Abstract
Background.
The transient development of perilesional edema (PE) around ≥1 calcification (defined as 1 episode) occurs in about 50% of the patients with recurrent seizures in calcified neurocysticercosis (NCC). We determined the long-term clinical and radiological course of persons undergoing PE episodes.
Methods.
Twenty-one persons with NCC who experienced ≥1 PE episode were followed for a median of 10.6 years (range, 0.4–29.2 years). Clinical evaluations and magnetic resonance imaging (MRI) were performed at the time of suggestive symptoms and during routine follow-up.
Results.
PE episodes were documented 78 times, involving 50 of 729 calcifications. Episodes reoccurred in all but 3 persons. The pattern, rate, and number of episodes were variable, commonly chronic, and not significantly associated with time from treatment, number of calcifications, or sex. Seizure was the most common symptom, but almost 30% of episodes were asymptomatic and detected by MRI during routine follow-up. Persons with delayed recurrent episodes were significantly older (age, 42.3 vs 28.8 years; P = .045). Seizures continued to occur in 37.5%, and 2 persons had a severe disabling clinical course.
Conclusions.
The number and timing of PE episodes in individuals with calcified NCC are variable and commonly chronic, sometimes recurring over decades. A minority of patients developed significant disability.
Keywords: Taenia solium, neurocysticercosis, epilepsy, brain calcifications, brain inflammation
Calcification of degenerating Taenia solium granulomas (calcifications) in the brain commonly occurs as a normal consequence of disease progression or following cysticidal treatment. Calcifications are the most common radiological finding among cases of neurocysticercosis (NCC) in T. solium–endemic populations, with a frequency of detection of 10%–20% in the general population in regions of endemicity [1]. Among patients with T. solium calcifications and epilepsy, calcifications have been found to be the focus of seizure activation in 40%–60% [2, 3, 4, 5]. In studies where magnetic resonance imaging (MRI) was performed shortly after the abrupt onset of seizures or other symptoms, perilesional edema (PE) was frequently detected around ≥1 calcification [6, 7–13, 14–19, 20, 21]. In a prospective study of PE [1] among patients with brain calcifications and a history of seizures, MRI of 50% demonstrated newly developed PE around ≥1 calcification at the time of a seizure; PE was detected in 9% of matched asymptomatic controls from the same cohort, proving the association between PE and seizures.
Most of the evidence suggests that the PE around calcifications is due to episodic host inflammatory responses directed to parasite antigen in calcified lesions [8, 13]. The presence of inflammation in acute PE around calcified lesions is supported by direct demonstration in surgically excised involved calcifications [15, 17], upregulation of the translocator protein by positron emission tomography of the tissues surrounding calcifications undergoing acute PE [22], and increased levels of serum metalloproteases in symptomatic patients with T. solium calcifications [23, 24]. Inflammation is indirectly implicated by the appearance and/or increase of pericalcification gadolinium enhancement with MRI, which is likely caused by perturbation of the blood-brain barrier function by ongoing [25] or increased [26] host inflammation.
In the absence of specific clinical descriptions and long-term observations, the prognosis of these patients remains unclear. We determined the clinical course and characteristics of PE in calcified NCC in a cohort of 21 persons who had experienced ≥1 documented PE episode.
METHODS
From 1985 until June 2014, 101 patients with a proven or probable diagnosis of NCC were enrolled in a clinical protocol approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases (protocol 85-I-127) with objectives to evaluate, diagnose, treat, and follow participants with NCC for as long as possible. All patients signed the protocol consent. All patients had a diagnosis of definite or probable NCC, underwent an extensive history, physical, and neurological evaluation, including computed tomography (CT) and MRI of the brain; spinal imaging, for patients with extraparenchymal NCC; Western blot serological testing for cysticercosis, performed by the Centers for Disease Control and Prevention; and other tests to optimize care, including electroencephalography, when clinically indicated. Patients were discovered under the following situations: (1) evaluation of newly referred patients at enrollment and subsequent clinical and radiological follow-up; (2) routine follow-up of patients with only calcifications, usually with a prior or current history of seizures; and (3) evaluation of patients followed with other forms of NCC who had or developed calcifications after cysticidal treatment or as a consequence of the natural evolution of parenchymal cysts.
Detection and Diagnosis of Perilesional Edema Around Calcified Lesions
Calcification within lesions was documented by CT (ie, detection of a bright signal similar to bone within a degenerating cyst) in most instances but alternatively by susceptibility-weighted imaging or gradient echo MRI sequences before or at the time of a PE occurrence. PE was arbitrarily defined as the presence of an area of edema at least twice the diameter of the calcification, as assessed by fluid attenuated inversion recovery (FLAIR) or by a T2 MRI closely associated with a calcification and with new or increased pericalcification enhancement. Its presence was assessed by ≥1 author and confirmed by diagnostic staff neuroradiologists. PE around large calcifications was also included when the edema was appreciable, even if it was less than twice the calcification’s diameter. By definition, PE episodes/occurrences are transient. In patients followed serially or in those with prior available MRIs, PE was required to be absent in imaging studies predating a particular occurrence. PE had to become undetectable or be greatly reduced in size over time to be considered a separate occurrence and episode. Gross changes in the degree of enhancement were visually assessed using axial T1 postgadolinium or T1 3-dimensional (3D) sagittal postgadolinium sequences. Gliosis around a calcification was defined as an unchanged increased FLAIR signal over time. Gross changes in the degree of enhancement were visually assessed using axial T1 postgadolinium or T1 3D sagittal postgadolinium sequences. Gliosis around a calcification was defined as an unchanged increased FLAIR signal over time.
Definition of Episode and Occurrence
An episode was defined as the new appearance of perilesional edema around ≥1 calcification as ascertained by MRI, beginning at the time of newly recognized PE surrounding ≥1 calcification and/or the onset of new symptoms and ending with the disappearance of all PEs. An episode may not have been accompanied by newly detected symptoms. In most instances, imaging was prompted by the onset of new clinical symptoms, most commonly seizures.
The description, imaging characteristics of each PE calcification, and duration of its associated edema in 1 specific episode was designated as an occurrence. The separate definition for a calcification and its associated edema over time as an occurrence allows analysis of features and characterization of all calcifications with PE, even when multiple calcifications were involved at the same time or during different episodes. The duration of PE was defined as the interval between the date of the MRI documenting an episode to the date of the MRI in which the size of the edema in a specific calcification was no longer present or, in some instances, barely discernable. An involved calcification was defined as any calcification that caused an occurrence.
Assessment of Suspected Episodes
Evaluation included clinical and neurological examinations; assessment for potential causes of seizures or symptoms, including review of antiepileptic drugs and their blood concentrations, if measureable; MRI as soon as possible following the onset of suggestive symptoms; electroencephalography when clinically indicated; routine hematological and chemical laboratory testing; and other testing as medically required. Referred patients underwent MRI and, if findings of a recent CT were not available, CT. Patients with seizures initiated antiseizure medication and were monitored appropriately for treatment adherence and maintenance of therapeutic blood levels. The timing and number of post-episode MRIs were variable, but an attempt was made to document the duration of perilesional edema, if logistically feasible. Patients were followed as clinically indicated. Asymptomatic patients were routinely reevaluated annually or semiannually, as outlined above, including a routine MRI.
Non–PE-Associated Seizures
A seizure not associated with PE was defined when PE was not present 17 days after a clinical event. This interval was selected because PE was present in all patients for at least 18 days after the clinical onset of symptoms.
Time Line of Episodes
The date of the MRI documenting the first episode was defined as time 0. The dates of treatment and enrollment and the duration of follow-up at the National Institutes of Health (NIH) were referenced to time 0, allowing comparison of the timing of episodes for all persons, including those without clinical symptoms. A number of patients had extensive documentation before enrollment at the NIH; these data were included in the analysis if an episode could be confirmed by MRI/CT.
Symptoms accompanying PE episodes were determined for all patients who were followed at the NIH and for the episode preceding enrollment, if it occurred within the prior year and the symptoms were known or recalled in sufficient detail.
Seizure Assessment
Seizure diagnosis and classification of seizure type followed the definitions of the International League Against Epilepsy [27].
Statistical Methods
Between-group comparisons were performed with nonparametric tests, such as the Mann-Whitney U test. χ2 statistics with contingency tables or Fisher’s exact tests (for comparisons between 2 groups) were used for comparing proportions and frequencies. Kaplan Meier statistics were used for survival analysis of time to PE episodes. Data were treated as nonparametric or were log transformed for parametric statistical analysis. Statistical analysis was performed using Prism software (GraphPad Software, La Jolla, CA)
RESULTS
Characteristics of Patients
Ninety-nine patients enrolled in the NIH neurocysticercosis protocol (85-I-0127) from 1985 until June 2014 (2 patients recruited from Peru for the study of PE were excluded) had an established diagnosis of neurocysticercosis that was based on previously published criteria [28]. Twenty-one patients had ≥1 documented PE episode, and 78 patients did not. There were no significant differences in age at enrollment (median age 30.7 years among patients with PE episodes vs 34 years among those with no episodes), female sex (66.7% of patients with PE vs 47.4% of patients without PE), or median duration of follow-up at the NIH (8.5 years among patients with PE episodes vs 6.8 years among those with no episodes).
All 21 patients had prior and/or current imaging diagnostic or suggestive of neurocysticercosis. At the time of the first PE episode, 20 had only calcifications, and the remaining patient had both calcifications and a cystic lesion that was successfully treated later. At enrollment, 16 (76.2%) had NCC-positive results on serologic testing. Three persons had other extraparenchymal manifestations of NCC, consisting of a calcified Sylvian fissure cyst in the first patient, 2 surgically removed fourth ventricular cysts and a lateral ventricular cyst in the second patient, and a lateral ventricular cyst resulting in an entrapment in the third patient. The latter patient also developed hydrocephalus. Most (19 of 21 [90.5%]) had >1 calcified lesion. One of the 2 persons with typical single calcifications had negative results on serologic testing for cysticercosis and was considered to have NCC because of the presence of a calcification and symptoms typical of a patient born and raised in a region of endemicity. All but 1 patient (ie, 20 [95.2%]) had a history and exposure suggestive of NCC. Thirteen (61.9%) were born or exposed in Latin America, 6 (28.6%) were born or exposed in Asia, 1 (4.8%) was exposed in Africa, and 1 (4.8%) had no known exposure outside of the United States. Three (14.3%) were expatriates or travelers to NCC-endemic countries. The patient without known exposure had a single degenerating lesion that calcified and had 2 Western blots positive for cysticercosis [29]. Including PE episodes before enrollment at the NIH, patients were followed for 228 patient-years, with a median follow-up duration of 10.6 years/patient (range, 0.4–29.2 years/patients). At the NIH, patients were followed for 180 patient-years, with a median follow-up duration of 8.5 years/patient (range, 0.3–28.8 years/patient).
Clinical Manifestations of PE Episodes
The presence and nature of accompanying symptoms could be determined in 65 of 78 PE episodes (83.3%; Table 1). A majority of evaluable episodes (46 of 65 [70.8%]) were associated with clinical symptoms. Seizure, occurring in 50.8%, was the most common symptom (focal, 19 evaluable episodes [29.2%]; generalized, 12 [18.5%]; and focal with generalization, 2 [3.1%]). Focal neurological complaints other than seizures were seen in 11 (16.9%). Although headache was a common complaint, it usually accompanied other symptoms. Headaches were the only symptom in only 2 instances (3.1%). A majority of patients (13 of 21 [61.9%]) experienced ≥1 asymptomatic episode, which comprised 29.2% of all episodes (19 of 65; Table 1). Repeated occurrences involving a same calcification tended to result in similar symptoms. In 15 of 78 episodes (19.2%), ≥2 calcifications demonstrated perilesional edema simultaneously (multiple occurrences). While all 21 patients with PE had at some point at least 1 episode of PE with associated neurological symptoms, a majority (13 [61.9%]) also experienced ≥1 asymptomatic episode, which comprised 29.2% of all episodes (19 of 65; Table 1).
Table 1.
Demographics, Characterization of Episodes and Associated Symptoms
| Variable | Value |
|---|---|
| Demographic characteristics | |
| Age at enrollment, y | 30.7 (19.7–69.8) |
| Female sex, persons, % | 66.7 |
| Site of birth or exposure, persons, % | |
| Latin America | 61.9 |
| Asia | 28.6 |
| Africa | 4.8 |
| No foreign exposure | 4.8 |
| Expatriates or travelers with >1 mo of exposure in NCC-endemic area, % | 14.3 |
| Follow-up duration, y | |
| At NIH only | 8.5 (0.3–28) |
| At NIH and elsewhere | 10.6 (0.4–29.2) |
| Episodes | |
| ≥1, persons, no. | 21 |
| Total no. | 78 |
| Evaluable for symptoms within 1 y of enrollment | 65/78 (83.3) |
| Recurrent | 18/21 (85.7) |
| No./person | 3 (0.1–5) |
| Incidence, no./evaluable year (range) | 0.42 (0.1–5) |
| Any asymptomatic | 13/21 (61.9) |
| 1 symptomatic | 7/13 (53.8) |
| 2 symptomatic | 6/13 (46.2) |
| Multiple occurrences | 15/78 (19.2) |
| Clinical symptoms of episodes | |
| Symptomatic | 46/65 (70.8) |
| Asymptomatic | 19/65 (29.2) |
| Focal seizures | 19/65 (29.2) |
| Generalized seizures | 12/65 (18.5) |
| Focal neurological | 11/65 (16.9) |
| Focal seizures with generalization | 2/65 (3.1) |
| Headaches | 2/65 (3.1) |
Data are no. of persons with the characteristic/no. evaluated (%) or median value (range), unless otherwise indicated.
Abbreviations: NCC, neurocysticercosis; NIH, National Institutes of Health.
Multiple PE Episodes in the Same Patient
Most patients (18 of 21 [85.7%]) experienced multiple PE episodes (median, 3 episodes; range, 1–14 episodes), and only 3 patients experienced a single episode without any recurrences (Table 1). In 15 of 78 episodes (19.2%), >1 calcification demonstrated perilesional edema simultaneously. The incidence of episodes was 0.42 per evaluable year. (range, 0.1–5 episodes/evaluable year).
Duration and Time Course of Episodes
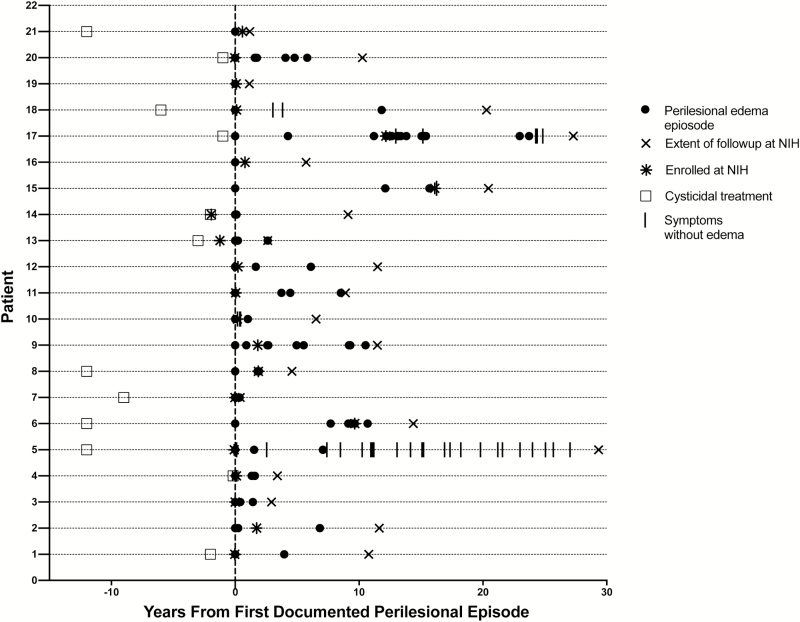
The duration of PE was determined for 24 occurrences in 11 individuals for whom the spacing of multiple MRIs allowed a reasonable determination. Overall, 24 PE occurrences persisted for a median duration of 40 days, with the duration never being <18 days (range, 18–421 days). Most occurrences were short lived, with a median duration of 35 days (range, 18–77 days; 18 occurrences in 7 individuals). However, in 4 patients there were 6 prolonged occurrences, with a median duration of 238 days (range, 98–421 days). A marked decrease in enhancement over time following an occurrence that included complete loss of enhancement did not predict an absence of future episodes or occurrences. The time of PE episodes and non–PE-associated seizures, the time of cysticidal treatment, and the extent of observation at the NIH for the 21 patients with PE episodes are shown in relation to the first documented PE episode in Figure 1. The pattern and chronology of PE episodes varied (Figure 2). Whereas 3 patients had 1 episode, others experienced a few episodes and then ceased having them, and some experienced many episodes over the period of observation. The pattern of PE episodes may have been influenced in 3 cases (patients 3, 13, and 17) by the tapering or stopping of corticosteroid therapy, possibly precipitating episodes [16] or by use of methotrexate, which was administered in 1 case (patient 17) and anecdotally prevented seizures for the next 8 years [30].
Figure 1.
Time course of episodes plotted from the date of the first documented perilesional episode (vertical line) in 21 patients. Each horizontal line is the time course for 1 patient. Some referral records allowed documentation of perilesional edema (PE) episodes before enrollment. Persons whose treatment is not denoted either received no treatment or had inadequate documentation. Abbreviation: NIH, National Institutes of Health.
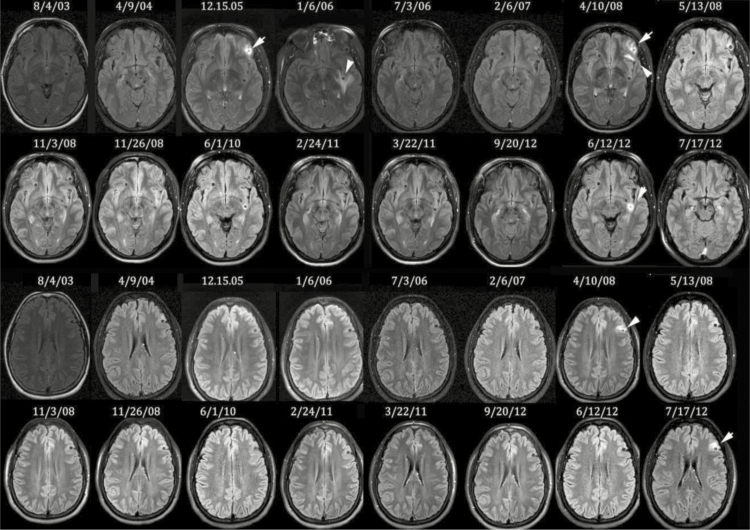
Figure 2.
Course of perilesional edema episodes/occurrences over time in 1 patient. Serial fluid-attenuated inversion recovery magnetic resonance images obtained over approximately 9 years in 1 patient focused on 2 involved calcifications (one shown in the upper 2 panels and the other shown in the lower 2 panels). The course of follow-up was more extensive than shown, and not all occurrences are presented.
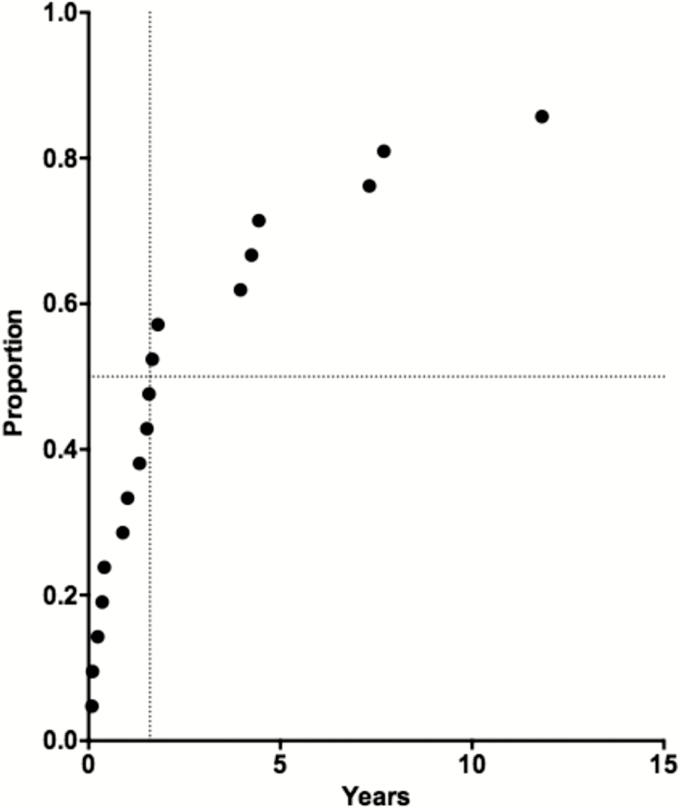
Time to the First Recurrent Episode
The time to the first recurrent PE episode after the initial PE episode is shown in Figure 3. One or more recurrent PE episodes occurred in 18 of 21 subjects. Patients could be reasonably divided into 2 groups: 12 (66.7%) had a recurrent PE episode within 1.8 years (median, 0.96 years; range, 0.09–1.8 years), whereas 6 (33.3%) had delayed recurrences (median, 5.9 years; range, 4–12 years). Those with earlier recurrences were significantly younger (median age, 28.8 vs 42.3 years; P = .025, by the Mann-Whitney test) at enrollment. However, there were no significant differences between the groups in the number of episodes or occurrences, the number of total calcifications or involved calcifications, sex, age at the time of their initial symptoms, or treatment of viable cysts.
Figure 3.
Kaplan-Meier survival curve of the time from the first documented episode to the first recurrent episode. Only 18 of 21 persons had a recurrence. The 6 outliers with the longest time to the recurrence were compared to the remaining 12.
The date of cysticidal treatment was known for 11 patients (Figure 1). Because PE episodes might occur more frequently in recently degenerated cysts in which the interval between cysticidal treatment and the initial PE episode was short, compared with the frequency in those in which the interval was longer, we tested for this possibility in this subset. There was no correlation between the duration between cysticidal treatment and the onset of the initial PE episode.
Involved Calcifications
Fifty of 729 calcifications (6.9%; Table 2) were involved in 107 PE occurrences (median, 3 occurrences/patient; median, 5 calcifications/patient; and median, 2 involved calcifications/patient). The location of involved calcifications is reviewed in Table 2.
Table 2.
Characterization of Calcifications, Location of Involved Calcifications, and Clinical State at Last Assessment
| Variable | Value |
|---|---|
| Calcifications | |
| Total no. | 729 |
| Involved, no. (%) | 50 (6.9) |
| Total/patient, no., median (range) | 5 (1–280) |
| Involved/patient, no., median (range) | 2 (1–11) |
| Total documented occurrences, no.a | 107 |
| Occurrences/person, no., median (range) | 3 (1–32) |
| Location of involved calcifications, % | |
| Right frontal | 20 |
| Left frontal | 26 |
| Right parietal | 14 |
| Left parietal | 10 |
| Right occipital | 6 |
| Left occipital | 12 |
| Right temporal | 0 |
| Left temporal | 8 |
| Right other | 2 |
| Left other | 2 |
| Clinical state at last assessment, patients, no. (%) | |
| Evaluableb | 16 (76.2) |
| Seizures within past 2 years | 6 (37.5) |
| Taking antiseizure medication | 10 (62.5) |
| Headaches | 6 (37.5) |
| Depression | 2 (12.5) |
| Dementia | 1 (6.25) |
| Extraparenchymal sequelae | 2 (12.5) |
| Disability from removal of degenerating cyst | 1 (6.25) |
| Mesial temporal sclerosis | 1 (6.25) |
Data are for each calcification with ≥1 occurrence.
On protocol, seen or contacted within past 4 years.
Possible Associations Between Calcifications and Frequencies of Occurrences or Episodes
There was a significant correlation between the number of occurrences and the total number of calcifications (P = .046, by the Spearman correlation test) but not for the number of episodes and the total number of calcifications (P = .426, by the Spearman correlation test). However, the numbers of occurrences and episodes were significantly correlated with the number of involved calcifications (P = .001 and P = .008, respectively, by the Spearman correlation test). The frequencies of PE occurrences and episodes in the treated group (60 occurrences and 40 episodes among 11 persons) were not significantly different (by the Mann-Whitney test) from those in the untreated group (32 episodes and 31 occurrences among 7 persons). Although the frequencies of occurrences and episodes were greater among females, there was no significant correlation (by the Mann-Whitney test) between sex or age and the frequencies of occurrences (86 among 14 females vs 21 among 7 males) or episodes (62 among 14 females vs 18 among 7 males). Similarly, despite a greater frequency of episodes among patients with positive results of serologic testing for cysticercosis (66 episodes among 16 patients), compared with patients with negative results (11 among 5), the difference was not statistically significant (by the Mann-Whitney test). There was a trend toward a greater frequency of occurrences if results of serologic analysis were positive (95 occurrences among 16 patients with positive results vs11 among 5 with negative results; P = .096, by the Mann-Whitney test).
General Clinical Course
Ongoing symptoms, long-term sequelae, and disability among patients at the end of the observation period (June 2014) are summarized in Table 2. A majority of patients fared moderately well in the long term. Seizures within the prior 2 years and headaches were the most common symptoms, each occurring in a little more than one third of patients (37.5%). Ongoing treatment with antiseizure medications was required in 62.5%. Five of 21 patients (23.8%) also had non–PE-related seizures, which contributed to morbidity in the 2 patients with the most-severe clinical courses. One patient transitioned exclusively to many non–PE-related seizures, while the other patient also developed mesial temporal sclerosis, loss of brain mass, dementia, and perilesional gliosis involving many of the patient’s numerous calcifications.
DISCUSSION
This is the first detailed description and characterization of the natural history of PE in calcified NCC in persons followed over an extended period. Most patients experienced recurrent episodes. In 76.2% of these patients, PE episodes were essentially the only documented pathophysiological process associated with seizures.
The clinical course of disease was highly variable, ranging from a single episode involving a single calcification to many episodes encompassing multiple involved calcifications, often with ≥2 simultaneous PE occurrences, resulting in lifelong incapacitating epilepsy. Although the clinical course was varied, it was commonly chronic. Of the 10 patients who were under our observation for at least 10 years, half continued to experience recurrent PE episodes throughout the period.
As summarized in Table 2, the continuing symptoms, clinical state, and residual disability of patients with PE at the end of the observation period was variable, but on the whole most patients did reasonably well. Patient 17 experienced many PE episodes and developed dementia, pericalcification gliosis [23, 24], and mesial temporal sclerosis [31, 32], all likely complications of epilepsy from NCC. Although most patients with PE episodes had seizures related to that pathophysiology, 5 patients, including 2 with the most-severe clinical course, also experienced non–PE-related seizures, possibility further contributing to their severe clinical course.
The duration of PE around individual calcifications was surprisingly variable. While the median duration in 18 of 24 (75%) was 35 days, the median duration in the remaining 6 (25%) was 238 days, with some lasting >1 year. Symptoms in the latter group waxed and waned in parallel with the changes in the extent of the perilesional edema.
A surprising 29.2% of all episodes were detected in asymptomatic individuals, over 3 times greater than the frequency found in our previous prospective study [1]. This was almost certainly due to our practice of obtaining MRIs during routine clinic visits and for evaluation of other manifestations of NCC in patients who also had parenchymal calcifications. All patients with asymptomatic PE episodes also had PE associated with overt seizures, so they had both symptomatic and asymptomatic PE episodes. A logical explanation is that PE episodes/occurrences detected by imaging are more common than appreciated clinically but at times fail to cause appreciable symptoms. The association of seizures and PE was conclusively established in a prior randomized, controlled, observational study in which 50% of patients experiencing a seizure demonstrated PE, while only 9% of a matched control group from the same cohort showed PE [1]. Patients were routinely administered therapeutic doses of antiepileptic drugs, which could also have suppressed clinical symptoms.
The present study indicates that PE episodes are frequently recurrent and chronic, commonly reoccurring over ≥1 decade. Because the available evidence indicates that PE episodes are caused by periodic bouts of increased inflammation centered on calcifications, there is a reasonable possibility that immunosuppressive treatments may be able to prevent and treat seizures caused by this pathophysiological mechanism [30]. However, optimal management has not been defined, and in the case of corticosteroid use, withdrawal can potentially worsen perilesional edema [16]. Clearly, further study is needed to define optimal therapy.
Notes
Financial support. This work was supported by intramural National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Nash TE, Pretell EJ, Lescano AG, et al. ; Cysticercosis Working Group in Peru. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol 2008; 7:1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh G, Sachdev MS, Tirath A, Gupta AK, Avasthi G. Focal cortical-subcortical calcifications (FCSCs) and epilepsy in the Indian subcontinent. Epilepsia 2000; 41:718–26. [DOI] [PubMed] [Google Scholar]
- 3. Cukiert A, Puglia P, Scapolan HB, Vilela MM, Marino Júnior R. Congruence of the topography of intracranial calcifications and epileptic foci. Arq Neuropsiquiatr 1994; 52:289–94. [DOI] [PubMed] [Google Scholar]
- 4. Murthy JM, Reddy VS. Clinical characteristics, seizure spread patterns and prognosis of seizures associated with a single small cerebral calcific CT lesion. Seizure 1998; 7:153–7. [DOI] [PubMed] [Google Scholar]
- 5. Rathore C, Thomas B, Kesavadas C, Abraham M, Radhakrishnan K. Calcified neurocysticercosis lesions and antiepileptic drug-resistant epilepsy: a surgically remediable syndrome? Epilepsia 2013; 54:1815–22. [DOI] [PubMed] [Google Scholar]
- 6. Nash TE, Del Brutto OH, Butman JA, et al. Calcific neurocysticercosis and epileptogenesis. Neurology 2004; 62:1934–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Del Brutto OH. Prognostic factors for seizure recurrence after withdrawal of antiepileptic drugs in patients with neurocysticercosis. Neurology 1994; 44:1706–9. [DOI] [PubMed] [Google Scholar]
- 8. DelBrutto OH, Campos X. Discontinuation of antiepileptic drugs in patients with calcified neurocysticercosis. J Epilepsy 1996; 9:231–3. [Google Scholar]
- 9. White AC., Jr Neurocysticercosis: a major cause of neurological disease worldwide. Clin Infect Dis 1997; 24:101–13; quiz 114–5. [DOI] [PubMed] [Google Scholar]
- 10. Antoniuk SA, Bruck I, Dos Santos LH, et al. Seizures associated with calcifications and edema in neurocysticercosis. Pediatr Neurol 2001; 25:309–11. [DOI] [PubMed] [Google Scholar]
- 11. Park SY, Barkovich AJ, Weintrub PS. Clinical implications of calcified lesions of neurocysticercosis. Pediatr Infect Dis J 2000; 19:581–3. [DOI] [PubMed] [Google Scholar]
- 12. Poeschl P, Janzen A, Schuierer G, Winkler J, Bogdahn U, Steinbrecher A. Calcified neurocysticercosis lesions trigger symptomatic inflammation during antiparasitic therapy. AJNR Am J Neuroradiol 2006; 27:653–5. [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta RK, Kumar R, Chawla S, Pradhan S. Demonstration of scolex within calcified cysticercus cyst: its possible role in the pathogenesis of perilesional edema. Epilepsia 2002; 43:1502–8. [DOI] [PubMed] [Google Scholar]
- 14. Gupta RK, Kathuria MK, Pradhan S. Magnetisation transfer magnetic resonance imaging demonstration of perilesional gliosis–relation with epilepsy in treated or healed neurocysticercosis. Lancet 1999; 354:44–5. [DOI] [PubMed] [Google Scholar]
- 15. Ooi WW, Wijemanne S, Thomas CB, Quezado M, Brown CR, Nash TE. Short report: a calcified Taenia solium granuloma associated with recurrent perilesional edema causing refractory seizures: histopathological features. Am J Trop Med Hyg 2011; 85:460–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mejia R, Nash TE. Corticosteroid withdrawal precipitates perilesional edema around calcified Taenia solium cysts. Am J Trop Med Hyg 2013; 89:919–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nash TE, Bartelt LA, Korpe PS, Lopes B, Houpt ER. Calcified neurocysticercus, perilesional edema, and histologic inflammation. Am J Trop Med Hyg 2014; 90:318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nash TE, Patronas NJ. Edema associated with calcified lesions in neurocysticercosis. Neurology 1999; 53:777–81. [DOI] [PubMed] [Google Scholar]
- 19. Sheth TN, Lee C, Kucharczyk W, Keystone J. Reactivation of neurocysticercosis: case report. Am J Trop Med Hyg 1999; 60:664–7. [DOI] [PubMed] [Google Scholar]
- 20. Garg RK, Karak B, Mohan Kar A. Neuroimaging abnormalities in Indian patients with uncontrolled partial seizures. Seizure 1998; 7:497–500. [DOI] [PubMed] [Google Scholar]
- 21. Nash TE, Pretell J, Garcia HH. Calcified cysticerci provoke perilesional edema and seizures. Clin Infect Dis 2001; 33:1649–53. [DOI] [PubMed] [Google Scholar]
- 22. Fujita M, Mahanty S, Zoghbi SS, et al. PET reveals inflammation around calcified Taenia solium granulomas with perilesional edema. PLoS One 2013; 8:e74052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gupta RK, Awasthi R, Rathore RK, et al. Understanding epileptogenesis in calcified neurocysticercosis with perfusion MRI. Neurology 2012; 78:618–25. [DOI] [PubMed] [Google Scholar]
- 24. Verma A, Prasad KN, Nyati KK, et al. Association of MMP-2 and MMP-9 with clinical outcome of neurocysticercosis. Parasitology 2011; 138:1423–8. [DOI] [PubMed] [Google Scholar]
- 25. Sheth TN, Pillon L, Keystone J, Kucharczyk W, Pilon L. Persistent MR contrast enhancement of calcified neurocysticercosis lesions. AJNR Am J Neuroradiol 1998; 19:79–82. [PMC free article] [PubMed] [Google Scholar]
- 26. Marzal M, Guerra-Giraldez C, Paredes A, et al. ; Cysticercosis Working Group in Peru. Evans blue staining reveals vascular leakage associated with focal areas of host-parasite interaction in brains of pigs infected with Taenia solium. PLoS One 2014; 9:e97321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anonymous Guidelines for epidemiologic studies on epilepsy. commission on epidemiology and prognosis, international league against epilepsy. Epilepsia 1993; 34:592–6. [DOI] [PubMed] [Google Scholar]
- 28. Del Brutto OH, Rajshekhar V, White AC, Jr, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology 2001; 57:177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis 1989; 159:50–9. [DOI] [PubMed] [Google Scholar]
- 30. Mitre E, Talaat KR, Sperling MR, Nash TE. Methotrexate as a corticosteroid-sparing agent in complicated neurocysticercosis. Clin Infect Dis 2007; 44:549–53. [DOI] [PubMed] [Google Scholar]
- 31. Bianchin MM, Velasco TR, Wichert-Ana L, et al. Neuroimaging observations linking neurocysticercosis and mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Res 2015; 116:34–9. [DOI] [PubMed] [Google Scholar]
- 32. Bianchin MM, Velasco TR, Santos AC, Sakamoto AC. On the relationship between neurocysticercosis and mesial temporal lobe epilepsy associated with hippocampal sclerosis: coincidence or a pathogenic relationship? Pathog Glob Health 2012; 106:280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]