To the Editor—We thank Hueston and colleagues for their comments about our manuscript and for sharing their results [1]. Indeed, antibody-dependent enhancement (ADE) of virus infection is a complex phenomenon. The mechanism is triggered by the attachment of immune complexes to Fcγ receptors, leading to an increased number of virus-infected cells (extrinsic ADE) and/or to the modulation of the antiviral signaling pathway (intrinsic ADE) [2].
In dengue, the relevance of ADE in driving the severe outcomes of the disease have been demonstrated in experimental studies conducted in vitro and in vivo [2, 3]. Additionally, it has been established that secondary infection with a heterologous dengue virus (DENV) serotype is a risk factor for the development of severe disease [4]. Another unique example of ADE in mediating severe outcomes is the fact that infants born to DENV-immune mothers might develop severe dengue during a primary infection when maternally transferred dengue antibodies have waned to below protective levels [5].
In children and adults, the spectrum of the clinical manifestations of Zika virus (ZIKV) infection is normally much less symptomatic when compared to dengue, and there is no equivalent to severe dengue (dengue hemorrhagic fever and dengue shock syndrome). However, ZIKV has the ability to infect embryos and fetuses inside the uterus, causing devastating pathology. The mechanisms underlying this severe outcome of ZIKV infection remain unknown, and several studies have focused on investigating whether ADE might have contributed to the expanded ZIKV pathogenesis [1, 6–8]. Collectively, these studies have confirmed (in vitro and in vivo) that ADE of ZIKV infection by dengue-specific antibodies not only facilitates viral uptake, as demonstrated by our study and by others, but also modifies antiviral mechanisms, resulting in increased ZIKV replication, as interestingly explored by Hueston and colleagues [1].
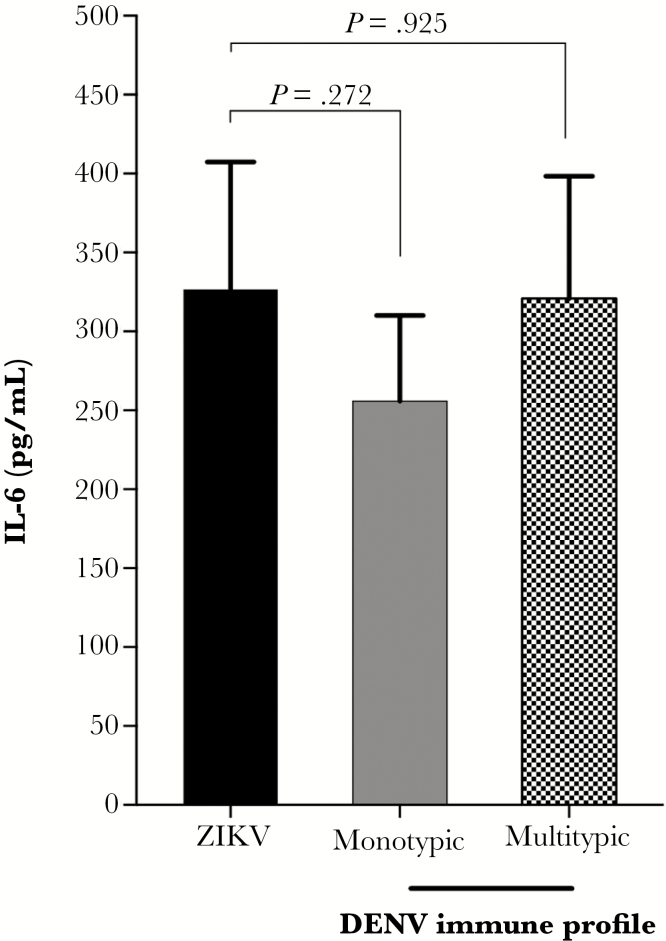
Notably, these studies have used different cell types to explore intrinsic and extrinsic ADE properties. The FcγRII-expressing K562 cell line does not produce type I interferon (IFN) and, thus, is not suitable for studying intrinsic ADE, as correctly pointed out by Hueston and colleagues [1]. Instead, this cell line has been widely used to measure extrinsic ADE properties [2]. We acknowledge that measuring the production of inflammatory mediators—as suggested by Hueston et al—would be very informative. In fact, we observed no differences in interleukin 6 (IL-6) production between K562 cells infected with ZIKV in the absence of antibodies or in the presence of a panel of sera with different dengue immune profile (monotypic and multitypic) (Figure 1). In contrast, Hueston et al demonstrated increased IL-6 levels on human macrophages infected with ZIKV preincubated with dengue-immune sera [1]. These dissimilar findings probably reflect variations on the production of inflammatory mediators among different cell types under ADE conditions [2]. Of note, both experiments were based on a single short time point after infection (24 and 48 hours postinfection for Hueston et al and our experimental system, respectively); thus, a complete time course experiment after infection would probably represent a better picture of K562 cell inflammatory responses. However, investigating intrinsic ADE and expression of different cytokines was beyond the scope of our manuscript.
Figure 1.
Antibody-dependent enhancement of Zika virus (ZIKV) infection by dengue virus (DENV)–specific antibodies and levels of interleukin 6 (IL-6). FcγRII-expressing K562 cells were infected with ZIKV PE/243 in the absence of antibodies or in the presence of a panel of serum samples from pregnant women with different dengue immune status, as determined by plaque reduction neutralization test: monotypic (DENV-3) (n = 10) and multitypic (DENV-3 and DENV-4) (n = 10). Cell culture supernatants were collected 48 hours postinfection, and levels of IL-6 were determined by Citometric bead array (BD CBA Human Th1/Th2/Th17 Cytokine Kit) following the manufacturer’s instructions. Mann-Whitney test was used to determine statistical significance. Statistical analysis was performed using Graph Pad Prism software, version 7.0a.
It is notable that considerable progress has been made in a short amount of time toward understanding the role of ADE of ZIKV infection by dengue antibodies. However, it remains necessary to determine the relevance of the ADE mechanism in the epidemiological context [9, 10], particularly by analyzing how previous dengue immunity affects virus transmission and the development of congenital ZIKV syndrome. Studies addressing this issue will have important implications for ZIKV and DENV vaccine development.
Notes
Financial support. This work was supported by the Brazilian Federal Agency for Support and Evaluation of Graduate Education; Center for Vaccine Research, University of Pittsburgh; Fogarty Training Program (grant number D43TW006592 Pitt GIDRTP/ 323 NIH to P. M. S. C.); National Council for Scientific and Technological Development (grant number 482915/2010–2 MCT/CNPq-321 14/2010); Strategic Program to Support Health Research/PAPES VI (grant number 322 407697/2012–8); and National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant number U19 AI56541).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hueston L, Ramirez R, Mahalingam S. Enhancement of Zika infection by dengue virus-specific antibody is associated with low levels of antiviral factors. J Infect Dis 2017;216:614–6. [DOI] [PubMed] [Google Scholar]
- 2. Halstead SB. Dengue antibody-dependent enhancement: knowns and unknowns. Microbiol Spectr 2014; 2. doi:10.1128/microbiolspec.AID-0022-2014. [DOI] [PubMed] [Google Scholar]
- 3. Ng JK, Zhang SL, Tan HC et al. First experimental in vivo model of enhanced dengue disease severity through maternally acquired heterotypic dengue antibodies. PLoS Pathog 2014; 10:e1004031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol 2013; 158:1445–59. [DOI] [PubMed] [Google Scholar]
- 5. Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg 1988; 38:411–9. [DOI] [PubMed] [Google Scholar]
- 6. Castanha PMS, Nascimento EJM, Braga C et al. Dengue virus-specific antibodies enhance Brazilian Zika virus infection. J Infect Dis 2017; 215:781–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bardina SV, Bunduc P, Tripathi S et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 2017; 356:175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dejnirattisai W, Supasa P, Wongwiwat W et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with Zika virus. Nat Immunol 2016; 17:1102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Halstead SB. Biologic evidence required for Zika disease enhancement by dengue antibodies. Emerg Infect Dis 2017; 23:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahalingam S, Teixeira MM, Halstead SB. Zika enhancement: a reality check. Lancet Infect Dis 2017; 17:686–8. [DOI] [PubMed] [Google Scholar]