Summary
Human bocavirus messenger RNA detection in nasopharyngeal specimens from children with community-acquired pneumonia (CAP) but not in asymptomatic children undergoing elective outpatient surgery supports the pathogenic role for this virus in CAP and may be a more specific target for diagnostic testing.
Keywords: Human bocavirus, pneumonia, asymptomatic shedding, mRNA, detection
Abstract
Background
The role of human bocavirus (HBoV) in respiratory illness is uncertain. HBoV genomic DNA is frequently detected in both ill and healthy children. We hypothesized that spliced viral capsid messenger RNA (mRNA) produced during active replication might be a better marker for acute infection.
Methods
As part of the Etiology of Pneumonia in the Community (EPIC) study, children aged <18 years who were hospitalized with community-acquired pneumonia (CAP) and children asymptomatic at the time of elective outpatient surgery (controls) were enrolled. Nasopharyngeal/oropharyngeal specimens were tested for HBoV mRNA and genomic DNA by quantitative polymerase chain reaction.
Results
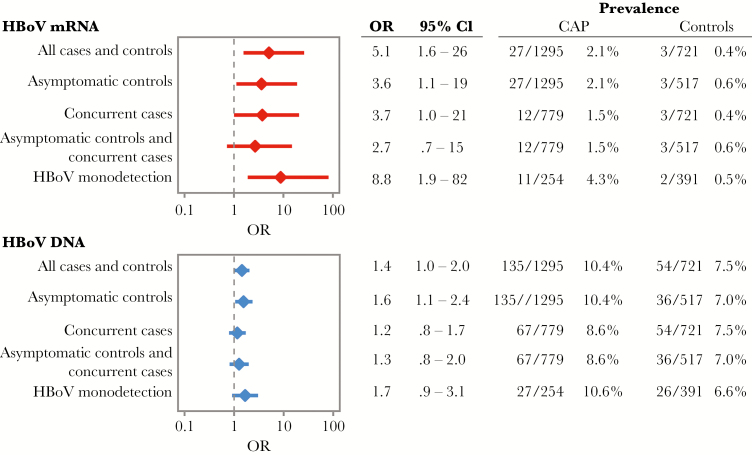
HBoV DNA was detected in 10.4% of 1295 patients with CAP and 7.5% of 721 controls (odds ratio [OR], 1.4 [95% confidence interval {CI}, 1.0–2.0]); HBoV mRNA was detected in 2.1% and 0.4%, respectively (OR, 5.1 [95% CI, 1.6–26]). When adjusted for age, enrollment month, and detection of other respiratory viruses, HBoV mRNA detection (adjusted OR, 7.6 [95% CI, 1.5–38.4]) but not DNA (adjusted OR, 1.2 [95% CI, .6–2.4]) was associated with CAP. Among children with no other pathogens detected, HBoV mRNA (OR, 9.6 [95% CI, 1.9–82]) was strongly associated with CAP.
Conclusions
Detection of HBoV mRNA but not DNA was associated with CAP, supporting a pathogenic role for HBoV in CAP. HBoV mRNA could be a useful target for diagnostic testing.
Human bocavirus (HBoV), a DNA virus in the family Parvoviridae, was first identified in nasopharyngeal aspirates from children with respiratory tract infections (RTIs) [1]. Four genotypes have been recognized. HBoV type 1 has been detected in up to approximately 20% of respiratory tract specimens from children with RTI, while HBoV types 2–4 have been detected in stool and respiratory tract specimens from patients with diarrhea and RTI [2–4]. The diagnosis of HBoV infections has generally relied on amplification of viral genomic DNA from routine respiratory tract specimens [5, 6]. HBoV genomic DNA can be shed (continuously or intermittently) for up to 12 months following acute infections, often at low loads, which complicates epidemiologic studies [6–14]. Published studies have provided conflicting information on a potential association of HBoV with acute pediatric RTI. While several studies reported an association of HBoV detection with acute otitis media and RTI, including community-acquired pneumonia (CAP), especially in young children, others have found similar detection rates in controls [2, 6, 9, 15–18].
Importantly, most studies have not differentiated acute infections from prolonged shedding. More recently, it has been suggested that an increased HBoV load in respiratory specimens, viremia, and seroconversion can be used to differentiate acute infections from prolonged shedding [6, 7, 9, 19–22]. In addition, a recent study in a limited number of children with RTI (n = 133) and asymptomatic controls (n = 28) demonstrated that spliced HBoV capsid messenger RNA (mRNA), as a measure for active viral replication, was more strongly associated with RTI, particularly lower RTI, than detection of HBoV genomic DNA [23]. Similarly, detection of mRNA has been used as a marker for acute infections with other parvoviruses [24–26]. On the basis of these findings, we hypothesized that spliced viral capsid mRNA produced during active replication may be a better marker than HBoV genomic DNA of acute RTI associated with HBoV infection. In this study, we tested our hypothesis by performing polymerase chain reaction (PCR) analysis for both spliced capsid mRNA and genomic DNA on nasopharyngeal (NP) and oropharyngeal (OP) swab specimens from 1295 children hospitalized with CAP and 721 children undergoing elective outpatient surgery who were asymptomatic at enrollment (controls). All participants were enrolled in the Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study, a large multicenter, prospective study of patients hospitalized with CAP [27].
METHODS
Study Population
We included children aged <18 years who were hospitalized with CAP and enrolled in the CDC EPIC study at Primary Children’s Hospital, Salt Lake City (Utah site), and at the Monroe Carell Jr Children’s Hospital, Vanderbilt University, Nashville (one of the Tennessee sites) [27]. The Memphis, Tennessee, site was excluded from this analysis as this site did not enroll controls. Children hospitalized with clinical CAP (ie, acute infection as defined by fever/chills or hypothermia, leukocytosis, or leukopenia, in combination with acute respiratory illness) and radiographic CAP (based on detection of consolidation, other infiltrate, or pleural effusion) were enrolled from January 2010 to June 2012 [27]. Children undergoing elective outpatient surgery were enrolled as controls from February 2011 (at the Utah site) or March 2011 (at the Nashville, Tennessee, site; hereafter the “Tennessee site”) to June 2012. Detailed laboratory and clinical information was collected per the EPIC study protocol (Supplementary Methods) [27]. All patients, controls, or their caregivers were asked to provide informed consent or assent as appropriate. The study protocol was approved by the institutional review board at each institution and the CDC.
Controls were excluded if they had fever or respiratory symptoms ≤14 days before or at enrollment, had received live attenuated influenza vaccine ≤7 days before enrollment, or were undergoing otolaryngologic surgery at the time of enrollment.
Respiratory Pathogen Detection
Viral and bacterial respiratory pathogens were sought per the EPIC study protocol as described previously (Supplementary Methods) [27].
Nucleic Acid Extraction and Stability of Banked NP and OP Specimens
Specimens were refrigerated upon collection and, within a mean of 24 hours after collection, were frozen at −70°C and stored until the time of extraction (February 2010–July 2013 for the Utah site and September 2014 for the Tennessee site). Specimen stability was assessed as described in the Supplementary Methods.
HBoV Spliced Capsid mRNA Detection and Quantification
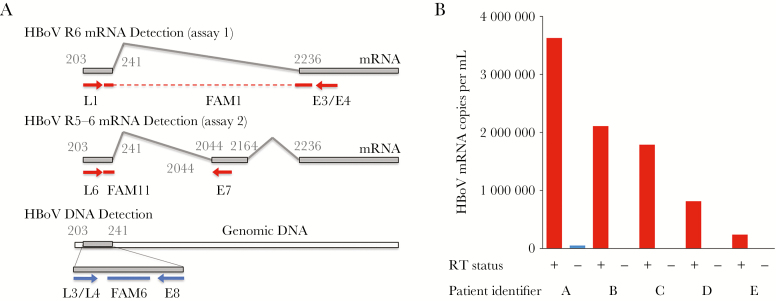
HBoV VP1/VP2 capsid mRNA splice variant R6 (assay 1) [28] of HBoV types 1–4 was amplified using primer HBoV-L1 (0.25 µM), an equimolar mix of primers HBoV-E3 and HBoV-E4 (1 µM each), probe HBoV-FAM1 (0.2 µM; Supplementary Table 1), 5 µL of total nucleic acid, and the QuantiTect Probe reverse transcription (RT)–PCR kit (Qiagen; Figure 1A) [28]. Assay 2 is described in the Supplementary Methods. Analytical sensitivity and quantitative results were calculated using in vitro–transcribed HBoV capsid mRNA as an external standard. To test for cross-amplification of HBoV DNA, the 5 specimens from the Utah site with the greatest HBoV DNA load (range, 5 × 108–3 × 109 copies/mL) were tested for HBoV transcript R6 with and without a reverse transcription step (no amplification is expected without the reverse transcriptase step if genomic DNA is not cross-amplified).
Figure 1.
Detection of human bocavirus (HBoV) DNA and spliced capsid messenger RNA (mRNA). A, Assay design for detection of HBoV genomic DNA, viral capsid mRNA transcript R6 (assay 1), and capsid mRNA transcripts R3–5 (assay 2), modified from material published elsewhere [28]. The capsid mRNA detection assays span 2 different splice sites of alternate capsid mRNAs; nucleotide numbering from NC_007455. B, Five specimens with the highest viral DNA load at the Utah site were tested with (red) and without (blue) reverse transcriptase (RT), using the capsid mRNA detection assay. Only minimal cross-amplification was seen in the 3 specimens with the highest DNA load at levels that were 2.7 × 104–1.6 × 105-fold lower than the DNA loads in the corresponding specimens and 72–1300-fold lower than with the RT step.
HBoV Genomic DNA Detection and Quantification
HBoV genomic DNA was amplified using an equimolar mix of primers HBoV-L3 and HBoV-L4 (0.25 µM each), primer HBoV-E8 (1 µM), and detection probe HBoV-FAM6 (0.2 µM; Supplementary Table 1), 5 µL of total nucleic acid, and Custom Hot Start PCR master mix (Promega) to detect all 4 HBoV types without differentiating them. Quantitative PCR (qPCR) was performed on a 7900HT thermocycler (Applied Biosystems). Melting analysis was performed following amplification. Specimens with a melting peak of 67°C were considered to have positive test results. An external standard curve created using 10-fold serial dilutions of plasmids pHBoV1 was used to determine analytical sensitivity and quantify HBoV genomic DNA copies.
Statistical Analyses
Descriptive statistics were used to characterize the study cohort. Categorical variables were compared using the χ2 test or the Fisher exact test, as appropriate. Normally distributed continuous variables were compared using the t test, and nonnormally distributed continuous variables were compared using the Wilcoxon-Mann-Whitney U test. For bivariate comparisons, odds ratios (ORs) and exact 95% confidence intervals (CIs) were calculated. Adjusted ORs (aORs) were derived from multivariable stepwise logistic regression models, using a P value of .05 as an entry and exit criterion.
Stratified analyses were conducted among patients who were positive for spliced capsid HBoV mRNA, positive for HBoV DNA and negative for spliced capsid mRNA, and negative for HBoV, using 1-way analysis of variance. In our primary and stratified analyses, we included all enrolled controls.
To assess for potential biases, we performed a number of sensitivity analyses: (1) an analysis limited to controls who did not develop fever or respiratory symptoms in the 14 days after enrollment (to exclude individuals with incubating infection; Supplementary Methods), (2) an analysis limited to patients enrolled between February/March 2011 and June 2012 (concurrently with all controls, to address seasonal differences), (3) an analysis limited to controls with no subsequent symptoms after enrollment and to season-matched patients, and (4) an analysis limited to controls with no subsequent symptoms and to season-matched patients with no other respiratory pathogens detected per the EPIC study protocol (ie, those with HBoV monodetection).
All statistical comparisons were performed in a 2-sided fashion with an α of 0.05. The analyses were conducted using Stata 14.1 (StataCorp, College Station, TX) and R 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Population
Specimens were available from 745 children with CAP and 330 controls from Utah and from 550 children with CAP and 391 controls from Tennessee. Enrollment statistics and demographic information for children with CAP and controls are shown in Table 1. Patients and controls in the 2 sites were generally similar (Supplementary Table 2). Controls were older than patients (median age, 4 years [interquartile range {IQR}, 1–9 years] vs 2 years [IQR, 1–6 years]; P < .001) and were more likely to be male (67% vs 54%; P < .001). Overall, 32% of children with CAP and 12% of controls had underlying medical conditions (P < .001).
Table 1.
Demographic, Clinical, and Human Bocavirus (HBoV) Epidemiologic Data for Children With Community-Acquire Pneumonia (CAP) and Controls Enrolled at Utah and Tennessee Sites of the Etiology of Pneumonia in the Community Study
| Characteristic | CAP Group (n = 1295) |
Control Group (n = 721) |
P |
|---|---|---|---|
| Age, y | 2 (1–6) | 4 (1–9) | <.001 |
| Female sex | 599 (46) | 238 (33) | <.001 |
| Race | |||
| Non-Hispanic white | 677 (52) | 583 (81) | <.001 |
| Non-Hispanic black | 157 (12) | 93 (13) | .61 |
| Hispanic | 326 (25) | 84 (12) | <.001 |
| Underlying conditiona | 418 (32) | 77 (11) | <.001 |
| Time from symptom onset to specimen collection, d | 5 (3–8) | NA | NA |
| Symptoms | |||
| Fever | 1189 (92) | NA | NA |
| Cough | 1210 (93) | NA | NA |
| Dyspnea | 887 (68) | NA | NA |
| Wheezing | 693 (54) | NA | NA |
| Abdominal pain | 302 (23) | NA | NA |
| Diarrhea | 396 (31) | NA | NA |
| Pathogen detection | |||
| Virus | 925 (71) | 185 (26) | <.001 |
| Bacteria | 208 (16) | 2 (0) | <.001 |
| Codetection | |||
| Virus or bacteria | 326 (25) | 24 (3) | <.001 |
| Virus-virus | 255 (20) | 24 (3) | <.001 |
| Virus-bacteria | 92 (7) | 0 (0) | <.001 |
| Bacteria-bacteria | 9 (1) | 0 (0) | .03 |
| HBoV DNA prevalence | 135 (10) | 54 (8) | .03 |
| HBoV capsid mRNA prevalence | 27 (2) | 3 (0.4) | .002 |
Data are median value (interquartile range) or no. (%) of participants.
Abbreviations: mRNA, messenger RNA; NA, not applicable.
aIncluded asthma or reactive airway disease, chromosomal disorders such as Down syndrome, chronic kidney disease, chronic liver disease, congenital heart disease, diabetes mellitus, immunosuppression (due to a chronic condition or long-term use of medication, cancer [excluding skin cancer], or human immunodeficiency virus infection with a CD4+ T-cell count of >200 cells/mm3), neurologic disorder (including seizure disorder, cerebral palsy, and scoliosis), preterm birth (defined as a gestational age of <37 weeks at birth for children <2 years of age at the time of hospitalization), and splenectomy. Additional details regarding the prevalence of specific conditions are provide elsewhere [27].
Stability of Banked NP and OP Specimens and Analytical Performance of HBoV qPCR Assays
Retesting of stored RNA (maximum storage duration, 41 months) from 21 specimens positive for respiratory syncytial virus demonstrated specimen stability (Supplementary Results and Supplementary Table 3).
HBoV genomic DNA was reproducibly detected at 10 copies per reaction (HBoV types 1 and 2) and 100 copies per reaction (HBoV types 3 and 4), whereas HBoV capsid mRNA was reproducibly detected at 100 copies per reaction (R6 and R3–5 capsid mRNA, types 1–4). Cross-amplification studies identified low-level amplification with the 3 specimens with the greatest HBoV DNA load (1.7 × 109, 2.8 × 109, and 3.5 × 109 copies/mL) but not with the 2 specimens with the next-greatest HBoV DNA load (Figure 1B). Cross-amplification resulted in false-positive capsid mRNA levels that were 72–1300-fold lower than true R6 transcript copy numbers (with reverse transcriptase). This minimal cross-amplification of genomic DNA in the 3 specimens with the greatest HBoV DNA load is unlikely to have significantly contributed to capsid mRNA detection.
Seasonality of HBoV Detection
In children with CAP, there were several peaks of HBoV capsid mRNA detection (March–July 2010, October–December 2010, and May–August 2011; Supplementary Figure 1). HBoV DNA was detected year-round, with peaks that matched those observed for capsid mRNA (Supplementary Figure 1C).
Detection of HBoV Capsid mRNA and Association With CAP
Overall, HBoV capsid mRNA was detected in 27 of 1295 children with CAP (2.1%) and 3 of 721 controls (0.4%; OR, 5.1 [exact 95% CI, 1.6–26] for the association between HBoV capsid mRNA detection and CAP; Figure 2). Overall HBoV prevalence was lower at the Tennessee site (1.3% in patients and 0.3% in controls) than at the Utah site (2.7% in patients and 0.6% in controls; Supplementary Figure 2). In the sensitivity analyses, the associations between HBoV and CAP were not significantly different than for the entire cohort (Figure 2). The association between HBoV capsid mRNA detection and CAP was strongest when limited to participants with no other pathogens detected (4.3% of children with CAP and 0.5% of controls; OR, 8.8 [95% CI, 1.9–82]). After stratification by enrollment site, HBoV monodetection was significantly associated with CAP at the Utah site (OR, 11.4 [95% CI, 1.5–509]) but did not reach statistical significance for the Tennessee site (OR, 5.3 [95% CI, .4–277]; P = .14, by the Fisher exact test), likely because of the small numbers of HBoV capsid mRNA detections (3 of 133 patients and 1 of 229 controls; Supplementary Figure 2) in Tennessee.
Figure 2.
Sensitivity analysis of association of human bocavirus (HBoV) DNA and capsid messenger RNA (mRNA) detection with community-acquired pneumonia (CAP) in children enrolled at Primary Children’s Hospital (Utah) and Monroe Carell Jr Children’s Hospital at Vanderbilt (Tennessee). Monodetection denotes that no other respiratory pathogens were detected, per Etiology of Pneumonia in the Community Study results [27]. Asymptomatic and concurrent analysis is limited to asymptomatic controls and patients enrolled after January 2011 for Utah patients and after February 2011 for Tennessee patients. Concurrent analysis is limited to patients enrolled after January 2011. Asymptomatic analysis is limited to controls confirmed to remain symptom free for 14 days. Numbers indicate the size of each group, given the different stratifications. CI, confidence interval; OR, odds ratio.
In multivariable logistic regression with adjustment for age, enrollment month, and detection of other respiratory viruses, detection of HBoV capsid mRNA remained strongly associated with CAP (aOR, 7.6 [95% CI, 1.5–38.4]). In the subanalyses by site, aORs for the Utah and Tennessee sites were similar despite the lower prevalence at the Tennessee site (Supplementary Figure 2).
Detection of HBoV Genomic DNA and Association With CAP
Overall, 135 of 1295 children with CAP (10.4%) and 54 of 721 controls (7.5%) were positive for HBoV DNA (OR, 1.4 [95% CI, 1.0–2.0]; Figure 2). HBoV DNA prevalence was lower at the Tennessee site (6.4% in patients and 6.4% in controls) than at the Utah site (13.4% in patients and 8.8% in controls; Supplementary Figure 2). In the sensitivity analyses, the associations between HBoV and CAP were not significantly different than for the entire cohort (Figure 2). After adjustment for age, enrollment month, and detection of other respiratory viruses, detection of HBoV genomic DNA was not significantly associated with CAP (aOR, 1.2 [95% CI, .6–2.4]). Site-specific results are shown Supplementary Figure 2.
Quantification of HBoV Genomic DNA and Capsid mRNA
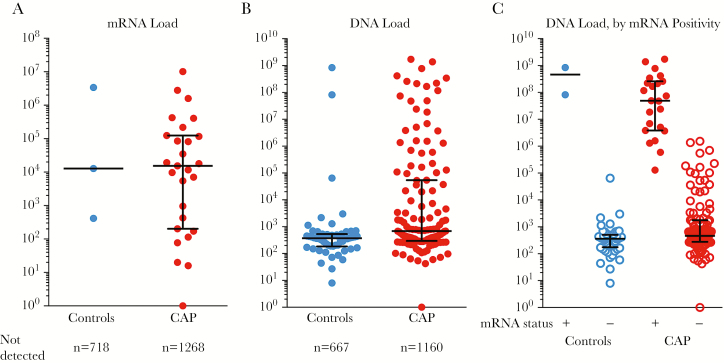
Among children with detectable capsid mRNA, the median mRNA load was 1.5 × 104 copies/mL in children with CAP and 1.3 × 104 copies/mL in controls (P = .74, by the Mann-Whitney test; Figure 3A). Among children with detectable HBoV genomic DNA, median viral loads were higher in those with CAP (6.8 × 102 copies/mL [range, 1 × 100–1.7 × 109 copies/mL]) than in controls (3.7 × 102 copies/mL [range, 8 × 100–8.4 × 108 copies/mL]; P < .001). In addition, the proportion of children with high HBoV DNA loads (>105 copies/mL) was greater in children with CAP than in controls (30 of 1295 [2.3%] vs 2 of 721 [0.3%]; OR, 6.1 [95% CI, 1.5–25.7]; Figure 3B). When stratified by capsid mRNA status, median HBoV DNA loads were 5–6 orders of magnitude higher for capsid mRNA–positive children with CAP (7.4 × 107 copies/mL) and controls (4.6 × 108 copies/mL) than for capsid mRNA–negative children with CAP (4.8 × 102 copies/mL) and controls (3.7 × 102 copies/mL; Figure 3C).
Figure 3.
Human bocavirus (HBoV) capsid messenger RNA (mRNA) and DNA load in nasopharyngeal and oropharyngeal swab specimens from children with community-acquired pneumonia (CAP) and controls. A, At approximately 4 × 104 copies/mL, the median HBoV capsid mRNA load in children with CAP was similar to that in controls. B, Median DNA load was approximately 2-fold higher in children with CAP (622 copies/mL) than in controls (375 copies/mL), but this difference was not statistically significant. However, a subset of children with CAP, as well as 2 controls, had HBoV DNA loads of 1 × 106–1 × 109 copies/mL. C, When stratifying HBoV DNA loads by HBoV capsid mRNA positivity, median HBoV DNA loads were 100000–1000000-fold higher in capsid mRNA–positive children with CAP (7.4 × 107 copies/mL) and controls (4.6 × 108 copies/mL) than in capsid mRNA–negative children with CAP (4.8 × 102 copies/mL) and controls (3.7 × 102 copies/mL). Conversely, HBoV DNA loads were similar in capsid mRNA– negative patients and controls and capsid mRNA–positive patients and controls. Children with no HBoV nucleic acid detected are not shown.
Clinical and Laboratory Features of HBoV Capsid mRNA–Positive Children With CAP
We compared children with CAP who were HBoV capsid mRNA positive to those who were HBoV DNA positive but capsid mRNA negative and to those without evidence of HBoV (neither capsid mRNA nor DNA positive; Table 2). Capsid mRNA–positive children with CAP were younger (median, 1.3 years [IQR, 0.8–1.5]) than HBoV DNA–positive children with CAP (median, 1.9 years [IQR, 0.9–5.9 years]; P = .01) or HBoV-negative patients (median, 4 years [IQR, 1–9 years]; P < .01). Capsid mRNA–positive children with CAP were more likely to have underlying conditions and less likely to have other respiratory pathogens codetected (Table 2).
Table 2.
Demographic and Clinical Data for Children With Community-Acquired Pneumonia, Stratified by Human Bocavirus Capsid Messenger RNA (mRNA) and DNA Detection Patterns
| Characteristic | mRNA Positive (n = 27) |
DNA Positive, mRNA Negative (n = 112) |
HBoV Negative (n = 1157) |
|---|---|---|---|
| Age, y | 1.3 (0.8–1.5) | 2.0 (1.0–5.8) | 2.6 (1.0–6.8) |
| Female sex | 11 (41) | 56 (50) | 532 (46) |
| Underlying conditiona | 11 (41) | 29 (26) | 371 (32) |
| Symptom onset to specimen collection, d | 4 (3–5) | 5 (3–8) | 5 (3–8) |
| Pathogen codetectionb | |||
| Virus | 14 (52) | 84 (75) | 827 (71) |
| Bacteria | 3 (11) | 23 (21) | 183 (16) |
Data are median value (interquartile range) or no. (%) of participants.
aSee Table 1 and for definition and additional information [27].
bNumbers only reflect detection of viruses and bacteria per the EPIC study protocol and exclude HBoV.
As HBoV infections are more prevalent in young children, we limited the analysis to children aged <5 years (n = 1279; 885 children with CAP and 394 controls). The association between HBoV and CAP was greater for detection of capsid mRNA (OR, 5.5 [95% CI, 1.3–48]) than for DNA (OR, 1.6 [95% CI, 1.0–2.5]). This difference was greater when limiting the analysis to HBoV monodetections (capsid mRNA: OR, 15.9 [95% CI, 2.2–692]; DNA: OR, 2.5 [95% CI, 1.0–6.1]).
DISCUSSION
We showed that detection of HBoV capsid mRNA was strongly associated with CAP (aOR, 7.6 [95% CI, 1.5–38.4]) in 2 geographically distinct cohorts of children hospitalized with CAP, compared with that in asymptomatic children undergoing elective outpatient surgery. This association was strongest when HBoV but no other respiratory pathogen was detected (OR, 9.6 [95% CI, 2.2–42.7]), especially in young children, although comparison of ORs is complicated by wide 95% CIs in these sensitivity analyses. Even though HBoV prevalence at the Tennessee site was approximately 3-fold lower than at the Utah site, the strength of the association between HBoV and CAP was similar at both sites and consistent across a number of sensitivity analyses. In contrast, detection of HBoV genomic DNA was not significantly associated with CAP after adjustment for age, season, and codetection of other pathogens. While high HBoV DNA load was associated with CAP, there was extensive overlap in viral loads of patients and controls. In contrast, HBoV DNA loads were consistently higher in capsid mRNA–positive children than in capsid mRNA–negative children. The presence of HBoV capsid mRNA, as a sign of active viral replication, may be a better diagnostic target than HBoV DNA for the diagnosis of active infection.
Since its identification a decade ago, the etiologic role of HBoV in acute RTI in children has been controversial. Numerous patients with severe or fatal pneumonia have been described in whom HBoV was the sole pathogen detected [29–35]. However, frequent detection in asymptomatic children and common codetection with other respiratory viruses have led to questions regarding its pathogenic role [6, 9, 36–38]. Studies linking HBoV with respiratory disease have been criticized for relying on cross-sectional designs, convenience samples, and inadequate control groups and for failing to confidently exclude alternate pathogens as the cause of symptoms [9, 36]. Potential confounders include incomplete matching on age, sampling method and time of year/season, different protocols for testing of other respiratory pathogens, and selection of controls with increased risk for HBoV infection [6, 9, 10, 39, 40]. Much of the controversy is related to difficulties in identifying acute HBoV infections and excluding other potential etiologies. Because HBoV can be shed for up to 12 months and can be reactivated upon infection with other pathogens, detection of genomic HBoV DNA alone is not likely to be suitable to identify acute and clinically relevant infections [6–8, 10, 14, 16, 29, 41]. In this context, in this study the weak association of HBoV DNA in the unadjusted analysis with CAP (OR, 1.4 [95% CI, 1.0–2.0]), which was further attenuated in the adjusted analysis (aOR, 1.2 [95% CI, .6–2.4]), is consistent with the prior literature and demonstrates the limitations of viral DNA as target.
Detection of viremia, seroconversion, and high viral loads have been proposed as alternate approaches to assessing the impact of HBoV detection; each method has limitations. Viremia has been detected during acute infections in some studies but has not been widely studied [38]. Seroconversion, defined as immunoglobulin M positivity, an increase in immunoglobulin G (IgG) titer, or an increase in IgG avidity, can identify acute infections but requires serial collection of blood specimens, limiting its clinical utility [9, 42, 43]. High HBoV loads in upper respiratory tract specimens have been associated with acute infections or more-severe disease, and thresholds have been proposed (eg, 104 copies/mL) [9, 19, 20, 36, 38, 44]. However, viral loads in patients are highly variable and overlap significantly with those in controls, limiting the usefulness of this approach [2, 16, 19, 21]. In addition, defining thresholds is difficult, even in homogenous specimens, such as plasma or serum, as viral loads differ between test methods, requiring complex standardization [45, 46]. It is not surprising that viral load determination has led to inconsistent results in nonhomogenous specimens, such as respiratory secretions [47].
A recent longitudinal study by Martin et al demonstrated that incident infections, defined as infections involving primary shedding of HBoV DNA, are associated with respiratory symptoms in infants, suggesting that HBoV is a true pathogen [14]. In a small study, Christensen et al detected HBoV capsid mRNA in the respiratory tract of 33 of 133 HBoV DNA–positive children with RTI but 0 of 28 HBoV DNA–positive asymptomatic children, suggesting capsid mRNA may be a marker of acute symptomatic infection [23]. Serial testing of a single patient demonstrated that viral capsid mRNA was detectable only during acute infection (ie, within 10 days of onset of symptoms), while HBoV genomic DNA persisted for an additional period of approximately 6 weeks. Studies with other parvoviruses (ie, human parvovirus B19 and canine parvovirus type 2) also indicated that capsid mRNA detection might be a marker for incident infections [24–26]. Our results support this notion, demonstrating a strong association of HBoV capsid mRNA with CAP, especially in children who tested negative for a large battery of other respiratory pathogens. In contrast to the earlier study of HBoV capsid mRNA, the present study is much larger, includes parallel testing for HBoV DNA and capsid mRNA, and used combined nasopharyngeal and oropharyngeal swab specimens. In the present study, all patients had radiographically confirmed CAP, and controls were surveyed for the development of respiratory symptoms in the weeks following specimen collection. Consistent with acute infection, detection of HBoV capsid mRNA was also correlated with higher viral DNA loads in both the present and previous studies (Figure 3C) [48]. Taken together, this large, prospective, multicenter case control study expands on the previous study by confirming a stronger association of HBoV capsid mRNA detection than HBoV genomic DNA detection with CAP.
Detection of viral capsid mRNA offers several advantages as a test for diagnosing recent HBoV infections. It can be performed on upper respiratory tract specimens without the need for viral load measurement. In addition, in the absence of standardized serologic tests, RT-PCR detection of HBoV capsid mRNA can be more easily implemented for epidemiologic studies.
Strengths of the present study include its large size, multisite design, strict definition of pneumonia, and thorough search for other respiratory pathogens by use of sensitive and comprehensive methods [27]. Therefore, alternate etiologies could be excluded with some confidence. By using specimens and data from a large, prospective multicenter study of children hospitalized with radiographically confirmed CAP and controls and by conducting adjusted and sensitivity analyses, this study reduces methodologic limitations that compromised previous studies.
Remaining limitations include the potential for cross-detection of DNA in the HBoV capsid mRNA assay. To address this, we designed the RT-PCR assay for HBoV capsid mRNA assay to span a splice site that removes approximately 2 kb of HBoV RNA. As a result, cross-detection of HBoV DNA is almost eliminated (Figure 1). Performance of HBoV capsid mRNA detection tests could be further improved to eliminate cross-detection of viral DNA and optimize analytical and clinical sensitivity. Although we used a multisite design, controls were only available from 2 of the EPIC study sites and were not enrolled during the entire study period. We performed sensitivity analyses that demonstrated that these limitations did not bias results. Controls were older than children with CAP, but we adjusted for this in the analysis. In future studies, age-matched controls will help further reduce the potential confounding effect of age. Children were enrolled in the EPIC study during a 30-month period so that this study only covered 3 respiratory seasons. Despite the size of the EPIC study, we only detected 27 patients with HBoV mRNA (2.1% prevalence), potentially leading to small-sample-size bias (for comparison, the prevalences of other respiratory viruses in EPIC were 28% for respiratory syncytial virus, 27% for rhinovirus, 13% for metapneumovirus, 11% for adenovirus, 7% for parainfluenza virus, 7% for influenza virus, and 5% for coronavirus). Last, there is the potential for unmeasured confounders. Future studies across additional geographic regions and respiratory seasons will be required to confirm results.
In summary, our results provide further evidence that HBoV is strongly associated with CAP. Results correlating HBoV capsid mRNA detection with CAP were highly consistent between 2 geographically independent and climatically diverse locations across the United States, with an approximately 3-fold difference in overall HBoV prevalence. In addition to strengthening the association between detection of HBoV replication and CAP, our results suggest that detection of viral replication through RT-PCR might provide a better target for diagnosing primary HBoV disease and a tool for studying the etiologic role of HBoV.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank the patients enrolled in the EPIC study, their parents, and their families.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Centers for Disease Control and Prevention.
Financial support. This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (awards 1KL2TR001065 and UL1TR001067), the Primary Children’s Hospital Foundation, the ARUP Institute for Clinical and Experimental Pathology, and the Centers for Disease Control and Prevention (award U181P00030).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A 2005; 102:12891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jartti T, Hedman K, Jartti L, Ruuskanen O, Allander T, Söderlund-Venermo M. Human bocavirus-the first 5 years. Rev Med Virol 2012; 22:46–64. [DOI] [PubMed] [Google Scholar]
- 3. Koseki N, Teramoto S, Kaiho M et al. Detection of human bocaviruses 1 to 4 from nasopharyngeal swab samples collected from patients with respiratory tract infections. J Clin Microbiol 2012; 50:2118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Risku M, Kätkä M, Lappalainen S, Räsänen S, Vesikari T. Human bocavirus types 1, 2 and 3 in acute gastroenteritis of childhood. Acta Paediatr 2012; 101:e405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kantola K, Sadeghi M, Antikainen J et al. Real-time quantitative PCR detection of four human bocaviruses. J Clin Microbiol 2010; 48:4044–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schildgen O, Müller A, Allander T et al. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev 2008; 21:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin ET, Fairchok MP, Kuypers J et al. Frequent and prolonged shedding of bocavirus in young children attending daycare. J Infect Dis 2010; 201:1625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blessing K, Neske F, Herre U, Kreth HW, Weissbrich B. Prolonged detection of human bocavirus DNA in nasopharyngeal aspirates of children with respiratory tract disease. Pediatr Infect Dis J 2009; 28:1018–9. [DOI] [PubMed] [Google Scholar]
- 9. Williams JV. Deja vu all over again: Koch’s postulates and virology in the 21st century. J Infect Dis 2010; 201:1611–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. von Linstow ML, Høgh M, Høgh B. Clinical and epidemiologic characteristics of human bocavirus in Danish infants: results from a prospective birth cohort study. Pediatr Infect Dis J 2008; 27:897–902. [DOI] [PubMed] [Google Scholar]
- 11. Brieu N, Guyon G, Rodière M, Segondy M, Foulongne V. Human bocavirus infection in children with respiratory tract disease. Pediatr Infect Dis J 2008; 27:969–73. [DOI] [PubMed] [Google Scholar]
- 12. Wagner JC, Pyles RB, Miller AL, Nokso-Koivisto J, Loeffelholz MJ, Chonmaitree T. Determining persistence of bocavirus DNA in the respiratory tract of children by pyrosequencing. Pediatr Infect Dis J 2016; 35:471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Byington CL, Ampofo K, Stockmann C et al. Community surveillance of respiratory viruses among families in the Utah Better Identification of Germs-Longitudinal Viral Epidemiology (BIG-LoVE) Study. Clin Infect Dis 2015; 61:1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin ET, Kuypers J, McRoberts JP, Englund JA, Zerr DM. Human Bocavirus 1 Primary Infection and Shedding in Infants. J Infect Dis 2015; 212:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nokso-Koivisto J, Pyles RB, Miller AL, Jennings K, Loeffelholz M, Chonmaitree T. Role of human bocavirus in upper respiratory tract infections and acute otitis media. J Pediatric Infect Dis Soc 2014; 3:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fry AM, Lu X, Chittaganpitch M et al. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis 2007; 195:1038–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rezes S, Söderlund-Venermo M, Roivainen M et al. Human bocavirus and rhino-enteroviruses in childhood otitis media with effusion. J Clin Virol 2009; 46:234–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beder LB, Hotomi M, Ogami M et al. Clinical and microbiological impact of human bocavirus on children with acute otitis media. Eur J Pediatr 2009; 168:1365–72. [DOI] [PubMed] [Google Scholar]
- 19. Zhao B, Yu X, Wang C et al. High human bocavirus viral load is associated with disease severity in children under five years of age. PLoS One 2013; 8:e62318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Christensen A, Nordbø SA, Krokstad S, Rognlien AG, Døllner H. Human bocavirus in children: mono-detection, high viral load and viraemia are associated with respiratory tract infection. J Clin Virol 2010; 49:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou L, Zheng S, Xiao Q et al. Single detection of human bocavirus 1 with a high viral load in severe respiratory tract infections in previously healthy children. BMC Infect Dis 2014; 14:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng LS, Yuan XH, Xie ZP et al. Human bocavirus infection in young children with acute respiratory tract infection in Lanzhou, China. J Med Virol 2010; 82:282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Christensen A, Døllner H, Skanke LH, Krokstad S, Moe N, Nordbø SA. Detection of spliced mRNA from human bocavirus 1 in clinical samples from children with respiratory tract infections. Emerg Infect Dis 2013; 19:574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elia G, Cavalli A, Desario C et al. Detection of infectious canine parvovirus type 2 by mRNA real-time RT-PCR. J Virol Methods 2007; 146:202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Söderlund-Venermo M, Hokynar K, Nieminen J, Rautakorpi H, Hedman K. Persistence of human parvovirus B19 in human tissues. Pathol Biol (Paris) 2002; 50:307–16. [DOI] [PubMed] [Google Scholar]
- 26. Corcioli F, Zakrzewska K, Rinieri A et al. Tissue persistence of parvovirus B19 genotypes in asymptomatic persons. J Med Virol 2008; 80:2005–11. [DOI] [PubMed] [Google Scholar]
- 27. Jain S, Williams DJ, Arnold SR et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen AY, Cheng F, Lou S et al. Characterization of the gene expression profile of human bocavirus. Virology 2010; 403:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jula A, Waris M, Kantola K et al. Primary and secondary human bocavirus 1 infections in a family, Finland. Emerg Infect Dis 2013; 19:1328–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ursic T, Steyer A, Kopriva S, Kalan G, Krivec U, Petrovec M. Human bocavirus as the cause of a life-threatening infection. J Clin Microbiol 2011; 49:1179–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edner N, Castillo-Rodas P, Falk L, Hedman K, Söderlund-Venermo M, Allander T. Life-threatening respiratory tract disease with human bocavirus-1 infection in a 4-year-old child. J Clin Microbiol 2012; 50:531–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Körner RW, Söderlund-Venermo M, van Koningsbruggen-Rietschel S, Kaiser R, Malecki M, Schildgen O. Severe human bocavirus infection, Germany. Emerg Infect Dis 2011; 17:2303–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ursic T, Krivec U, Kalan G, Petrovec M. Fatal human bocavirus infection in an 18-month-old child with chronic lung disease of prematurity. Pediatr Infect Dis J 2014. [DOI] [PubMed] [Google Scholar]
- 34. Brebion A, Vanlieferinghen P, Déchelotte P, Boutry M, Peigue-Lafeuille H, Henquell C. Fatal subacute myocarditis associated with human bocavirus 2 in a 13-month-old child. J Clin Microbiol 2014; 52:1006–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sadeghi M, Kantola K, Finnegan DP et al. Possible involvement of human bocavirus 1 in the death of a middle-aged immunosuppressed patient. J Clin Microbiol 2013; 51:3461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pellett PE. Indictment by association: once is not enough. J Infect Dis 2015; 212:509–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shi T, McLean K, Campbell H, Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: A systematic review and meta-analysis. J Glob Health 2015; 5:010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Broccolo F, Falcone V, Esposito S, Toniolo A. Human bocaviruses: Possible etiologic role in respiratory infection. J Clin Virol 2015; 72:75–81. [DOI] [PubMed] [Google Scholar]
- 39. Lu QB, Wo Y, Wang HY et al. Epidemic and molecular evolution of human bocavirus in hospitalized children with acute respiratory tract infection. Eur J Clin Microbiol Infect Dis 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lu X, Gooding LR, Erdman DD. Human bocavirus in tonsillar lymphocytes. Emerg Infect Dis 2008; 14:1332–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Korppi M. Polymerase chain reaction in respiratory samples alone is not a reliable marker of bocavirus infection. Pediatr Pulmonol 2014; 49:515–6. [DOI] [PubMed] [Google Scholar]
- 42. Söderlund-Venermo M, Lahtinen A, Jartti T et al. Clinical assessment and improved diagnosis of bocavirus-induced wheezing in children, Finland. Emerg Infect Dis 2009; 15:1423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meriluoto M, Hedman L, Tanner L et al. Association of human bocavirus 1 infection with respiratory disease in childhood follow-up study, Finland. Emerg Infect Dis 2012; 18:264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deng Y, Gu X, Zhao X et al. High viral load of human bocavirus correlates with duration of wheezing in children with severe lower respiratory tract infection. PLoS One 2012; 7:e34353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hayden RT, Yan X, Wick MT et al. Factors contributing to variability of quantitative viral PCR results in proficiency testing samples: a multivariate analysis. J Clin Microbiol 2012; 50:337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hayden RT, Shahbazian MD, Valsamakis A et al. Multicenter evaluation of a commercial cytomegalovirus quantitative standard: effects of commutability on interlaboratory concordance. J Clin Microbiol 2013; 51:3811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Van Wesenbeeck L, Meeuws H, D’Haese D et al. Sampling variability between two mid-turbinate swabs of the same patient has implications for influenza viral load monitoring. Virol J 2014; 11:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Proença-Modena JL, Gagliardi TB, Paula FE et al. Detection of human bocavirus mRNA in respiratory secretions correlates with high viral load and concurrent diarrhea. PLoS One 2011; 6:e21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.