Abstract
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common enzymatic disorder in humans and appears to be protective against falciparum severe malaria. Controversially, it is also thought that Plasmodium vivax has driven the recent selection of G6PD alleles. We use an experimental approach to determine whether G6PD-MahidolG487A variant, a widespread cause of severe G6PD deficiency in Southeast Asia, provides a barrier against vivax malaria. Our results show that the immature reticulocytes (CD71+) targeted by P. vivax invasion are enzymatically normal, even in hemizygous G6PD-Mahidol G487A mutants; thus, allowing the normal growth, development, and high parasite density in severely deficient samples.
Keywords: glucose-6-phosphate dehydrogenase deficiency, Plasmodium vivax, reticulocytes
Mutations in the X-linked glucose-6-phosphate dehydrogenase (G6PD) gene are the most common and widespread cause of human enzymopathy; they are associated with stress-induced hemolysis, resulting in a range of mild to life-threatening clinical conditions. The degree of phenotypic G6PD deficiency (G6PDd) depends on the genetic variant and the zygosity of the carrier [1], and it is most commonly due to an instability of the G6PD enzyme [2] or an altered functionality of the protein active site expressed in mature erythrocytes [3].
G6PDd is protective against severe malaria caused by Plasmodium falciparum [4] as documented by epidemiological evidence [5] and in vitro studies on parasite development [6]. Recent studies suggest that by reducing levels of parasitemia, the selection of G6PD mutations has also been driven by P. vivax, the other major cause of human malaria [7]. However, this is in contradiction to an earlier study showing higher P. vivax parasitemias in G6PDd young children as compared to G6PD normal [8] in the same population. Here, we conduct the first ever ex vivo experiments to determine whether the G6PD-Mahidol mutation (487G>A) in hemizygous and homozygous status poses a barrier to the invasion and the normal development of vivax malaria parasites.
EXPERIMENTAL PROCEDURES
Ethics Statement
The clinical infected red blood cell samples examined in this study were collected under the following ethical guidelines in the approved protocols: OXTREC 45–09 (University of Oxford, Centre for Clinical Vaccinology and Tropical Medicine, UK) and MUTM 2008–215 (Ethics Committee, Faculty of Tropical Medicine, Mahidol University, Thailand).
G6PD Characterization
Blood samples were collected at the Thailand–Myanmar border among subjects of Karen ethnicity where the prevalence of G6PD can reach 15%; the major variant found in the population is Mahidol (487G>A) (Supplementary Figure 1).
Blood samples were screened by fluorescent spot test (R&D Diagnostics, Greece) and genotyped for the Mahidol variant using a polymerase chain reaction–restriction fragment length polymorphism protocol [9]. Quantitative phenotypic characterization of samples was performed by gold standard spectrophotometric assay using Trinity Kits (Trinity, Ireland) and flow-cytometric assay according to Shah et al. [10].
Human Parasites
Thirty-four clinical isolates of P. vivax were collected from malaria patients receiving treatment at clinics run by the Shoklo Malaria Research Unit on the northwestern border of Thailand. The project was explained to all the patients before they provided informed consent prior to collection of blood by venipuncture. Whole blood (5 mL) was collected in lithium heparin collection tubes. These samples were cryopreserved in Glycerolyte 57 Solution (Baxter) after leukocyte depletion using a nonwoven fabric filter (Antoshin Pte Ltd). After thawing, the parasites present in the packed cells (1.5 mL per isolate) were cultured to the schizont stage in 12 mL McCoy 5A medium supplemented with 2.4 g/L D-glucose, 40 mg/mL gentamycin sulfate, and 20% heat-inactivated human AB serum, in an atmosphere of 5% O2 at 37.5°C.
Immunomagnetic Erythrocyte Sorting
The selection of the CD71+ reticulocytes was performed using the magnetic-activated cell sorting system (Miltenyi Biotec). A total of 2 mL blood at 50% hematocrit in phosphate-buffered saline was passed through an LS column (Miltenyi), the purity of the positive and negative fractions was monitored by flow cytometry using Thiazole orange (Sigma-Aldrich) staining. The yield of CD71+ cells is upper 90% of purity.
Scanning Electron Microscopy
Electron microscopy was conducted on reticulocytes and normocytes after immunomagnetic sorting using the methods outlined in Malleret et al. [11].
Optical Microscopy
Microscopic enumeration of infected red blood cells was performed using thin blood smears stained with Giemsa. A minimum of 4000 red blood cells were counted (20 fields at 100× magnification). Live cell subvital staining of reticulocytes and parasites was done using Giemsa.
Statistical Analyses
Statistical analyses were performed using Graph Pad Prism (5.1). Mean values were compared, and P values were calculated using paired t tests.
RESULTS AND DISCUSSION
Unlike P. falciparum, P. vivax only invades reticulocytes. Importantly, P. vivax targets a specific population of immature reticulocytes (CD71+), which are primarily found in the bone marrow [12]. Our hypothesis was that selection of G6PD mutations by P. vivax might be mediated by phenotypic deficiency in those young reticulocytes, whereby the parasite would be unable to invade or normally replicate in red blood cells (RBCs) whose intracellular capacity of respond to stress was decreased by lack of G6PD activity.
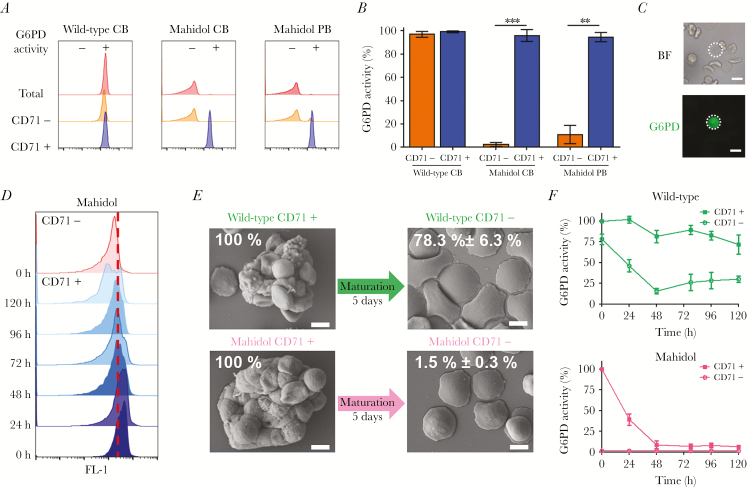
We first characterized the G6PD phenotypes of CD71+ reticulocytes in blood samples of Karen donors who were either wild-type genotype or hemi/homozygous for the Mahidol mutation. Analyses by flow cytometry and fluorescent microscopy showed that immature reticulocytes have a normal G6PD phenotype, irrespective of the genetic background of the individual (Figure 1A–1C). We studied the biochemical kinetics of immunomagnetically sorted and ex vivo matured CD71+ and CD71– erythrocytes from wild-type and G6PD-Mahidol mutant patients and showed that CD71+ reticulocytes from hemizygous mutants maintain normal G6PD activity throughout the period that the reticulocyte is receptive to P. vivax merozoite invasion [12] (Figure 1D–1F). Upon maturation to normocytes, only genetically wild-type G6PD RBCs retained normal activity. Earlier studies showed that in G6PDd individuals with African mutations, the young erythrocytes produced in response to drug-induced hemolysis had normal enzymatic activity and therefore were not susceptible to hemolysis during the continued administration of oxidative drug [13]; further evidence indicated that G6PD activity was normal in young RBCs of the A variant but very low in young RBCs of the Mediterranean variant [14]. This is the first study to show that CD71+ reticulocytes in G6PD-Mahidol mutants have normal G6PD enzymatic activity.
Figure 1.
CD71+ reticulocytes are phenotypically G6PD normal in subjects with Mahidol Mutation. A, G6PD activity detected by flow cytometry in erythrocytes, CD71– and CD71+ fraction of wild-type and G6PD-Mahidol mutant cord blood and in G6PD-Mahidol mutant peripheral blood. B, Quantification of G6PD activity (mean values ± SDs) for 4 wild-type cord blood, 3 G6PD-Mahidol–deficient cord blood, and 3 G6PD-Mahidol peripheral blood samples. C, G6PD activity detected by fluorescence microscopy in CD71– fraction of Mahidol mutant cord blood (scale bar represents 5 μm). D, G6PD activity time course detected by flow cytometry in CD71+ and CD71– erythrocytes. The red dotted line represents the limit of positivity for G6PD (separation between methemoglobin and oxyhemoglobin peaks of green fluorescence). E, Morphology of immunomagnetically sorted CD71+ and CD71– erythrocytes from wild-type and G6PD-Mahidol–mutant patients visualized by scanning electron microscopy. Scale bar represents 5 μm. F, G6PD kinetic activity in CD71+ and CD71– for wild-type and G6PD-Mahidol patients (n = 4) measured by flow cytometry during 5 days of culture at 37.5°C and 5% CO2 in McCoy medium 20% AB serum. The MFI of oxyhemoglobin for CD71+ reticulocytes at time 0 was assigned to be 100% of G6PD activity for each sample.
Abbreviations: G6PD, glucose-6-phosphate dehydrogenase; MFI, mean fluorescence intensity.
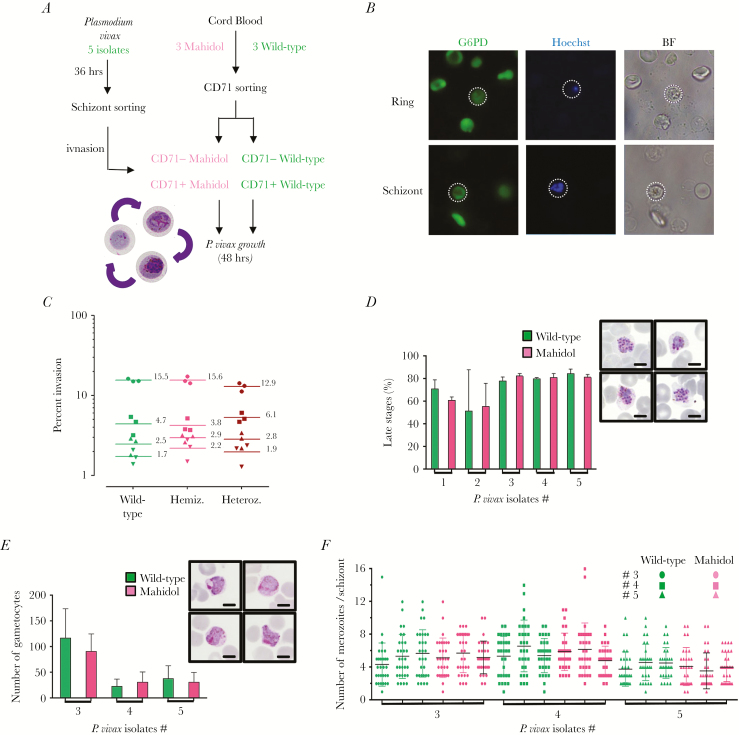
To prove that these Mahidol CD71+ reticulocytes are indeed receptive to invasion and capable of supporting the full asexual and sexual erythrocytic life cycle, we conducted a number of ex vivo invasion and maturation assays using freshly isolated P. vivax (Figure 2A). Our results show that P. vivax merozoites invade Mahidol CD71+ reticulocytes equally as well as wild-type CD71+ reticulocytes (Figure 2B and 2C), and that full erythrocytic life cycle of P. vivax takes place at the same rate (Figure 2D and 2E), resulting in a similar number of merozoites per schizont (Figure 2F). The development of healthy male and female gametocytes occurs in Mahidol CD71+ reticulocytes at similar proportions as those seen in G6PD wild-type cells (Figure 2E). Thus, there seems to be no major biological impediment to the development of P. vivax in Mahidol CD71+ reticulocytes [15]. If there is indeed a protective effect of G6PDd-Mahidol against vivax malaria parasitemia, it may be due to immune host response rather than any intrinsic erythrocytic barriers.
Figure 2.
Normal development of Plasmodium vivax in G6PD-Mahidol–mutant reticulocytes. A, Schematic of P. vivax growth assay in G6PD wild-type and G6PD-Mahidol–mutant reticulocytes. B, G6PD activity detected by fluorescence microscopy in ring and schizont stages 3 hours and 40 hours, respectively, after inoculation into nascent reticulocytes. C, P. vivax invasion in adult blood from 3 different genotypes (wild-type, and Mahidol homozygote and heterozygote) with 4 different field isolates. D, Proportion of late parasite stages after 48 hours of culture in G6PD wild-type and G6PD-Mahidol mutant (n = 3 for each genotype with 5 different P. vivax isolates; 48 hr postinvasion). In insert, Giemsa-stained thin film showing segmented vivax schizonts in G6PD-Mahidol–mutant reticulocytes (54 hr postinvasion). The scale represents 5 μm. E, Plasmodium vivax gametocyte frequency in wild-type and G6PD-Mahidol reticulocytes. In insert, Giemsa-stained thin film showing vivax gametocytes in G6PD-Mahidol–mutant reticulocytes (54 hr postinvasion). The scale represents 5 μm. F, Number of vivax merozoites per schizonts in wild type and G6PD-Mahidol–mutant CD71+ reticulocytes (n = 3 for each genotype with 3 different P. vivax isolates).
Quite often, it is assumed that malaria has been a major force in driving the evolution of human RBC mutations; while this seems to be the case for P. falciparum, the picture is less clear for P. vivax. The progressive loss of enzymatic activity upon maturation, especially in G6PD-deficient RBCs but also in the normal ones, might have contributed to shift the preference of plasmodia toward the invasion of younger RBCs. G6PD deficiency might then be among a variety of host mutations that may have driven the selection of parasites that prefer to invade younger RBCs; in particular, reticulocytes.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgements. We thank the staff and patients attending the Mae Sot Malaria Clinic, and also clinics associates with the Shoklo Malaria Research Unit (SMRU), Tak Province, Thailand. We thank Daniel Parker for creating the map of Supplementary Figure 1.
Financial support. This work was supported with a Young Investigator grant (BMRC YIG Grant No: 13/1/16/YA/009, to B. M.) under the Agency for Science, Technology and Research (A*STAR, Singapore) and had a joint appointment with the National University of Singapore, funded from the Singapore National Medical Research Council (NMRC/CBRG/0047/2013). L. R. was supported by funding from the Singapore Immunology Network (SIgN) and the Horizontal Programme on Infectious Diseases under the Agency for Science, Technology and Research (A*STAR, Singapore). B. R. was funded by the University of Otago, New Zealand. Shoklo Malaria Research Unit is part of the Mahidol Oxford University Research Unit, supported by the Wellcome Trust of Great Britain. G. B. and C. S. C. were supported by the Wellcome Trust of Great Britain (Programme Grant 089179).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008; 371:64–74. [DOI] [PubMed] [Google Scholar]
- 2. Huang Y, Choi MY, Au SW, Au DM, Lam VM, Engel PC. Purification and detailed study of two clinically different human glucose 6-phosphate dehydrogenase variants, G6PD(Plymouth) and G6PD(Mahidol): Evidence for defective protein folding as the basis of disease. Mol Genet Metab 2008; 93:44–53. [DOI] [PubMed] [Google Scholar]
- 3. Pandolfi PP, Sonati F, Rivi R, Mason P, Grosveld F, Luzzatto L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J 1995; 14:5209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luzzatto L, Seneca E. G6PD deficiency: a classic example of pharmacogenetics with on-going clinical implications. Br J Haematol 2014; 164:469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruwende C, Khoo SC, Snow RW et al. . Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature 1995; 376:246–9. [DOI] [PubMed] [Google Scholar]
- 6. Luzzatto L, Sodeinde O, Martini G. Genetic variation in the host and adaptive phenomena in Plasmodium falciparum infection. Ciba Found Symp 1983; 94:159–73. [DOI] [PubMed] [Google Scholar]
- 7. Louicharoen C, Patin E, Paul R et al. . Positively selected G6PD-Mahidol mutation reduces Plasmodium vivax density in Southeast Asians. Science 2009; 326:1546–9. [DOI] [PubMed] [Google Scholar]
- 8. Kruatrachue M, Charoenlarp P, Chongsuphajaisiddhi T, Harinasuta C. Erythrocyte glucose-6-phosphate dehydrogenase and malaria in Thailand. Lancet 1962; 2:1183–6. [DOI] [PubMed] [Google Scholar]
- 9. Huang CS, Hung KL, Huang MJ, Li YC, Liu TH, Tang TK. Neonatal jaundice and molecular mutations in glucose-6-phosphate dehydrogenase deficient newborn infants. Am J Hematol 1996; 51:19–25. [DOI] [PubMed] [Google Scholar]
- 10. Shah SS, Diakite SA, Traore K et al. . A novel cytofluorometric assay for the detection and quantification of glucose-6-phosphate dehydrogenase deficiency. Sci Rep 2012; 2:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malleret B, Xu F, Mohandas N et al. . Significant biochemical, biophysical and metabolic diversity in circulating human cord blood reticulocytes. PLOS One 2013; 8:e76062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malleret B, Li A, Zhang R et al. . Plasmodium vivax: restricted tropism and rapid remodeling of CD71-positive reticulocytes. Blood 2015; 125:1314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beutler E, Dern RJ, Alving AS. The hemolytic effect of primaquine. IV. The relationship of cell age to hemolysis. J Lab Clin Med 1954; 44:439–42. [PubMed] [Google Scholar]
- 14. Piomelli S, Corash LM, Davenport DD, Miraglia J, Amorosi EL. In vivo lability of glucose-6-phosphate dehydrogenase in GdA- and GdMediterranean deficiency. J Clin Invest 1968; 47:940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoshida A, Roth EF Jr. Glucose-6-phosphate dehydrogenase of malaria parasite Plasmodium falciparum. Blood 1987; 69:1528–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.