No single mechanism explains excess chronic disease risk among people aging with human immunodeficiency virus (HIV) infection. Future HIV drug discovery will likely require attention to multiple biomarkers or an index. Inflammation biomarkers are valuable for research but not yet ready for use in the clinic.
Keywords: Biomarker, index, inflammation, HIV, therapeutic discovery
Abstract
Despite achieving human immunodeficiency virus type 1 (HIV-1) RNA suppression below levels of detection and, for most, improved CD4+ T-cell counts, those aging with HIV experience excess low-level inflammation, hypercoagulability, and immune dysfunction (chronic inflammation), compared with demographically and behaviorally similar uninfected individuals. A host of biomarkers that are linked to chronic inflammation are also associated with HIV-associated non–AIDS-defining events, including cardiovascular disease, many forms of cancer, liver disease, renal disease, neurocognitive decline, and osteoporosis. Furthermore, chronic HIV infection may interact with long-term treatment toxicity and weight gain after ART initiation. These observations suggest that future biomarker-guided discovery and treatment may require attention to multiple biomarkers and, possibly, weighted indices. We are clinical trialists, epidemiologists, pragmatic trialists, and translational scientists. Together, we offer an operational definition of a biomarker and consider how biomarkers might facilitate progress along the translational pathway from therapeutic discovery to intervention trials and clinical management among people aging with or without HIV infection.
The accelerated drug development that transformed human immunodeficiency virus (HIV) infection from a fatal condition to a manageable disease was enabled by a single biomarker of HIV infection, HIV-1 RNA. HIV-1 RNA allowed pharmaceutical companies to screen millions of compounds for activity in preclinical studies, provided a surrogate outcome for trials, and yielded a clear pathway to drug approval. HIV-1 RNA levels now guide antiretroviral therapy (ART). However, while many individuals infected with HIV achieve viral suppression, ART does not cure or completely reverse HIV-associated injury; rather, it has converted HIV infection from an acutely fatal disease to a chronic illness.
Despite viral suppression and, in most cases, a strong CD4+ T-cell count response, people aging with HIV infection experience chronic inflammation (ie, ongoing low-level inflammation, hypercoagulability, and immune dysfunction). After adjustment for established risk factors and as compared to uninfected individuals, they experience excess risk for some non–AIDS-defining cancers and liver, neurocognitive, cardiovascular, renal and bone diseases. Many biomarkers of chronic inflammation predictive of morbidity and mortality after ART initiation remain significantly higher than levels before HIV infection [1]. These observations suggest new questions: why are well-controlled HIV-infected individuals more susceptible to these diseases as compared to uninfected individuals, and how should their risk be managed? These questions likely require new biomarkers or indices.
In this viewpoint, we offer special considerations in the use and interpretation of biomarkers among HIV-infected individuals experiencing heightened risk of noncommunicable, aging-associated, diseases in the ART era. We also consider how biomarkers and indices might further facilitate progress along the pathway from therapeutic discovery bench to bedside for people aging with HIV infection.
BIOMARKERS AND INDICES: DEFINITION AND EVALUATION
Strimbu and Tavel [2] defined biomarkers as “objective indications of a medical state observed from outside the patient—which can be measured accurately and reproducibly” (p. 1). Biomarkers may be unique to a particular condition (eg, HIV RNA), reflect distinct physiologic systems (eg, lymphocyte subsets), or reflect generalized health status (eg, weight loss). Biomarkers may be considered individually or grouped into indices, and associations of interest may be concurrent or predictive. The key questions when evaluating their utility address the state or pathway of interest and how clearly the biomarker or index signifies the state or pathway.
A detailed description of the evaluation of biomarkers and indices is offered elsewhere [3, 4]. In brief, once the state or pathway is measured, one can evaluate correspondence between the biomarker and the intended state or pathway by calculating the sensitivity, specificity, and positive and negative predictive values. If the biomarker is continuous, cut points can be selected using receiver operating characteristic curves [5]. Accuracy is often summarized using the C statistic [6]. Risk reclassification metrics can help determine the value of adding an additional biomarkers to an established index [7]. Generalizability is the ability to accurately use a biomarker for the outcome of interest across settings [3]. Many factors can alter the accuracy and generalizability of a biomarker (see below). Practical concerns in the use of biomarkers include cost, availability, and performance.
Considerations in Using Biomarkers in the Context of Clinical Trials
Because clinical outcomes typically require large sample sizes and extended periods of follow-up, the use of rapidly responsive, quantifiable biomarkers as surrogate outcomes has been advocated. For a biomarker or an index to qualify as a surrogate outcome it must predict, with accuracy and generalizability, the outcome of interest [3] and must completely respond to the intervention under study intervention: mediated changes in the biomarker must translate into changes in the actual risk of the outcome of interest [8, 9]. Even though CD4+ T-cell count and HIV-1 RNA load were imperfect surrogates, they were used successfully in the development of ART. Some biomarkers are clearly not reliable surrogate biomarkers: CD4+ T-cell count and levels of T-cell–activation biomarkers improved with interventions such as interleukin 2 without meaningful reduction in morbidity or mortality [10]. Other biomarkers may be too slowly responsive: carotid intima-media thickness may take years to respond to therapeutic intervention [11]. In contrast, biomarkers of endothelial dysfunction or vascular inflammation, such as FDG/PET-CT or flow-mediated dilatation, may respond within weeks to months. Biomarker outcomes in small pilot clinical trials need to be stable and reproducible within individuals. Variability in levels of biomarkers such as interleukin 6 (IL-6) and D-dimer mandates larger sample size to detect response [12–15], whereas biomarkers of immune activation with less intra-individual variability in level (eg, T-cell activation) require fewer subjects to detect important differences.
SPECIAL CONSIDERATIONS IN THE STUDY OF HIV
As we explore non–HIV-specific biomarkers in HIV-infected individuals, it is important to consider how HIV may alter their interpretation. We address (1) the return to health after ART initiation, (2) HIV-associated comorbid behaviors and conditions, and (3) progressive frailty.
Return to Health
ART that successfully suppresses HIV-1 RNA is associated with a return-to-health phenomenon that may confound biomarker interpretation. Driven in part by decreased metabolic requirements, most individuals gain weight with ART [16]. As among those without HIV infection, gains in visceral adiposity, in particular, are associated with elevations in cholesterol, insulin, and glucose levels. However, obesity following ART initiation may also have different health implications than among individuals aging without HIV infection [17]. Synthetic liver function improves with ART, manifesting as an increase in levels of cholesterol and other liver synthetic biomarkers, including some related to inflammation and coagulation. Other biomarker changes suggest widespread improvements in organ systems, including bone marrow and kidneys [18].
Comorbid Behaviors and Conditions
The greater prevalence of smoking, harmful alcohol use, coinfections, and comorbidities often seen in HIV-infected individuals as compared to healthy uninfected individuals [19] is likely associated with greater inflammatory burden. Furthermore, underlying liver disease, renal impairment, stress, coinfections [20], and specific antiretroviral regimens [21] may alter the production, metabolism, and clearance of biomarkers. In this regard, cohorts comparing HIV-infected and uninfected individuals with similar demographic characteristics and health behaviors have provided important insight: differences in inflammatory biomarkers by HIV serostatus became more apparent after adjustment for important comorbidities [22].
Inconsistent chronic inflammatory responses of the biomarkers C-reactive protein (CRP) and D-dimer may be attributable to chronic liver disease, common among HIV-infected individuals. The level of CRP, produced in the liver, was lower than expected, given the degree of associated inflammation among individuals in the FRAM study who were coinfected with HIV and hepatitis C virus [23]. Expected declines in IL-6 and D-dimer levels with ART initiation were paralleled by increased CRP levels in 2 cohorts [24]. Several liver-produced coagulation factor levels (including anticoagulants) were lower in untreated participants in initial studies of the SMART trial, resulting in greater thrombin production and higher D-dimer levels; this reversed with treatment [25].
Frailty
Frailty is a geriatric syndrome of vulnerability to adverse health outcomes, characterized by impairment in mobility, balance, strength, cognition, nutrition, endurance, and physical activity [26]. Frailty contributes to and is exacerbated by other geriatric syndromes [27]. Many studies suggest that frailty occurs earlier and more often among HIV-infected individuals [28]. Frailty is both driven by and exacerbates chronic inflammation [26, 29], and the presence of frailty should be considered when interpreting inflammatory biomarker levels [30]. Furthermore, frailty is strongly associated with increasing age, immunodeficiency, and immune dysfunction [26, 28–30]; thus, frailty may modify the interpretation of biomarkers. For example, a strongly positive correlation between activated T cells and T-regulatory cells among frail, HIV-infected men but an inverse correlation among nonfrail and uninfected men likely reflects altered immune regulation and, subsequently, may impact biomarker levels [31].
INDICES IN TRANSLATIONAL RESEARCH
Combining multiple biomarkers, each imperfectly reflective of related mechanisms of injury, may decrease noise in measurement and improve our ability to study chronic inflammation. This approach has been used to develop genetic and inflammatory risk indices [32]. Indeed, the central role of inflammation highlights the potential relevance of an inflammatory index (incorporating IL-6 and soluble tumor necrosis factor (TNF) receptor 1) during HIV infection, where a study among injection drug users found a strong association with frailty and mortality [33]. However, a biomarker may demonstrate stronger associations simply because it is part of a final common pathway rather than directly reflective of the targeted mechanisms of injury. For example, elevated levels of transaminases, indicative of liver damage, may be more strongly predictive of fibrosis than biomarkers of visceral fat or microbial translocation, even if visceral adiposity is triggering microbial translocation, increased transaminases, and liver fibrosis.
While other prognostic indices have been developed among HIV-infected individuals, the Veterans Aging Cohort Study (VACS) index has been the most widely validated [34–36]. A weighted combination of routine clinical biomarkers (eg, CD4+ T-cell count, HIV RNA load, hemoglobin level, platelet count, aspartate and alanine transaminase levels, creatinine level, and hepatitis C virus serologic findings), the index is strongly correlated with biomarkers of chronic inflammation and predictive of all-cause mortality, cardiovascular events, fragility fractures, medically significant falls, cognitive dysfunction, sarcopenia, and physical performance. Of note, HIV-infected individuals have higher scores than uninfected comparators, suggesting excess physiologic frailty.
BIOMARKERS AND THERAPEUTIC DISCOVERY
General Considerations
Biomarkers help identify targets for intervention and facilitate screening of compounds for activity against these targets. The more targeted a biomarker is for the mechanism of injury, the more rapidly this process can occur. Because current mechanisms of end-organ injury among HIV-infected individuals receiving ART are multifaceted and not unique to HIV infection, it has been challenging to identify a single biomarker or interventional target.
Biomarkers are being used to identify interventional targets of chronic inflammation. Several of these biomarkers predict comorbid diseases with stronger associations than in uninfected populations [37, 38], suggesting that immune activation and inflammation play a more important role among HIV-infected individuals than in the general population. A greater understanding of the interrelationships between these mechanisms, feedback loops, and common pathways would help prioritize interventional targets. Biomarkers may demonstrate different relationships among men and women [39], and pathways of injury among those aging with HIV infection may differ in resource-limited settings, where infectious complications remain a common cause of death. Development and validation of biomarkers that are feasible and specific for these settings are imperative [40].
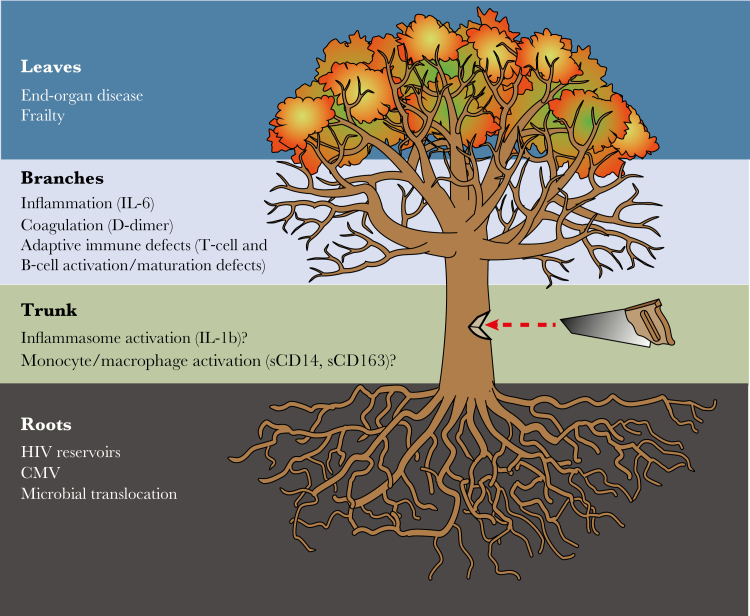
One might liken the optimal process for identifying therapeutic targets to cutting down a tree (Figure 1). One could attempt to identify targets in a piecemeal fashion by cutting off each individual “root” driving the inflammatory state (eg, HIV-associated microbial translocation) or cutting off each “branch” (eg, hypercoagulability). However, piecemeal approaches may fail to reverse pathology if all important “root” causes are not blunted and/or downstream pathways reversed. Identifying the “trunk”—a central pathway activated by all major drivers and giving rise to all parallel pathways—would be optimal. Applying systems biology and assessing multiple parallel inflammatory pathways in the context of trials of immune-based interventions may help identify promising central pathways. Similarly, this approach may identify unintended negative consequences on parallel pathways when targeting individual “roots” or “branches,” informing the next generation of pilot trials.
Figure 1.
Can we find the tree trunk? One might liken the optimal process for identifying therapeutic targets to cutting down a tree. One could attempt to identify targets in a piecemeal fashion by cutting off each individual “root” driver of the inflammatory state (eg, human immunodeficiency virus [HIV]–associated microbial translocation) or by cutting off each “branch” (eg, hypercoagulability). However, piecemeal approaches may fail to reverse pathology if all important “root” causes are not blunted and/or downstream pathways reversed. Identifying the “trunk”—a central pathway activated by all major drivers and giving rise to all parallel pathways—would be optimal. Applying systems biology and assessing multiple parallel inflammatory pathways in the context of trials of immune-based interventions may help identify promising central pathways. CMV, cytomegalovirus; IL-1β, interleukin 1β; IL-6, interleukin 6; sCD14, soluble CD14; sCD163, soluble CD163.
An example of how biomarkers used in pilot interventional trials of immune-based interventions can improve our understanding of the system affected by the intervention comes from a recent trial of intensification with the CCR5 inhibitor maraviroc in individuals with ART-suppressed HIV infection [41]. As CCR5 signaling was thought to contribute to immune activation, the a priori hypothesis of the investigators was that immune activation would decline during maraviroc intensification, but the trial actually demonstrated increased immune activation during maraviroc intensification. By measuring secondary effects of CCR5 inhibition (ie, an increase in circulating CCR5 ligands as a consequence of blocking ligand-receptor binding and cellular internalization), the investigators identified a plausible unintended consequence of CCR5 blockade: CCR5 ligands might increase and bind to alternative coreceptors on myeloid cells and T cells, driving immune activation. Thus, the next generation of studies designed to reduce immune activation by blocking chemokine receptors is using agents that block >1 chemokine receptor (ie, the CCR5/CCR2 inhibitor cenicriviroc) to mitigate some of the unintended negative consequences of isolated CCR5 blockade [42].
Concomitant Viral Infections
Immune-based interventions can also have secondary effects on viral coinfections that themselves can contribute to the inflammatory state. For example, because treating asymptomatic cytomegalovirus (CMV) replication reduces persistent immune activation in treated HIV infection [43], any antiinflammatory intervention that decreased CMV-specific immune responses (and thereby increased CMV replication) might actually increase immune activation (or at least attenuate the primary antiinflammatory effect). Conversely, reducing chronic immune activation may decrease viral shedding, resulting in favorable secondary and, perhaps, more-durable effects of immune-based interventions. Consequently, several trials of potent immune-based interventions (eg, sirolimus and ruxolitinib) in the AIDS Clinical Trials Group are now measuring shedding of chronically coinfecting viruses to capture these effects. Such studies will help us understand how best to intervene on the “tree” without making the “roots” stronger.
Microbial Translocation
Biomarkers of microbial translocation, including lipopolysaccharide and soluble CD14, are associated with poor outcomes and have uncovered mechanisms of gut mucosal involvement in HIV infection. Despite modest success in nonhuman primates [44], attempts to attenuate microbial translocation in HIV-infected individuals have been unsuccessful [45]. Interventional studies targeting the gut through probiotics are underway. Indoleamine 2,3-dioxygenase-1 activity and gut epithelial barrier dysfunction (for which zonulin and intestinal fatty acid–binding protein levels are surrogate biomarkers) also predict increased mortality during treated HIV infection, even among those with high CD4+ T-cell counts [46], and represent additional interventional targets.
Immune Dysfunction and Systemic Inflammation
Biomarkers of immune dysfunction have long been a priority in HIV research. Beyond CD4+ T-cell counts, these include skewing of monocyte subpopulations, functional T-cell subsets (eg, T-helper type 1 [Th1], Th2, Th17, and T-regulatory cells), and both activated and dysfunctional CD8+ T cells. Biomarkers of systemic inflammation, including IL-6, CRP, TNF-α, and soluble TNF-α receptors, have been used to identify individuals at higher risk for poor outcomes with ART, including mortality [47]. Clinical trials targeting inflammation are underway with interventions such as statins, metformin, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, direct inhibitors of inflammatory cytokines, and even anticoagulants [48]. Measuring parallel inflammatory pathways and “roots” driving the inflammatory state in the context of these trials will be important to determine whether the intervention is truly targeting the “trunk” or only a single “branch” (Figure 1).
Hypercoagulability
HIV-infected individuals are at an increased risk of venous thrombosis [49, 50]. The level of D-dimer (a biomarker of ongoing thrombin generation) is also increased and is an exceptionally strong indicator of future risk for cardiovascular disease and mortality, even in well-controlled HIV infection [25]. The D-dimer level is also elevated in simian immunodeficiency virus–infected pig-tailed macaques [51]. At autopsy, these monkeys show extensive in situ thrombosis in multiple organ systems. Trials of direct inhibitors of coagulation, such as the ongoing TACTICAL trial [48], will be required to determine the degree to which hypercoagulability is a cause or consequence of immune activation in treated HIV infection.
BIOMARKERS AND CLINICAL TRIALS
Usefulness of Biomarkers in Pilot Trials of Immune-Based Interventions
Several of the biomarkers highlighted above have been successfully used in early clinical trials of immune-based interventions. These pilots suggest that such interventions may mitigate pathways thought to be important in driving excess risk of disease in treated HIV infection. This strategy can help prioritize promising interventions that should advance to more-expensive trials focused on clinical outcomes and can screen out interventions that fail to improve even the more intermediate biomarkers. For example, recent pilot studies of statins demonstrated significant reductions in monocyte/macrophage activation biomarkers, inflammation, tissue factor expression, and surrogate biomarkers of cardiovascular disease. These positive impacts on biomarkers and surrogate biomarkers helped motivate funding for a large clinical trial of statins that investigated clinical end points [52]. On the other hand, a recent clinical trial of 2 doses of aspirin [53] revealed that, although the intervention clearly reduced thromboxane levels (a direct surrogate biomarker of cyclooxygenase inhibition), it failed to reduce levels of immune activation biomarkers that predict disease in treated HIV infection or a surrogate biomarker of cardiovascular disease. Thus, aspirin is not moving forward in clinical trials focusing on clinical end points. A similar approach is being taken in the aging and frailty fields, where commonly used drugs with antiinflammatory properties are first being studied in pilot trials of elderly uninfected individuals to see whether the drugs influence inflammatory biomarkers. Interventions that reduce levels of inflammatory biomarkers in pilot trials would then move forward in larger clinical trials investigating whether they reduce the risk of frailty [54].
Notably, the use of biomarkers in these earlier stage trials to pick promising interventions and to inform in an iterative way the most-sensible interventional targets (as discussed above) is an inexact science. None of the biomarkers of immune activation and inflammation currently in use have been validated as true surrogate biomarkers in the context of HIV infection. As discussed above, true surrogacy can only be established when a treatment that specifically improves the biomarker of interest translates into a measurable improvement in health in a clinical trial. The inflammation field is on the cusp of demonstrating this in uninfected populations in the CANTOS trial [55, 56], where the interleukin 1β–inhibitor canakinumab was shown to reduce the risk of cardiovascular events and mortality from lung cancer. Presumably, treatment-mediated reductions in levels of biomarkers of the interleukin 1β pathway in that study can now be linked to an improvement in health, validating novel surrogate biomarkers in that population. That said, there is no guarantee that the same surrogate biomarkers will predict disease in the HIV-infected population, where the “roots” driving the inflammatory state are distinct and the relative contributions of discrete inflammatory pathways to disease may be different. Thus, validating surrogate biomarkers of inflammatory drivers of end-organ disease will be needed in large clinical studies of HIV-infected individuals.
Whether biomarkers of chronic inflammation will prove successful as surrogates in the complex milieu of aging and HIV infection remains to be seen. An early opportunity to identify surrogate biomarkers will come from the REPRIEVE trial. If statins reduce the risk not just of cardiovascular events, but also of events less likely to be mediated by hyperlipidemia (eg, infections), biomarkers of statin-mediated antiinflammatory effects may emerge as surrogates in future studies.
Indices as Surrogates
Given the multifaceted, complex, recursive, and overlapping mechanisms of immune dysfunction, viral coinfection, chronic inflammation, hypercoagulability, and microbial translocation, an index of biomarkers validated for all-cause mortality and shown to be responsive to candidate interventions could be a useful surrogate. Indices, such as the VACS and the inflammatory index, potentially have the benefit of weighting included biomarkers in light of their independent association with all-cause mortality and can include biomarkers reflective of both benefits and harms of treatment. It remains to be seen whether either index is responsive to interventions targeting chronic inflammation.
BIOMARKERS AND CLINICAL MANAGEMENT
Clinical applications of biomarkers or indices include risk assessment, treatment guidance (choice of treatment and measuring response), and prognosis. Some biomarkers may be used for several purposes, and others may be useful for only one. For example, CD4+ T-cell count is not currently considered a useful criterion for switching ART among those with suppressed HIV RNA, but it does have an independent impact on prognosis. Many biomarkers are being considered in large epidemiological studies and clinical trials as candidates for use in clinical management. To date, no clear winners have emerged, in part because biomarkers are, in general, difficult to implement clinically [57]. The VACS index is calculated based on Clinical Laboratory Improvement Amendments–certified biomarkers and is being used in some clinical settings in the United States. Nevertheless, the biomarkers that most strongly predict mortality in resource-limited settings may be different than those that are most prognostic in resource-rich settings. It is important to validate clinical biomarkers and indices in both settings.
EMERGING AREAS OF STUDY
It is reasonable to assume that important and informative biomarkers will emerge from new large-scale -omic approaches in studies of HIV infection and aging, as they have outside this field. Genomic, transcriptomic, and proteomic approaches are in progress, and metabolomics studies are certain to follow. Work is also underway to further improve the responsiveness of the VACS index by converting it to a continuous measure and adding additional routine clinical biomarkers (eg, albumin level and body mass index). Progress will require innovative approaches to the complex mix of contributing factors, including the impact of HIV infection on the immune system, lifestyle and behavioral traits, varied underlying pathology, and powerful and variable drug treatments present in HIV-infected populations.
LIMITATIONS OF CANDIDATE BIOMARKERS, INDICES, AND SURROGATE BIOMARKERS
The mechanisms of adverse effects from treatment are often different from those of beneficial effects. In the case of statins, improved values of biomarkers or surrogate biomarkers would need to be separately weighed against known adverse effects of statins, including muscle and liver injury. Also, interventions may interact with multiple mechanisms of disease (pleiotropy), not all of which are captured by a single biomarker. Finally, even well accepted surrogates may not completely reflect the disease or primary mechanism in question. Nevertheless, drug companies require reliable surrogate biomarkers to rapidly screen large numbers of compounds for activity that may reasonably warrant their investigation in pilot studies and, eventually, randomized controlled trials. Identification of novel surrogate biomarkers is a critical step in developing the next generation of effective interventions.
CONCLUSION
No single mechanism of injury explains the residual excess risk among HIV-infected individuals, compared with uninfected persons. Rather, the excess risk appears to reflect multiple, overlapping, and reinforcing mechanisms. The next wave of clinical discovery will likely require multiple biomarkers and/or an index to help reduce measurement noise, appropriately weight components, improve responsiveness, and account for beneficial and harmful effects of therapeutic interventions. Criteria for selecting biomarkers for HIV research depend upon the questions, mechanisms, and outcomes being investigated. Developing surrogates is an especially important area of research that should explore both individual biomarkers and indices. While biomarkers for the inflammatory state have proven crucial in our understanding of the pathology of chronic HIV infection, none of these are yet ready for clinical application.
Notes
Financial support. This work was supported by the National Institute on Alcohol Abuse and Alcoholism (grants U10 AA013566, U24 AA020794, and U01 AA020790 to A. C. J.), the National Institute on Aging (grants K23 AG050260 and R01 AG054366 to K. M. E.), and the National Institute of Allergy and Infectious Diseases (grants AI 068636 [to A. L.] and R01 HL125032 [to R. P. T.]), National Institutes of Health.
Potential conflicts of interest. K. M. E. receives research funding (to the University of Colorado) from Gilead Sciences and has served as a medical consultant for Theratechnologies. R. P. T. receives travel support from Virology Education for organizing meetings on HIV research and has served as a consultant for Tibotec/Centocor Ortho Biotech/J&J, Abbott/AbbVie, and Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wada NI, Jacobson LP, Margolick JB et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strimbu K, Tavel JA. What are biomarkers?Curr Opin HIV AIDS 2010; 5:463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med 1999; 130:515–24. [DOI] [PubMed] [Google Scholar]
- 4. Cook NR. Comments on ‘Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond’ by M. J. Pencina et al. , Statistics in Medicine (DOI: 10.1002/sim.2929). Stat Med 2008; 27:191–5. [DOI] [PubMed] [Google Scholar]
- 5. Centor RM. A visicalc program for estimating the area under a receiver operating characteristic (ROC) curve. Med Decis Making 1985; 5:139–48. [DOI] [PubMed] [Google Scholar]
- 6. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15:361–87. [DOI] [PubMed] [Google Scholar]
- 7. Cook NR. Assessing the Incremental Role of Novel and Emerging Risk Factors. Curr Cardiovasc Risk Rep 2010; 4:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gilbert PB, Gabriel EE, Huang Y, Chan IS. Surrogate Endpoint Evaluation: Principal Stratification Criteria and the Prentice Definition. J Causal Inference 2015; 3:157–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saraf S, Mathew T, Roy A. Statistical Validation of Surrogate Endpoints: Another Look at the Prentice Criterion and Other Criteria. J Biopharm Stat 2015; 25:1234–46. [DOI] [PubMed] [Google Scholar]
- 10. Abrams D, Levy Y, Losso MH et al. Interleukin-2 therapy in patients with HIV infection. N Engl J Med 2009; 361:1548–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stein JH, Ribaudo HJ, Hodis HN et al. A prospective, randomized clinical trial of antiretroviral therapies on carotid wall thickness. AIDS 2015; 29:1775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grund B, Baker JV, Deeks SG et al. INSIGHT SMART/ESPRIT/SILCAAT Study Group Relevance of Interleukin-6 and D-Dimer for Serious Non-AIDS Morbidity and Death among HIV-Positive Adults on Suppressive Antiretroviral Therapy. PLoS One 2016; 11:e0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hunt PW, Shulman NS, Hayes TL et al. The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood 2013; 121:4635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breen EC, Reynolds SM, Cox C et al. Multisite comparison of high-sensitivity multiplex cytokine assays. Clin Vaccine Immunol 2011; 18:1229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fahey JL, Aziz N, Spritzler J et al. Need for an external proficiency testing program for cytokines, chemokines, and plasma markers of immune activation. Clin Diagn Lab Immunol 2000; 7:540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koethe JR, Jenkins CA, Lau B et al. North American AIDS Cohort Collaboration on Research and Design Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses 2016; 32:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herrin M, Tate JP, Akgün KM et al. Weight Gain and Incident Diabetes Among HIV-Infected Veterans Initiating Antiretroviral Therapy Compared With Uninfected Individuals. J Acquir Immune Defic Syndr 2016; 73:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tate JP, Justice AC, Hughes MD et al. Performance of the refined VACS Risk Index during the first 12 months of antiretroviral therapy among US and European subjects. 15th International Workshop on HIV Observational Databases2011. [Google Scholar]
- 19. Park LS, Hernández-Ramírez RU, Silverberg MJ, Crothers K, Dubrow R. Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis. AIDS 2016; 30:273–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Floris-Moore M, Howard AA, Lo Y, Schoenbaum EE, Arnsten JH, Klein RS. Hepatitis C infection is associated with lower lipids and high-sensitivity C-reactive protein in HIV-infected men. AIDS Patient Care STDS 2007; 21:479–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelesidis T, Tran TT, Stein JH et al. Changes in Inflammation and Immune Activation With Atazanavir-, Raltegravir-, Darunavir-Based Initial Antiviral Therapy: ACTG 5260s. Clin Infect Dis 2015; 61:651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knudsen TB, Ertner G, Petersen J et al. Plasma Soluble CD163 level independently predicts all-cause mortality in HIV-1-infected individuals. J Infect Dis 2016; 214:1198–204. [DOI] [PubMed] [Google Scholar]
- 23. Reingold J, Wanke C, Kotler D et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr 2008; 48:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palella FJ Jr, Gange SJ, Benning L et al. Inflammatory biomarkers and abacavir use in the Women’s Interagency HIV Study and the Multicenter AIDS Cohort Study. AIDS 2010; 24:1657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baker JV, Brummel-Ziedins K, Neuhaus J et al. INSIGHT SMART Study Team HIV replication alters the composition of extrinsic pathway coagulation factors and increases thrombin generation. J Am Heart Assoc 2013; 2:e000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walston J, Hadley EC, Ferrucci L et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006; 54:991–1001. [DOI] [PubMed] [Google Scholar]
- 27. Tassiopoulos K, Abdo M, Wu K et al. Frailty is strongly associated with increased risk of recurrent falls among older HIV-infected adults. AIDS 2017; 31:2287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Althoff KN, Jacobson LP, Cranston RD et al. Multicenter AIDS Cohort Study Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci 2014; 69:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Erlandson KM, Wu K, Koletar SL et al. Association between frailty and components of the frailty phenotype with modifiable risk factors and antiretroviral therapy. J Infect Dis 2017; 215:933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang W, Nilles TL, Johnson JR, Margolick JB. Regulatory T cells, frailty, and immune activation in men who have sex with men in the multicenter AIDS cohort study. J Gerontol A Biol Sci Med Sci 2015; 70:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Varadhan R, Yao W, Matteini A et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci 2014; 69:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Piggott DA, Varadhan R, Mehta SH et al. Frailty, inflammation, and mortality among persons aging with HIV infection and injection drug use. J Gerontol A Biol Sci Med Sci 2015; 70:1542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tate JP, Justice AC, Hughes MD et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS 2013; 27:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Justice AC, Modur SP, Tate JP et al. NA-ACCORD and VACS Project Teams Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr 2013; 62:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VACS index information and VACS calculator. Updated July 2017. http://medicine.yale.edu/intmed/vacs/welcome/vacsindexinfo.aspx. Accessed 27 July 2017.
- 37. Gupta SK, Kitch D, Tierney C, Melbourne K, Ha B, McComsey GA. AIDS Clinical Trials Group Study A5224s Team Markers of renal disease and function are associated with systemic inflammation in HIV infection. HIV Med 2015; 16:591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Erlandson KM, Wu K, Koletar SL et al. Association between frailty and components of the frailty phenotype with modifiable risk factors and antiretroviral therapy. J Infect Dis 2017; 215:933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mathad JS, Gupte N, Balagopal A et al. Sex-related differences in inflammatory and immune activation markers before and after combined antiretroviral therapy initiation. J Acquir Immune Defic Syndr (1999) 2016; 73:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee S, Byakwaga H, Boum Y et al. Immunologic pathways that predict mortality in HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis 2017; 215:1270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hunt PW, Shulman NS, Hayes TL et al. The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood. 2013; 121:4635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thompson M, Saag M, DeJesus E et al. A 48-week randomized phase 2b study evaluating cenicriviroc versus efavirenz in treatment-naive HIV-infected adults with C-C chemokine receptor type 5-tropic virus. AIDS. 2016; 30:869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hunt PW, Martin JN, Sinclair E et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011; 203:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pandrea I, Xu C, Stock JL et al. Antibiotic and antiinflammatory therapy transiently reduces inflammation and hypercoagulation in acutely SIV-infected pigtailed macaques. PLoS Pathog 2016; 12:e1005384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tenorio AR, Chan ES, Bosch RJ et al. ; A5286 Team Rifaximin has a marginal impact on microbial translocation, T-cell activation and inflammation in HIV-positive immune non-responders to antiretroviral therapy - ACTG A5286. J Infect Dis 2015; 211:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hunt PW, Sinclair E, Rodriguez B et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tenorio AR, Zheng Y, Bosch RJ et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Minneapolis Medical Research Foundation. Targeted anticoagulation therapy to reduce inflammation and cellular activation in long-term HIV disease (TACTICAL-HIV), NCT02339415, 15 September 2017. https://clinicaltrials.gov/ct2/show/NCT02339415. Accessed 6 December 2017.
- 49. Bibas M, Biava G, Antinori A. HIV-associated venous thromboembolism. Mediterr J Hematol Infect Dis 2011; 3:e2011030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rasmussen LD, Dybdal M, Gerstoft J et al. HIV and risk of venous thromboembolism: a Danish nationwide population-based cohort study. HIV Med 2011; 12:202–10. [DOI] [PubMed] [Google Scholar]
- 51. Pandrea I, Cornell E, Wilson C et al. Coagulation biomarkers predict disease progression in SIV-infected nonhuman primates. Blood 2012; 120:1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. NIAID. Evaluating the use of pitavastatin to reduce the risk of cardiovascular disease in HIV-infected adults (REPRIEVE), NCT02344290. https://clinicaltrials.gov/ct2/show/NCT02344290?term=reprieve&rank=3. Accessed 6 December 2017.
- 53.O’Brien MP, Hunt PW, Kitch DW, et al. A randomized placebo controlled trial of aspirin effects on immune activation in chronically human immunodeficiency virus-infected adults on virologically suppressive antiretroviral therapy. Open Forum Infect Dis 2017; 4(1):ofw278. [DOI] [PMC free article] [PubMed]
- 54. Manini TM, Anton SD, Beavers DP et al. ENRGISE Pilot Study Investigators Enabling reduction of low-grade inflammation in seniors pilot study: concept, rationale, and design. J Am Geriatr Soc 2017; 65:1961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377(12):1119–31. [DOI] [PubMed]
- 56.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ; CANTOS Trial Group. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390(10105):1833–42. [DOI] [PubMed]
- 57. Sturgeon C, Hill R, Hortin GL, Thompson D. Taking a new biomarker into routine use–a perspective from the routine clinical biochemistry laboratory. Proteomics Clin Appl 2010; 4:892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]