Summary
The immunogenicity of the varicella-zoster virus vaccine in elderly nursing home residents has an inverse relationship with serum levels of C-reactive protein and heart failure status. Furthermore, heart failure in this population is associated with markers of T-cell immunosenescence.
Keywords: Vaccination, Varicella-zoster, inflammation, heart failure, immunosenescence
Abstract
Background
Elderly long-term care residents often exhibit a myriad of risk factors for immune dysfunction, including chronic inflammation and multiple comorbid conditions, which undoubtedly contribute to their enhanced susceptibility to infection. Hence, understanding the factors required for optimal vaccine responsiveness is critical.
Methods
We examined 187 elderly nursing home residents (aged 80–102 years) and 50 community-dwelling seniors (aged 60–75 years) immunized with the live-attenuated varicella-zoster virus (VZV) vaccine. Specifically, we examined whether vaccine responsiveness was associated with serum C-reactive protein (CRP), tumor necrosis factor, interleukin 1β, 6, and 10, leukocyte telomere length, chronic disease status, and frailty.
Results
Elderly participants had significantly higher levels of CRP, tumor necrosis factor, and interleukin 6 and shorter leukocyte telomere length. Vaccine responsiveness was inversely related to the CRP level in elderly participants, but not seniors, and those with congestive heart failure were less likely to achieve a 2-fold response (odds ratio, 0.08). The latter relationship is probably due to immunosenescence, because heart failure was associated with increased senescent CD4+ T cells, and reduced naive and effector and central memory CD8+ T cells.
Conclusions
In summary, these data improve our understanding of vaccine responsiveness for those in long-term care, suggesting that certain risk factors are associated with a greater likelihood of vaccine failure.
Primary infection with varicella-zoster virus (VZV) usually occurs in young children, resulting in chickenpox. After illness, VZV becomes latent in the dorsal root ganglion, a cluster of nerve cell bodies in a dorsal root of the spinal nerve, and remains dormant until reactivation [1]. Reactivation often leads to herpes zoster (HZ), also known as shingles, which is characterized by a painful skin rash lasting days to weeks [2, 3]. Pain persisting >90–120 days after HZ rash onset is known as postherpetic neuralgia [4] and can require analgesic and neuroactive drugs for pain management as well as hospitalization [3].
VZV-specific immunity wanes with age [5–7], which is probably the major contributing factor for the elevated incidence of HZ and postherpetic neuralgia in older adults [8]. Elderly residents of long-term care are of particular concern with regard to development HZ, owing not only to their advanced age but also to a high degree of immunosenescence, malnutrition, and existing chronic conditions [9]. Lelic et al [10] showed that elderly nursing home residents do indeed have reduced VZV-specific immunity before and after vaccination, even though their vaccine response did not differ from that in community-dwelling older adults when measured as a fold change relative to baseline. That said, variability in the magnitude of this response was significantly greater in the elderly, suggesting the presence of underlying factors that influence the vaccine response.
In the previous study, Lelic et al [10] also showed that the frequency of cytomegalovirus-specific CD4+ T cells and regulatory T cells, 2 lymphocyte subsets that potently suppress viral immunity [11, 12], were inversely related to the vaccine response of elderly nursing home residents. Other studies in community-dwelling adults found that chronic conditions and comorbid conditions such as diabetes [13] and depression [14], and changes in serum cytokines [15], significantly influence VZV vaccine responsiveness. Other risk factors or mechanisms that influence the response to VZV vaccination in older adults, whether community dwelling or in long-term care, remain to be discovered.
In the current study, we sought to expand our understanding of the risk factors related to VZV vaccine responsiveness in elderly long-term care residents. Specifically, we investigated the levels of serum C-reactive protein (CRP), tumor necrosis factor (TNF), interleukin 6, 1β, and 10 (IL-6, IL-1β, and IL-10), leukocyte telomere length, disease status, and frailty, comparing those relationships with vaccine responsiveness to findings in community-dwelling seniors (60-75 years).
MethodS
VZV Vaccine Study: Participants and Design
This study is based on participants from a previously published trial comparing VZV vaccine immunogenicity in community-dwelling seniors and elderly nursing home residents ([10]; Clinical Trials registration: NCT01328548). For this study, nursing home residents (n = 187) from 18 facilities in Hamilton and Toronto (Ontario, Canada) were recruited between January 2012 and February 2014; they were between 80 and 102 years of age (median, 89 years), 81% female, and all had ≥1 comorbid condition. Community-dwelling seniors (n = 50) from the same geographic location were recruited between the months of February and May 2012, and were between 60 and 75 years of age (median age, 68 years), 64% female, and had no more than 1 comorbid condition.
Frailty, defined using a frailty index score [10, 16], and comorbidity status, defined using a major condition score [10], were included in analyses, as well as the following comorbid conditions: congestive heart failure (CHF; prevalence in elderly nursing home residents, 13%), chronic pulmonary disease (11%), dementia (67%), diabetes (with or without end-organ damage, 23%), hemiplegia (12%), and peripheral vascular disease (64%); comorbid conditions with a prevalence <10% in the nursing home residents were not included. Written informed consent was obtained from all participants or their legally appointed guardians. The study protocol and consent procedures were approved by the McMaster Research Ethics Board and the participants’ nursing homes.
All participants were subcutaneously administered the live-attenuated Oka strain of VZV (≥19400 plaque-forming units; Zostavax, Merck Group). Heparinized venous blood was obtained before vaccination (baseline) and 6 weeks later (follow-up) and processed within 8 hours of blood collection; serum was collected only at baseline and stored at −20°C. Peripheral blood mononuclear cells (PBMCs) were isolated using a validated protocol [17] and were cryopreserved in vapor-phase liquid nitrogen (160°C) in 10% dimethyl sulfoxide/human AB serum (Lonza) freezing medium.
Interferon γ Enzyme-Linked Immunospot Assay
Vaccine responses were determined by interferon (IFN) γ enzyme-linked immunospot assay, as described elsewhere [10]. Briefly, cryopreserved PBMCs were thawed at 37°C, washed and resuspended in complete medium. Cells (5 × 105) were added to Multiscreen-IP membrane plates (Millipore) coated with anti-human IFN-γ and stimulated with VZV antigen for 20 hours at 37°C; VZV antigen was prepared from lysed, UV light–inactivated VZV-infected MRC-5 cells. Plates were subsequently incubated with biotinylated anti-human IFN-γ, followed by streptavidin-alkaline phosphatase. Spots were enumerated by CTL ImmunoSpot Image Analyzer system and counting software (Cellular Technologies), and VZV-specific responses were reported as absolute VZV spot-forming cells per 106 PBMCs. Fold-change responses were calculated as the VZV-specific spot-forming cell count at follow-up relative to the baseline count.
Serum Cytokines, CRP, and Telomere Length Quantification
Serum cytokines TNF, interleukin (IL)-6, IL-1β and IL-10 were measured using the Milliplex MAP Human High Sensitivity T Cell kit (Millipore) according to manufacturer’s recommendations. CRP was measured in serum (1/500 diluted) by sandwich enzyme-linked immunosorbent assay using the monoclonal capture and detection antibody clones C5 (ab8279; 1 μg/mL) and C6 (ab24462; 1 μg/mL) (Abcam), respectively, and using native, purified CRP as a standard (Aviva Systems Biology). Cytokines and CRP were natural log-transformed in regression analyses to minimize the effect of outliers.
Telomere length was measured in heparinized peripheral blood. Briefly, genomic DNA was extracted from whole blood using the DNeasy Blood Mini Kit (Qiagen). Telomere length was measured using a validated quantitative polymerase chain reaction assay [18] at the Genetic and Molecular Epidemiology Laboratory in Hamilton, Ontario, and reported as the telomere to single-copy gene (T/S) ratio.
Analysis of Immunosenescence in Elderly Participants With CHF
To examine the effects of CHF on immunosenescence, we analyzed a large cohort of nursing home residents in whom T-cell immunosenescence markers were previously quantified [19–21]. This cohort includes 1072 participants from 32 nursing homes in 4 Canadian cities (including Hamilton, Ontario) and is similar to the current cohort with regard to median age (86 years), sex ratio (72% female), and CHF prevalence (14%). Written informed consent was obtained from all participants or their legally appointed guardians, and the study protocol and consent procedures were approved by the McMaster Research Ethics Board and the participants’ nursing homes. The following CD4+ and CD8+ T-cell subsets were enumerated in cryopreserved PBMCs by flow cytometry: naive, CD45RA+CCR7+; central memory, CD45RACCR7+; effector memory, CD45RA−CCR7−; terminally differentiated, CD45RA+CCR7−; and senescent, CD28−CD57+. Each subset was reported as the percentage of CD3+ cells and natural-log-transformed to minimize the effects of outliers. To allow log-transformation of those subsets whose frequency was zero, 0.1% was added to all subsets.
Statistics
All analyses were performed using R software, version 3.3.2 (R Foundation for Statistical Computing). Pairwise comparisons were performed by means of Wilcoxon rank sum test or Spearman correlation test. Associations with disease status were determined by using multiple logistic regression, adjusting for age and sex. For associations with continuous log2 VZV fold-change responses or having a ≥2-fold VZV vaccine response, multiple linear regression and logistic regression were performed, respectively, adjusting for age, sex, and log2 baseline VZV response. These models were performed in the elderly and senior cohorts separately, and the resulting trends were compared afterward; adjustment for the baseline response was deemed necessary, because it is significantly associated with the overall fold-change vaccine response in both elderly persons (β = −.48; P < .001) and seniors (β = −.47; P < .001), and such adjustment improved the fitness of the above models, as determined by the Akaike information criterion (data not shown). For tests of immunosenescence markers, linear mixed models were used, fitting age, sex, and CHF as fixed effects and nursing home residence as a random effect. Type II P values were calculated using the Wald χ2 test.
RESULTS
Elevated Serum Cytokine and CRP Levels in Elderly Nursing Home Residents
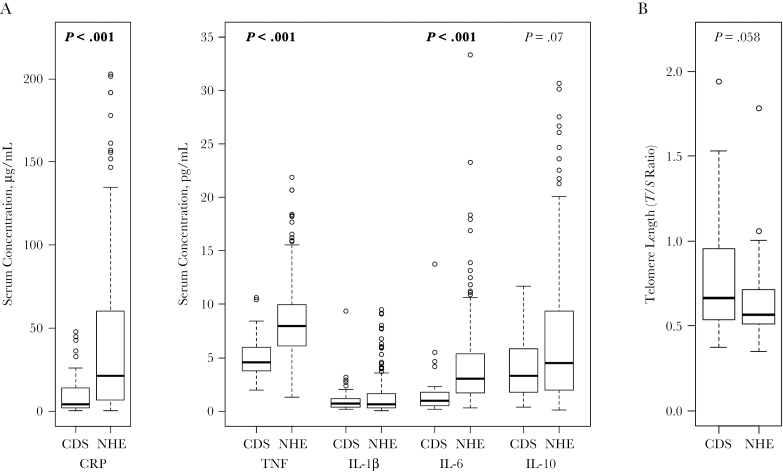
The levels of TNF, IL-1β, IL-6, IL-10, and CRP were measured in the serum of elderly nursing home residents (n = 187) and community-dwelling seniors (n = 50). Elderly participants were older (median age [range], 89 [80–102] vs 68 [60–75] years), were more frail (median frailty index, 0.31 vs 0.03; P < .001), and had a higher comorbidity score (2 vs 0; P < .001). As expected, the levels of serum cytokines and CRP were also higher, as follows: CRP (median [interquartile range], 21.2 μg/mL [6.7–57.2] vs 4.1 μg/mL [1.9–13.3]; P < .001), TNF (7.9 pg/mL [6.1–10.0 ] vs 4.6 pg/mL [3.7–5.9]; P < .001), IL-6 (3.1 pg/mL [1.7–5.3] vs 0.95 pg/mL [0.49–1.76]; P < .001) (Figure 1A). Leukocyte telomere length was also measured in a subset of participants (elderly, n = 35; seniors, n = 39), and although the median length was shorter in the elderly (T/S ratio, median [interquartile range], 0.57 [0.51–0.71] vs 0.66 [0.54–0.96]), this difference did not reach statistical significance (P = .058) (Figure 1B).
Figure 1.
Serum levels of inflammatory mediators are significantly higher in elderly nursing home residents. A, Serum C-reactive protein (CRP), tumor necrosis factor (TNF), and interleukin 1β, 6, and 10 (IL-1β, IL-6, and IL-10) were measured in the nursing home elderly (NHE; n = 187) and community-dwelling senior (CDS; n = 50) cohorts. B, Telomere length was measured in leukocytes from a subset of NHE (n = 35) and CDS (n = 39) donors. Significance was determined by means of Wilcoxon rank sum test, and only results with P values <.10 are shown.
The levels of serum cytokines and CRP were also compared between elderly participants with or without the following diseases, using logistic regression: CHF (prevalence, 13%), peripheral vascular disease (64%), dementia (67%), chronic pulmonary disease (11%), diabetes (23%), and hemiplegia (12%). These diseases were selected because they exhibited a prevalence >10% in our nursing home elderly cohort; no disease had >2% prevalence in the senior cohort; hence, seniors were not included in the analysis. Elderly participants with diabetes had significantly lower levels of CRP (natural-log-transformed mean [standard error (SE)], 2.24 μg/mL [0.13] vs 3.13 μg/mL [0.12]; P = .002), those with hemiplegia had significantly lower levels of TNF (1.85 [0.04] vs 2.06 [0.04]; P = .045), and those with peripheral vascular disease had significantly higher levels of TNF (2.12 [0.05] vs 1.90 [0.06]; P = .004). No associations were observed between the levels of serum cytokines/CRP and frailty or comorbidity score, or between telomere length and disease status, frailty, or comorbidity score.
Association of CRP and Disease Status With VZV Vaccine Response in Elderly but Not Senior Participants
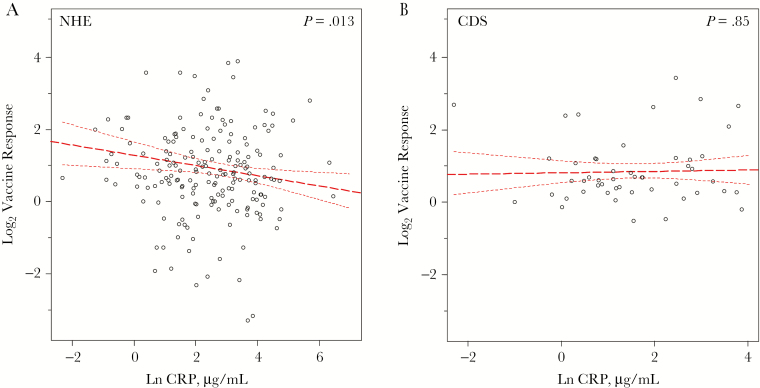
Lelic et al [10] have shown elsewhere that the immunogenicity of the VZV vaccine does not differ between the elderly nursing home residents and community-dwelling seniors (fold change in elderly participants, median [interquartile range], 1.8 [1.2–3.2]; fold change in seniors, 1.5 [1.2–2.3]); however, the variance in this response was significantly greater in the elderly (F test, P < .001), indicating the presence of underlying contributing factors. To ascertain whether serum cytokine and CRP levels were associated with the log2 VZV vaccine response, we performed multiple linear regression, adjusting for age, sex, and log2 VZV baseline response. Adjusting for the baseline response was critical, given that it is strongly correlated with the follow-up response (ρ = 0.7; P < .001). Of all molecules tested, only CRP was found to predict the VZV vaccine response; an inverse relationship was observed in the elderly nursing home residents (β = −.14 [SE, .06], P = .01; Figure 2A), but not in community-dwelling seniors (Figure 2B). No associations with telomere length were observed.
Figure 2.
Circulating C-reactive protein (CRP) levels predict the response to varicella-zoster virus (VZV) vaccination in the nursing home elderly (NHE) but not the community-dwelling senior (CDS) cohort. The log2 fold-change response to VZV vaccination at 6-week follow-up was correlated with natural-log-transformed (Ln) CRP levels in NHE (n = 187) (A) and CDS (n = 50) (B) donor groups. Slope, 95% confidence intervals (upper and lower dashed lines), and significance were determined using multiple linear regression, with adjustments for age, sex, and log2 baseline vaccine response, in separate models for NHE and CDS participants.
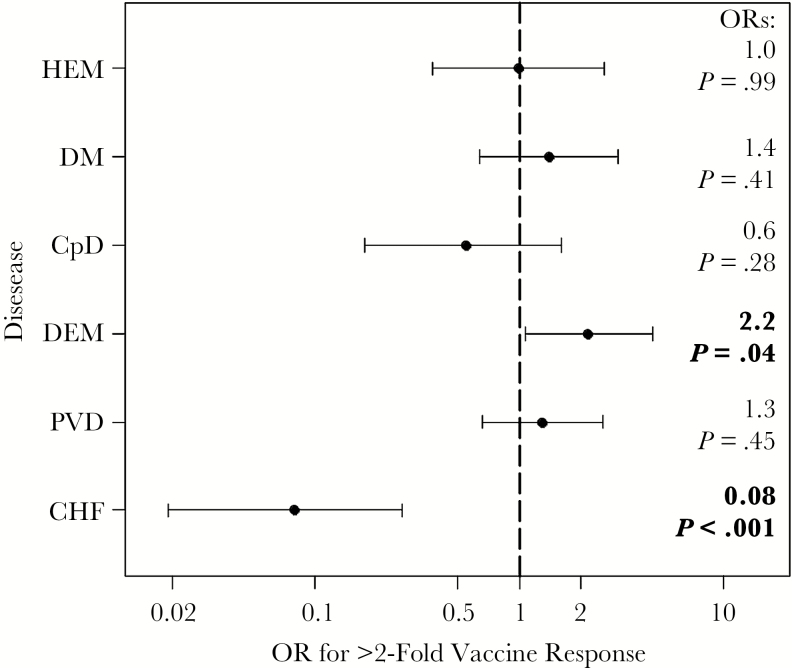
We also sought to determine whether disease- status was a significant predictor of the VZV vaccine response. Elderly participants with CHF were at a significantly lower odds of having a ≥2-fold VZV vaccine response (odds ratio [95% confidence interval], 0.08 [.019–.27]; P < .001), whereas those with dementia had significantly higher odds (2.2 [1.1–4.5]; P = .04) (Figure 3). Both of these associations remained significant when CRP was also adjusting for, as did the above association with CRP with adjustment for disease status.
Figure 3.
Elderly individuals (n = 187) with congestive heart failure (CHF) are likely to have a poor varicella-zoster virus (VZV) vaccine response. The odds of achieving a ≥2-fold VZV vaccine response was determined by multiple logistic regression, with adjustments for age, sex, and log2 baseline VZV response, for the following diseases: CHF, peripheral vascular disease (PVD), dementia (DEM), chronic pulmonary disease (CPD), diabetes mellitus (DM), and hemiplegia (HEM). Odds ratios (ORs) and P values are listed at right.
Increased T-Cell Markers of Immunosenescence in Elderly Residents With CHF
Immunosenescence of the T-cell compartment plays a prominent role in the response of older individuals to vaccination [22]. Hence, to further investigate the mechanism of CHF on VZV vaccine responsiveness, we analyzed a large cohort of elderly nursing home residents (median age [range], 86 [65–102] years), comparing the frequency of peripheral blood CD4+ and CD8+ T-cell subsets (naive, effector and central memory, terminally differentiated, and exhausted) between individuals with (n = 148) or without (n = 924) CHF (Table 1). Adjusting for age, sex, and nursing home residence, we found that participants with CHF had a significantly greater frequency of senescent CD4+ T cells (mean [SE], 4.5% [0.2%] vs 3.4% [0.2%]; P = .02), and a lower frequency of naive (1.2% [0.03%] vs 1.4% [0.03%]; P = .03), effector memory (6.8% [0.2%] vs 8.0% [0.2%]; P = .008) and central memory (0.51% [0.01%] vs 0.70% [0.02%]; P < .001) CD8+ T cells.
Table 1.
Association of CHF With Elevated T-Cell Immunosenescence in Elderly Nursing Home Residentsa
| T Cells | T-Cell Frequency, Mean (SE), % | β Coefficient (SE) | P Valueb | |
|---|---|---|---|---|
| CHF (n = 148) | No CHF (n = 924) | |||
| CD4+ | ||||
| Naive | 15.7 ± 1.0 | 15.9 ± 0.4 | −0.04 ± 0.08 | .645 |
| Effector memory | 32.7 ± 1.0 | 32.0 ± 0.4 | 0.03 ± 0.04 | .433 |
| Central memory | 11.8 ± 0.5 | 13.2 ± 0.2 | −0.11 ± 0.06 | .069 |
| Terminally differentiated | 12.4 ± 0.8 | 10.6 ± 0.3 | 0.11 ± 0.07 | .113 |
| Senescent | 4.5 ± 0.5 | 3.4 ± 0.2 | 0.29 ± 0.13 | .024c |
| CD8+ | ||||
| Naive | 1.2 ± 0.08 | 1.4 ± 0.04 | −0.14 ± 0.06 | .027c |
| Effector memory | 6.8 ± 0.4 | 8.0 ± 0.2 | −0.18 ± 0.07 | .008c |
| Central memory | 0.51 ± 0.04 | 0.70 ± 0.02 | −0.22 ± 0.06 | <.001c |
| Terminally differentiated | 12.0 ± 0.7 | 11.1 ± 0.3 | 0.05 ± 0.07 | .471 |
| Senescent | 9.1 ± 0.6 | 8.1 ± 0.3 | 0.07 ± 0.1 | .488 |
Abbreviations: CHF, congestive heart failure; SE, standard error.
aThe frequencies of naive, effector and central memory, terminally differentiated and senescent CD4+ and CD8+ T cells were measured in the peripheral blood of individuals with or without CHF.
b P values represent significance based on linear mixed-model regression analyses natural-log-transformed T-cell frequencies, with adjustment for age, sex, and nursing home residence.
cSignificant at P < .05.
DISCUSSION
Most of our knowledge on the factors that influence vaccine responsiveness in older adults come from studies of influenza vaccination. Interestingly, though there is overwhelming evidence indicating that the response to vaccination is reduced in older adults compared with younger persons [23], it is not clear whether responses of elderly nursing home residents are further compromised [24–26]. That being said, numerous factors have been reported that significantly influence influenza vaccine immunogenicity in that population, in particular, malnutrition [27–29], vaccine dose [30], and disease status [26, 29]. We have shown that elderly long-term care residents exhibit VZV vaccine responses similar to those in older adults, responses correlated with cytomegalovirus-reactive CD4+ T-cell and regulatory T-cell frequency [10]; unfortunately, little else is known. Identifying factors related vaccine immunogenicity in elderly nursing home residents will allow us to discriminate those at risk of vaccine failure and target interventions accordingly—for example, pharmacologically, using mammalian target of rapamycin (mTOR) inhibitors [31], nutritionally, by complex, fortified supplements [32], or through exercise [33].
A major finding of the current study was that serum levels of CRP, but not TNF, IL-1β, IL-6, or IL-10, were inversely related to vaccine responsiveness in the elderly nursing home population; no associations with serum CRP or cytokines were observed for community-dwelling seniors. As a canonical biomarker often used to estimate systemic inflammatory status, this finding was not surprising. Chronic, low-grade inflammation is widely considered to have a negative impact on vaccine responsiveness [34], supported by associations reported for circulating proinflammatory molecules, such as endotoxin [35], interleukin 12 [36], and neopterin [37]. Although the mechanism of this phenomenon is not completely understood, it may be related to an inflammation-dependent expansion of immunosuppressive regulatory T cells [38]. Lelic et al [10] previously showed that regulatory T-cell frequency at baseline was also inversely correlated to VZV vaccine responsiveness in our elderly cohort, but we cannot comment on whether expansion occurs after vaccination and whether circulating inflammatory levels modify this process.
We also found a significant effect of disease status in determining vaccine responsiveness in nursing home residents; in particular, having CHF greatly reduced the odds (odds ratio, 0.08) of having a ≥2-fold vaccine response, whereas having dementia actually improved the odds (2.2). The association between heart failure and vaccine immunogenicity is well documented, and, interestingly, the effect is bidirectional. On the one hand, influenza vaccination has been shown to reduce rates of hospitalization [39, 40] and death related to heart failure [41], the mechanism of which is thought to function by reducing or completely preventing the proatherogenic responses related to influenza infection (ie, proinflammatory cytokine secretion and leukocyte recruitment to the vascular intima) [42]. On the other hand, similar to our findings, individuals with acute or CHF exhibit reduced cell-mediated immunity [43, 44] and seroconversion [43, 45] in response to influenza vaccination.
Although the mechanism of this effect is less well known, findings in mice [46] and humans [47] suggest that heart failure induces an immense expansion of T cells, which contributes to an overall reduction of the naive T-cell pool and, therefore, a greater degree of immunosenescence. We further investigated this mechanism by examining T-cell immunosenescence markers in a separate cohort of elderly nursing home residents. Specifically, we compared the frequency of CD4+ and CD8+ naive, senescent, terminally differentiated and effector and central memory T cells in individuals with or without CHF. Indeed, the differences observed were consistent with T-cell immunosenescence; CD4+ senescent cells (CD28−CD57+) were elevated, whereas CD8+ naive (CD45RA+CCR7+), central memory (CD45RA−CCR7+), and effector memory (CD45RA−CCR7−) were reduced in individuals with CHF. Although these results and those of others would suggest that CHF accompanies T-cell immunosenescence in older adults, thereby correlating with impaired vaccine responses, further investigation is required to establish causality in these relationships.
In summary, we have shown that circulating CRP and CHF are significant predictors of the VZV vaccine response in elderly nursing home residents. Furthermore, CHF in this population seems to be related to T-cell immunosenescence, which would explain the observed reduction in vaccine immunogenicity. Our findings add to a body of literature suggesting that targeted interventions aiming to boost vaccine immunogenicity could be a viable approach to protect those at risk of vaccine failure. This would be particularly relevant for residents of long-term care, given their enhanced susceptibility to infection and related comorbid conditions.
Notes
Acknowledgments. M. B. L. is the Michael G DeGroote Chair in Infectious Diseases, and D. M. E. B. is the Canada Research Chair in Aging and Immunity.
Financial support. This work was supported by the Labarge Optimal Aging Initiative and the Canadian Institutes of Health Research (grant to D. M. E. B.), and the original study was funded by Merck Vaccines. The Bowdish laboratory is supported by the McMaster Immunology Research Centre and the M. G. DeGroote Institute for Infectious Disease Research.
Potential conflicts of interest. The original study was funded by Merck Vaccines. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the antelope valley varicella active surveillance project data. Vaccine 2013; 31:1680–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O’Connor KM, Paauw DS. Herpes zoster. Med Clin North Am 2013; 97:503–22, ix. [DOI] [PubMed] [Google Scholar]
- 3. Shapiro M, Kvern B, Watson P, Guenther L, McElhaney J, McGeer A. Update on herpes zoster vaccination: a family practitioner’s guide. Can Fam Physician 2011; 57:1127–31. [PMC free article] [PubMed] [Google Scholar]
- 4. Coplan PM, Schmader K, Nikas A0, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain 2004; 5:344–56. [DOI] [PubMed] [Google Scholar]
- 5. Levin MJ, Oxman MN, Zhang JH, et al. ; Veterans Affairs Cooperative Studies Program Shingles Prevention Study Investigators Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis 2008; 197:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levin MJ, Schmader KE, Pang L, et al. Cellular and humoral responses to a second dose of herpes zoster vaccine administered 10 years after the first dose among older adults. J Infect Dis 2016; 213:14–22. [DOI] [PubMed] [Google Scholar]
- 7. Irwin MR, Levin MJ, Carrillo C, et al. Major depressive disorder and immunity to varicella-zoster virus in the elderly. Brain Behav Immun 2011; 25:759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alicino C, Trucchi C, Paganino C, et al. Incidence of herpes zoster and post-herpetic neuralgia in Italy: results from a 3-years population-based study. Hum Vaccin Immunother 2017; 13:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drinka PJ. How should nursing homes use vaccine to prevent zoster? J Am Med Dir Assoc 2007; 8:419–20. [DOI] [PubMed] [Google Scholar]
- 10. Lelic A, Verschoor CP, Lau VW, et al. Immunogenicity of varicella vaccine and immunologic predictors of response in a cohort of elderly nursing home residents. J Infect Dis 2016; 214:1905–10. [DOI] [PubMed] [Google Scholar]
- 11. Xing Q, Hu D, Shi F, Chen F. Role of regulatory T cells in patients with acute herpes zoster and relationship to postherpetic neuralgia. Arch Dermatol Res 2013; 305:715–22. [DOI] [PubMed] [Google Scholar]
- 12. Clement M, Marsden M, Stacey MA, et al. Cytomegalovirus-specific IL-10-producing CD4+ T cells are governed by type-I IFN-induced IL-27 and promote virus persistence. PLoS Pathog 2016; 12:e1006050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hata A, Inoue F, Yamasaki M, et al. Safety, humoral and cell-mediated immune responses to herpes zoster vaccine in subjects with diabetes mellitus. J Infect 2013; 67:215–9. [DOI] [PubMed] [Google Scholar]
- 14. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis 2013; 56:1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qi Q, Cavanagh MM, Le Saux S, et al. Defective T memory cell differentiation after varicella zoster vaccination in older individuals. PLoS Pathog 2016; 12:e1005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rockwood K, Mogilner A, Mitnitski A. Changes with age in the distribution of a frailty index. Mech Ageing Dev 2004; 125:517–9. [DOI] [PubMed] [Google Scholar]
- 17. Disis ML, dela Rosa C, Goodell V, et al. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J Immunol Methods 2006; 308:13–8. [DOI] [PubMed] [Google Scholar]
- 18. Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 2009; 37:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnstone J, Parsons R, Botelho F, et al. T-cell phenotypes predictive of frailty and mortality in elderly nursing home residents. J Am Geriatr Soc 2017; 65:153–9. [DOI] [PubMed] [Google Scholar]
- 20. Johnstone J, Parsons R, Botelho F, et al. Immune biomarkers predictive of respiratory viral infection in elderly nursing home residents. PLoS One 2014; 9:e108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnstone J, Millar J, Lelic A, et al. Immunosenescence in the nursing home elderly. BMC Geriatr 2014; 14:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McElhaney JE, Kuchel GA, Zhou X, Swain SL, Haynes L. T-cell immunity to influenza in older adults: a pathophysiological framework for development of more effective vaccines. Front Immunol 2016; 7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006; 24:1159–69. [DOI] [PubMed] [Google Scholar]
- 24. Sato M, Saito R, Tanabe N, et al. Antibody response to influenza vaccination in nursing home residents and healthcare workers during four successive seasons in Niigata, Japan. Infect Control Hosp Epidemiol 2005; 26:859–66. [DOI] [PubMed] [Google Scholar]
- 25. Iorio AM, Alatri A, Camilloni B, Neri M, Baglio G, Donatelli I. Antibody response to 1995–1996 influenza vaccine in institutionalized and non-institutionalized elderly women. Gerontology 1999; 45:31–8. [DOI] [PubMed] [Google Scholar]
- 26. Muszkat M, Friedman G, Dannenberg HD, et al. Response to influenza vaccination in community and in nursing home residing elderly: relation to clinical factors. Exp Gerontol 2003; 38:1199–203. [DOI] [PubMed] [Google Scholar]
- 27. Sagawa M, Kojimahara N, Otsuka N, Kimura M, Yamaguchi N. Immune response to influenza vaccine in the elderly: association with nutritional and physical status. Geriatr Gerontol Int 2011; 11:63–8. [DOI] [PubMed] [Google Scholar]
- 28. Miyagawa K, Hayashi Y, Kurihara S, Maeda A. Co-administration of l-cystine and l-theanine enhances efficacy of influenza vaccination in elderly persons: nutritional status-dependent immunogenicity. Geriatr Gerontol Int 2008; 8:243–50. [DOI] [PubMed] [Google Scholar]
- 29. Bellei NC, Carraro E, Castelo A, Granato CF. Risk factors for poor immune response to influenza vaccination in elderly people. Braz J Infect Dis 2006; 10:269–73. [DOI] [PubMed] [Google Scholar]
- 30. Cools HJ, Gussekloo J, Remmerswaal JE, Remarque EJ, Kroes AC. Benefits of increasing the dose of influenza vaccine in residents of long-term care facilities: a randomized placebo-controlled trial. J Med Virol 2009; 81:908–14. [DOI] [PubMed] [Google Scholar]
- 31. Mannick JB, Del Giudice G, Lattanzi M, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med 2014; 6:268ra179. [DOI] [PubMed] [Google Scholar]
- 32. Langkamp-Henken B, Wood SM, Herlinger-Garcia KA, et al. Nutritional formula improved immune profiles of seniors living in nursing homes. J Am Geriatr Soc 2006; 54:1861–70. [DOI] [PubMed] [Google Scholar]
- 33. Woods JA, Keylock KT, Lowder T, et al. Cardiovascular exercise training extends influenza vaccine seroprotection in sedentary older adults: the immune function intervention trial. J Am Geriatr Soc 2009; 57:2183–91. [DOI] [PubMed] [Google Scholar]
- 34. Alter G, Sekaly RP. Beyond adjuvants: Antagonizing inflammation to enhance vaccine immunity. Vaccine 2015; 33suppl 2:B55–9. [DOI] [PubMed] [Google Scholar]
- 35. Bukhari SSI, Phillips BE, Wilkinson DJ, et al. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am J Physiol Endocrinol Metab 2015; 308:E1056–65. [DOI] [PubMed] [Google Scholar]
- 36. Furman D, Jojic V, Kidd B, et al. Apoptosis and other immune biomarkers predict influenza vaccine responsiveness. Mol Syst Biol 2013; 9:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Naylor C, Lu M, Haque R, et al. ; PROVIDE study teams Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine 2015; 2:1759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baeyens A, Saadoun D, Billiard F, et al. Effector T cells boost regulatory T cell expansion by IL-2, TNF, OX40, and plasmacytoid dendritic cells depending on the immune context. J Immunol 2015; 194:999–1010. [DOI] [PubMed] [Google Scholar]
- 39. Wang CS, Wang ST, Lai CT, Lin LJ, Lee CT, Chou P. Reducing major cause-specific hospitalization rates and shortening hospital stays after influenza vaccination. Clin Infect Dis 2004; 39:1604–10. [DOI] [PubMed] [Google Scholar]
- 40. Seo YB, Choi WS, Baek JH, et al. Effectiveness of the influenza vaccine at preventing hospitalization due to acute exacerbation of cardiopulmonary disease in Korea from 2011 to 2012. Hum Vaccin Immunother 2014; 10:423–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kopel E, Klempfner R, Goldenberg I. Influenza vaccine and survival in acute heart failure. Eur J Heart Fail 2014; 16:264–70. [DOI] [PubMed] [Google Scholar]
- 42. Bhatt AS, DeVore AD, Hernandez AF, Mentz RJ. Can vaccinations improve heart failure outcomes? contemporary data and future directions. JACC Heart Fail 2017; 5:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vardeny O, Sweitzer NK, Detry MA, Moran JM, Johnson MR, Hayney MS. Decreased immune responses to influenza vaccination in patients with heart failure. J Card Fail 2009; 15:368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Albrecht CM, Sweitzer NK, Johnson MR, Vardeny O. Lack of persistence of influenza vaccine antibody titers in patients with heart failure. J Card Fail 2014; 20:105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McElhaney JE, Herre JM, Lawson ML, Cole SK, Burke BL, Hooton JW. Effect of congestive heart failure on humoral and ex vivo cellular immune responses to influenza vaccination in older adults. Vaccine 2004; 22:681–8. [DOI] [PubMed] [Google Scholar]
- 46. Bansal SS, Ismahil MA, Goel M, et al. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ Heart Fail 2017; 10:e003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xydonas S, Parissis J, Lioni L, et al. Immunosenescence in patients with chronic systolic heart failure. J Cardiovasc Med 2016; 17:624–30. [DOI] [PubMed] [Google Scholar]