plasmodium falciparum; P. vivax, Plasmodium vivax.
aDefined as the proportion of qPCR-positive infections that were defined as clinical malaria (based on measured or self-reported fever and LM positivity).
bPopulation attributable fraction (PAF) of measured or self-reported fever caused by LM-positive infections
cDefined as a hemoglobin level of <8 g/dL.
dAdjusted for age and sex.
There was no significant association between measured fever and P. falciparum infection in 2006 (odds ratio [OR], 1.56; 95% confidence interval [CI], .60–4.01; P = .37). In 2010, this association was weak (OR, 2.24; 95% CI, 1.04–4.83; P = .039), and it was very strong in 2014 (OR, 4.46; 95% CI, 2.02–9.88; P < .001). Measured fever was not associated with P. vivax infection.
The proportion of participants presenting with an enlarged spleen decreased from 30.2% to 1.3% (Table 2). In 2006 and 2010, having an infection approximately doubled the odds of presenting with an enlarged spleen (2006: OR, 2.36 [P < .001]; 2010: OR, 1.65 [P = .06]); in 2014, this association was even stronger (OR, 7.99; P < .001). The proportion of participants with moderate-to-severe anemia (defined as a hemoglobin level of <8 g/dL) halved between 2006 and 2014 (P < .001; Table 2).
Transmission Potential
P. falciparum gametocytes were detected in 60.7% of individuals with blood-stage parasitemia during 2010 and in 43.3% during 2014 (P < .001). This resulted in a population gametocyte prevalence of 11.1% in 2010 and 3.9% in 2014 (P < .001; Table 1 and Figure 2C and 2D). P. vivax gametocytes were detected in 48.9% of infected individuals during 2010 and in 22.6% during 2014 (P < .001), resulting in a population prevalence of 6.2% and 4.4%, respectively (P = .009; Table 1 and Figure 2C and 2D). P. falciparum gametocyte densities decreased 5-fold between 2010 and 2014 (2010: 85.5 transcripts/μL [95% CI, 58.2–125.4]; 2014: 18.2 transcripts/μL [95% CI, 9.9–33.5]). Little change of P. vivax gametocyte densities was observed (2010: 13.6 transcripts/μL [95% CI, 10.0–18.4]; 2014: 23.7 transcripts/μL [95% CI, 15.2–37.0]).
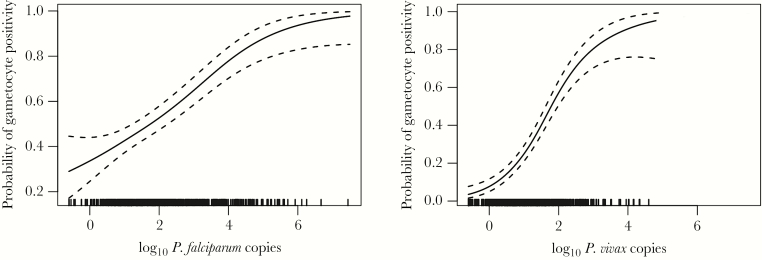
Both the proportion gametocyte positive and gametocyte densities closely correlated with blood-stage parasite densities, especially for P. vivax. Each 10-fold increase in parasite density increased the odds of detecting gametocytes 1.64-fold (95% CI, 1.42–1.90; P < .001) for P. falciparum and 3.77-fold (95% CI, 2.98–4.78; P < .001) for P. vivax (Figure 5). Among gametocyte-positive samples, each 10-fold increase in parasite density resulted in a 1.66-fold (95% CI, 1.33–2.08) and 3.77-fold (95% CI, 2.98–4.77) increase in P. falciparum and P. vivax gametocyte densities, respectively (P < .001).
Figure 5.
Probability to detect Plasmodium falciparum (left) and Plasmodium vivax (right) gametocytes versus copy numbers (by quantitative polymerase chain reaction analysis). Data are general additive model predictions with 95% confidence intervals.
The proportion of gametocyte carriers that had blood-stage parasites detected by LM decreased from 2010 to 2014. In 2010, 54.3% of P. falciparum gametocyte carriers were LM positive for blood-stage parasites, and only 37.1% were positive in 2014 (P = .004). Among P. vivax gametocyte carriers, 84.1% were LM positive for asexual blood-stage parasites in 2010, but only 39.7% were positive in 2014 (P < .001). A total of 90.4% of P. falciparum and 92.6% of P. vivax gametocyte carriers were asymptomatic.
Spatial Heterogeneity
In multivariate analysis, catchment was associated with P. falciparum infection in all 3 surveys (Supplementary Table 3). In 2006, P. falciparum prevalence ranged from 35.1% to 45.5% (P = .005). More-pronounced differences were observed in 2010. Prevalence was lowest in Utu (8.0%) but 2-fold higher in Mugil (15.1%) and 3-fold higher in Malala (25.5%; P < .001).
In 2014, P. falciparum prevalence was 4.7% in Utu as compared to 8.6% and 12.3% in Mugil and Malala, respectively (P < .001). At the village level, substantial P. falciparum spatial heterogeneity was observed (Supplementary Table 1). In 9 villages, P. falciparum prevalence was low (range, 0%–5.6%), while in 8 villages, it ranged from 8.3% to 22.2%. The diversity of parasite populations remained high, even when prevalence was low. In 6 of the low-prevalence villages, ≥2 isolates were genotyped by pfmsp2, and within each village, different clones were detected (Supplementary Table 4).
Catchment was not associated with P. vivax prevalence in either survey (Supplementary Table 3). In 2006, prevalence was 40.8% in Utu, 43.9% in Mugil, and 39.6% in Malala (P = .386). In 2010, prevalence was lowest in Utu (10.6%) and Malala (11.7%) but higher in Mugil (15.1%; P = .130). In 2014, prevalence was 15.7% in Utu, 19.7% in Mugil, and 21.8% in Malala (P = .0502).
DISCUSSION
Along the north coast of PNG, continuous control of malaria over 8 years has led to a 12- and 6-fold decrease of P. falciparum and P. vivax prevalence, respectively, detected by LM. Using a highly sensitive qPCR to diagnose infections, the continuous decrease in P. falciparum prevalence was confirmed, whereas the P. vivax prevalence increased between 2010 and 2014. Parasite densities of both species have decreased considerably, and thus an increasing proportion of infections were asymptomatic and submicroscopic.
Gametocyte densities and the probability to detect gametocytes—and, thus, human-to-mosquito transmission potential—were closely correlated to blood-stage parasite density. Because of the lower parasite densities, gametocytes were detected in a lower proportion of infections in 2014 than in 2010. However, because of the increase in the proportion of submicroscopic infections, remaining gametocyte carriers became more difficult to identify. For both species, the majority of gametocyte carriers (determined by reverse-transcription qPCR) were LM positive for asexual parasites in 2010, but in 2014 approximately two thirds of gametocyte carriers presented with submicroscopic infections. Over 90% of gametocyte carriers were asymptomatic, and such individuals thus present a challenge for malaria control and elimination. In PNG, most febrile cases presenting at health centers are diagnosed by LM or rapid diagnostic test and antimalarial treatment is given to positive individuals. The increasing proportion of asymptomatic and submicroscopic infections thus remain untreated, yet such infections of both P. falciparum and P. vivax have been shown to frequently infect mosquitos [8–10].
Decreasing levels of transmission also appeared to have an impact on acquisition of immunity to P. falciparum. In malaria-endemic countries, the attack rate in children increases with age [32], and in parallel individuals acquire immunity gradually, with the speed of acquisition depending on the transmission intensity. As a result, clinical malaria and parasite prevalence peaks in children and then decreases as immunity is acquired [33]. In 2006, very high parasite densities were observed in young children, followed by a rapid decline with increasing age. This age-associated decline was less marked in 2014, and the peak P. falciparum prevalence shifted from children to adolescents, reflecting delayed acquisition of immunity, similar to trends observed for clinical malaria in Africa [34]. In parallel, the odds of presenting with fever when infected with P. falciparum increased 4-fold between 2006 and 2014, further suggesting a reduced level of (clinical) immunity.
Individuals with low levels of immunity are expected to present with higher parasite densities, and the risk of developing clinical malaria increases. However, over the 8-year period, parasite densities for both species decreased considerably, and the proportion of individuals with clinical malaria decreased 3-fold. Parasite densities are determined not only by acquired immunity, but also by the age of the infection on the day of sampling (Supplementary Figure 2). Densities in the peripheral blood peak in the first phase of the infection, and when not treated they can persist for weeks or months at low densities [3]. When transmission is lower, fewer new infections are acquired and persisting infections are thus on average older and densities lower. For example, the molecular force of P. vivax blood-stage infection in PNG children decreased from 15 clones/year [35] to 5 clones/year between 2006 and 2010 [19]. In 2014, the contribution of older low-density infections appeared to be far greater than the contribution of infections with possibly higher initial parasite densities caused by lower levels of immunity. In addition, 6-fold less treatment was administered in 2014 as compared to 2006, further contributing to a large number of old, low-density chronic infections.
The overall decrease in prevalence was accompanied by increasing heterogeneity of P. falciparum prevalence at the village level, indicating foci of residual transmission. In contrast to clonal P. falciparum outbreaks observed in the highlands of PNG [36], Solomon Islands [37], and South America [38], the parasite populations in the 2014 survey remained genetically diverse, even in villages with very low prevalences. This could indicate that residual infections were imported from villages with higher transmission, where a genetically diverse population is maintained.
In contrast to the constant decline in P. falciparum prevalence, P. vivax prevalence has increased since 2010. In PNG, in 2008–2010, 80% of all blood-stage P. vivax infections in children were caused by relapses [18, 19]. As a consequence, for P. vivax, mosquito-to-human transmission levels and parasite prevalence rates in the population are less correlated than for P. falciparum. In 2014, P. vivax densities were very low, and almost 80% were single-clone infections. Both factors suggest high proportions of relapses. Relapses often consist of a single clone [39–41], and often consist of clones that are homologous or related to the initial blood-stage parasite infection [40, 42]. They thus carry a reservoir of antigens the immune system has been exposed to recently, and even young children with limited acquired immunity may be able to control such infections [43]. An increasing proportion of infections caused by relapses of previously acquired infections could thus explain the low P. vivax densities across all ages in 2014 and the increase in P. vivax prevalence from 2010 to 2014. It is possible that the 2014 survey captured a phase during which the vast P. vivax hypnozoite reservoir that had accumulated in the population from years of high transmission had not yet been exhausted and that the prevalence had thereby increased temporarily.
In conclusion, the rapid decline in transmission in a population maintaining a relatively high level of clinical immunity resulted in a large proportion of very-low-density infections. Increasing proportions of submicroscopic infections when prevalence is lower has been found across countries both for P. falciparum and P. vivax [3, 4]. Few studies have assessed the speed of change in the same population. In the Brazilian Amazon, a 9-fold decrease of P. vivax prevalence over 3 years was accompanied by an increase in the proportion of submicroscopic infections, from 44% to 73%, and almost all of them carried gametocytes [12]. The present finding of an increasing proportion of gametocyte carriers being submicroscopic—despite an overall lower proportion gametocyte positive—is thus likely a general pattern in countries where transmission levels are decreasing.
Novel strategies are therefore needed to effectively target the asymptomatic low-density reservoir of Plasmodium infections. For P. falciparum, mass screen and treat (MSAT) approaches would require highly sensitive diagnostics tools. For P. vivax, in which hypnozoite carriers cannot be detected with any current diagnostic test, MSAT is not an appropriate intervention [19], and other approaches will need to be developed. The large asymptomatic and submicroscopic reservoirs thus represent a challenge to the goal of a malaria-free Asia-Pacific for the foreseeable future. An in-depth understanding of their contribution to maintaining transmission and better tools and surveillance strategies to efficiently identify and target these infections are thus urgently needed.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the International Centers of Excellence for Malaria Research (grant U19 AI089686), the TransEPI consortium, funded by the Bill and Melinda Gates Foundation; the NHMRC (grant 1021544; early career fellowship 1016443 to L. J. R.); the Victorian State Government, through operational infrastructure support; the Australian Government NHMRC IRIISS; the Swiss National Science Foundation (fellowship P2BSP3_151880 to C. K.); the Medical Research Council (population health scientist fellowship to M. W.); and the NHMRC (senior research fellowship to I.M.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Molecular Approaches to Malaria Meeting, Lorne, Australia, February 2016; Annual Meeting of the American Society for Tropical Medicine and Hygiene, Atlanta, Georgia, November 2016.
References
- 1. Bhatt S, Weiss DJ, Cameron E et al. . The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015; 526:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferreira MU, Castro MC. Challenges for malaria elimination in Brazil. Malar J 2016; 15:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okell LC, Bousema T, Griffin JT, Ouédraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun 2012; 3:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng Q, Cunningham J, Gatton ML. Systematic review of sub-microscopic P. vivax infections: prevalence and determining factors. PLoS Negl Trop Dis 2015; 9:e3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Imwong M, Nguyen TN, Tripura R et al. . The epidemiology of subclinical malaria infections in South-East Asia: findings from cross-sectional surveys in Thailand-Myanmar border areas, Cambodia, and Vietnam. Malar J 2015; 14:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Guidelines for the treatment of malaria. 3rd ed Geneva: World Health Organization, 2015. [Google Scholar]
- 7. Kiattibutr K, Roobsoong W, Sriwichai P et al. . Infectivity of symptomatic and asymptomatic Plasmodium vivax infections to a Southeast Asian vector, Anopheles dirus. Int J Parasitol 2017; 47:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ouédraogo AL, Gonçalves BP, Gnémé A et al. . Dynamics of the human infectious reservoir for malaria determined by mosquito feeding assays and ultrasensitive malaria diagnosis in Burkina Faso. J Infect Dis 2016; 213:90–9. [DOI] [PubMed] [Google Scholar]
- 9. Vallejo AF, García J, Amado-Garavito AB, Arévalo-Herrera M, Herrera S. Plasmodium vivax gametocyte infectivity in sub-microscopic infections. Malar J 2016; 15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coleman RE, Kumpitak C, Ponlawat A et al. . Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in western Thailand. J Med Entomol 2004; 41:201–8. [DOI] [PubMed] [Google Scholar]
- 11. Galatas B, Bassat Q, Mayor A. Malaria parasites in the asymptomatic: looking for the hay in the haystack. Trends Parasitol 2016; 32:296–308. [DOI] [PubMed] [Google Scholar]
- 12. Barbosa S, Gozze AB, Lima NF et al. . Epidemiology of disappearing Plasmodium vivax malaria: a case study in rural Amazonia. PLoS Negl Trop Dis 2014; 8:e3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Waltmann A, Darcy AW, Harris I et al. . High rates of asymptomatic, sub-microscopic Plasmodium vivax infection and disappearing Plasmodium falciparum malaria in an area of low transmission in Solomon Islands. PLoS Negl Trop Dis 2015; 9:e0003758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin E, Kiniboro B, Gray L et al. . Differential patterns of infection and disease with P. falciparum and P. vivax in young Papua New Guinean children. PLoS One 2010; 5:e9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris I, Sharrock WW, Bain LM et al. . A large proportion of asymptomatic Plasmodium infections with low and sub-microscopic parasite densities in the low transmission setting of Temotu Province, Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malar J 2010; 9:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michon P, Cole-Tobian JL, Dabod E et al. . The risk of malarial infections and disease in Papua New Guinean children. Am J Trop Med Hyg 2007; 76:997–1008. [PMC free article] [PubMed] [Google Scholar]
- 17. Baird KJ, Maguire JD, Price RN. Diagnosis and treatment of Plasmodium vivax malaria. Adv Parasitol 2012; 80:203–70. [DOI] [PubMed] [Google Scholar]
- 18. Betuela I, Rosanas-Urgell A, Kiniboro B et al. . Relapses contribute significantly to the risk of Plasmodium vivax infection and disease in Papua New Guinean children 1-5 years of age. J Infect Dis 2012; 206:1771–80. [DOI] [PubMed] [Google Scholar]
- 19. Robinson LJ, Wampfler R, Betuela I et al. . Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med 2015; 12:e1001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kasehagen LJ, Mueller I, McNamara DT et al. . Changing patterns of Plasmodium blood-stage infections in the Wosera region of Papua New Guinea monitored by light microscopy and high throughput PCR diagnosis. Am J Trop Med Hyg 2006; 75:588–96. [PMC free article] [PubMed] [Google Scholar]
- 21. Mueller I, Widmer S, Michel D et al. . High sensitivity detection of Plasmodium species reveals positive correlations between infections of different species, shifts in age distribution and reduced local variation in Papua New Guinea. Malar J 2009; 8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barry AE, Schultz L, Senn N et al. . High levels of genetic diversity of Plasmodium falciparum populations in Papua New Guinea despite variable infection prevalence. Am J Trop Med Hyg 2013; 88:718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reimer LJ, Thomsen EK, Koimbu G et al. . Malaria transmission dynamics surrounding the first nationwide long-lasting insecticidal net distribution in Papua New Guinea. Malar J 2016; 15:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hetzel MW, Morris H, Tarongka N et al. . Prevalence of malaria across Papua New Guinea after initial roll-out of insecticide-treated mosquito nets. Trop Med Int Health 2015; 20:1745–55. [DOI] [PubMed] [Google Scholar]
- 25. Koepfli C, Robinson LJ, Rarau P et al. . Blood-stage parasitaemia and age determine Plasmodium falciparum and P. vivax gametocytaemia in Papua New Guinea. PLoS One 2015; 10:e0126747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosanas-Urgell A, Mueller D, Betuela I et al. . Comparison of diagnostic methods for the detection and quantification of the four sympatric Plasmodium species in field samples from Papua New Guinea. Malar J 2010; 9:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Falk N, Maire N, Sama W et al. . Comparison of PCR-RFLP and Genescan-based genotyping for analyzing infection dynamics of Plasmodium falciparum. Am J Trop Med Hyg 2006; 74:944–50. [PubMed] [Google Scholar]
- 28. Koepfli C, Ross A, Kiniboro B et al. . Multiplicity and diversity of Plasmodium vivax infections in a highly endemic region in Papua New Guinea. PLoS Negl Trop Dis 2011; 5:e1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arnott A, Barnadas C, Senn N et al. . High genetic diversity of Plasmodium vivax on the north coast of Papua New Guinea. Am J Trop Med Hyg 2013; 89:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wampfler R, Mwingira F, Javati S et al. . Strategies for Detection of Plasmodium species Gametocytes. Plos One 2013; 8:e76316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith T, Hii JL, Genton B et al. . Associations of peak shifts in age–prevalence for human malarias with bednet coverage. Trans R Soc Trop Med Hyg 2001; 95:1–6. [DOI] [PubMed] [Google Scholar]
- 32. Port GR, Boreham PFL, Bryan JH. The Relationship of Host Size to Feeding by Mosquitos of the Anopheles-Gambiae Giles Complex (Diptera, Culicidae). B Entomol Res 1980; 70:133–44. [Google Scholar]
- 33. Smith T, Beck HP, Kitua A et al. . Age dependence of the multiplicity of Plasmodium falciparum infections and of other malariological indices in an area of high endemicity. Trans R Soc Trop Med Hyg 1999; 93 Suppl 1:15–20. [DOI] [PubMed] [Google Scholar]
- 34. Mogeni P, Williams TN, Fegan G et al. . Age, Spatial, and Temporal Variations in Hospital Admissions with Malaria in Kilifi County, Kenya: A 25-Year Longitudinal Observational Study. PLoS Med 2016; 13:e1002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koepfli C, Colborn KL, Kiniboro B et al. . A high force of plasmodium vivax blood-stage infection drives the rapid acquisition of immunity in papua new guinean children. PLoS Negl Trop Dis 2013; 7:e2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mueller I, Kaiok J, Reeder JC, Cortés A. The population structure of Plasmodium falciparum and Plasmodium vivax during an epidemic of malaria in the Eastern Highlands of Papua New Guinea. Am J Trop Med Hyg 2002; 67:459–64. [DOI] [PubMed] [Google Scholar]
- 37. Ballif M, Hii J, Marfurt J et al. . Monitoring of malaria parasite resistance to chloroquine and sulphadoxine-pyrimethamine in the Solomon Islands by DNA microarray technology. Malar J 2010; 9:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baldeviano GC, Okoth SA, Arrospide N et al. . Molecular Epidemiology of Plasmodium falciparum Malaria Outbreak, Tumbes, Peru, 2010–2012. Emerg Infect Dis 2015; 21:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen N, Auliff A, Rieckmann K, Gatton M, Cheng Q. Relapses of Plasmodium vivax infection result from clonal hypnozoites activated at predetermined intervals. J Infect Dis 2007; 195:934–41. [DOI] [PubMed] [Google Scholar]
- 40. Imwong M, Boel ME, Pagornrat W et al. . The first Plasmodium vivax relapses of life are usually genetically homologous. J Infect Dis 2012; 205:680–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin JT, Juliano JJ, Kharabora O et al. . Individual Plasmodium vivax msp1 variants within polyclonal P. vivax infections display different propensities for relapse. J Clin Microbiol 2012; 50:1449–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim JR, Nandy A, Maji AK et al. . Genotyping of Plasmodium vivax reveals both short and long latency relapse patterns in Kolkata. PLoS One 2012; 7:e39645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cole-Tobian JL, Michon P, Biasor M et al. . Strain-specific duffy binding protein antibodies correlate with protection against infection with homologous compared to heterologous plasmodium vivax strains in Papua New Guinean children. Infect Immun 2009; 77:4009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.