Summary
Systemic chemotherapy for various malignancies leads to long-term increases in CD4+ T-cell–associated HIV-1 RNA and DNA burden in some individuals. HIV-infected cytomegalovirus/Epstein-Barr virus–specific CD4 + T cells may also contribute to maintenance of the HIV DNA reservoir following chemotherapy.
Keywords: HIV-1, chemotherapy, stem cell transplantation, lymphoma, cytomegalovirus infection
Abstract
Background
Systemic chemotherapies for various malignancies have been shown to significantly, yet transiently, decrease numbers of CD4+ T lymphocytes, a major reservoir for human immunodeficiency virus type 1 (HIV-1) infection. However, little is known about the impact of cytoreductive chemotherapy on HIV-1 reservoir dynamics, persistence, and immune responses.
Methods
We investigated the changes in peripheral CD4+ T-cell–associated HIV-1 DNA and RNA levels, lymphocyte activation, viral population structure, and virus-specific immune responses in a longitudinal cohort of 15 HIV-1–infected individuals receiving systemic chemotherapy or subsequent autologous stem cell transplantation for treatment of hematological malignancies and solid tumors.
Results
Despite a transient reduction in CD4+ T cells capable of harboring HIV-1, a 1.7- and 3.3-fold increase in mean CD4+ T-cell–associated HIV-1 RNA and DNA, respectively, were observed months following completion of chemotherapy in individuals on antiretroviral therapy. We also observed changes in CD4+ T-cell population diversity and clonal viral sequence expansion during CD4+ T-cell reconstitution following chemotherapy cessation. Finally, HIV-1 DNA was preferentially, and in some cases exclusively, detected in cytomegalovirus (CMV)– and Epstein-Barr virus (EBV)–responsive CD4+ T cells following chemotherapy.
Conclusions
Expansion of HIV-infected CMV/EBV-specific CD4 + T cells may contribute to maintenance of the HIV DNA reservoir following chemotherapy.
Despite the ability of combination antiretroviral therapy (ART) to suppress human immunodeficiency virus (HIV) to undetectable blood levels, viral reservoirs persist [1–3]. Consequently, the main challenge in achieving HIV eradication is to eliminate these persistent, infected cells [1, 4]. Systemic cytoreductive chemotherapy used to treat various malignancies has been shown to significantly, yet transiently, decrease circulating and tissue-based CD4+ T lymphocytes and monocyte-derived cells [5–9]. These are the primary cells infected with HIV, and it is plausible that chemotherapy-induced elimination of cells capable of harboring HIV leads to a reduction in the number of latently infected cells. Furthermore, many of the first-line chemotherapeutic regimens for lymphomas involve the use of rituximab, a monoclonal antibody that targets CD20-expressing B cells and can disrupt the architecture of B-cell follicles [10–18]. CD4+ T follicular helper (TFH) cells within these follicular zones are thought to be a major source of HIV persistence and residual transcription in the setting of ART [19–22]. However, the effects of systemic cancer chemotherapies, with or without concomitant rituximab, on HIV persistence are poorly understood, and there are few data regarding the effects of chemotherapy on HIV-specific immune responses, immune activation, or viral evolution.
Prior work has suggested that integration of HIV into genes that regulate the cell cycle, including oncogenes, may result in preferential proliferation of these cells, leading to enrichment of such HIV integrants [23, 24]. However, there is a paucity of information regarding whether extrinsic drivers of cell proliferation may lead to persistence of the HIV reservoir. The reduction in CD4+ T cells in the setting of cytoreductive chemotherapy and subsequent cellular proliferation during postchemotherapy immune reconstitution [7] offers a window into the mechanisms by which HIV-infected cell pools persist or even expand over time with the potential to provide unique insights into reservoir dynamics. As a result, we investigated the changes in peripheral CD4+ T cell–associated HIV DNA and RNA levels, viral diversity, HIV- and other virus-specific immune responses, and plasma markers of inflammation in the setting of cytoreductive chemotherapy for solid tumor and hematological malignancies. In addition, the contribution of Epstein-Barr virus (EBV)– and cytomegalovirus (CMV)–responsive CD4+ T cells to the peripheral blood HIV-1 burden was determined as these viruses may reactivate during cancer therapy, triggering CD4+ T-cell proliferation.
METHODS
Study Subjects, Sample Collection, and Processing
Medical record information, plasma, and peripheral blood mononuclear cells (PBMCs) were obtained from a prospective cohort of HIV-infected individuals with a malignancy requiring systemic chemotherapy from 2012 until 2015 at Dana-Farber/Harvard Cancer Center (DFHCC)–affiliated institutions. The DFHCC Office for Human Research Studies approved the study and written signed informed consent was obtained from all participants. Baseline samples were collected prior to or immediately following cycle 1 of chemotherapy initiation whenever possible (baseline time point). Samples were also collected between chemotherapy cycles (if unable to obtain baseline time points for hematological malignancies and for all Kaposi sarcoma [KS] and solid tumor patients who received ongoing, continuous therapy cycles); after cessation of chemotherapy once absolute neutrophil counts reached >1000 cells/μL or higher (postchemotherapy time point 1); and approximately every 6–9 months thereafter if available (postchemotherapy time points 2, 3, etc). CD4+ T-cell counts and percentages and viral load measurements were obtained from routine clinical testing at the time of sample draw. PBMCs were obtained from whole blood by Ficoll-Hypaque gradient centrifugation following removal of plasma.
CD4+ T-Cell–Associated HIV-1 DNA and RNA Quantification
Purified, CD4+ T cells were isolated from PBMCs using negative selection magnetic bead separation (STEMCELL Technologies). DNA and RNA were extracted from CD4+ T cells using the AllPrep Kit (Qiagen, Valencia, California) and quantified using a sensitive and specific real-time polymerase chain reaction (PCR) assay as described previously [25, 26]. Unspliced cell-associated RNA (usRNA) was quantified using a real-time PCR assay using the same primers and probes as for HIV-1 DNA; HIV-1 DNA and usRNA levels were normalized to cell number using a conserved region of the CCR5 gene as previously described [25, 26].
Assessment of Surface Markers of Lymphocyte Activation and Proliferation and HIV-Specific T-Cell Responses
Thawed PBMCs were stained with Fixable Blue Dead Cell Stain, anti-CD3 (Invitrogen, Eugene, Oregon), anti-CD4 (eBioscience, San Diego, California), anti-CD8, anti-CD38, anti–HLA-DR, anti-CD57, anti-CD25, and anti-CD69 (BD Biosciences, San Jose, California). Following surface staining, cells were fixed, permeabilized, and stained for intracellular anti–Ki-67 (BD), a cell cycling marker. Samples were analyzed on a BD LSRII flow cytometer using FACSDiva software (BD). Cytometer settings were kept consistent by tracking laser voltages using UltraRainbow Fluorescent Particles (Spherotech, Lake Forest, Illinois). Compensation settings were assessed using BD CompBead particles (BD). Samples were also analyzed using FlowJo (Tree Star, Ashland, Oregon). Only live cells were included in the flow cytometric analyses. Interferon gamma (IFN-γ) enzyme-linked immunospot (ELISpot) assays were performed on PBMCs using a comprehensive HLA-specific peptide panel as described previously [27]. An antigen-specific PBMC response was considered positive only if it was ≥5 spot-forming units/106 PBMCs, and at least 4 times the mean background.
HIV-1 Envelope Single-Genome Analysis
Single genome amplification and sequencing of near full-length envelope sequences from longitudinal time points were performed based on an existing protocol [28]. Maximum likelihood (ML) trees of near full-length envelope sequences were constructed to compare sequence divergence using the PhyML/PAUP plugins for Geneious Pro (Biomatters) after statistical selection of best-fit models of nucleotide substitution (jModelTest). Large gaps and sequence segments with ambiguous nucleotide alignments were stripped prior to model selection and phylogenetic analysis. ML trees including sequences from all samples from all participants were constructed to rule out contamination.
CMV/EBV-Specific Responses and CD4+ T-Cell DNA
To identify CD4+ T cells specific for EBV or CMV for subsequent fluorescence-activated cell sorting (FACS), we adapted a previously described method [29] for use on postchemotherapy lymphocytes. Following PBMC thawing, cells were resuspended at a final concentration of 1.5 × 106/mL in R10-IL2 (RPMI/10% fetal bovine serum/penicillin-streptomycin/L-glutamine/IL-2 at 50 U) in FACS tubes at no more than 2 mL of culture per tube. CMV and EBV lysates (Virusys lot G1232161 and lot H1245002) were added to tubes at a final concentration of 10 μg/mL for each. Negative and positive control experiments involving media alone or αCD3/αCD28 beads were also performed. Two hours following addition of CMV and EBV lysates, bovine serum albumin was added to each tube to a final concentration of 5 μg/mL. Following 18 hours, cells were washed with phosphate-buffered saline and resuspended in 200 μL of complete medium as detailed above followed by surface staining with anti-CD14 (APC-Cy7), anti-CD16 (APC-Cy7), anti-CD19 (APC-Cy7), anti-CD3 (BV510), anti-CD4 (BV785), anti-CD8 (PE-Cy7), and Live-Dead Blue (UV). Cells were then washed and fixed with Perm A, and washed and resuspended in Perm B with intracellular staining antibodies IFN-γ (fluorescein isothiocyanate) and interleukin 2 (IL-2) (PE-cf594). Cells were then analyzed and sorted by CD3+/CD4+ cytokine-positive (defined by detectable IL-2 and/or IFN-γ) or cytokine-negative subsets using a FACSAria sorter. The gating strategy is shown in Supplementary Figure 6. Cells were then pelleted and DNA was extracted using the QiaAMP DNA Mini Kit (Qiagen) for HIV quantification.
Data Analysis
Phylogenic and statistical analyses were performed using Geneious version R7 (Biomatters) and Prism 6.0 (GraphPad Software). MEGA 6.0 was used to calculate mean genetic phylogenetic differences between single genome alignments. SPSS (IBM) version 20 was used to calculate no-parametric analyses (Wilcoxon rank-sum test). Comparisons between each time-point were performed using 2-sided tests without adjustment for the number of repeated measures given limited sample sizes.
RESULTS
CD4+ T-Cell–Associated HIV-1 DNA and RNA Levels Increase Following Cancer Chemotherapy in Antiretroviral-Treated Individuals
To determine the impact of cytoreductive therapies on HIV reservoirs and immune responses, we established a longitudinal cohort of 15 HIV-1–infected individuals with a diagnosis of malignancy requiring systemic chemotherapy (n = 13) or subsequent autologous hematopoietic stem cell transplantation (HSCT) (n = 2). Large-volume peripheral blood collection before, during and following cancer therapy was collected. Table 1 shows clinical details for each participant. A majority of individuals had a diagnosis of diffuse large B-cell lymphoma and were treated with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) or EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin) with the addition of rituximab for a minimum of 5 cycles. In some instances, salvage therapy was required for insufficient first-line responses. Another common group consisted of participants with KS who received systemic liposomal doxorubicin. Longitudinal samples were also obtained from 2 individuals who received autologous HSCT for refractory lymphoma; one remained on ART throughout the study interval, whereas the other stopped ART at the time of HSCT and subsequently died from cancer-related causes.
Table 1.
Human Immunodeficiency Virus–Infected Participants Who Received Systemic Chemotherapy for Malignancies, Underwent Longitudinal Sampling, and Were Included in the Analyses
| Participant | Sex | Diagnosis | Chemotherapy Regimen | On ART During Chemotherapy or HSCT | Peak VL on Reservoir Study (HIV 1 RNA Copies/mL) | Timing of Baseline Sample (Days Prior to or Following First Chemotherapy Cycle) |
|---|---|---|---|---|---|---|
| 1 | F | DLBCL | R-EPOCH, R-CODOX-M/ IVAC | No | 1630000 | 124 |
| 2 | M | DLBCL | R-CHOP | Yes | 539 | –7 |
| 3 | M | DLBCL | R-CHOP | Yes | 134 | 152 |
| 4 | M | DLBCL | R-CHOP | Yes | <50 | 0 |
| 5 | M | DLBCL | R-EPOCH | Yes | <50 | 65 |
| 6 | M | DLBCL | EPOCH, itMTX, DHAP | Yes | <50 | 12 |
| 7 | M | NHL/Hodgkin | ABVD | Yes | <50 | 119 |
| 8 | M | Hodgkin | ABVD | Yes | 61 | –1 |
| 9 | M | KS | Doxorubicin, paclitaxel | Yes | 269000a | 0 |
| 10 | M | KS | Doxorubicin | Yes | <50 | 3 |
| 11 | M | MCD/KS | Rituximab | Yes | 321 | 0 |
| 12 | M | RCC | Carboplatin + gemcitabine | Yes | <50 | 131 |
| 13 | M | RCC | Pazopanib, everolimus | Yes | <50 | 126 (pazopanib), –391 (everolimus) |
| 14 | M | Hodgkin | Autologous HSCT | Yes | 246 | –10 (pre-HSCT) |
| 15 | M | PTCL | Autologous HSCT | No | 1660000b | –120 (pre-HSCT) |
Abbreviations: ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; ART, antiretroviral therapy; DHAP, dexamethasone, cytarabine, cisplatin; DLBCL, diffuse large B-cell lymphoma; HIV, human immunodeficiency virus; HSCT, hematopoietic stem cell transplantation; itMTX, intrathecal methotrexate; KS, Kaposi sarcoma; MCD, multicentric Castleman disease; NHL, non-Hodgkin lymphoma; PTCL, peripheral T-cell lymphoma; RCC, renal cell carcinoma; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-CODOX-M/IVAC, rituximab, cyclophosphamide, doxorubicin, vincristine, methotrexate/ifosfamide, etoposide, cytarabine; R-EPOCH, rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; VL, viral load.
aParticipant started ART at first chemotherapy infusion, VL decreased to 32 copies/mL during subsequent chemotherapy cycles.
bParticipant discontinued ART at the time of autologous HSCT (pretransplant VL = 22 copies/mL).
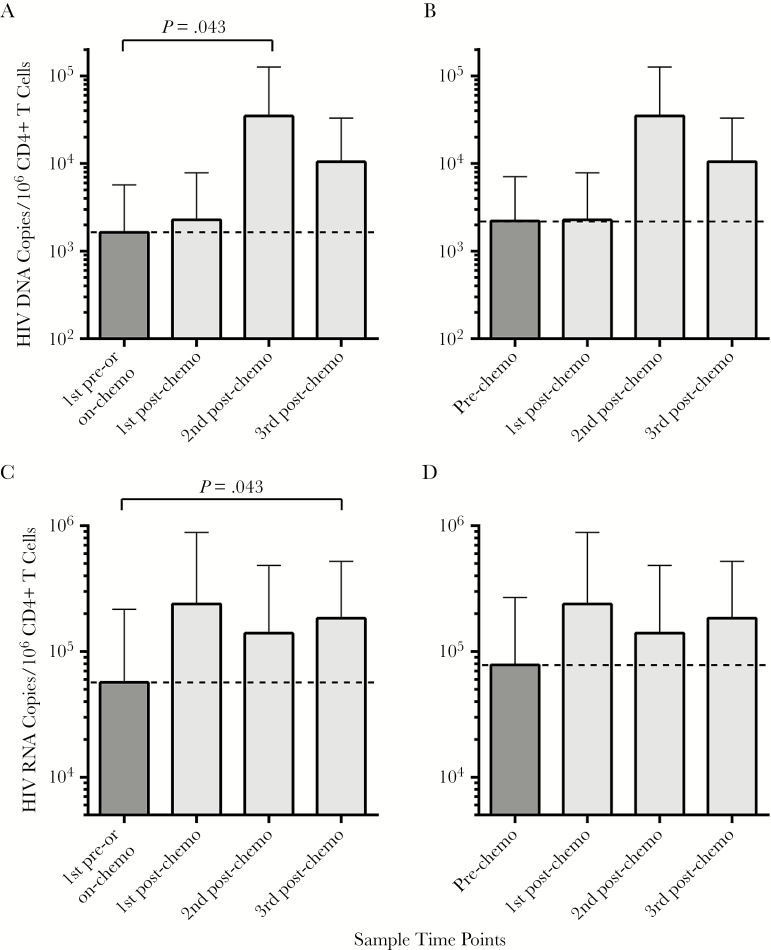
HIV-1 DNA and RNA levels measured directly in CD4+ T cells before initiation of chemotherapy (or at the first collection time point during chemotherapy if no pretreatment time point was available) were compared with levels following completion of chemotherapy in individuals with hematological malignancies (n = 8) or KS (n = 2) who remained on ART throughout cancer treatment or KS. Of note, there were no significant differences in mean HIV nucleic acid levels measured at baseline and the first collection time point (all P > .8). However, significantly higher levels of CD4+ T-cell–associated DNA and RNA were observed during the second and third postchemotherapy time points, respectively, compared with the first pre- or on-chemotherapy values (P = .043 for both analyses; Figure 1). A similar pattern emerged in sensitivity analyses comparing CD4+ T-cell–associated HIV-1 nucleic acid levels from participants who had samples available only from baseline time points (n = 7). A >1 log10 increase in HIV-1 DNA copies/106 CD4+ T cells was observed between the prechemotherapy and second postchemotherapy time points (approximately 6–9 months following completion of chemotherapy), but this difference was not statistically significant (all P > .05; Figure 1).
Figure 1.
CD4+ T cell–associated human immunodeficiency virus (HIV) DNA and RNA levels prior to and following completion of chemotherapy for hematologic malignancies and Kaposi sarcoma. Significant increases in both HIV DNA (A) and HIV RNA (C) in paired analyses were observed following completion of chemotherapy (postchemotherapy time points) compared with prechemotherapy or the first sampling time point while receiving cancer treatment (n = 10). Statistical significance was lost if only prechemotherapy time points are compared with those after completion of therapy for both DNA (B) and RNA (D), but paired sample size was smaller (n = 7), and up to a 1 log increase in DNA levels was still observed. Bars represent mean values and standard error. Paired, nonparametric Wilcoxon tests were used.
All individuals included in reservoir analyses were taking ART consistently during the time of HIV-1 DNA and RNA sampling. Four individuals had transient viral “blips” during or following therapy (Table 1), with the highest blip measured equal to 539 HIV-1 RNA copies/mL. A majority of individuals were fully suppressed. As expected, peripheral blood CD4+ T cell counts declined following chemotherapy initiation, and increased to similar or higher levels months to years following completion of treatment. Except for cell-associated RNA from participant 4, cellular HIV-1 DNA and RNA levels remained stable or increased following chemotherapy completion (Supplementary Figure 1). Viral loads, CD4+ T-cell counts, and CD4+ T-cell HIV-1 DNA and RNA levels from participants receiving treatment for solid tumors (KS and renal cell carcinoma) for whom longitudinal samples were available are shown in Supplementary Figure 2. Although 1 participant with KS showed a >3 log increase in cell-associated RNA, there was no consistency in the magnitude or direction of change in cell-associated HIV-1 DNA and RNA over time among participants overall. Interestingly, decreasing levels of CD4+ T-cell HIV-1 DNA and RNA were observed in 1 of 2 recipients who underwent autologous HSCT (participant 15) who stopped ART at the time of transplant and experienced a 6 log10 increase in HIV-1 following transplantation. The other autologous HSCT patient, who remained on ART (participant 14), did not have a marked change in HIV nucleic acid levels prior to and following transplantation (Supplementary Figure 3).
Impact of Chemotherapy on Markers of T-Cell Activation and Proliferation, and HIV-1 Immune Responses
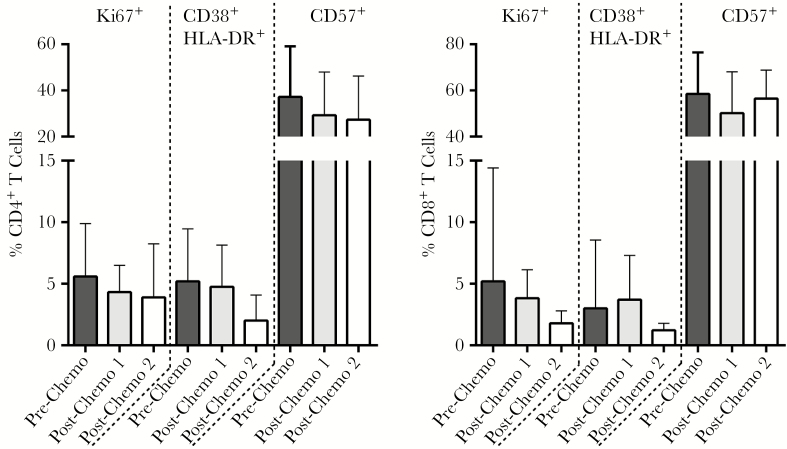
Except for participant 1, who was not taking ART during cancer treatment, cellular markers of CD4+ and CD8+ T-cell activation (cells co-expressing HLA-DR and CD38) and proliferation (Ki67) either remained stable or decreased following completion of treatment (Figure 2; Supplementary Figure 4). Overall, however, there were no significant differences between time points with respect to any of the surface markers. ELISpot assays of total PBMC responses to overlapping HIV-1 peptides were performed on samples from those subjects with sufficient cell numbers and longitudinal sampling to quantify changes in HIV-1–specific immune responses (n = 5) (Supplementary Figure 5; Supplementary Table 1). Overall responses to HIV-1 peptide pools before, during, or after chemotherapy were mixed. However, the CMV/EBV/influenza antigen (CEF) responses increased dramatically in 2 participants (4 and 7, on ART throughout treatment) following completion of chemotherapy despite consistent high responses to mitogen controls at all time points. All participants tested had detectable EBV or CMV immunoglobulin G (IgG) levels, but no detectable CMV or EBV plasma DNA at the time of postchemotherapy sampling.
Figure 2.
Mean percentages of intracellular Ki67 expression and surface expression of CD38, HLA-DR, and CD57 on CD4+ and CD8+ T cells prior and following completion of chemotherapy in participants with lymphoma or Kaposi sarcoma. Nonsignificant decreases in T-cell activation (CD38+ HLA-DR+ dual positive) and proliferation (Ki67+) were observed by the second postchemotherapy sampling time point. Bars represent mean values and standard error.
Oligoclonality of Single-Genome HIV-1 Envelope Sequences Following Chemotherapy
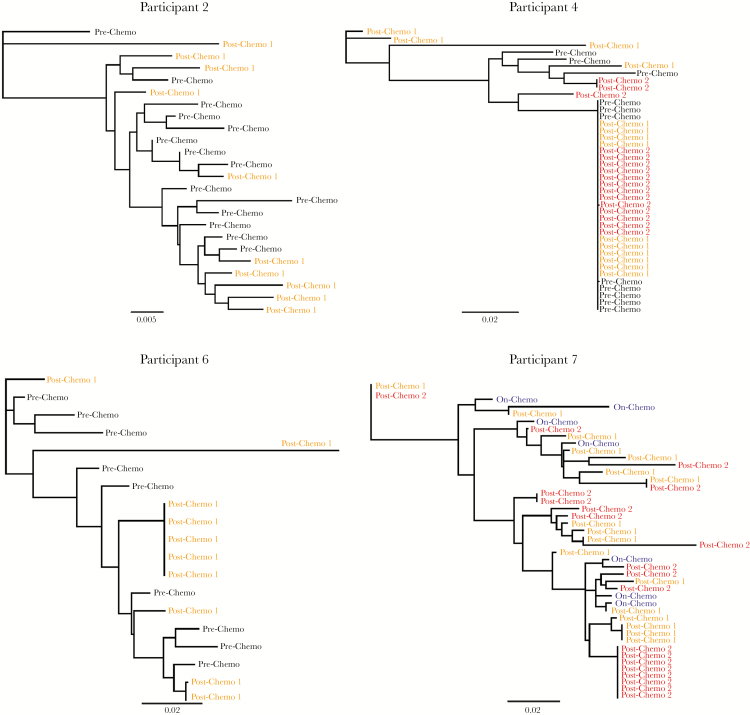
Single-genome amplification (SGA) of HIV env sequences from proviral DNA in peripheral blood CD4+ T cells was performed on samples from participants 2, 4, 6, and 7 with lymphoma who received ART and autologous HSCT participant 14. These individuals had sufficient numbers of cells at each time point to perform SGA. Maximum likelihood phylogenetic trees of prechemotherapy, on-chemotherapy, or postchemotherapy sequences are shown in Figure 3. Clustering of identical or nearly identical sequences following completion of chemotherapy was observed in 2 participants (6 and 7). SGA of proviral HIV-1 DNA from CD4+ T cells from participant 4, who had no detectable HIV RNA by clinical assay, revealed extensive mono- or oligophyletic clustering of sequences before and after chemotherapy; the number of clonal sequences increased substantially following cancer therapy. No clonal clustering was observed before or after chemotherapy from participant 2 or from autologous HSCT in participant 14, who remained on ART throughout transplantation (data not shown). When excluding one outlier sequence from participant 6, we identified lower mean genetic distances (D) in the last vs first time points of 3 individuals with emergence of clonal sequences (Figure 3).
Figure 3.
Maximum likelihood phylogenetic trees of single-genome human immunodeficiency virus type 1 (HIV-1) envelope DNA sequences from pre- or on-chemotherapy and postchemotherapy CD4+ T cells in patients with lymphoma on stable antiretroviral therapy throughout chemotherapy. Oligoclonal clustering of sequences was observed only following completion of chemotherapy in participants 4 and 6, with an increase in the number of near-identical envelope sequences following chemotherapy in participant 7. Phylogenetic trees were generated using bootstrapped, generalized time-reversible models. Bars represent genetic distance. Mean genetic distances for each participant time point are as follows: participant 2 prechemotherapy D = 0.019, postchemotherapy D = 0.032; participant 4 prechemotherapy D = 0.015, first postchemotherapy D = 0.032, second postchemotherapy D = 0.012; participant 6 prechemotherapy D = 0.018, postchemotherapy D = 0.019; participant 7 first on-chemotherapy D = 0.046, postchemotherapy D = 0.033. When excluding the single postchemotherapy outlier sequence from participant 6, the prechemotherapy D = 0.024 and postchemotherapy D = 0.013.
HIV-1 DNA Preferentially Persists in EBV/CMV-Responsive CD4+ T Cells Following Chemotherapy and Autologous HSCT
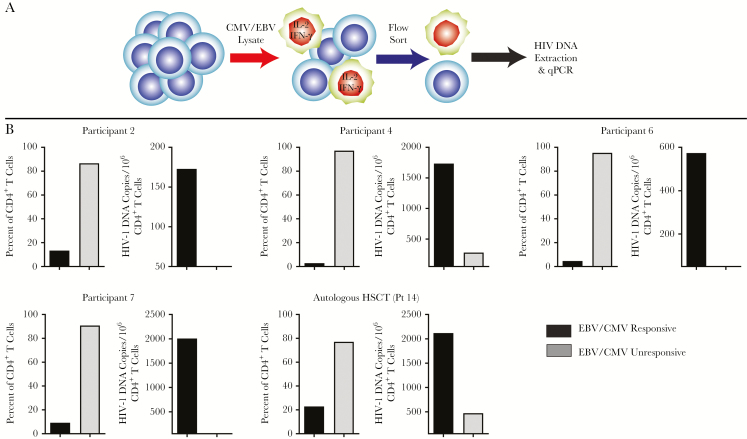
Given the clonal expansion of HIV envelope sequences described above and the increasing HIV DNA and RNA levels following chemotherapy, we sought to identify whether these sequences were arising from antigen-specific cell populations. The HIV DNA burden in CD4+ T cells responsive to EBV or CMV antigens as indicated by intracellular IFN-γ and/or IL-2 production in postchemotherapy samples from participants with available SGA data was determined. CMV and EBV lysates were pooled to maximize the yield of cells responsive to antigens from either of the viruses. The percentages of EBV/CMV-responsive CD4+ T cells producing IFN-γ and/or IL-2 varied from 3% to 23%. However, the level of proviral HIV-1 DNA was greatest in EBV/CMV-responsive cells, with little to no HIV-1 DNA detected in CMV/EBV-unresponsive lymphocytes (Figure 4). Cytokine-producing cells from CD3/CD28 stimulation controls did not show the same degree of enrichment of HIV DNA sequences. As above, all of participants tested had detectable EBV or CMV IgG levels, but no detectable CMV or EBV plasma DNA at the time of postchemotherapy sampling. We attempted to sequence proviral HIV-1 DNA from sorted cells, but were unable to obtain intact sequences due to the fixation involved in cell sorting.
Figure 4.
Functional CD4+ T-cell responses to Epstein-Barr virus (EBV)/cytomegalovirus (CMV) lysate stimulation and CD4+ T cell–associated human immunodeficiency virus (HIV) DNA levels within responsive and unresponsive cells. CD4+ T cells were exposed to CMV/EBV lysates followed by fluorescence-activated cell sorting and enumeration of cells that produced interleukin 2 (IL-2) and/or interferon gamma (IFN-γ) (A). HIV DNA extracted and enumerated by quantitative polymerase chain reaction (qPCR) was found to be highest in cells responding to CMV/EBV antigen despite these cells being the least frequent (B).
DISCUSSION
This study led to several novel insights into HIV-1 reservoirs and the nature of viral persistence. For example, despite a transient, and at times profound, reduction in CD4+ T cells capable of harboring HIV, CD4+ T-cell–associated viral nucleic acid increased in several individuals on ART following completion of repeated cycles of chemotherapy for various malignancies. Oligoclonal clustering of HIV-1 envelope sequences following completion of chemotherapy in certain participants was also observed, suggesting a change in population structure during CD4+ T-cell reduction or clonal expansion during postchemotherapy immune reconstitution in the setting of ART. Our data also suggest that clonal expansion and maintenance of the HIV reservoir following cytoreductive chemotherapy may be due, at least in part, to CMV- or EBV-responsive CD4+ T cells. To our knowledge, we are the first to show that following cancer chemotherapy, HIV-1 DNA is preferentially, and in some cases exclusively, detected in CD4+ T cells that produce IFN-γ and/or IL-2 in response to EBV/CMV antigens.
Chemotherapy is an intriguing context in which to study HIV persistence, as numerous cycles of therapy lead to reductions in the total numbers of CD4+ T cells and other hematopoietic cells that may harbor latent HIV [5–7]. As a result, it is plausible that total body HIV reservoirs decrease during chemotherapy through a stochastic reduction of infected cells. One prior study of lymphoma patients receiving systemic chemotherapy and one of otherwise healthy HIV-infected individuals who received cyclophosphamide monotherapy did not show a significant decrease or increase in HIV DNA or RNA levels following completion of therapy [30, 31]. In contrast, we observed an increase in both CD4+ T-cell–associated HIV DNA and RNA months to years following completion of therapy. These data do not discount, however, potential transient reductions in absolute numbers of infected CD4+ T cells in blood or various tissues because of the temporary loss of cells harboring HIV during chemotherapy administration. On the other hand, measuring HIV-1 DNA and RNA in CD4+ T cells has the potential to inform on clonal viral reservoir expansion. Interestingly, we did not observe increased markers of lymphocyte activation or proliferation following completion of chemotherapy or conditioning for autologous stem cell transplant, suggesting that tissues may be a major source of lymphocyte expansion or redistribution during immune reconstitution.
Several mechanisms may be involved in changes to the population structure of HIV-1 envelope sequences observed in 3 of 4 chemotherapy-treated participants with available data following completion of chemotherapy. First, it is possible that the overall HIV diversity decline was directly due to chemotherapy eliminating a portion of the infected lymphoid cells. Further studies will also be needed to clarify whether a change in the viral population structure in the periphery can reflect tissue based reservoir size and diversity. The enrichment in HIV sequences may also be related to antigen-driven proliferation, selective survival of these antigen-specific cell populations, or to preexisting enrichment in these populations prior to chemotherapy. For example, it is possible that observed HIV-1 reservoir expansion and increased envelope clonality was a result of preferential expansion of residual infected cells following chemotherapy containing HIV-1 DNA integrated into genes that regulate the cell cycle [23, 24]. Integration site analysis was out of the scope of this study, but further investigation is certainly warranted.
Our data suggest that proliferative stimuli other than HIV-1, such as CMV or EBV antigens, may lead, at least in part, to expansion of these cells. Self-limited reactivation of CMV (50%) and EBV (22%) occurs frequently in the setting of chemotherapy for hematological malignancies [32, 33]. Prior studies have also demonstrated that (1) genital CMV shedding is associated with higher levels of CD4+ T-cell activation and HIV-1 DNA levels [34, 35]; (2) detectable CMV DNA obtained from PBMCs is correlated with a slower DNA decay in the setting of ART [36]; and (3) EBV DNA levels are associated with higher cell-associated HIV-1 DNA and RNA levels [37]. No detectable plasma CMV or EBV DNA was identified in these patients at the time of sampling, but all had antibodies to at least 1 of these viruses. Furthermore, IFN-α– and/or IL-2–producing cells in response to CMV/EBV antigens represented a minority of the CD4+ T-cell population. However, a large majority of HIV-1 DNA was observed in the CMV/EBV-responsive cells, with DNA exclusively recovered from CMV/EBV-responsive cells in 1 patient. It is possible that proliferation of CMV/EBV-responsive CD4+ T cells from subclinical antigen exposure in blood or tissue led to selective expansion of cells that also happen to harbor HIV. It is interesting to note that data from a prior study suggest that HIV preferentially exists in HIV-responsive CD4+ T cells [38]. However, these studies were conducted in individuals who did not undergo chemotherapy-related cytotoxicity, and it is certainly possible that HIV-specific CD4+ T cells in our study also preferentially harbored HIV.
Most participants with lymphoma in our cohort received multiple doses of rituximab, which targets CD20+ B cells [13]. The depletion of B cells may disrupt the local architecture at sites that are critical to reservoir maintenance. HIV largely resides in organized lymphoid tissues outside of the peripheral circulation. More specifically, TFH cells located within lymph node B-cell follicles are highly enriched in HIV-1 DNA, are highly permissible to HIV infection, and produce high levels of replication competent virus upon ex vivo stimulation [19, 21, 22]. It is therefore possible that rituximab therapy may expose tissue HIV-1 reservoirs for elimination. Six of our participants received repeated doses of rituximab but did not have long-term reductions in peripheral blood HIV RNA or DNA levels. We did not have the opportunity to examine HIV-1 burden in tissues, however, and studies involving the tissue responses to anti–B-cell therapies are needed.
This study was limited by the small sample size, heterogeneous makeup of the cohort, and the variable timing of follow-up samples, as dictated by clinical convenience. Furthermore, many potential participants identified were off ART and/or diagnosed with HIV at the time of presentation of their malignancy, thereby limiting the inclusions of many individuals on suppressive ART prior to the initiation of cancer therapy. Finally, given the presence of comorbidities related to the underlying malignancies in these patients, we were unable to obtain sufficient PBMCs to complete all studies on all participants, and were unable to perform more invasive tissue sampling. Nonetheless, we identified several important findings relevant to the understanding of HIV persistence as described above, and critical insights regarding antigen specificity of the reservoir may be further investigated in more diverse cohorts.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. Marisol Romero-Tejeda helped perform ELISpot experiments.
Financial support. This work was supported by federal funds from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant number K23AI098480 to T. J. H.); the AIDS Clinical Trials Group Virology Support Laboratory (grant number UM1 068636); and the Foundation for AIDS Research ARCHE grant.
Potential conflicts of interest. R. T. G. receives educational grants to his institution from Gilead, ViiV, and Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Deeks SG, Lewin SR, Ross AL et al. ; International AIDS Society Towards a Cure Working Group International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med 2016; 22:839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siliciano JD, Kajdas J, Finzi D et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003; 9:727–8. [DOI] [PubMed] [Google Scholar]
- 3. Chun TW, Justement JS, Moir S et al. Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: implications for eradication of virus. J Infect Dis 2007; 195:1762–4. [DOI] [PubMed] [Google Scholar]
- 4. Deeks SG, Autran B, Berkhout B et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol 2012; 12:607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mackall CL, Fleisher TA, Brown MR et al. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood 1994; 84:2221–8. [PubMed] [Google Scholar]
- 6. Mackall CL, Fleisher TA, Brown MR et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood 1997; 89:3700–7. [PubMed] [Google Scholar]
- 7. Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Stem Cells 2000; 18:10–8. [DOI] [PubMed] [Google Scholar]
- 8. Jason M, Andrews BS, Colvin M, Friou GJ. In vitro effects of 4-hydroperoxycyclophosphamide on the morphology and function of human peripheral blood mononuclear phagocytic cells (macrophages). Cancer Res 1984; 44:3936–41. [PubMed] [Google Scholar]
- 9. Mackiewicz U, Brelińska-Peczalska R, Konys J. The influence of cyclophosphamide on lymph node and peripheral blood lymphocytes in mice. Arch Immunol Ther Exp (Warsz) 1978; 26:375–80. [PubMed] [Google Scholar]
- 10. Afonso A. Immune reconstitution in patients wiht B-non Hodgkin lymphoma (B-NHL) treated with rituximab, chemotherapy or both—a cohort study. Haematologica 2010; 95:1559. [Google Scholar]
- 11. Barnes JA, Lacasce AS, Feng Y et al. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt’s lymphoma: a retrospective analysis. Ann Oncol 2011; 22:1859–64. [DOI] [PubMed] [Google Scholar]
- 12. Dunleavy K, Little RF, Pittaluga S et al. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood 2010; 115:3017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Irie E, Shirota Y, Suzuki C et al. Severe hypogammaglobulinemia persisting for 6 years after treatment with rituximab combined chemotherapy due to arrest of B lymphocyte differentiation together with alteration of T lymphocyte homeostasis. Int J Hematol 2010; 91:501–8. [DOI] [PubMed] [Google Scholar]
- 14. Kaplan LD, Lee JY, Ambinder RF et al. Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lymphoma: AIDS-Malignancies Consortium Trial 010. Blood 2005; 106:1538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Drugs 2003; 63:803–43. [DOI] [PubMed] [Google Scholar]
- 16. Sparano JA, Lee JY, Kaplan LD et al. ; AIDS Malignancy Consortium Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood 2010; 115:3008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spina M, Jaeger U, Sparano JA et al. Rituximab plus infusional cyclophosphamide, doxorubicin, and etoposide in HIV-associated non-Hodgkin lymphoma: pooled results from 3 phase 2 trials. Blood 2005; 105:1891–7. [DOI] [PubMed] [Google Scholar]
- 18. Spina M, Simonelli C, Tirelli U. Phase II trial of CHOP plus rituximab in patients with HIV-associated non-Hodgkin’s lymphoma. J Clin Oncol 2007; 25:e7. [DOI] [PubMed] [Google Scholar]
- 19. Lindqvist M, van Lunzen J, Soghoian DZ et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest 2012; 122:3271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Banga R, Procopio FA, Noto A et al. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med 2016; 22:754–61. [DOI] [PubMed] [Google Scholar]
- 21. Perreau M, Savoye AL, De Crignis E et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med 2013; 210:143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fukazawa Y, Lum R, Okoye AA et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 2015; 21:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maldarelli F, Wu X, Su L et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 2014; 345:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wagner TA, McLaughlin S, Garg K et al. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 2014; 345:570–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malnati MS, Scarlatti G, Gatto F et al. A universal real-time PCR assay for the quantification of group-M HIV-1 proviral load. Nat Protoc 2008; 3:1240–8. [DOI] [PubMed] [Google Scholar]
- 26. Henrich TJ, Gallien S, Li JZ, Pereyra F, Kuritzkes DR. Low-level detection and quantitation of cellular HIV-1 DNA and 2-LTR circles using droplet digital PCR. J Virol Methods 2012; 186:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Altfeld MA, Trocha A, Eldridge RL et al. Identification of dominant optimal HLA-B60- and HLA-B61-restricted cytotoxic T-lymphocyte (CTL) epitopes: rapid characterization of CTL responses by enzyme-linked immunospot assay. J Virol 2000; 74:8541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keele BF, Giorgi EE, Salazar-Gonzalez JF et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 2008; 105:7552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amyes E, Hatton C, Montamat-Sicotte D et al. Characterization of the CD4+ T cell response to Epstein-Barr virus during primary and persistent infection. J Exp Med 2003; 198:903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cillo AR, Krishnan S, McMahon DK, Mitsuyasu RT, Para MF, Mellors JW. Impact of chemotherapy for HIV-1 related lymphoma on residual viremia and cellular HIV-1 DNA in patients on suppressive antiretroviral therapy. PLoS One 2014; 9:e92118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bartlett JA, Miralles GD, Sevin AD et al. ; ACTG 380 Study Team Addition of cyclophosphamide to antiretroviral therapy does not diminish the cellular reservoir in HIV-infected persons. AIDS Res Hum Retroviruses 2002; 18:535–43. [DOI] [PubMed] [Google Scholar]
- 32. Kuo CP, Wu CL, Ho HT, Chen CG, Liu SI, Lu YT. Detection of cytomegalovirus reactivation in cancer patients receiving chemotherapy. Clin Microbiol Infect 2008; 14:221–7. [DOI] [PubMed] [Google Scholar]
- 33. Ogata M, Satou T, Kawano R et al. High incidence of cytomegalovirus, human herpesvirus-6, and Epstein-Barr virus reactivation in patients receiving cytotoxic chemotherapy for adult T cell leukemia. J Med Virol 2011; 83:702–9. [DOI] [PubMed] [Google Scholar]
- 34. Gianella S, Massanella M, Richman DD et al. ; California Collaborative Treatment Group 592 Team Cytomegalovirus replication in semen is associated with higher levels of proviral HIV DNA and CD4+ T cell activation during antiretroviral treatment. J Virol 2014; 88:7818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gianella S, Anderson CM, Vargas MV et al. Cytomegalovirus DNA in semen and blood is associated with higher levels of proviral HIV DNA. J Infect Dis 2013; 207:898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weibel SG, Anderson C, Var SR et al. Detectable CMV in PBMC is associated with slower HIV DNA decay during suppressive ART [abstract 375]. In: Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 13–16 February 2017. [Google Scholar]
- 37. Gianella S, Anderson CM, Var SR et al. Replication of human herpesviruses is associated with higher HIV DNA levels during antiretroviral therapy started at early phases of HIV infection. J Virol 2016; 90:3944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Douek DC, Brenchley JM, Betts MR et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature 2002; 417:95–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.