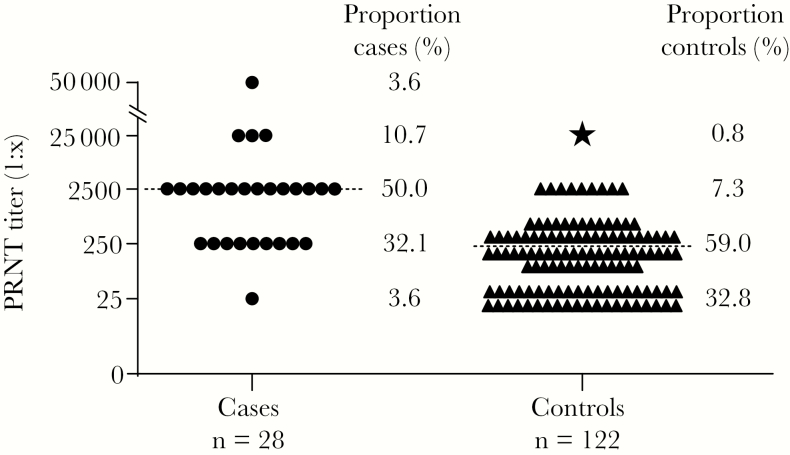
ZIKV-specific neutralizing antibody titers were significantly higher in 28 mothers of children with microcephaly than in 122 controls from northeastern Brazil, suggesting an unusually strong immunological stimulus and potential utility of maternal antibody titers to corroborate congenital ZIKV infection.
Keywords: Zika virus, microcephaly, neutralization test, Brazil, parturient
Abstract
Reliable diagnosis of congenital Zika virus (ZIKV) infection is challenging. Here, we assessed ZIKV-specific neutralizing antibodies in 28 mothers of children with microcephaly (cases) and 122 controls from northeastern Brazil using plaque reduction neutralization tests. ZIKV-specific antibody titers were significantly higher in cases than in controls (t test, P < .0001). We identified a putative case of congenital Zika syndrome retrospectively by unusually high ZIKV-specific antibody titers. High ZIKV-specific antibody titers in cases were unrelated to prior dengue virus infection. Our data suggest a strong immunological stimulus from prolonged placental or transplacental ZIKV shedding and potential utility of maternal antibody titers to corroborate congenital ZIKV infection.
During the 2015–2016 Zika virus (ZIKV) epidemic, a 20-fold increase in the incidence of neonatal microcephaly was observed in northeastern Brazil and attributed to congenital ZIKV infection [1]. Reliable diagnosis of congenital ZIKV infection is technically challenging. First, high-resolution imaging is not always available. Additionally, the alterations caused by ZIKV can be similar to those caused by other infections (eg, toxoplasmosis and syphilis) [2]. Second, clinical diagnosis of ZIKV infection is difficult because up to 80% of courses in adults are asymptomatic and symptoms overlap with those elicited by co-circulating arboviruses, such as the ubiquitous dengue virus (DENV) [3]. Again, clinical diagnostics cannot ascertain congenital ZIKV infection. Third, qualitative detection of ZIKV RNA or ZIKV-specific antibodies in maternal serum can corroborate infection of the mother but cannot confirm congenital infection. Similarly, demonstration of ZIKV-specific immunoglobulin G (IgG) in neonatal serum cannot prove congenital infection, as maternal IgG passes the placental barrier [4]. In contrast, detection of ZIKV-specific IgM or RNA in umbilical cord blood or cerebrospinal fluid (CSF) can substantiate fetal infection. Unfortunately, the sensitivity of these tests is low. In the only available case-control study, ZIKV-specific IgM was detectable only in 27% of neonatal sera and in 36% of CSF specimens from 32 cases of microcephaly [5]. None of the neonatal sera and only 4 of the CSF specimens tested positive for ZIKV RNA [5]. The low sensitivity of IgM tests may be associated with insufficient fetal IgM production due to immature immune responses or interference from maternal IgG [6]. In addition, usability of these tests to confirm fetal infection may be limited by low quantities of maternal IgM that may reach fetal circulation in cases of severe placental inflammation [4].
In many cases of suspected ZIKV-associated malformations, proof of congenital infection is thus impossible. The inability to ascertain congenital infection challenges definite estimates of the absolute risk of congenital malformations upon ZIKV infection during pregnancy and targeted antiviral intervention strategies, once these become available.
Here, we investigated whether the magnitude of neutralizing antibody responses in maternal sera can be used to substantiate congenital ZIKV infection.
METHODS
We compared 28 mothers of microcephaly cases (hereafter termed cases) and 122 mothers of children born without microcephaly (hereafter termed controls) from Salvador, northeastern Brazil, sampled between 29 October 2015 and 9 December 2016. The institutional research ethics board of the Federal University of Bahia Climério de Oliveira approved sampling and testing under protocol number 1.408.49. All study participants provided written informed consent before sampling, which was conducted upon delivery in the university maternity ward. Microcephaly was diagnosed when the head circumference was 2 standard deviations below that of the corresponding gestational age, based on intergrowth charts from the World Health Organization, in addition to clinical and imaging data. For all cases, no excessive alcohol consumption during pregnancy was documented. Due to limited resources, no other infectious or noninfectious causes of microcephaly could be evaluated.
To test for exposure to the hyperendemic DENV, we performed a competitive enzyme-linked immunosorbent assay (ELISA), relying on a mutant envelope (E) antigen of DENV designed to be robust against cross-reactivity with ZIKV-specific antibodies [7]. Neutralizing antibody titers against ZIKV were measured by plaque reduction neutralization tests (PRNTs) in sera that previously tested positive for ZIKV IgG using a commercially available ELISA (Euroimmun, Lübeck, Germany). In brief, 2 µL of heat-inactivated serum (56°C, 30 minutes) were diluted in DMEM with 1% fetal calf serum (FCS) at 1:25, 1:250, 1:2500 and 1:25000. Serum dilutions were incubated with 50 plaque-forming units (PFU) of ZIKV outbreak strain H/PF/2013 at 37°C for 60 minutes followed by a second incubation on Vero cells 37°C for 60 minutes in 12-well plates, followed by an agarose/Dulbecco’s Modified Eagle Medium overlay (2% FCS, 0.6% final agarose concentration). Further serum dilutions of 1:50000 and 1:100000 were performed if needed to determine end-point titers. Cells were incubated for 4 days before formaldehyde fixation, staining, and plaque counting. Serum titers reducing ZIKV PFU by ≥50% in any dilution were considered positive.
RESULTS
The study comprised 150 parturients, including 28 cases and 122 controls. The age distribution of cases and controls showed no statistically significant difference (median, 28.8 [range, 15–44] years for cases and 29.4 [range, 17–44] years for controls; t test, P = .693). In PRNTs, the median serum end-point dilution still showing neutralization of ZIKV was 10-fold higher in cases at 1:2500, compared to controls at 1:250 (Figure 1). The difference of neutralizing antibody titers between cases and controls was statistically highly significant (t test, P < .0001). As shown in Table 1, the proportion of cases yielding very high PRNT titers of up to 1:50000 serum end-point dilution was 14.3%, compared to only 0.8% of controls (Fisher exact test, P = .004). A similar discrepancy between groups was observed in the next-highest titer category of 1:2500 serum end-point dilution, which occurred significantly more frequently in cases at 50.0% compared to controls at 7.3% (Fisher exact test, P < .0001). Taken together, relatively higher PRNT titers of ≥1:2500 were between 7 and 17 times more likely to occur in cases than in controls (relative risk ratios, 6.7–17.4). Conversely, relatively lower PRNT titers of 1:250 and 1:25 were significantly more frequently observed in controls (Fisher exact test, P = .012 and P = .0009, respectively). Despite the overall stark differences in the distribution of ZIKV-specific PRNT titers between cases and controls, some overlap in end-point titers occurred, illustrated by 35.7% of cases showing relatively lower end-point titers of ≤1:250. Whether relatively lower titers in cases were associated with a different etiology of microcephaly than congenital ZIKV infection or whether the lower titers were due to interindividual variation in ZIKV-specific antibody responses [8] remains to be determined. However, end-point titers of ≤1:250 occurred significantly more frequently in controls at 91.8% (χ2 = 43.6, P < .00001), emphasizing the significant difference in PRNT titers between cases and controls (Figure 1).
Figure 1.
Differences in plaque reduction neutralization test (PRNT) titers between cases and controls. PRNT titers indicating serum end-point dilutions still showing ≥50% reduction of Zika virus plaque-forming units. Titers were logarithmized before plotting for clarity of presentation. Dashed horizontal lines, medians; circles, cases; triangles, controls; star, datum point of a control (ZK033) with an unusually high Zika virus PRNT titer, retrospectively identified as a putative congenital Zika syndrome. The proportion of cases and controls yielding respective end-point titers is indicated to the right of symbols. The y-axis was extended manually between 1:25000 and 1:50000 serum dilutions for clarity of presentation, as indicated by slashed lines.
Table 1.
Neutralization Titers of Cases and Controls
| PRNT End-point Titer | Group | PRNT + |
PRNT – |
Proportion PRNT + per titer category, % | Relative Risk | (95% CI) | P Valuea |
|---|---|---|---|---|---|---|---|
| ≥1:25000 | Cases | 4 | 24 | 14.3 | 17.4 | (2.0–150.0) | .0044 |
| Controls | 1 | 121 | 0.8 | ||||
| 1:2500 | Cases | 14 | 14 | 50.0 | 6.7 | (3.2–14.1) | <.0001 |
| Controls | 9 | 113 | 7.3 | ||||
| 1:250 | Cases | 9 | 19 | 32.1 | 0.5 | (.3–.9) | .0120 |
| Controls | 72 | 50 | 59.0 | ||||
| 1:25 | Cases | 1 | 27 | 3.6 | 0.1 | (.02–.8) | .0009 |
| Controls | 40 | 82 | 32.8 |
Cases are mothers of microcephaly cases; controls are mothers of children born without microcephaly.
Abbreviations: CI, adjusted Wald confidence interval; PRNT, plaque reduction neutralization test.
aTwo-tailed significance level, calculated using Fisher exact tests. All statistical analyses were done using GraphPad Prism version 5 (GraphPad Software, La Jolla, California).
ZIKV-specific PRNT titers can be higher in secondary than in primary flavivirus infections [8]. The most relevant flavivirus in Brazil is DENV, circulating widely in Salvador since 1995 [9]. Therefore, we sought evidence for exposure to DENV in all of the cases and 59 controls, for which sufficient serum volumes were available (48.4% of all controls). Exposure to DENV was significantly more frequent in controls at 94.9% (56/59) than in cases at 75.0% (21/28; Fisher exact test, P = .011). Moreover, no significant difference was observed between the PRNT titers of DENV-positive vs DENV-negative cases (t test, P = .858). This suggested that prior DENV infection did not cause the higher ZIKV-specific PRNT titers in cases.
The ZIKV-specific PRNT titer from one control termed ZK033 was unusually high at 1:25000 (shown with a star in Figure 1), a titer observed only in cases in our study. Consistent with the classification of the study participant as a control, the neonate of ZK033 was born in February 2016 without signs of microcephaly. However, the neonate presented cardiomyopathy associated with a situs inversus. The 25-year-old study participant recalled having an episode of acute febrile illness with rash, pruritus, myalgia and arthralgia during the first trimester of pregnancy. This may suggest maternal ZIKV infection. Retrospective contact with the study participant revealed that the infant died in June 2016 from severe cardiomyopathy and hydrocephalus. A putative congenital Zika syndrome (CZS) in this infant despite normal head circumference at birth would be compatible with the hydrocephalus and explain the unusually high PRNT titer. Unfortunately, we could not substantiate this hypothesis due to lack of imaging data and clinical follow-up of the neonate.
DISCUSSION
Our data strongly suggest differences in the magnitude of ZIKV-specific maternal antibody titers in cases of congenital ZIKV infection, compared to controls. Our data thus corroborated ZIKV infection as the cause of severe neurological malformation in most cases of microcephaly in this study. However, high PRNT titers can result from other factors than congenital ZIKV infection alone. As an alternative hypothesis, cases may differ from controls in their prior exposure to heterologous flaviviruses, leading to higher PRNT titers [8]. However, the magnitude and homogeneity of the observed titer differences, together with our DENV test results, do not support this hypothesis. Of note, our DENV seroprevalence estimate is consistent with reports showing DENV seroprevalence of around 90% in northeastern Brazil [3], suggesting validity of our results. Hypothetically, cases and controls may have differed in the DENV serotypes they were exposed to prior to ZIKV infection, and distinct DENV serotypes may differentially cross-stimulate ZIKV PRNT titers. However, cases were not older than controls, suggesting that cases may have been exposed to the same DENV epidemics as controls in the past. Finally, high PRNT titers of up to 1:150000 were observed in several individuals during the ZIKV outbreak on the island of Yap in 2007 [8]. Straightforward comparison of PRNT titers between laboratories is impossible due to variation resulting from the choice of cell lines, viral strain, and assay protocol [10]. However, the magnitude of titers observed during the Yap outbreak illustrates that our results should neither be extrapolated to an absolute PRNT threshold suggestive of congenital infection, nor to a diagnostic protocol. Future studies capitalizing on our pilot data would greatly benefit from standardized protocols and reagents to allow comparable test results between laboratories, including antisera of defined reactivity.
We postulate that the high maternal ZIKV antibody titers observed could be compatible with unusually intense ZIKV exposure of the mother, resulting either from transplacental fetomaternal shedding or from placental infection. In case series and infection models of congenital ZIKV infection, ZIKV is consistently detected in fetal tissues, whereas detection in placental tissue is variable [11, 12]. This may suggest predominantly transplacental shedding to the mother. This interpretation is consistent with our data showing higher ZIKV PRNT titers in mothers of microcephaly cases, whereas titers in ZIKV-infected mothers of children without microcephaly were consistently lower. Presence of ZIKV in blood is normally limited to 1–2 weeks after symptom onset [8]. Our interpretation is consistent with the unusually long maternal viremia of up to 14 weeks that has been observed in small case series reporting congenital ZIKV infection [12, 13]. Nevertheless, case-control studies that aim at differentiating cases of ZIKV congenital disease from maternal ZIKV infection without infection of the fetus are needed to provide further evidence for this hypothesis. In light of the potentially prolonged systemic circulation of ZIKV, pregnant women in general and mothers of congenitally infected fetuses in particular may be at a higher risk to develop severe clinical symptoms. Additionally, it would be relevant to investigate whether these women may represent an epidemiologically relevant source of ZIKV for secondary infections.
Our study is limited by lack of routine screening for genetic causes of microcephaly and lack of exhaustive testing for all pathogens capable of causing neonatal malformations. Another limitation that could be addressed in further studies is the determination of individual infection histories with distinct DENV serotypes by PRNT to determine their differential impact on ZIKV-specific PRNT titers and on the pathogenesis of microcephaly [14]. Furthermore, our data on a potential case of CZS developing months after delivery illustrate a risk of incompletely classifying cases and controls when using microcephaly at birth as the main criterion for case designations and the importance of clinical follow-up in studies on CZS.
Geographical and temporal variation of ZIKV infection rates affect estimates of the absolute risk of microcephaly upon maternal ZIKV infection [1]. Additionally, technical limitations of virological diagnostics greatly contribute to the variability of available estimates ranging from 0.03% to 17.1% [15]. Our results suggest that despite the interindividual variability in immune responses, the magnitude of the maternal ZIKV-specific neutralizing antibody response may prove useful to corroborate congenital ZIKV infection, contributing to reliable epidemiological estimates of ZIKV-associated congenital disease. Further studies will be needed to evaluate the time-course of maternal neutralizing antibody responses to identify whether a high maternal PRNT titer can be used as an early marker of congenital infection aiding potential antiviral intervention strategies.
Notes
Financial support. This work was supported by the German Centre for Infection Research through the ZIKApath project, an intramural funding programme at the University of Bonn (BONFOR), and the European Union’s Horizon 2020 research and innovation programme through the ZIKAlliance project (grant agreement number 734548).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. de Oliveira WK, de Franca GVA, Carmo EH, Duncan BB, de Souza Kuchenbecker R, Schmidt MI. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: a surveillance-based analysis. Lancet 2017; 390:861–70. [DOI] [PubMed] [Google Scholar]
- 2. Coyne CB, Lazear HM. Zika virus—reigniting the TORCH. Nat Rev Microbiol 2016; 14:707–15. [DOI] [PubMed] [Google Scholar]
- 3. Braga C, Luna CF, Martelli CM et al. Seroprevalence and risk factors for dengue infection in socio-economically distinct areas of Recife, Brazil. Acta Trop 2010; 113:234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ben-Hur H, Gurevich P, Elhayany A, Avinoach I, Schneider DF, Zusman I. Transport of maternal immunoglobulins through the human placental barrier in normal pregnancy and during inflammation. Int J Mol Med 2005; 16:401–7. [PubMed] [Google Scholar]
- 5. de Araújo TVB, Rodrigues LC, de Alencar Ximenes RA et al. investigators from the Microcephaly Epidemic Research Group; Brazilian Ministry of Health; Pan American Health Organization; Instituto de Medicina Integral Professor Fernando Figueira; State Health Department of Pernambuco Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis 2016; 16:1356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Glezen WP. Effect of maternal antibodies on the infant immune response. Vaccine 2003; 21:3389–92. [DOI] [PubMed] [Google Scholar]
- 7. Rockstroh A, Moges B, Barzon L et al. Specific detection of dengue and Zika virus antibodies using envelope proteins with mutations in the conserved fusion loop. Emerg Microbes Infect 2017; 6: doi:10.1038/emi.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lanciotti RS, Kosoy OL, Laven JJ et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barreto FR, Teixeira MG, Costa Mda C, Carvalho MS, Barreto ML. Spread pattern of the first dengue epidemic in the city of Salvador, Brazil. BMC Public Health 2008; 8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas SJ, Nisalak A, Anderson KB et al. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: how alterations in assay conditions impact performance. Am J Trop Med Hyg 2009; 81:825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nguyen SM, Antony KM, Dudley DM et al. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog 2017; 13:e1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meaney-Delman D, Oduyebo T, Polen KN et al. U.S. Zika Pregnancy Registry Prolonged Viremia Working Group Prolonged detection of Zika virus RNA in pregnant women. Obstet Gynecol 2016; 128:724–30. [DOI] [PubMed] [Google Scholar]
- 13. Suy A, Sulleiro E, Rodó C et al. Prolonged Zika virus viremia during pregnancy. N Engl J Med 2016; 375:2611–3. [DOI] [PubMed] [Google Scholar]
- 14. Castanha PM, Nascimento EJ, Cynthia B et al. Dengue virus (DENV)-specific antibodies enhance Brazilian Zika virus (ZIKV) infection. J Infect Dis 2016; 215:781–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jaenisch T, Rosenberger KD, Brito C, Brady O, Brasil P, Marques ET. Risk of microcephaly after Zika virus infection in Brazil, 2015 to 2016. Bull World Health Organ 2017; 95:191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]