Genotypes of Oryza sativa, O. glaberrima and Triticum aestivum were investigated for leaf gas-exchange parameters in relation to anatomical characteristics. Species-dependent relationships provide leads for growing rice like dryland cereals.
Keywords: Drought, leaf anatomy, mesophyll conductance, rice, stomatal conductance, transpiration efficiency, wheat
Abstract
Increasing leaf transpiration efficiency (TE) may provide leads for growing rice like dryland cereals such as wheat (Triticum aestivum). To explore avenues for improving TE in rice, variations in stomatal conductance (gs) and mesophyll conductance (gm) and their anatomical determinants were evaluated in two cultivars from each of lowland, aerobic, and upland groups of Oryza sativa, one cultivar of O. glaberrima, and two cultivars of T. aestivum, under three water regimes. The TE of upland rice, O. glaberrima, and wheat was more responsive to the gm/gs ratio than that of lowland and aerobic rice. Overall, the explanatory power of the particular anatomical trait varied among species. Low stomatal density mostly explained the low gs in drought-tolerant rice, whereas rice genotypes with smaller stomata generally responded more strongly to drought. Compared with rice, wheat had a higher gm, which was associated with thicker mesophyll tissue, mesophyll and chloroplasts more exposed to intercellular spaces, and thinner cell walls. Upland rice, O. glaberrima, and wheat cultivars minimized the decrease in gm under drought by maintaining high ratios of chloroplasts to exposed mesophyll cell walls. Rice TE could be improved by increasing the gm/gs ratio via modifying anatomical traits.
Introduction
Rice (Oryza sativa L.) is a major staple crop that adapts strongly to fully inundated conditions. Compared with other cereal crops, such as wheat (Triticum aestivum L.) grown on dry uplands, rice cultivation requires massive amounts of fresh water for irrigation. To minimize the total water requirement for rice cultivation, several water-saving regimes have already been introduced, such as alternate wetting and drying irrigation (Bouman and Tuong, 2001; Zhang et al., 2008) and controlled soil drying during grain filling (Yang et al., 2003; Yang and Zhang, 2006). However, these regimes are largely management measures to reduce water use for rice production. Solving the problem of the intrinsically high water requirement of rice would need breeding or genetic engineering approaches to develop new drought-tolerant rice genotypes with increased water use efficiency (WUE).
Genetic diversity in drought tolerance has long been explored to develop cultivars of O. sativa for diverse growing conditions: irrigated lowland, rain-fed lowland, and upland environments. More recently, genotypes suitable for aerobic environments (moderately dry conditions, not inundated lowland or dry upland conditions), i.e. aerobic rice (Bouman et al., 2005; Singh et al., 2008), have been developed. However, aerobic rice cannot replace lowland (‘paddy’) rice in most of the rice-growing areas because of its lower grain yield and is an option only for farmers in rain-fed lowlands with limited or erratic rainfall (Atlin et al., 2006). In addition, African rice (Oryza glaberrima Steud.) has evolved as a cultivated species in parallel with O. sativa, and is more drought-resistant (Sarla and Swamy, 2005). Natural interspecific hybrids between O. sativa and O. glaberrima are cultivated in West Africa (Nuijten et al., 2009). The adaptation of rice genotypes to a wider range of edaphic conditions indicates the possibility of growing rice in the same way as other dryland cereal crops such as wheat. Also belonging to the C3-crop type, wheat has a relatively higher WUE than rice (Kemanian et al., 2005; Haefele et al., 2009). Compared with rice, wheat shows higher plasticity in root morphological and anatomical adaptation to water-deficit conditions (Kadam et al., 2015). Further comparative analysis of the physiology and anatomy of rice and wheat in response to drought will facilitate the identification of the general traits and mechanisms required for breeding drought-tolerant yet high-yielding rice varieties (Praba et al., 2009).
Photosynthesis is the key process of primary metabolism, and its capacity can influence plant performance and productivity (Lawlor and Tezara, 2009; Pinheiro and Chaves, 2011). Photosynthesis under drought, despite being affected by Rubisco velocity, is often limited by the CO2 concentration at carboxylation sites (Cc) inside the chloroplast, which is determined by CO2 diffusion components, i.e. stomatal conductance (gs) and mesophyll conductance (gm) (Evans et al., 2009). Stomata regulate CO2 diffusion into, and water diffusion out of, plant leaves (Chaves et al., 2002). Under water-deficit conditions, plants close stomata to prevent major water loss; this, consequently, reduces photosynthesis via decreased influx of CO2 (Pinheiro and Chaves, 2011). In the long-term response to water deficit, gs can be influenced by leaf anatomical traits such as stomatal density and size, which can vary to acclimate to the environment (Xu and Zhou, 2008; Franks and Beerling, 2009). As the inevitable consequence of CO2 entry through leaf stomata is water loss through transpiration, the stomata-related environmental adaptation may also affect plant instantaneous transpiration efficiency (TE), i.e. the ratio of net photosynthesis rate (An) to transpiration rate. In general, higher gs results in a lower TE (Flexas et al., 2008; Gu et al., 2012).
Mesophyll conductance (gm) has been viewed as the diffusion of CO2 from sub-stomatal cavities to the sites of carboxylation in the chloroplasts (Flexas et al., 2008). In contrast to gs, increasing gm increases An at no cost of increased transpiration, because the CO2 diffusion pathway involving gm is not shared with the diffusion pathway of transpired H2O. Therefore, increasing gm not only increases An but also increases TE (Barbour et al., 2010; Flexas et al., 2013). However, long-term drought stress reduces gm (Scartazza et al., 1998; Gu et al., 2012; Han et al., 2016). Along the CO2 diffusion pathway inside leaves, the conductance through the liquid phase (gliq) is the most limiting factor for CO2 diffusion in the mesophyll in many species (Gorton et al., 2003; Flexas et al., 2008; Flexas et al., 2012). Growing evidence indicates that (i) genotypic differences in gm exist within a given species (Evans and Vellen, 1996; Gu et al., 2012; Jahan et al., 2014), and (ii) leaf mesophyll structure and anatomical properties are important determinants of gm. Mesophyll thickness (Tm), surface area of chloroplasts exposed to the intercellular airspace (Sc), and mesophyll cell wall thickness (Tw) are suggested to be the most important structural components determining gm (Evans et al., 1994; Evans et al., 2009; Scafaro et al., 2011; Tosens et al., 2012b; Tomás et al., 2013), as these parameters determine pathways for CO2 permeation into chloroplasts (Terashima et al., 2011). Therefore, investigating relationships between leaf anatomy and photosynthetic features could improve understanding of leaf structural features required to enhance drought tolerance in rice.
In this study, the photosynthetic diffusion components (gs and gm) and possibly related leaf anatomical properties were examined in three species, O. sativa, O. glaberrima and T. aestivum. gs was measured from gas exchange, and combined gas exchange and chlorophyll fluorescence measurements were used to estimate gm. Leaf anatomical properties were examined through light and transmission electron microscopy. The objectives of this study were: (i) to evaluate the variation in photosynthetic capacity among the contrasting types of rice species as well as between rice and wheat in response to a long-term drought, and (ii) to assess the leaf anatomical determinants of gs and gm and how those determinants underpin genotypic differences in the response of gs, gm, and TE under drought.
Materials and methods
Plant materials and treatments
Six cultivars from O. sativa, one cultivar from O. glaberrima, and two cultivars from T. aestivum were used for the study of photosynthetic and leaf anatomical properties. The O. sativa cultivars represented rice types bred for lowland, aerobic or upland cultivation systems, respectively, whereas wheat cultivars were selected based on their drought tolerance (Table 1). The tolerance and susceptibility classifications were based on previous reports (Thanh et al., 1999; George et al., 2002; Sarla and Swamy, 2005; Atlin et al., 2006; Liu et al., 2006; Praba et al., 2009). Seeds of rice and wheat cultivars were obtained from the International Rice Research Institute (IRRI) and from the International Maize and Wheat Improvement Center (CIMMYT), respectively.
Table 1.
Description of rice and wheat cultivars used in the study
| Species | Cultivar | Abbrev. | Cultivar type | Drought tolerance |
|---|---|---|---|---|
| Oryza sativa | IR 64-21 | IR64 | High-yielding lowland cultivar | Susceptible |
| IR 77298-14-1-2::IRGC 117374-1 | II | Lowland cultivar | Susceptible to moderately tolerant | |
| NSIC RC 9 | Apo | High-yielding aerobic cultivar | Moderately tolerant | |
| NSIC RC 192 | 148 | High-yielding aerobic cultivar | Moderately tolerant | |
| UPL Ri7 | UPL7 | Improved upland cultivar | Drought tolerant | |
| Salumpikit | Sal | Traditional upland cultivar | Drought tolerant | |
| Oryza glaberrima | CG14 | CG14 | Cultivated African rice | Drought tolerant |
| Triticum aestivum | SeriM82 | S82 | High-yielding irrigated cultivar | Moderately susceptible |
| Weebill4 | We4 | Dryland-adapted cultivar | Drought tolerant |
Pot experiments were conducted at Yangzhou University, Jiangsu Province, China (32°30′ N, 119°25′ E). Rice seeds were sown in saturated soil after pre-germination, and wheat seeds were sown directly in moist soil. The pots were placed in an open field sheltered from rain by a mobile transparent polyethylene shelter. Each pot (30 cm in height, 25 cm in diameter, 14.72 liters in volume) contained 20 kg of sandy loam soil from the Yangzhou University rice/wheat rotated experimental field. One day before sowing, 1.1 g CO(NH2)2 (urea) and 0.5 g KH2PO4 were pre-mixed through the soil per pot. Extra nitrogen (2.5 g urea for rice and 1.5 g urea for wheat per pot) were split-applied during different plant growth stages.
We imposed three levels of soil moisture, i.e. control (CT), mild drought stress (MD), and more severe drought stress (SD). Across all species and treatments, three replications were maintained and pots were placed in a randomized design. Soil water potential was monitored by inserting a tension meter (Institute of Soil Sciences, Chinese Academy of Sciences, Nanjing, China) at 15 cm soil depth throughout the experiments. Because rice and wheat have different growth duration and are naturally adapted to different moisture environments during domestication histories (Praba et al., 2009), different timings and intensity of stress impositions were applied (Table 2). When tension-meter readings reached the lower limit in each stress level, tap water was added until the upper limit of the target stress was reached. Once stress was imposed, the target stress levels were maintained to ensure that leaves for measurements (see below) were initiated and developed under stress, until all the measurements were completed.
Table 2.
Description of cultivation and water regimes for rice and wheat cultivars
CT, control; MD, mild drought; SD, more severe drought.
| Species | Sowing time | Density (seedlings per pot) |
Soil pH | Initial soil moisture |
Stress imposed time (leaves)a |
Water stress level | Final leaf numberb | ||
|---|---|---|---|---|---|---|---|---|---|
| CT | MD | SD | |||||||
|
O. sativa,
O. glaberrima |
June 2013 | 3 | 5.5–6.0 | Saturated | 5 | Inundated | 0 to −5 kPa | −20 to −40 kPa | 14–15 |
| T. aestivum | November 2013 | 8 | Not adjusted | 0 to −5 kPa | 4 | 0 to −5 kPa | −20 to −40 kPa | −50 to −70 kPa | 10–11 |
a Leaf number on the main stem.
b The final leaf number for the flag leaf on the main stem; this number was 18 for cv. Sal of O. sativa.
Gas exchange and chlorophyll fluorescence measurements
An open gas exchange system integrated with a fluorescence chamber head (Li-Cor 6400XT; Li-Cor Inc., Lincoln, NE, USA) was used to simultaneously measure gas exchange (GE) and chlorophyll fluorescence (CF) parameters. During flowering, the penultimate leaf on the main shoot from each replication per treatment was used for measurements. To avoid the effect of fluctuation in outdoor environment on GE measurement, all measurements were taken in a climate chamber with air temperature at 28 °C (23 °C for wheat), 65% relative humidity and a photosynthetic photon flux density at the leaf surface of 1200 μmol m–2 s–1 (artificial light source). All measurements were made at a leaf temperature of 25 °C and the leaf-to-air vapor pressure difference (VPD) was kept between 1.0 and 1.6 kPa. Light and CO2 response curves were measured under both ambient (21%) and low (2%) O2 conditions. The low O2 condition was created by using a gas mixture of 2% O2 and 98% N2, and the infrared gas analyser calibration was adjusted for O2 composition of the gas mixture according to the manufacturer’s instructions.
For the CO2 response curve, under both O2 conditions, the leaf was consecutively exposed to an incident irradiance (Iinc) of 1000 µmol m−2 s−1 with different levels of CO2: 50, 90, 150, 250, 400, 700, 1000, and 1500 µmol mol−1. For the light response curve, under the 21% O2 condition, the CO2 was kept constant at 400 µmol mol−1 and Iinc was increased in the order of 30, 50, 80, 120, 200, 500, 1000, and 1600 µmol m−2 s−1. Under 2% O2, in order to ensure non-photorespiratory conditions, the CO2 was kept constant at 1000 µmol mol−1 with Iinc levels of 30, 50, 80, 120, and 200 µmol m−2 s−1. The measurement flow rate was 400 μmol s−1. CO2 exchange rates were corrected for CO2 leakage into and out of the leaf cuvette, based on measurements using the same flow rate on boiled leaves across a range of CO2 levels, and intercellular CO2 levels (Ci) were then recalculated (Flexas et al., 2007).
The steady-state fluorescence (Fs) was measured at each light or CO2 step. Then a multiphase flash method (Loriaux et al., 2013) was applied to determine F′m (the maximum fluorescence during the saturating light pulse). The apparent photosystem II electron (e−) transport efficiency for each irradiance or CO2 step was calculated as Ф2=(F′m–Fs)/F′m (Genty et al., 1989).
Estimation of diffusion components
Total conductance to CO2 (gtot) was calculated based on stomatal and mesophyll conductance according to 1/gtot=1/gs+1/gm. For the analysis, stomatal conductance for CO2 (gs) was obtained as stomatal conductance for water vapour divided by 1.6, from the data points measured under ambient condition (400 µmol mol−1 CO2, 1000–1500 µmol m−2 s−1 irradiance, and 25 °C) of the light response curve under 21% O2.
In order to compare gm across species, genotypes, and treatments, the value of gm assumed as constant was estimated using the NRH-A variant method (Yin and Struik, 2009):
| (1) |
where Γ* is the CO2 compensation point in the absence of day respiration (Rd) and J is the linear electron transport rate used for CO2 fixation and photorespiration. Equation (1) provides a model to estimate gm by a curve fitting that minimizes the difference between measured and estimated An (Yin and Struik, 2009). In the model, Γ* is calculated from the Rubisco specificity factor (Sc/o) as Γ*=0.5O/Sc/o, where O is O2 partial pressure (mbar). Sc/o at a given temperature is expected to be constant for a specific species; however, the value at 25°C did not differ much between rice and wheat (Makino et al., 1988; Yin et al., 2009; Gu et al., 2012), so a single value for Sc/o at 25 °C (3.13 mbar µbar−1) was adopted from Makino et al. (1988) and Yin et al. (2009). Data obtained from low Iinc levels of the light response curve and high Ca levels of the CO2 response curve measured at 21% O2 were used for the curve fitting. Model inputs (J and Rd) were estimated as described by Yin et al. (2009). In brief, using data of light-limited range under non-photorespiratory conditions (i.e. the light response curve under 2% O2 with 1000 µmol mol−1 CO2 plus points from >500 µmol mol−1Ca levels of CO2 response curve under 2% O2), a linear regression can be performed for the observed An against IincФ2/4. The day respiration (Rd) is the intercept of the linear regression, and the slope of the regression yields the estimated lumped parameter s (Yin et al., 2009). Then J at each Ci can be calculated using J=sIincФ2.
Light and transmission electron microscopy
Stomatal density and size were determined using the silicon rubber impression technique (Smith et al., 1989). Stomatal features from this method did not differ from those based on glancing sections cut from fresh leaf sample. The method and impression material are described by Giday et al. (2013). Imprints were taken from both adaxial and abaxial surfaces of the area where GE and CF were measured, and were later on smeared with nail polish in the mid-area between the central vein and the leaf edge, for approximately 20 min. The thin film was carefully peeled off the imprint with no stretching and mounted on a glass slide with a drop of water to improve contrast. Stomatal density (D) was determined by counting the maximum numbers of stomata from ten randomly selected non-overlapping rectangular fields of view per leaf under the light microscope (Axio Imager D2, Carl Zeiss, Germany). Stomatal size (S) was calculated by multiplying guard cell length (L) by width (W). All parameters were determined as the average of both sides of the leaf. Specific stomatal conductance (sgs) was determined by dividing gs by D (Ohsumi et al., 2007). An integrative parameter stomatal area index (SAI) was calculated by multiplying density by size and expressed in mm2 stomata mm−2 leaf. Another integrative parameter, so-called maximum stomatal diffusive conductance (gsmax), was also calculated, according to Franks and Beerling (2009):
| (2) |
where d is the diffusivity of water vapour in air, v is the molar volume of air, amax is the maximum area of the open stomatal pore, and l is the stomatal pore depth for fully open stomata. The values for standard gas constants d and v at 25 °C are 2.82 × 10–5 m2 s−1 and 24.5 m3 mol−1, respectively. amax was calculated as π(p/2)2, where p is the stomatal pore length. p was calculated as L/2 according to Franks and Beerling (2009). l was taken as equal to W/2, assuming guard cells inflate to circular cross section.
For the anatomical measurement of mesophyll cells, leaflet samples were collected from the leaves where GE and CF were measured. Half of the leaves were fixed and further used for anatomical study. Tissue fixation and preparation for light and transmission electron microscopy (TEM) studies followed the method of Scafaro et al. (2011) with small modification. Small leaf samples (4 × 1.5 mm2) from each cultivar×water treatment combination were cut parallel to the main vein avoiding large veins. The samples were infiltrated in a solution of 3% glutaraldehyde, 2% paraformaldehyde, and 0.1 M phosphate buffer (pH 7.2) for at least 48 h. Samples were further post-fixed in 1% osmium tetroxide for 2 h, followed by dehydration in an ethanol series (30, 50, 70, 80, 90, 95, and 100%). Ethanol was replaced by 1,2-epoxypropane, and the samples were further embedded with Spurr’s resin (London Resin Company, London, UK) and cured in an oven at 70 °C for 12 h. Transverse sections for light and TEM studies were cut using an ultra-microtome (Ultra-cut R, Leica, Germany). Leaf cross sections 1 µm thick were cut for the light microscopy study, and sections 70 nm thick were cut for the TEM study. Light sections were stained with safranin (1%) for 20 s and followed with methyl purple (1%) for 20 s, then viewed and photographed under a light microscope (Axio Imager D2, Carl Zeiss, Germany). Electron sections were doubly stained with uranyl acetate for 30 min and lead citrate for 15 min, and then photographed under a transmission electron microscope (Tecnai 12, Philips, The Netherlands).
Analyses of images were performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA) (Abràmoff et al., 2004). All mesophyll anatomical characteristics were determined in multiple sections, with 12–30 complete mesophyll cells per replication per treatment depending on the image quality. The analysis protocol followed the method of Evans et al. (1994). Mesophyll thickness (Tm) was measured as the length between the two epidermises of the leaf from light microscopy images. The surface area of mesophyll cells exposed to the intercellular airspace per leaf area (Sm) was calculated from light microscopy images as:
| (3) |
where w is the width of the section measured, Lm is the length of mesophyll exposed to the intercellular airspace, and F is the curvature correction factor (Thain, 1983; Evans et al., 1994). Although the mesophyll size differed between rice and wheat, mesophyll cells in both species are presented as lobed spheroids with a different orientation of the long axes to the veins (Chonan, 1970, 1972; Sage and Sage, 2009), and thus a difference of curvature correction factors between species was not taken into account in this study, and we adopted the value 1.55 from a previous rice study (Scafaro et al., 2011). Uncertainties were also analysed given that Barbour et al. (2016) recently assumed a value of 1.25 for wheat.
The chloroplast surface area exposed to the intercellular airspace per leaf area (Sc) was calculated as:
| (4) |
where L′c is the length of chloroplast exposed to the intercellular airspace and L′m is the corresponding length of mesophyll exposed to the intercellular airspace. L′c and L′m were determined from TEM images based on average values measured for 12–30 mesophyll cells (parameters obtained from TEM are marked by a prime (′) to distinguish them from Lm measured from light microscopy images). The ratio of the exposed surface area of chloroplast to the exposed surface area of mesophyll cell walls (Sc/Sm) was set equal to L′c/L′m (Tosens et al., 2012a,b).
In addition, thickness of the mesophyll cell wall (Tw) was also determined from TEM images. Of the thickness values measured for different sections of each mesophyll cell, only the lower half of the values were averaged as the Tw of that cell to minimize the artefact of inaccurate orientation of ultra-microtome sectioning. The average value of Tw from all mesophyll cells in each cultivar and treatment was used for further analysis.
N content measurements
The other half of the collected leaflets after GE and CF measurements were used to measure the leaf area and then oven dried at 70 °C to constant weight. Leaflets were weighed to determine leaf mass per unit area (LMA), and then ground to fine powder. Samples were analysed for N content by using an elementary analyser (Vario Macro cube, Elementar, Germany). Leaf nitrogen per unit area (Na) was calculated from these data as a measure of leaf physiological status.
Statistical analyses
A one-way analysis of variance (ANOVA) was used to reveal the differences between cultivars in the studied characteristics. A two-way ANOVA was used to study the effect of genotypes, drought treatment, and their interaction on photosynthetic and anatomical parameters. Linear regression analyses were also conducted. These analysis were performed using the R programming language (http://www.R-project.org/). Non-linear fitting for Eq. (1) was carried out using the GAUSS method in PROC NLIN of SAS (SAS Institute Inc., Cary, NC, USA).
Results
Variation in photosynthesis
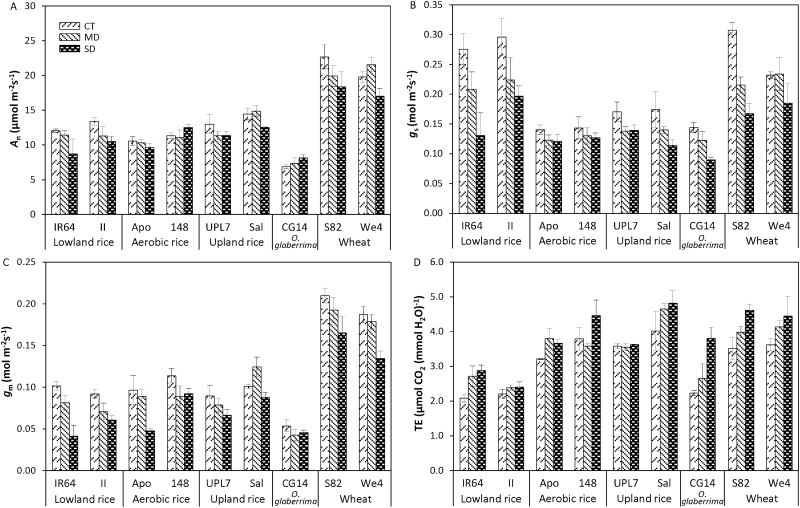
The net photosynthesis rate (An) varied almost threefold among species, with T. aestivum having a significantly higher An than O. sativa and O. glaberrima (cv. CG14) (Fig. 1A). Under the CT condition, An varied between 6.9 µmol m−2 s−1 for CG14 and 22.7 µmol m−2 s−1 for S82. Overall, drought decreased An strongly with no significant interaction between cultivars and water treatments (Supplementary Table S1 at JXB online). However, for CG14, drought increased An (Fig. 1A), and this was probably associated with an increase in Na by drought in this cultivar (Supplementary Fig. S1A). Across all cultivars and treatments, An was positively correlated with Na among wheat cultivars, but not among cultivars of O. sativa (Supplementary Fig. S1B).
Fig. 1.
Photosynthetic parameters obtained and estimated from GE and CF data under three treatments: control (CT), mild drought (MD), and more severe drought (SD). Values of (A) net photosynthetic rate (An), (B) stomatal conductance (gs), and (D) transpiration efficiency (TE) were obtained under the condition of 400 µmol mol−1 CO2, 1000–1500 µmol m−2 s−1 irradiance, and 25 °C. (C) Mesophyll conductance (gm) was calculated based on the NRH-A method (Yin and Struik, 2009). Bars represent standard errors of the mean for An, gs, and TE, and standard error of the estimate for gm.
Variation in gs and gm
Significant variation in stomatal conductance (gs) was observed both among species and within O. sativa (Supplementary Table S1). Notably, lowland rice and wheat cultivars had a much higher gs than other cultivars (Fig. 1B). gs decreased under drought in all cultivars, with mild interaction between cultivars and water treatments (0.05<P<0.1). The decrease in gs was stronger in IR64, Sal, CG14, and S82 than in other cultivars (Fig. 1B). Mesophyll conductance (gm) varied significantly among species, with wheat having a higher gm than O. sativa, and CG14 having the lowest value (Fig. 1C). In wheat, the drought effect on gm was only significant in We4 under SD condition (28% reduction). The differences in gm among O. sativa cultivars increased with an increase in stress level (P>0.05 under CT, P<0.05 under MD, and P<0.01 under SD). Only lowland rice cultivars had significant decreases in gm under the MD condition. Compared with gm in CT, a significant decrease in gm by SD was observed in lowland rice and aerobic rice cv. Apo, but not in other cultivars. The lowest decrease in gm was observed in Sal and CG14 (ca 14%), and the highest decrease was recorded in IR64 (59%).
Influences of gs and gm on An and transpiration efficiency
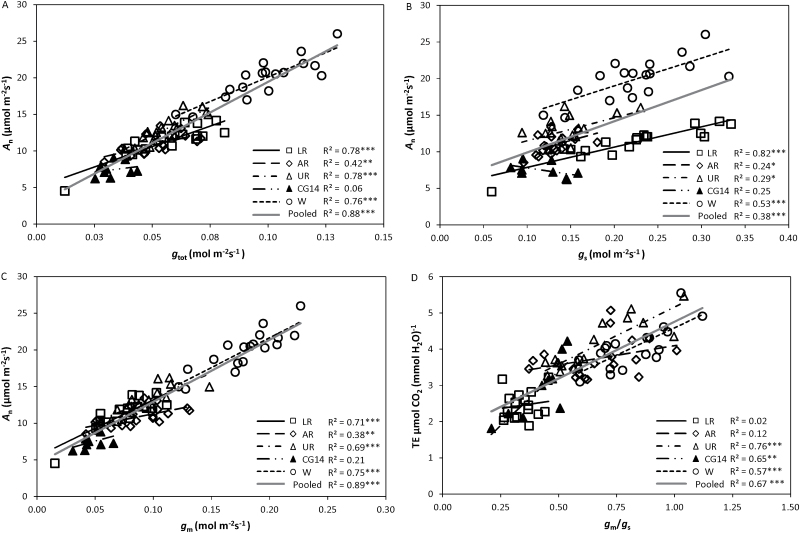
Significant positive correlations between An and total conductance (gtot) were observed among species and within each group (Fig. 2A), indicating the presence of major diffusion limitations to CO2 assimilation. Different individual effects of gs and gm on An were observed (Fig. 2B, C). The correlation between An and gs was highly significant in lowland rice, in the wheat group, and for all data combined (P<0.001), and weaker in aerobic and upland rice (P<0.05) and in O. glaberrima (P>0.05) (Fig. 2B). On the other hand, the correlation between An and gm was highly significant for all data combined as well as in most of the subgroups (Fig. 2C). Multiple regression analysis indicated that gs contributed more to the variation in An in lowland rice cultivars, whereas gm contributed more to the variation in An in aerobic rice, upland rice, and wheat cultivars (Supplementary Table S2).
Fig. 2.
The relationships (A) between photosynthetic rate (An) and total leaf conductance (gtot), (B) between An and stomatal conductance (gs), (C) between An and mesophyll conductance (gm), and (D) between transpiration efficiency (TE) and the ratio of mesophyll conductance to stomatal conductance (gm/gs). An, gs, and TE were obtained under 400 µmol mol−1 CO2, 1000–1500 µmol m−2 s−1 light intensity, and 25 °C. gm was calculated based on the NRH-A method (Yin and Struik, 2009). AR, aerobic rice; CG14, O. glaberrima; LR, lowland rice; UR, upland rice; W, wheat. Linear regressions were fitted for overall data and for each genotype group. The significance of each correlation is shown by asterisks: *P<0.05, **P<0.01, ***P<0.001.
Aerobic rice, upland rice, and wheat cultivars had higher TE than lowland rice cultivars (Fig. 1D). Under the CT condition, TE varied between 2.09 µmol CO2 (mmol H2O)−1 for IR64 and 4.02 µmol CO2 (mmol H2O)−1 for Sal. TE increased under drought, and the highest increase in TE was recorded for CG14 under the SD condition, with a 70% increase over the CT condition. A weak negative correlation between TE and gs was observed within each genotype group (Supplementary Fig. S2A), but the correlation between TE and gm was not significant in most groups (Supplementary Fig. S2B). However, TE was well explained by combined gs and gm (Supplementary Table S3) and was actually highly correlated to the gm/gs ratio (Fig. 2D), especially in upland rice, CG14, and wheat cultivars, although this relationship was less clear in lowland and aerobic rice cultivars. Both the gm/gs and the TE of aerobic rice, upland rice, and wheat cultivars were higher than those of lowland rice cultivars.
Anatomical determinants of gs
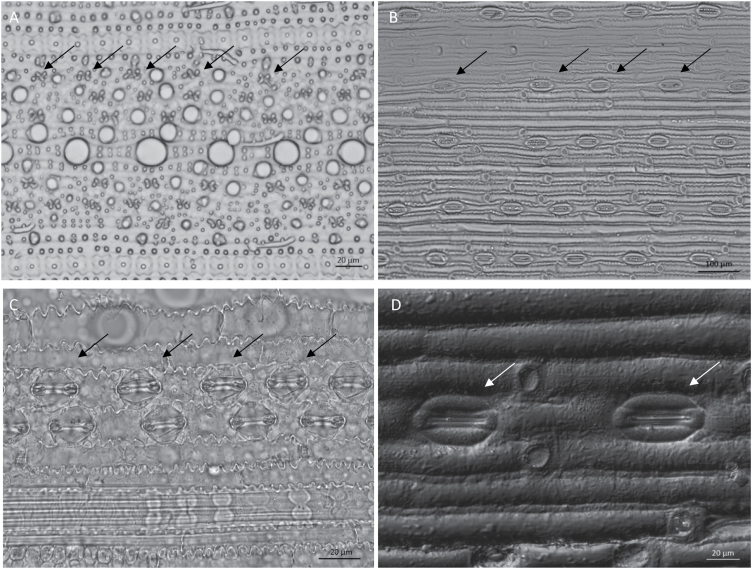
Wheat cultivars had significantly lower density and larger stomata than rice cultivars (Fig. 3 and Table 3). Stomatal density (D) varied significantly among rice cultivars, with lowland rice having higher D than aerobic and upland rice, and CG14 having the lowest value. In rice, D on the adaxial side (Dadaxial) was lower than that on the abaxial side (Dabaxial), and Dadaxial varied significantly among cultivars, whereas less variation was observed for Dabaxial (Table 3). Interestingly, the drought effect was significant only for Dadaxial but not for Dabaxial in both rice and wheat (Supplementary Table S1). Drought increased Dadaxial in most cultivars (Supplementary Fig. S3A).
Fig. 3.
Light micrographs illustrating leaf surfaces of rice (IR64; A, C) and wheat (S82; B, D) used to calculate stomatal density (A, B) and size (C, D). Arrows indicate stomata.
Table 3.
Leaf anatomical properties of O. sativa, O. glaberrima, and T. aestivum under the control (CT) condition
Mesophyll thicknesses (Tm), mesophyll surface area exposed to intercellular airspace (Sm), and stomatal size (S) and density (D) were determined from light microscope images. The ratio of the exposed chloroplast surface area to the exposed surface area of mesophyll cell walls (Sc/Sm) and mesophyll cell wall thickness (Tw) were determined from transmission electron microscope images. Chloroplast surface area exposed to intercellular airspace (Sc) was obtained by multiplying Sm by L′c/L′m. Data are means±standard error of three replicates. The significance of differences between cultivars was based on a one-way analysis of variance. The significance level of overall data/within O. sativa is shown by asterisks: *P<0.05, **P<0.01, ***P<0.001.
| Species | Genotype group |
Cultivar | Mesophyll | Stomata | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T m (µm) | S m (m m−2) | S c/Sm | S c (m m−2) | T w (nm) | S (µm2) | D (No. mm−2) | |||||
| Adaxial | Abaxial | Adaxial | Abaxial | ||||||||
| O. sativa | Lowland rice | IR64 | 70.9 ± 4.2 | 11.2 ± 0.2 | 0.77 ± 0.01 | 8.7 ± 0.3 | 126 ± 1 | 172.1 ± 2.1 | 186.7 ± 2.5 | 621.0 ± 7.6 | 732.7 ± 15.1 |
| II | 91.6 ± 0.6 | 10.8 ± 0.2 | 0.71 ± 0.02 | 7.6 ± 0.1 | 151 ± 2 | 194.7 ± 10.3 | 203.5 ± 4.0 | 547.1 ± 5.8 | 680.9 ± 15.1 | ||
| Aerobic rice | Apo | 73.6 ± 0.9 | 10.6 ± 0.3 | 0.68 ± 0.02 | 7.2 ± 0.4 | 136 ± 3 | 217.8 ± 11.0 | 215.6 ± 11.3 | 460.0 ± 26.1 | 678.3 ± 10.2 | |
| 148 | 70.1 ± 1.5 | 9.7 ± 0.3 | 0.72 ± 0.02 | 7.0 ± 0.2 | 140 ± 4 | 211.3 ± 7.0 | 213.0 ± 6.1 | 509.1 ± 17.9 | 639.4 ± 22.4 | ||
| Upland rice | UPL7 | 82.4 ± 1.7 | 10.9 ± 0.2 | 0.71 ± 0.02 | 7.7 ± 0.2 | 174 ± 2 | 188.7 ± 6.3 | 186.8 ± 5.4 | 475.6 ± 36.4 | 640.8 ± 41.6 | |
| Sal | 79.7 ± 0.7 | 11.5 ± 0.1 | 0.87 ± 0.02 | 10.1 ± 0.3 | 182 ± 1 | 185.3 ± 5.6 | 168.7 ± 4.5 | 467.4 ± 15.2 | 685.4 ± 21.7 | ||
| O. glaberrima | African rice | CG14 | 59.8 ± 1.8 | 11.9 ± 0.4 | 0.62 ± 0.03 | 7.4 ± 0.3 | 161 ± 4 | 183.5 ± 8.1 | 174.9 ± 11.6 | 361.5 ± 34.3 | 498.0 ± 52.7 |
| T. aestivum | Wheat | S82 | 164.7 ± 1.2 | 14.3 ± 1.1 | 0.81 ± 0.03 | 11.6 ± 1.0 | 110 ± 2 | 1592.4 ± 42.9 | 1755.5 ± 46.0 | 67.8 ± 0.6 | 58.7 ± 1.1 |
| We4 | 147.2 ± 2.2 | 14.9 ± 0.9 | 0.87 ± 0.01 | 13.0 ± 0.9 | 116 ± 2 | 1600.2 ± 32.9 | 1692.5 ± 72.7 | 61.7 ± 2.0 | 45.1 ± 1.8 | ||
| P-value | ***/*** | ***/* | ***/*** | ***/*** | ***/*** | ***/* | ***/** | ***/** | ***/ns | ||
Aerobic rice cultivars had clearly larger stomatal size (S) than other rice cultivars (Table 3). This high S resulted more from wider stomatal width (W) than from stomatal length (L) (data not shown). Drought decreased S, and the effect was similar on both leaf surfaces in all cultivars (Supplementary Fig. S3C, D). Cultivars CG14 and S82 had the strongest decrease in S in rice and wheat, respectively.
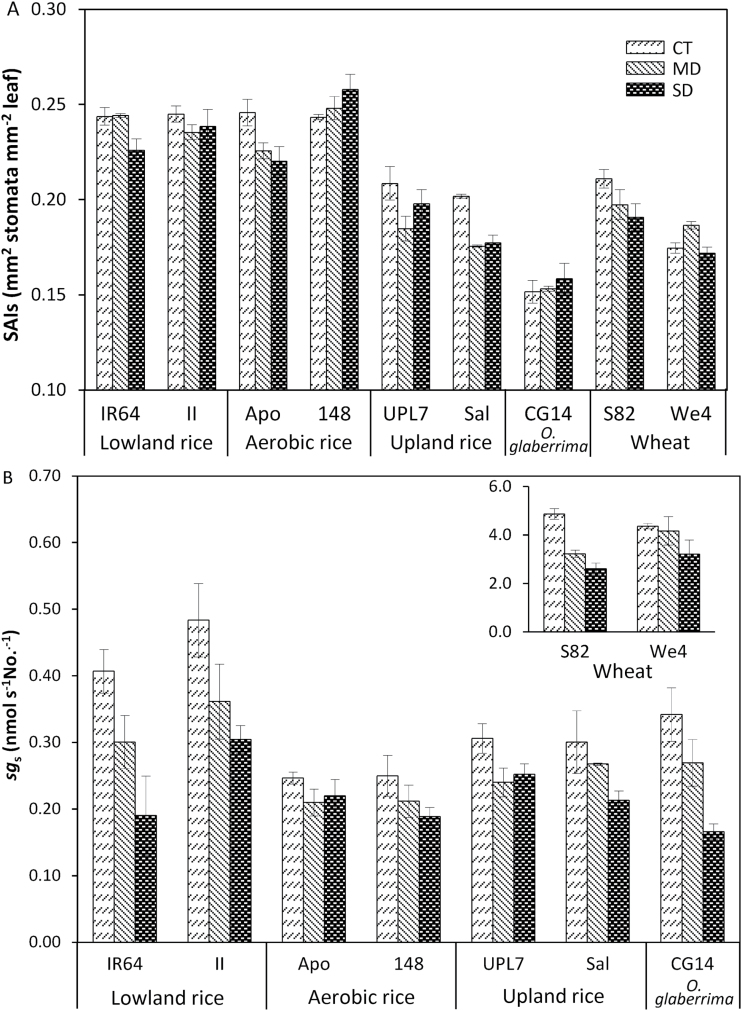
The sum of the stomatal area index on both sides of the leaf (SAIs) differed among the three species, with CG14 having the lowest SAIs and lowland, aerobic rice having higher SAIs than the other cultivars. The variation in SAIs within rice species was more associated with D (R2=0.58) than with S (R2=0.33). SAIs decreased under drought in most cultivars, except in 148, CG14 and We4 (Fig. 4A). Specific stomatal conductance (sgs) was much higher in wheat than in rice cultivars, and higher in lowland rice than in other rice cultivars under the CT condition (Fig. 4B). Drought decreased sgs in all cultivars, with IR64, CG14, and S82 showing the highest decrease among rice and wheat cultivars, respectively.
Fig. 4.
Response of (A) the summed stomatal area index on both sides of the leaf surface (SAIs) and (B) specific stomatal conductance (sgs) of rice and wheat cultivars to water treatments: control (CT), mild drought (MD), and more severe drought (SD).
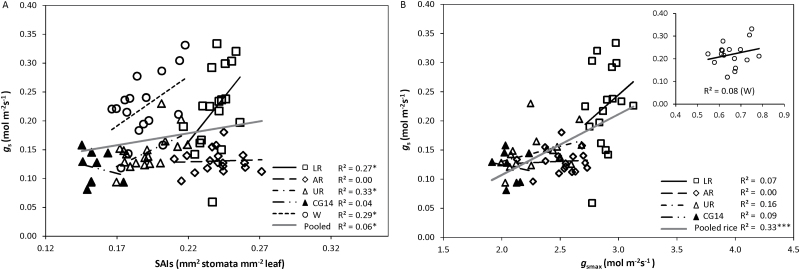
The variation in stomatal properties can partially explain the variation in gs among species and within species. gs was partially correlated with S or D, depending on genotype groups (Supplementary Fig. S4). A significant positive correlation was found between gs and SAIs for lowland and upland O. sativa as well as for wheat genotypes (Fig. 5A). The theoretical maximum stomatal conductance (gsmax) was higher than the measured gs in all cultivars and treatments, and was higher in rice than in wheat (Fig. 5B). The correlation between gs and gsmax was significant only for O. sativa, and not in wheat and each genotype group (Fig. 5B).
Fig. 5.
The relationships (A) between stomatal conductance (gs) and the summed stomatal area index (SAIs), and (B) between gs and the maximum stomatal diffusive conductance (gsmax). Values of gs were obtained and calculated under 400 µmol mol−1 CO2, 1000–1500 µmol m−2 s−1 light intensity, and 25 °C. AR, aerobic rice; CG14, O. glaberrima; LR, lowland rice; UR, upland rice; W, wheat. Linear regressions were fitted for overall data and for each genotype group. The significance of each correlation is shown by asterisks: *P<0.05, **P<0.01, ***P<0.001.
Anatomical determinants of gm
Estimation of the transverse sections of leaves by light microscopy revealed a similar general leaf structure among the three species (Fig. 6 left panels). Significant variations in some anatomical traits were observed among species and among cultivars within the O. sativa species (Table 3). Wheat mesophyll tissue was about twice as thick as that of O. sativa cultivars and almost three times as thick as that of CG14 (Fig. 6). No strong drought effect was observed on Tm (Supplementary Table S1). The variation observed in the surface area of mesophyll cells exposed to the intercellular airspace per leaf area (Sm) was mainly between rice and wheat (Table 3), with wheat having a higher Sm than all rice cultivars even when the curvature factor 1.25 was used for wheat (Supplementary Fig. S5B). Sm was significantly increased in wheat cultivars under drought (P<0.05), whereas no clear trend was observed in rice cultivars (Supplementary Fig. S5B).
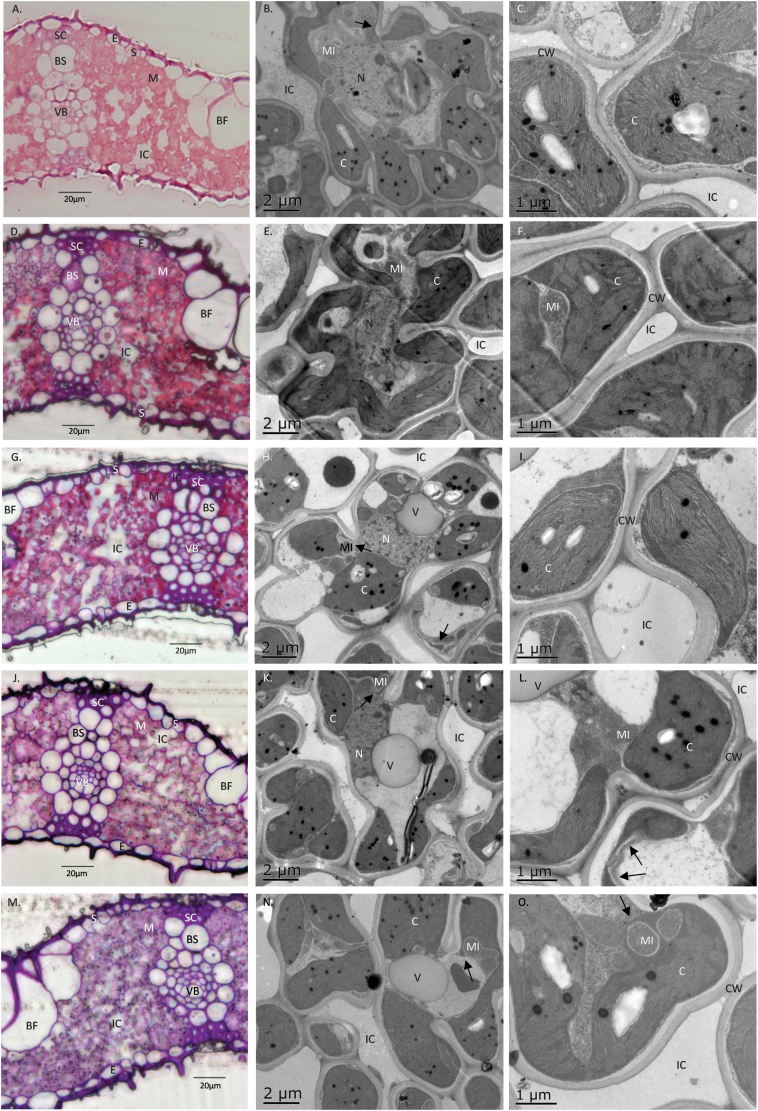
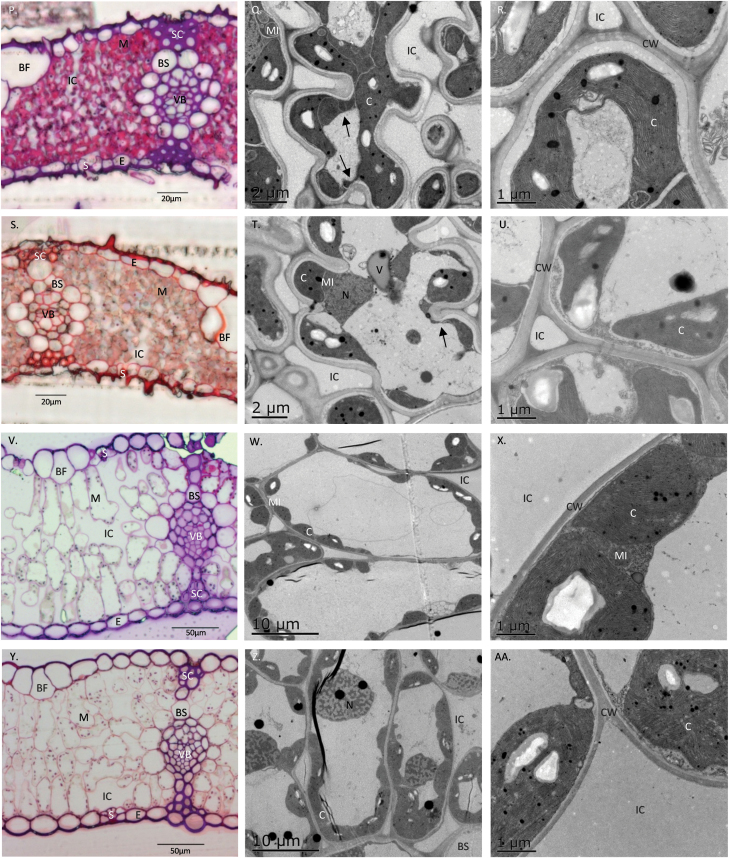
Fig. 6.
Light (left panels) and electron (middle and right panels) micrographs of leaf structure and anatomy for O. sativa cultivars (A–C, IR64; D–F, II; G–I, Apo; J–L, 148; M–O, UPL7; P–R, Sal), O. glaberrima (S–U, CG14), and T. aestivum cultivars (V–X, S82; Y–AA, We4). The left panels show the overall leaf transverse sections; the middle panels show mesophyll cell shape, chloroplast distribution, and lobe development; and the right panels show mesophyll cell walls. Arrows mark stromules. BF, bulliform cell; BS, outer bundle-sheath cell; C, chloroplast; CW, mesophyll cell wall; E, epidermis; IC, intercellular airspace; M, mesophyll cell; MI, mitochondria; N, nucleus; S, stoma; SC, sclerenchyma strand; VB, vascular bundle; V, vacuole.
In both rice and wheat, more than 60% of the exposed mesophyll cell surface area was covered by chloroplasts (Sc) (Table 3). Wheat cultivars and upland rice cultivar Sal had the highest Sc/Sm ratio, and CG14 had the lowest. A significant decrease in Sc/Sm was observed in IR64 and aerobic rice cultivars, whereas an increase was recorded in CG14 under the SD condition (Supplementary Fig. S5C). No strong negative effect on Sc/Sm was observed in upland rice and wheat cultivars under either drought condition (Supplementary Fig. S5C). Sc varied significantly among species and among rice cultivars, with Sal having a higher Sc value than other rice cultivars (Table 3). Wheat cultivars showed a clear increase in Sc under drought, whereas no clear Sc response was observed in rice cultivars (Supplementary Fig. S5D). A thinner mesophyll cell wall was observed in wheat cultivars than in all rice cultivars (Fig. 6), and drought increased Tw, with the most significant effect observed in IR64, Sal and We4 under the SD condition (Supplementary Fig. S5E). Tw was also observed to be the only anatomical parameter to correlate with Na, a parameter related to leaf physiological maturity (Supplementary Fig. S6).
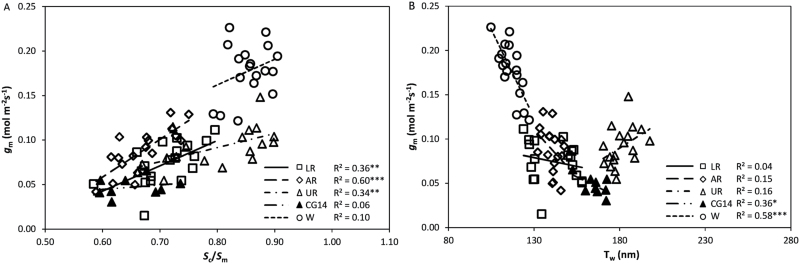
Contributions of leaf anatomical properties to the variation in gm were different in wheat and rice. A higher gm in wheat was associated with a thicker Tm, a higher Sm, a higher Sc and a thinner Tw compared with rice cultivars, and these associations were little altered by using a different curvature factor for calculating Sm in wheat (Supplementary Fig. S7). However, no clear relationship was observed between gm and Tm or between gm and Sm within O. sativa or within most genotype groups (Supplementary Fig. S7A, B). gm was positively correlated with Sc (Supplementary Fig. S7C; R2=0.14, P<0.01) and with Sc/Sm (Fig. 7A; R2=0.33, P<0.001) among rice cultivars. gm and Tw were negatively correlated among wheat cultivars (Fig. 7B), and this correlation only applied among rice cultivars if upland rice was excluded (R2=0.37, P<0.001). These correlations were also confirmed by multiple regression analysis, which indicated that Sc/Sm contributed mostly to the variation in gm within O. sativa, whereas Tw was the main determinant of gm in wheat (Supplementary Table S4).
Fig. 7.
The relationships (A) between mesophyll conductance (gm) and the ratio of the exposed chloroplast surface area to the exposed surface area of mesophyll cell walls (Sc/Sm), and (B) between gm and the thickness of the mesophyll cell wall (Tw). AR, aerobic rice; CG14, O. glaberrima; LR, lowland rice; UR, upland rice; W, wheat. Linear regressions were fitted for each genotype group. The significance of each correlation is shown by asterisks: *P<0.05, **P<0.01, ***P<0.001.
Discussion
Importance of gs and gm in determining An and TE
The photosynthetic capacity of leaves is closely related to their nitrogen content (Evans, 1989). In our study, the large variations in An cannot be explained by the small difference in leaf Na among and within species (Supplementary Fig. S1B). The close correlation between An and total leaf conductance (gtot) among species as well as within each group (Fig. 2A) suggests that the variation in CO2 diffusion limitation could explain the genetic variation in An. Further, An was correlated with CO2 diffusional conductance (Fig. 2B, C), especially with gm, in agreement with previous reports (Flexas et al., 2008; Warren, 2008). It is of note that aerobic rice, upland rice, and wheat showed higher dependences of An on gm than lowland rice (Supplementary Table S2).
TE was influenced mostly by gs within each group (Supplementary Table S3); and this is not surprising given that gs directly controls the transpirational water flow from leaf to the ambient air. However, gm can influence An with no direct impact on transpiration and therefore can influence TE (Barbour et al., 2010; Jahan et al., 2014). For this reason, it has previously been suggested that the gm/gs ratio is a potential target for improving TE under drought in several crops (Flexas et al., 2010; Galmés et al., 2011; Gu et al., 2012; Flexas et al., 2013) and that the ideal plants for arid environment have a high gm/gs ratio (Condon et al., 2004; Giuliani et al., 2013). The observed relationship between TE and gm/gs supports this (Fig. 2D). In the literature, a relationship between an intrinsic TE (An/gs) and gm/gs has often been shown to exclude any influence of VPD on the relationship, especially when data are collected from a wide range of sources (Flexas et al., 2013). Mathematically, this is equivalent to the relationship between An and gm (Fig. 2C). The somewhat more scattered relation in Fig. 2D than in Fig. 2C may reflect the influence of VPD, which varied between 1.0 and 1.6 kPa during our measurements. However, we found no systematic effect of such a small range of VPD on transpiration and TE (results not shown), and therefore we preferred to use TE for our illustration instead of intrinsic TE, which has a different biological unit and is more difficult to interpret in agronomic terms. In our case, a higher gm/gs ratio in aerobic rice, upland rice, and wheat was found to be associated with higher TE, whereas lowland rice cultivars had a low gm/gs ratio and low TE (Fig. 2D). Furthermore, there was a higher slope for the linear relationship in TE versus the gm/gs ratio in upland rice, O. glaberrima, and wheat (Fig. 2D), indicating that the sensitivity of TE in response to the gm/gs ratio was higher in drought-tolerant genotypes than in drought-susceptible genotypes.
Although gm is expected to have little instantaneous impact on transpiration, our data showed that like gs, gm decreased simultaneously under drought in all cultivars (Fig. 1B, C). This indicates that long-term drought during growth affected gm in our experiment, in line with reports for several species (Miyazawa et al., 2008; Flexas et al., 2009), including rice (Gu et al., 2012). When gs and gm had a rather parallel decrease, such as in lowland rice and We4 under the MD and the SD condition, or when gm showed a stronger decrease than gs, such as in aerobic rice and UPL7 under the SD condition, no benefit for TE was observed. Only cultivars with a small decrease in gm combined with a strong decrease in gs under drought showed strong increases in TE (e.g. Sal, O. glaberrima, and S82). Therefore, a strong response of gs combined with a small response of gm would be beneficial for water conservation and increasing TE under long-term drought.
Anatomical determinants of gs
Stomatal conductance (gs) is influenced by the structural traits and the opening of stomata (Xu and Zhou, 2008; Franks and Beerling, 2009; Galmés et al., 2013). In our study, D or S alone could not explain the variation in gs (Supplementary Fig. S4). S and D together determine the total pore area (stomatal area index, SAI) and the maximum stomatal conductance gsmax (Franks and Beerling, 2009; Galmés et al., 2013). Our results confirmed this with marginally significant correlations (P<0.05) when gs was plotted versus SAIs (Fig. 5A) or versus gsmax (Fig. 5B). The variation in SAIs or in gsmax within rice species was more associated with D than with S. Drought-tolerant types of aerobic rice, upland rice, and O. glaberrima had lower D than lowland rice (Table 3), in line with reports that mutants with reduced leaf stomatal density had enhanced the drought tolerance and WUE of plants (Yu et al., 2008; Franks et al., 2015). Furthermore, the difference in D was larger on the adaxial leaf surface than on the abaxial leaf surface, indicating that rice plants adapted to drier environments have developed fewer stomata on the upper surface to avoid direct water loss.
An increase in D under drought has been reported in several species (Martinez et al., 2007; Hamanishi et al., 2012), but this response has not been widely observed in other species (Xu and Zhou, 2008; Galmés et al., 2013). On the other hand, a decrease in S in response to drought was consistent in those studies. In our study, cultivars with a smaller S under drought were accompanied by a greater reduction in gs and sgs (e.g. IR64, Sal, CG14, and S82). This is in line with the idea that smaller stomata are capable of a faster response to drought, compared with larger stomata (Franks and Beerling, 2009).
The stomatal properties examined in our study concerned only the anatomical features. However, biochemical components such as abscisic acid also influence gs, especially under drought (Franks and Farquhar, 2007). Such biochemical components influence the opening of stomata and may be responsible for part of the variability observed in gs.
Anatomical determinants of gm
Leaf mesophyll thickness (Tm) and Sm are the critical structural components that impact upon gm (Evans et al., 1994, 2009; Terashima et al., 2011; Giuliani et al., 2013). In our study, wheat cultivars had a thicker Tm, a higher Sm, and a higher gm than rice cultivars. However, the difference in Tm or Sm was not associated with gm variation within each species (Supplementary Fig. S7A, B). Drought had a stronger effect on Sm in wheat than in rice cultivars. This higher increase in Sm in wheat cultivars might contribute to the smaller decline in gm under drought conditions (Galmés et al., 2013).
It has been suggested that mesophyll cell walls account for more than 25% of total mesophyll resistance (Evans et al., 2009; Terashima et al., 2011; Tholen and Zhu, 2011). Han et al. (2016) reported that the decreasing gm in cotton leaf under drought was associated with increased Tw. Variation in gm between O. sativa and wild rice species was suggested to be attributed to the thicker Tw in wild rice species (Scafaro et al., 2011). In our study, wheat cultivars had a thinner Tw than all rice cultivars. However, Tw varied significantly among rice cultivars and the difference was not associated with observed differences in gm (Fig. 7B). Increased Tw under drought was observed in our study, but this did not lead to a decreased gm, particularly in cultivars Sal and CG14. This suggests that Tw might not be the most dominant limiting factor for gm under drought in rice. On the other hand, it has generally been proposed that thick mesophyll cell walls are beneficial for plant drought tolerance (Scafaro et al., 2011; Giuliani et al., 2013). The thicker Tw found in drought-tolerant cultivars (e.g. upland rice and O. glaberrima) than in drought-sensitive cultivars might support this idea.
S c affects gm in many species (Tholen et al., 2008; Scafaro et al., 2011; Terashima et al., 2011; Tosens et al., 2012a; Tomás et al., 2013). Barbour et al. (2016) showed that the decrease in gm in aged wheat and maize leaves was accompanied by a decline in Sc/Sm. In our study, the difference in gm was explained somewhat more by Sc/Sm (Fig. 7A) than by Sc (Supplementary Fig. S7D), particularly in rice cultivars. In response to drought, gm decreased more in cultivars with clear decreased Sc/Sm (e.g. lowland and aerobic rice) and decreased less in cultivars with a relatively constant Sc/Sm ratio (e.g. upland rice and wheat) across water treatments. The increase in Sc/Sm observed in O. glaberrima (Supplementary Fig. S5C) might be a compensating strategy for the increase in Tw under drought (Tosens et al., 2012a), which resulted in a small decrease in gm. The high correlation between gm and Sc/Sm within rice (Fig. 7A) indicates that Sc/Sm might be very important in determining gm in rice.
It has been suggested that certain biochemical components such as aquaporins and carbonic anhydrase are gm-related (Miyazawa et al., 2008; Flexas et al., 2012). These characteristics were not investigated in our study. They might explain the part of the variability observed in gm that was not explained by anatomical features.
Conclusions
Stomatal conductance and mesophyll conductance explained most of the variability in An among species and within the species O. sativa. The higher gm/gs ratio in aerobic rice, upland rice, and wheat cultivars was associated with higher TE, compared with lowland rice cultivars and O. glaberrima. Our study revealed the genotypic variation in the TE versus gm/gs relationship among species and within the species O. sativa. There was a higher TE sensitivity in response to gm/gs in drought-tolerant groups (O. glaberrima, upland rice, and wheat) than in lowland and aerobic rice. In particular, upland rice, O. glaberrima, and wheat cultivars minimized the decrease in gm; this can simultaneously sustain photosynthesis and increase TE under drought.
Stomatal development was responsible for the variation in gs among contrasting rice types and between rice and wheat. SAIs and gsmax were correlated with the genetic variation of gs among three species. More specifically, stomatal density (one component affecting SAIs and gsmax) was more related to the genetic variation in gs among rice cultivars, suggesting that drought-tolerant rice cultivars might have developed a mechanism of stomatal acclimation to drier edaphic environments. Moreover, cultivars with smaller stomata had a stronger decrease in gs, and thus a higher increase in TE, under drought stress.
Previous studies indicated that thick Tm, high Sm, high Sc, and thin Tw were associated with high gm. These correlations were observed between wheat and rice but not among rice cultivars in our study. More importantly, for rice cultivars, the adverse impact of thick Tw can be neutralized by other anatomical factors such as a high Sc/Sm ratio. In particular, under drought, the maintained or increased Sc/Sm ratio in upland rice, O. glaberrima, and wheat cultivars resulted in a smaller decrease in gm in them than in lowland and aerobic rice cultivars.
In short, our results suggest that rice TE might be improved by modulating stomatal and mesophyll structural traits via breeding selection and/or genetic engineering.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Response of leaf nitrogen per unit area (Na) of rice and wheat cultivars to water treatments, relationship between photosynthetic rate (An) and Na, and relationship between mesophyll conductance (gm) and Na.
Fig. S2. Relationship between transpiration efficiency (TE) and stomatal conductance (gs), and between TE and mesophyll conductance (gm).
Fig. S3. Stomatal density (D) and size (S) from adaxial side and abaxial side of rice and wheat cultivars under three treatments.
Fig. S4. The relationships between stomatal conductance (gs) and stomatal density (D), and between gs and stomatal size (S).
Fig. S5. Mesophyll cell properties of wheat and rice leaves obtained from light and electron microscope images under three treatments.
Fig. S6. Relationships between mesophyll thickness (Tm) and Na, the surface area of mesophyll cells exposed to the intercellular airspaces per leaf area (Sm) and Na, ratio of the exposed surface area of chloroplast to the exposed surface area of mesophyll cell walls (Sc/Sm) and Na, the surface area of chloroplasts exposed to intercellular airspace per leaf area (Sc) and Na, and thickness of the mesophyll cell wall (Tw) and Na.
Fig. S7. The relationship between mesophyll conductance (gm) and mesophyll thickness (Tm), the surface area of mesophyll cells exposed to the intercellular airspaces per leaf area (Sm), and the surface area of chloroplasts exposed to intercellular airspace per leaf area (Sc).
Table S1. A two-way analysis of variance of genotype versus water stress for measured and estimated photosynthetic and anatomical parameters.
Table S2. Multiple regression analysis of light-saturated photosynthesis (An) as a function of gs and gm, based on data of three treatments.
Table S3. Multiple regression analysis of transpiration efficiency (TE) as a function of gs and gm, based on data of three treatments.
Table S4. Multiple regression analysis of mesophyll conductance (gm) as a function of Tw, Sc/Sm and Na, based on combined data of all three water treatments.
Supplementary Material
Acknowledgements
This work is part of the Growing Rice like Wheat research programme supported by an anonymous private donor who provided the financial support, via Wageningen University Fund, for the first author’s PhD fellowship. The experimental work was financed by the College of Agriculture, Yangzhou University, Yangzhou, China. We thank Zhiqing Wang, Qun Zhou and Zhenxiang Zhou for technical assistance during the experiments, Prof. Zhong Wang, Prof. Fei Xiong, Dr Xurun Yu, and Dr Dongliang Li for guidance with microscopy, Dr Junfei Gu for help with GE and CF measurements, and Dr Herman Berghuijs for helpful discussions. Dr G. van der Linden and Dr P. S. Bindraban are acknowledged for their valuable advice.
References
- Abràmoff MD, Magalhães PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics International 11, 36–42. [Google Scholar]
- Atlin G, Lafitte H, Tao D, Laza M, Amante M, Courtois B. 2006. Developing rice cultivars for high-fertility upland systems in the Asian tropics. Field Crops Research 97, 43–52. [Google Scholar]
- Barbour MM, Evans JR, Simonin KA, von Caemmerer S. 2016. Online CO2 and H2O oxygen isotope fractionation allows estimation of mesophyll conductance in C4 plants, and reveals that mesophyll conductance decreases as leaves age in both C4 and C3 plants. New Phytologist 210, 875–889. [DOI] [PubMed] [Google Scholar]
- Barbour MM, Warren CR, Farquhar GD, Forrester G, Brown H. 2010. Variability in mesophyll conductance between barley genotypes, and effects on transpiration efficiency and carbon isotope discrimination. Plant, Cell & Environment 33, 1176–1185. [DOI] [PubMed] [Google Scholar]
- Bouman B, Peng S, Castaneda A, Visperas R. 2005. Yield and water use of irrigated tropical aerobic rice systems. Agricultural Water Management 74, 87–105. [Google Scholar]
- Bouman B, Tuong TP. 2001. Field water management to save water and increase its productivity in irrigated lowland rice. Agricultural Water Management 49, 11–30. [Google Scholar]
- Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CP, Osório ML, Carvalho I, Faria T, Pinheiro C. 2002. How plants cope with water stress in the field. Photosynthesis and growth. Annals of Botany 89, 907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonan N. 1970. Studies on the photosynthetic tissues in the leaves of cereal crops. V. Comparison of the mesophyll structure among seedling leaves of cereal crops. Crop Science Society of Japan 39, 418–425. [Google Scholar]
- Chonan N. 1972. Differences in mesophyll structures between temperate and tropical grasses. Crop Science Society of Japan 41, 414–419. [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. 2004. Breeding for high water-use efficiency. Journal of Experimental Botany 55, 2447–2460. [DOI] [PubMed] [Google Scholar]
- Evans JR. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78, 9–19. [DOI] [PubMed] [Google Scholar]
- Evans JR, Kaldenhoff R, Genty B, Terashima I. 2009. Resistances along the CO2 diffusion pathway inside leaves. Journal of Experimental Botany 60, 2235–2248. [DOI] [PubMed] [Google Scholar]
- Evans JR, Vellen L. 1996Wheat cultivars differ in transpiration efficiency and CO2 diffusion inside their leaves. In: Ishii R, Horie T, eds. Crop research in Asia: achievements and perspective. Fukui, Japan: Asian Crop Science Association, 326–329. [Google Scholar]
- Evans JR, von Caemmerer S, Setchell BA, Hudson GS. 1994. The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Functional Plant Biology 21, 475–495. [Google Scholar]
- Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriquí M, Díaz-Espejo A, Douthe C, Dreyer E, Ferrio JP, Gago J. 2012. Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Science 193, 70–84. [DOI] [PubMed] [Google Scholar]
- Flexas J, Barón M, Bota J, et al. 2009. Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri×V. rupestris). Journal of Experimental Botany 60, 2361–2377. [DOI] [PubMed] [Google Scholar]
- Flexas J, Díaz-Espejo A, Berry JA, Cifre J, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbó M. 2007. Analysis of leakage in IRGA’s leaf chambers of open gas exchange systems: quantification and its effects in photosynthesis parameterization. Journal of Experimental Botany 58, 1533–1543. [DOI] [PubMed] [Google Scholar]
- Flexas J, Galmés J, Gallé A, Gulías J, Pou A, Ribas-Carbó M, Tomàs M, Medrano H. 2010. Improving water use efficiency in grapevines: potential physiological targets for biotechnological improvement. Australian Journal of Grape and Wine Research 16, 106–121. [Google Scholar]
- Flexas J, Niinemets U, Gallé A, et al. 2013. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynthesis Research 117, 45–59. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H. 2008. Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell & Environment 31, 602–621. [DOI] [PubMed] [Google Scholar]
- Franks PJ, Farquhar GD. 2007. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiology 143, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Beerling DJ. 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proceedings of the National Academy of Sciences, USA 106, 10343–10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, W Doheny-Adams T, Britton-Harper ZJ, Gray JE. 2015. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytologist 207, 188–195. [DOI] [PubMed] [Google Scholar]
- Galmés J, Conesa MÀ, Ochogavía JM, Perdomo JA, Francis DM, Ribas-Carbó M, Savé R, Flexas J, Medrano H, Cifre J. 2011. Physiological and morphological adaptations in relation to water use efficiency in Mediterranean accessions of Solanum lycopersicum. Plant, Cell & Environment 34, 245–260. [DOI] [PubMed] [Google Scholar]
- Galmés J, Ochogavía JM, Gago J, Roldán EJ, Cifre J, Conesa MÀ. 2013. Leaf responses to drought stress in Mediterranean accessions of Solanum lycopersicum: anatomical adaptations in relation to gas exchange parameters. Plant, Cell & Environment 36, 920–935. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990, 87–92. [Google Scholar]
- George T, Magbanua R, Garrity DP, Tubana BS, Quiton J. 2002. Rapid yield loss of rice cropped successively in aerobic soil. Agronomy Journal 94, 981–989. [Google Scholar]
- Giday H, Kjaer KH, Fanourakis D, Ottosen CO. 2013. Smaller stomata require less severe leaf drying to close: a case study in Rosa hydrida. Journal of Plant Physiology 170, 1309–1316. [DOI] [PubMed] [Google Scholar]
- Giuliani R, Koteyeva N, Voznesenskaya E, Evans MA, Cousins AB, Edwards GE. 2013. Coordination of leaf photosynthesis, transpiration, and structural traits in rice and wild relatives (genus Oryza). Plant Physiology 162, 1632–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorton HL, Herbert SK, Vogelmann TC. 2003. Photoacoustic analysis indicates that chloroplast movement does not alter liquid-phase CO2 diffusion in leaves of Alocasia brisbanensis. Plant Physiology 132, 1529–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Yin X, Stomph TJ, Wang H, Struik PC. 2012. Physiological basis of genetic variation in leaf photosynthesis among rice (Oryza sativa L.) introgression lines under drought and well-watered conditions. Journal of Experimental Botany 63, 5137–5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefele S, Siopongco J, Boling A, Bouman B, Tuong T. 2009. Transpiration efficiency of rice (Oryza sativa L.). Field Crops Research 111, 1–10. [Google Scholar]
- Hamanishi ET, Thomas BR, Campbell MM. 2012. Drought induces alterations in the stomatal development program in Populus. Journal of Experimental Botany 63, 4959–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JM, Meng HF, Wang SY, Jiang CD, Liu F, Zhang WF, Zhang YL. 2016. Variability of mesophyll conductance and its relationship with water use efficiency in cotton leaves under drought pretreatment. Journal of Plant Physiology 194, 61–71. [DOI] [PubMed] [Google Scholar]
- Jahan E, Amthor JS, Farquhar GD, Trethowan R, Barbour MM. 2014. Variation in mesophyll conductance among Australian wheat genotypes. Functional Plant Biology 41, 568–580. [DOI] [PubMed] [Google Scholar]
- Kadam NN, Yin X, Bindraban PS, Struik PC, Jagadish KS. 2015. Does morphological and anatomical plasticity during the vegetative stage make wheat more tolerant of water deficit stress than rice?Plant Physiology 167, 1389–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemanian AR, Stöckle CO, Huggins DR. 2005. Transpiration-use efficiency of barley. Agricultural and Forest Meteorology 130, 1–11. [Google Scholar]
- Lawlor DW, Tezara W. 2009. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Annals of Botany 103, 561–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liao D, Oane R, Estenor L, Yang X, Li Z, Bennett J. 2006. Genetic variation in the sensitivity of anther dehiscence to drought stress in rice. Field Crops Research 97, 87–100. [Google Scholar]
- Loriaux SD, Avenson TJ, Welles JM, McDermitt DK, Eckles RD, Riensche B, Genty B. 2013. Closing in on maximum yield of chlorophyll fluorescence using a single multiphase flash of sub-saturating intensity. Plant, Cell & Environment 36, 1755–1770. [DOI] [PubMed] [Google Scholar]
- Makino A, Mae T, Ohira K. 1988. Differences between wheat and rice in the enzymic properties of ribulose-1,5-bisphosphate carboxylase/oxygenase and the relationship to photosynthetic gas exchange. Planta 174, 30–38. [DOI] [PubMed] [Google Scholar]
- Martinez J, Silva H, Ledent J, Pinto M. 2007. Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). European Journal of Agronomy 26, 30–38. [Google Scholar]
- Miyazawa S-I, Yoshimura S, Shinzaki Y, Maeshima M, Miyake C. 2008. Deactivation of aquaporins decreases internal conductance to CO2 diffusion in tobacco leaves grown under long-term drought. Functional Plant Biology 35, 553–564. [DOI] [PubMed] [Google Scholar]
- Nuijten E, van Treuren R, Struik PC, Mokuwa A, Okry F, Teeken B, Richards P. 2009. Evidence for the emergence of new rice types of interspecific hybrid origin in West African farmers’ fields. PLoS ONE 4, e7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi A, Kanemura T, Homma K, Horie T, Shiraiwa T. 2007. Genotypic variation of stomatal conductance in relation to stomatal density and length in rice (Oryza sativa L.). Plant Production Science 10, 322–328. [Google Scholar]
- Pinheiro C, Chaves MM. 2011. Photosynthesis and drought: can we make metabolic connections from available data?Journal of Experimental Botany 62, 869–882. [DOI] [PubMed] [Google Scholar]
- Praba ML, Cairns J, Babu R, Lafitte H. 2009. Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. Journal of Agronomy and Crop Science 195, 30–46. [Google Scholar]
- Sage TL, Sage RF. 2009. The functional anatomy of rice leaves: implications for refixation of photorespiratory CO2 and efforts to engineer C4 photosynthesis into rice. Plant & Cell Physiology 50, 756–772. [DOI] [PubMed] [Google Scholar]
- Sarla N, Swamy BM. 2005. Oryza glaberrima: a source for the improvement of Oryza sativa. Current Science Bangalore 89, 955–963. [Google Scholar]
- Scafaro AP, Von Caemmerer S, Evans JR, Atwell BJ. 2011. Temperature response of mesophyll conductance in cultivated and wild Oryza species with contrasting mesophyll cell wall thickness. Plant, Cell & Environment 34, 1999–2008. [DOI] [PubMed] [Google Scholar]
- Scartazza A, Lauteri M, Guido M, Brugnoli E. 1998. Carbon isotope discrimination in leaf and stem sugars, water-use efficiency and mesophyll conductance during different developmental stages in rice subjected to drought. Functional Plant Biology 25, 489–498. [Google Scholar]
- Singh S, Ladha J, Gupta R, Bhushan L, Rao A. 2008. Weed management in aerobic rice systems under varying establishment methods. Crop Protection 27, 660–671. [Google Scholar]
- Smith S, Weyers J, Berry W. 1989. Variation in stomatal characteristics over the lower surface of Commelina communis leaves. Plant, Cell & Environment 12, 653–659. [Google Scholar]
- Terashima I, Hanba YT, Tholen D, Niinemets Ü. 2011. Leaf functional anatomy in relation to photosynthesis. Plant Physiology 155, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thain J. 1983. Curvature correction factors in the measurement of cell surface areas in plant tissues. Journal of Experimental Botany 34, 87–94. [Google Scholar]
- Thanh N, Thanh N, Zheng H, Dong N, Trinh L, Ali M, Nguyen H. 1999. Genetic variation in root morphology and microsatellite DNA loci in upland rice (Oryza sativa L.) from Vietnam. Euphytica 105, 53–62. [Google Scholar]
- Tholen D, Boom C, Noguchi K, Ueda S, Katase T, Terashima I. 2008. The chloroplast avoidance response decreases internal conductance to CO2 diffusion in Arabidopsis thaliana leaves. Plant, Cell & Environment 31, 1688–1700. [DOI] [PubMed] [Google Scholar]
- Tholen D, Zhu XG. 2011. The mechanistic basis of internal conductance: a theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion. Plant Physiology 156, 90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás M, Flexas J, Copolovici L, Galmés J, Hallik L, Medrano H, Ribas-Carbó M, Tosens T, Vislap V, Niinemets Ü. 2013. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. Journal of Experimental Botany 64, 2269–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosens T, Niinemets U, Vislap V, Eichelmann H, Castro Díez P. 2012a Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: how structure constrains function. Plant, Cell & Environment 35, 839–856. [DOI] [PubMed] [Google Scholar]
- Tosens T, Niinemets Ü, Westoby M, Wright IJ. 2012b Anatomical basis of variation in mesophyll resistance in eastern Australian sclerophylls: news of a long and winding path. Journal of Experimental Botany 63, 5105–5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CR. 2008. Stand aside stomata, another actor deserves centre stage: the forgotten role of the internal conductance to CO2 transfer. Journal of Experimental Botany 59, 1475–1487. [DOI] [PubMed] [Google Scholar]
- Xu Z, Zhou G. 2008. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. Journal of Experimental Botany 59, 3317–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang J. 2006. Grain filling of cereals under soil drying. New Phytologist 169, 223–236. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang J, Wang Z, Liu L, Zhu Q. 2003. Postanthesis water deficits enhance grain filling in two-line hybrid rice. Crop Science 43, 2099–2108. [Google Scholar]
- Yin X, Struik PC. 2009. Theoretical reconsiderations when estimating the mesophyll conductance to CO2 diffusion in leaves of C3 plants by analysis of combined gas exchange and chlorophyll fluorescence measurements. Plant, Cell & Environment 32, 1513–1524 (corrigendum 33, 1595). [DOI] [PubMed] [Google Scholar]
- Yin X, Struik PC, Romero P, Harbinson J, Evers JB, Van der Putten PE, Vos J. 2009. Using combined measurements of gas exchange and chlorophyll fluorescence to estimate parameters of a biochemical C3 photosynthesis model: a critical appraisal and a new integrated approach applied to leaves in a wheat (Triticum aestivum) canopy. Plant, Cell & Environment 32, 448–464. [DOI] [PubMed] [Google Scholar]
- Yu H, Chen X, Hong YY, Wang Y, Xu P, Ke SD, Liu HY, Zhu JK, Oliver DJ, Xiang CB. 2008. Activated expression of an Arabidopsis HD-START protein confers drought tolerance with improved root system and reduced stomatal density. The Plant Cell 20, 1134–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhang S, Yang J, Zhang J, Wang Z. 2008. Postanthesis moderate wetting drying improves both quality and quantity of rice yield. Agronomy Journal 100, 726–734. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.