Summary
Human immunodeficiency virus (HIV)–specific antibody levels can be used as inexpensively measured, high-throughput surrogate markers to estimate the total size of the HIV reservoir and to monitor viral replication or antigen expression in HIV-infected tissue and reservoir sanctuary sites.
Keywords: Antibodies, HIV persistence, latent reservoir, incidence assay
Abstract
Background.
Human immunodeficiency virus (HIV) antibodies are generated and maintained by ongoing systemic expression of HIV antigen. We investigated whether HIV antibody responses as measured by high-throughput quantitative and qualitative assays could be used to indirectly measure persistent HIV replication in individuals receiving antiretroviral therapy (ART).
Methods.
HIV antibody responses were measured over time in the presence or absence of suppressive ART and were compared to the HIV reservoir size and expression of antiviral restriction factors.
Results.
Among untreated individuals, including both elite controllers (ie, persons with a viral load of ≤40 copies/mL) and noncontrollers, antibody parameters were stable over time and correlated with the individual viral load. Viral suppression with ART led to a progressive decline in antibody responses after treatment induction that persisted for 5–7 years. Higher levels of HIV antibodies during suppressive therapy were associated with later initiation of ART after infection, with higher DNA and cell-associated RNA levels, and with lower expression of multiple anti-HIV host restriction factors.
Discussion.
These findings suggest that declining antibody levels during ART reflect lower levels of antigen production and/or viral replication in the persistent HIV reservoir. Results of relatively inexpensive and quantitative HIV antibody assays may be useful indirect markers that enable efficient monitoring of the viral reservoir and suppression during functional-cure interventions.
Despite undetectable levels of plasma RNA in human immunodeficiency virus (HIV)–infected individuals who receive antiretroviral therapy (ART), HIV persists in latently infected cells [1, 2] that are sequestered in lymphoid tissue and other anatomic compartments [3–6]. Functional-cure interventions have been developed to reduce or eliminate the latent reservoir and are moving into clinical studies, with the ultimate goal of achieving ART-free HIV remission. Assays to quantify the total burden of the HIV-infected reservoir will be essential in determining whether intervention methods are successful in reducing the reservoir size and potentially eradicating the virus. The lack of reliable, reproducible, and sensitive assays to evaluate the magnitude and character of the latent reservoir has been a key barrier in evaluating these interventions.
HIV antibody recency assays have been extensively used to calculate population incidence in cross-sectional serosurveys [7]. It is well documented that ART administration or spontaneous elite control of HIV replication results in reductions in HIV antibodies as measured by these assays [8–11]. In this study, we explore the use of recency assays to evaluate viral suppression in a diverse and well-characterized cohort of both untreated and treated HIV infection. Three different assays were used to measure the relative quantity and quality of HIV antibodies. These include the chemiluminescent Vitros anti-HIV1 + 2 assay, which is used to measure relative antibody concentration (by using a diluted plasma sample) and antibody avidity (by measuring a plasma sample incubated with a chaotropic agent and compared it to findings for sample that is unmanipulated), and the Sedia limiting antigen (LAg) assay was used to measure quantity and avidity at the same time.
Given the established association between antigenic burden and antibody production that has been noted in HTLV-1 and HIV [12–19], we hypothesized that the total size of the reservoir is directly associated with the degree of systemic antigen exposure. We also hypothesized that antigen expression contributes to the level of antibody production and avidity and that antibody levels can hence be used as a surrogate marker of the HIV reservoir during ART [20]. To more fully characterize the relationship between the HIV antigen burden and HIV antibody responses during ART, we compared antibody responses to both viral reservoir levels and expression of restriction factors in untreated individuals, elite controllers, and patients who initiated ART early or later after HIV infection and were followed over time.
METHODS
Individuals and Specimen Processing
Specimens from patients participating in the UCSF Options and SCOPE cohort studies were analyzed [9, 20–23] and are listed in Table 1. Patients provided written informed consent for study participation, according to the Declaration of Helsinki.
Table 1.
Characteristics of Patient Groups Included in the Analyses
| Analysis, Group | ART Initiation Criteria | Measurements, No. | Time Points, No., Median |
| Untreated, antibody correlation with viral load analysis [9] | NA | 274 | … |
| Untreated, longitudinal antibody analysis [9] | |||
| Elite controller group viral load, <80 copies/mL | NA | 50 | 5 |
| Viremic group viral load, > 80 copies/mL | NA | 25 | 3 |
| Longitudinal antibody analysis [21] | |||
| Early treated group | <6 mo after infection | 31 | 5 |
| Late-treated group | > 2 y after infection | 35 | 5 |
| Restriction factor analysis [22] | |||
| Early treated group | <1 y from infection | 23 | 1 |
| Late-treated group | >1 y from infection | 49 | 1 |
Abbreviations: ART, antiretroviral therapy; NA, not applicable.
Specimens from untreated participants were analyzed for the relationship between anti-HIV antibody responses and the viral load (n = 274) [9]. To determine variability of the assays over time, longitudinal anti-HIV antibodies were measured in both untreated viremic individuals (ie, 50 individuals with a viral load of ≥80 copies/mL and infected for >2 years; a median of 5 time points per individual were measured) and elite controllers (ie, 25 individuals with a viral load of ≤80 copies/mL; a median of 3 time points per individuals were measured) [9].
As previously reported, study participants were identified as having early HIV infection if they had 2 plasma specimens in which HIV-1 RNA levels were ≥3000 copies/mL and a negative or indeterminate HIV-1 antibody test result, if they tested positive for HIV antibodies but had a history of negative HIV-1 antibody test results during the past 6 months, or if they tested negative for HIV-1 antibodies within the past 12 months and had a reactive result of a standard HIV-1 antibody test but a result of a less sensitive (ie, “detuned”) HIV-1 antibody test suggesting infection within <6 months [24–26]. Standardized estimated infection dates were calculated from these data [20]. Antibodies were measured in HIV-infected adults with acute/early infection who started ART early (ie, within 6 months of the estimated infection date) or later (ie, >2 years after the estimated infection date) and had measurements of cell-associated HIV RNA and DNA performed longitudinally as previously reported [21]. In the early treated (ET) group (n = 31), antibody levels were measured at baseline (during acute/early HIV infection), 1 year following ART start, 3 and 5 years after ART start, and, among those who had received ART for ≥2 years, at the final observed time points. In the later treated (LT) group (n = 35), antibody responses were measured at baseline, 1 year following infection, at the final observed pre-ART time points, 1 year following ART start, and, among those who had received ART for ≥2 years, at the final observed time points [21]. In the ET and LT groups, cell-associated HIV DNA/RNA levels were measured only at time points during therapy (ie, 1 year after ART initiation and at the final time point during ART). Antibody responses were compared to viral loads from all time points, and cell-associated RNA and DNA levels at the final time point were analyzed.
Expression of host restriction factor genes was measured in 72 individuals with ART-suppressed HIV infection [22]. Peripheral blood mononuclear cells (PBMCs) and plasma specimens were collected from individuals with ART-suppressed HIV infection who were enrolled in the SCOPE and Options cohorts within 1–2 years of ART initiation, either during early infection (ie, <1 year after infection; range, 0.11–0.58 years; n = 23) or later infection (ie, >1 year after infection; n = 49).
HIV Antibody Recency Assays
The less sensitive and avidity-modified Vitros HIV 1 + 2 assays are based on a previously described [8] modification of the Vitros HIV 1 + 2 ECi/ECiQ Immunodiagnostic System, which is a chemiluminescence assay that is licensed for diagnosis of HIV infection (Ortho-Clinical Diagnostics, Rochester, NY). These assays provide a quantitative readout of envelope and p24-specific HIV antibodies relative to an assay calibrator. For the less sensitive Vitros system, the assay was modified by using a 1:400 dilution of a specimen in Vitros buffer B (Ortho-Clinical Diagnostics), and the reported signal-to-cutoff ratio is given for a diluted specimen relative to the calibrator. For the Vitros avidity-modified assay, one aliquot of a sample was diluted 1:10 in a chaotropic agent (guanidine), and another aliquot of the sample diluted 1:10 in phosphate-buffered saline. After incubation, signal-to-cutoff ratios of each dilution were determined, and an avidity index was calculated as the signal-to-cutoff ratio of the guanidine dilution divided by that of the phosphate-buffered saline dilution. The product as used here is still investigational, and the assay is not currently approved for clinical application.
The gp41-detecting limiting antigen (LAg) avidity enzyme immunoassay was performed as previously described [27]. Assay controls and HIV-positive specimens were diluted 1:101 in specimen diluent; 100 μL of calibrator, controls, or specimens was added to antigen-coated plates; and the plates were incubated. Plates were washed 4 times with 1× wash buffer to remove unbound antibodies. A buffer (pH 3.0) was added to each well to dissociate low avidity antibodies. Plates were developed, and the OD was read using a spectrophotometer (Molecular Devices Microplate Reader). The raw OD for each specimen was normalized using the calibrator OD on each plate as a ratio, with the normalized OD calculated as the OD of the specimen divided by the median OD of the calibrator.
Gene Expression Profiling
CD4+ T cells were enriched by negative selection using the EasySep Human CD4+ T Cell Enrichment Kit (Stemcell Technologies, Vancouver, Canada), according to the manufacturer’s instructions. Genomic DNA and total RNA were extracted from enriched CD4+ T cells (1 million–2 million cells), using the Allprep DNA/RNA/miRNA Universal Kit (Qiagen, Valencia, CA) with on-column DNase treatment (Qiagen RNase-Free DNase Set). RNA was transcribed into complementary DNA (cDNA), using random primers and the SuperScript Vilo cDNA Synthesis Kit (Invitrogen/Thermo Fisher, Waltham, MA) according to manufacturer’s instructions. Quantitative real-time polymerase chain reaction (PCR) analysis using custom-made TaqMan low-density arrays (Life Technologies) was used to comprehensively profile the relative expression of 42 established anti–HIV-1 host restriction factors, as previously described [22]. A panel of 6 housekeeping genes was included in the TaqMan low-density array plates (GAPDH, 18S, ACTB, PPIA, RPLP0, and UBC). GAPDH was identified as the most stably expressed gene from those 6 housekeeping genes among all samples, using the GeNorm algorithm. Therefore, raw cycle threshold numbers of amplified gene products were normalized to the housekeeping gene, GAPDH, to control for cDNA input amounts. Fold induction was determined using the comparative cycle threshold method [28].
HIV DNA and Cell-Associated RNA
Methods for quantification of HIV DNA and cell-associated RNA levels were previously published [21]; in brief, extracted PBMC DNA was measured using real-time PCR analysis targeting Gag DNA sequences; total amplifiable DNA (indicated cell input) was measured using real-time PCR analysis of a single-copy gene (the conserved region of the HLA-DQ alpha locus), as described elsewhere [29–32], and values were normalized per million PBMCs. Extracted cell-associated RNA levels were measured with the transcription-mediated amplification assay (Aptima; Gen-Probe), using modified PBMC extraction and transcription-mediated amplification of cell-associated HIV RNA [33, 34]. This yields HIV RNA values expressed as signal-to-cutoff ratios (range, 0–30; undetectable, <1.0; detectable, >1.0). Signal-to-cutoff ratios were normalized per million PBMCs, as with DNA.
Statistical Analysis
All data were compiled and analyzed in Prism 6.0 (GraphPad, La Jolla, CA). Antibody kinetics were determined by calculating the change in antibody responses over time. Group comparisons were performed using analysis of variance (the Kruskal-Wallis test). Plasma viral load, host restriction factor expression, cellular HIV RNA/DNA levels, and antibody responses were log transformed and analyzed using Pearson correlation. For the restriction factor analysis, P values were adjusted into false-discovery rates by the Benjamini and Hochberg controlling procedure, a commonly used method for analysis of large sets of biological data (available at: http://www.sdmproject.com/utilities/?show=FDR) [35].
Ethics Statements
Informed consent was obtained from patients. Human experimentation guidelines of the Department of Health and Human Services and/or those of the authors’ institution(s) were followed in the conduct of clinical research.
RESULTS
Anti-HIV Antibody Levels in Chronically Infected Individuals Correlate With Plasma HIV-1 RNA Levels
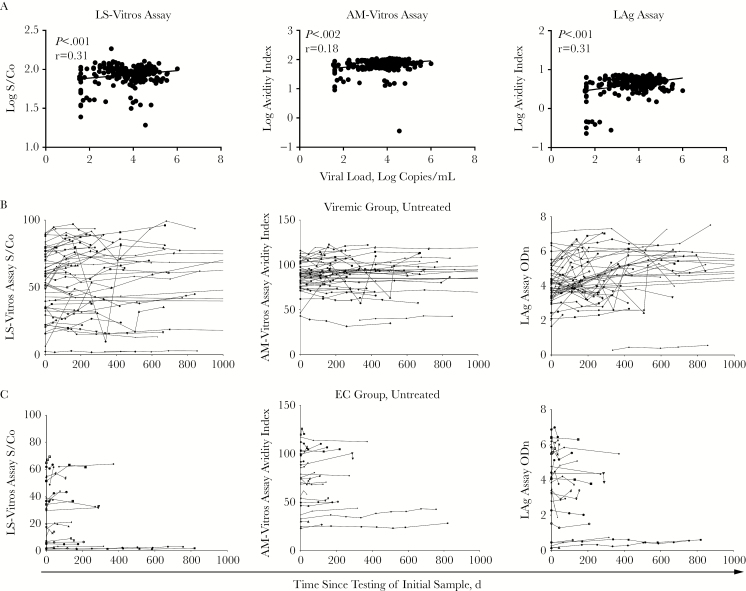
We set out to investigate the relationship between anti-HIV antibody and viral load measurements in untreated HIV-infected individuals. Antibody levels from untreated time points for chronically infected individuals were compared to the viral loads from the same time points. Antibody levels detected by all 3 assays showed modest but statistically significant correlations with viral load (r = 0.31 and P < .005 for the less sensitive Vitros assay, r = 0.18 and P = .002 for the avidity-modified Vitros assay, and r = 0.31 and P < .0001 for the LAg assay; Figure 1A).
Figure 1.
Antibody levels in chronically infected untreated individuals correlate with viral loads. A, The concentration of virus influences antibody production, and the log viral load was found to correlate with antibody levels. Antibody concentration assays (ie, the less sensitive Vitros [LS-Vitros] assay and the Sedia limiting antigen [LAg] assay) have higher correlations than the avidity-modified Vitros (AM-Vitros) assay. Pearson correlation results are reported. B, Longitudinal measurements in untreated individuals show stable antibody concentrations measurable by these assays. LS-Vitros and AM-Vitros assay measurements are more stable than LAg assay measurements. Antibody levels in viremic individuals are significantly higher than those in elite controllers (ECs; P < .001, by the t test). Viral loads undetectable by a standard assay and those determined to be <40 or <75 copies/mL were excluded from the analysis. ODn, normalized OD; S/Co, signal-to-cutoff ratio.
Antibody Levels Are Stable Over Time in Chronically Infected Individuals
Median variabilities in coefficients of variation for the less sensitive Vitros assay, the avidity-modified Vitros assay, and the LAg assay were 10%, 4%, and 15%, respectively, for the viremic chronic infected individuals and 10%, 3%, and 7%, respectively, for the elite controllers (Figure 1B). There was a statistically significant difference between the viremic and elite controller antibody levels detected by the less sensitive Vitros assay only, with median signal-to-cutoff ratios of 61.3 and 31.6, respectively (P = .02), for all longitudinal time points in viremic and suppressed groups (median avidity indexes of 0.91 and 0.74, respectively [P = not significant], were observed for the avidity-modified Vitros assay, and median normalized ODs of 4.4 and 4.0, respectively [P = not significant], were observed for the LAg assay).
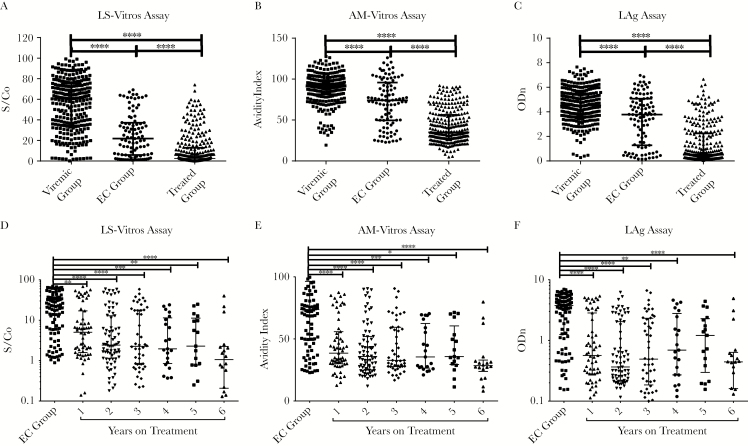
Antibody Levels Decline as Treatment Duration Increases
We compared antibody levels between chronic, untreated HIV-infected individuals, elite controllers, and individuals with ART-suppressed infection to determine the impact of viral replication or ART on antibody production. Median signal-to-cutoff ratios yielded by the less sensitive Vitros assay for untreated individuals, elite controllers, and treated individuals were 60.4, 21.9, and 2.5, respectively; median avidity indexes for the avidity-modified Vitros assay were 0.91, 0.74, and 0.31, respectively; and median normalized ODs for the LAg assay were 4.5, 3.8, and 0.5, respectively. There were higher relative antibody amounts and avidity in individuals with chronic viremic infection, compared with amounts for elite controllers or individuals with ART-suppressed infection (P < .005 for each pairwise comparison; Figure 2A–2C). All antibody levels decreased significantly in individuals with ART-suppressed infection, compared with those in elite controllers (P < .05 for all comparisons; Figure 2D–2F). We found that, in this cross-sectional analysis, antibody levels decreased as the duration of ART increased and that these measures were significantly lower than in elite controllers.
Figure 2.
Antibody levels reflect levels of viral replication and the duration of suppression due to antiretroviral therapy. A, The quality of human immunodeficiency virus (HIV) control impacts the concentration and avidity of antibodies. Antibody levels are significantly reduced in both elite controllers (ECs) and treated participants compared to viremic. Values in the treated group were significantly reduced as compared to those in the other groups. B, Antibody levels decrease with treatment duration and are significantly lower than those in ECs. Bars represent medians and interquartile ranges. Abbreviations: ODn, normalized OD; S/Co, signal-to-cutoff ratio. *P < .05; **P < .01; ***P < .001; ****P < .0001.
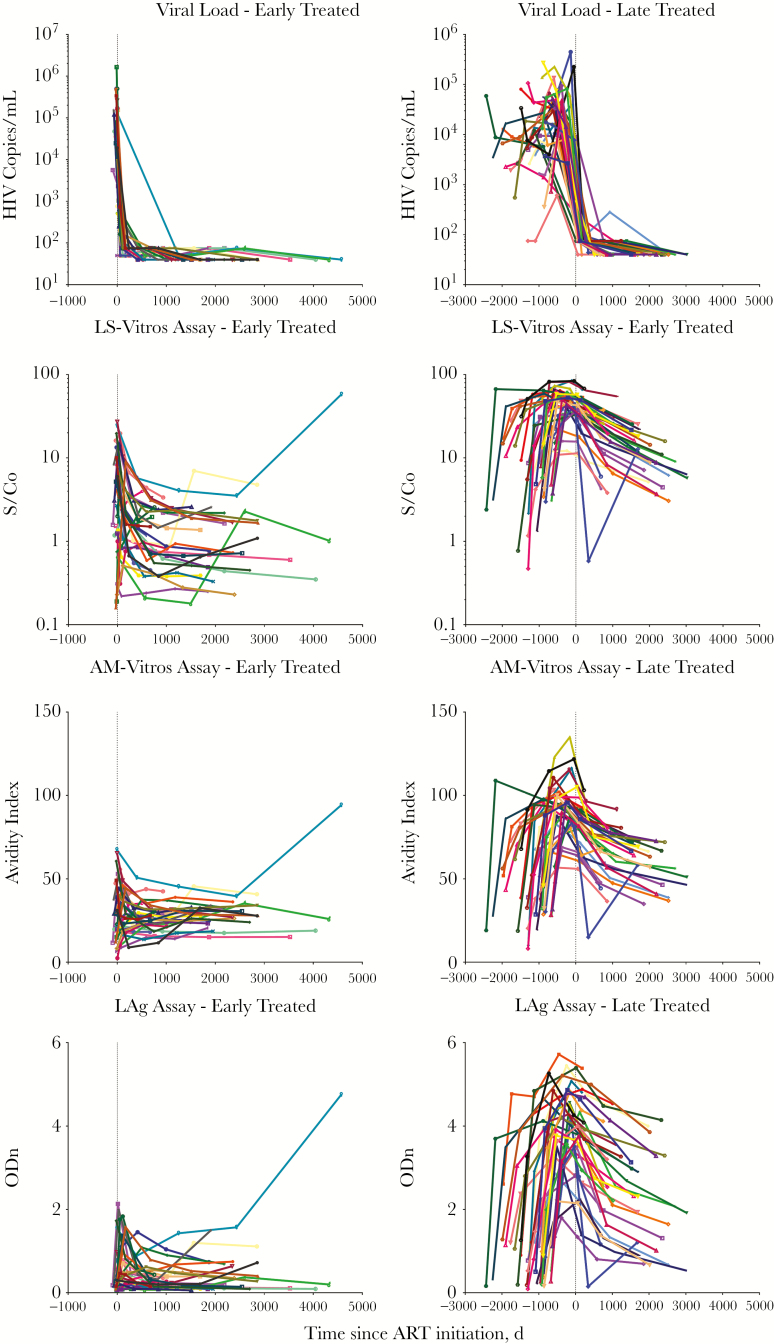
Decline in Antibody Responses in the ET and LT Groups
Antibody responses were measured in the ET and LT cohorts from pretreatment to ≥5 years after ART initiation. Participants from the ET and LT groups showed decreased antibody responses after treatment. On the day of treatment, antibody responses were significantly higher in the LT group as compared to the ET group (for the less sensitive Vitros assay, signal-to-cutoff ratios were 5.5 vs 0.27; for the avidity-modified Vitros assay, avidity indexes were 0.86 vs 0.28 AI; and for the LAg assay, normalized ODs were 3.88 vs 0.3; P < .005 for all comparisons). In longitudinal sample sets, decay rates were calculated between the first and last time points after treatment. For all assays, declines in antibody responses were slower in the ET group as compared to the LT group, with signal-to-cutoff ratio decreases of 9.5 × 10–5 and 6.3 × 10–4 per day (P < .001) for the less sensitive Vitros assay, avidity indexes decreases of 6.5 × 10–4 and 1.3 × 10–2 per day (P < .001) for the avidity-modified Vitros assay, and normalized OD decreases of 5.2 × 10–5 and 5.1 × 10–4 per day (P < .001) for the LAg assay (Figure 3).
Figure 3.
Antibody levels dramatically decline with viral load suppression after antiretroviral treatment (ART) initiation. During total viral suppression, antibody levels dramatically decline in both early and late-treated groups. Since antibody concentrations were higher in the late-treated group, the slope of the decline is much steeper. While concentrations in the early treated group seem to reach a new steady state or increase, those in the late-treated group continue to decline. Colors correspond to the same study participant throughout the antibody measurements. Abbreviations: AM-Vitros, avidity-modified Vitros assay; LAg, Sedia limiting antigen; LS-Vitros, less sensitive Vitros assay; ODn, normalized OD; S/Co, signal-to-cutoff ratio.
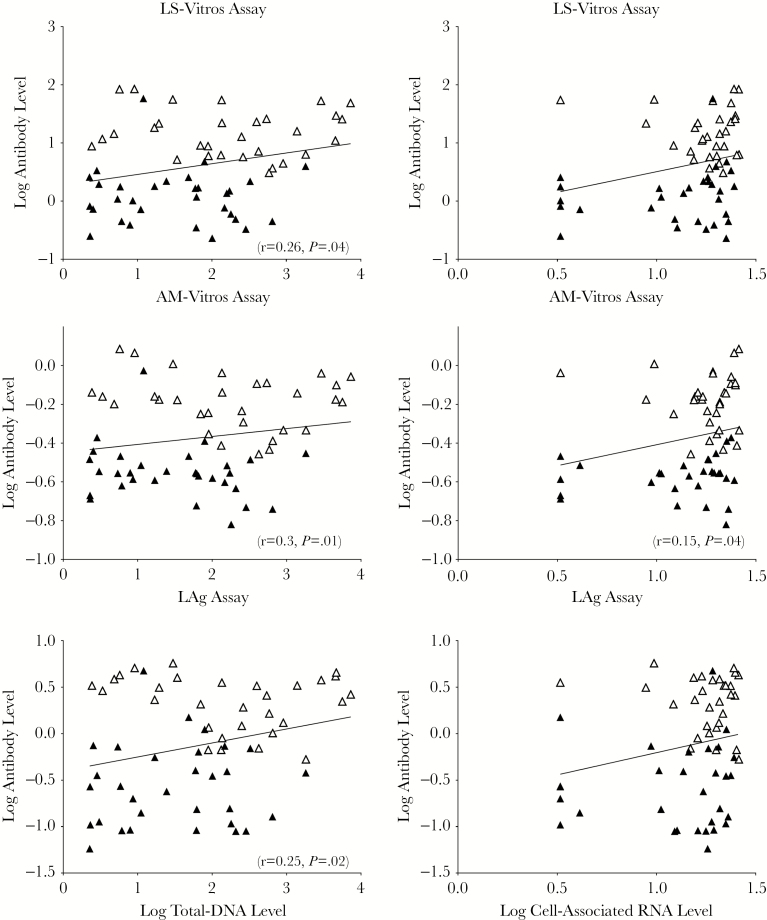
Viral Reservoir Levels Correlate With Antibody Responses
Levels of the viral reservoir (as determined by measuring levels of cell-associated RNA and DNA) significantly correlated with antibody responses in the ET and LT groups combined. DNA levels correlated with findings of the less sensitive Vitros assay (r = 0.26 and P = .02), the avidity-modified Vitros assay (r = 0.30 and P = .01), and the LAg assay (r = 0.25 and P = .04; Figure 4). Cell-associated RNA levels correlated with findings from the avidity-modified Vitros assay only (r = 0.27 and P = .04). Levels of the viral reservoir correlated with antibody responses for the ET and LT groups combined but not separately.
Figure 4.
Viral reservoir levels correlate with antibody levels. DNA and RNA were measured and compared to antibody concentrations [21]. Antibody and DNA levels correlate for all antibody assays, and cell-associated RNA and antibody levels correlated for the less sensitive Vitros (LE-Vitros) assay and the Sedia limiting antigen (LAg) assay but not for the avidity-modified Vitros (AM-Vitros) assay. There are no significant correlations with individual treatment groups. The open triangles are later-treated individuals, while the closed triangles are early treated individuals.
Antibody Levels Are Associated With Lower Levels of Expression of Anti-HIV Host Restriction Factors
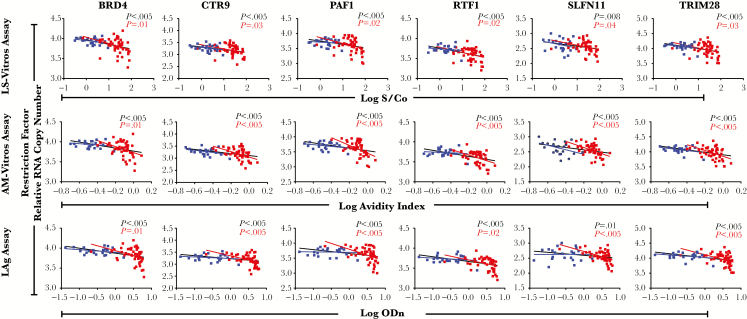
Of the 42 genes assessed, expression of 9 restriction factors had inverse correlations (r < –0.25 and P < .05, by the Benjamini-Hochberg false-discovery rate correction) with antibody levels for BRD4, CNP, CTR9, HERC5, PAF1, RTF1, SLFN11, TNFRSF10A, and TRIM28 (Supplementary Table 1). This correlation remained significant in 6 of 9 restriction factors in the subgroup analysis involving individuals from the LT group (P < .05; Figure 5).
Figure 5.
Antibody levels inversely correlate with human immunodeficiency virus restriction factor expression. Concentrations of BRD4, CTR9, PAF1, RTF1, SLFN11, and TRIM28 inversely correlate with antibody levels. Early treated (blue) and late-treated (red) individuals were analyzed separately, and P values are reported for the groups together (values are reported in black) and separate (red for late-treated individuals and blue for early treated individuals). The figures presented here are the significant correlations after false-discovery rate correction in all and late-treated groups in at least one of the antibody levels. Abbreviations: AM-Vitros, avidity-modified Vitros assay; LAg, Sedia limiting antigen; LS-Vitros, less sensitive Vitros assay; ODn, normalized OD; S/Co, signal-to-cutoff ratio.
DISCUSSION
In this longitudinal study of patients with well-characterized histories of HIV infection and treatment, we found that changes in HIV antibody responses over time during ART reflect the timing of treatment initiation, the degree of viral control over time, and the size of the persistent HIV reservoir. The antibody levels are stable, with higher antibody levels in viremic individuals, lower antibody levels in elite controllers, whereas antibody levels during ART-induced viral suppression are very low and/or consistently declining. These observations are consistent with the idea that viral replication or antigen expression is responsible for maintenance of antibody levels in both untreated individuals and individuals with viral suppression. Among patients taking continuous ART in our study, declining antibody levels corresponded to better viral control and a lower systemic viral burden (ie, lower cell-associated HIV DNA levels). In a few of our subjects, responses declined with ART but increased again later, as revealed by multiple-antibody assays—however, these patients maintained “undetectable” viral loads during the study. It seems likely that the antibody assays detected viral replication too low or too brief to be captured by standard, intermittent viral load measurements. Taken together, the data from this study suggest that monitoring HIV antibody responses over time in treated patients could provide information on the effectiveness of viral control over time (as hemoglobin A1C levels reflect consistent glycemic control in diabetes mellitus). These findings might have implications for adherence monitoring in a broad range of antiretroviral treatment and research programs, since antibody levels summarize responses to viral antigens during the periods between clinical viral load measurements.
The ability to identify the degrees of cumulative HIV replication or antigen expression taking place in reservoirs of ART recipients (or even untreated individuals) could be particularly important for ongoing research aiming to enhance intrinsic control of the HIV reservoir. Analysis of serial antibody levels could be used to assess pre-intervention control in patients receiving ART or to monitor changes in low-level viral burdens that may occur during viral breakthrough, as has been shown in other studies [12, 14], or during treatment interruption. A validated measure of tight viral control could be useful in identifying patients who were more or less likely to have low reservoirs during ART. Because of the tendency for antibody levels to continue declining without a plateau for many years during ART, single measurements of antibody levels at a point in time are unlikely to directly estimate the viral reservoir size. While we did see evidence of an association between antibody levels and available measurements of the reservoir levels in our pooled cohort of treated patients, the association was not found when the ET or LT groups were considered separately. If early treatment led independently to lower antibody levels [36–38] and to lower HIV DNA levels [21, 39], this would produce a spurious association. Further analysis of the timing of treatment and effects on reservoir size will require additional studies, larger sample numbers, and detailed analysis of alternative measurements of viral reservoir levels.
The source and persistence of HIV-specific antibodies are dependent on the location of the viral reservoir and antigenic stimulation of antibody-producing B or plasma cells. The follicular T-cell population has been identified as one of the preferred targets of HIV infection, and the B-cell follicle acts as a sanctuary site for these infected cells, where they evade direct killing by CD8+ T cells, as shown in simian immunodeficiency virus infection [6]. The close proximity of these infected cells to B cells allows for direct stimulation of antibody producing cells during HIV antigen expression in the follicular reservoir [40, 41]. One of the major limitations of this study was the lack of single-copy RNA levels, owing to the fact that large volumes of plasma that are required to detect measurable viral loads using these assays were not available for our participants. It would be important to investigate changes in low-level viral replication and the subsequent impact on antibody concentration, in addition to determining the kinetics of this change. Overall, antibody levels may provide amore comprehensive characterization of viral replication in the tissue sanctuaries, which may not be sufficiently measured by determining the high sensitivity viral load measurement alone. In preparation for clinical interventions, future studies investigating multiple viral reservoir measurements (eg, viral load analyses, cellular DNA and RNA analyses, tissue biopsies, quantitative viral growth assays, in vitro cell stimulation assessments, and antibody response assays) will help to identify the best ways to measure the total HIV replication burden.
While the clinical utility of these new applications for quantitative HIV antibody assays are not clear, these assays are already commercially available and could be readily adopted in many settings. In stark contrast with currently validated methods of measuring antiretroviral adherence, reservoir size, and low-level replication [42–46], the quantitative HIV antibody assays examined in this study use very small sample volumes (5–10 μL of plasma), can be completed in minutes to hours, and cost only a few dollars. These assays have been extensively validated, have been found to be minimally variable (5%–10%), can be controlled by using calibrators across runs to fine-tune assay precision, and could demonstrate usefulness in measuring long-term viral suppression through longitudinal analysis.
Previous work has shown that intrinsic viral restriction factors can influence viral replication and reservoir measurements [22]. For example, restriction factors BRDF4, PAF1 and TRIM28, associated with anti-HIV antibody levels in this study, suppress HIV transcription following viral integration. This supports the hypothesis that restriction factor suppression of viral replication in infected cells leads to lower HIV antigen expression and consequent reduction in host antibody production. Expression levels of these genes have been shown to be inversely correlated with cell-associated HIV RNA levels in individuals with ART-suppressed HIV infection; therefore, they may contribute to limiting the turnover of the latent reservoir. In this study, there was no correlation with restriction factors in the ET group, likely because ART was initiated before full seroconversion. More work will be needed to fully elucidate the utility of combinations of biomarkers of latent infection and immunologic mechanisms governing viral persistence.
In summary, in this study we showed that HIV antibodies may be a useful marker of persistent low-level viral replication during ART, and demonstrated how certain innate immune factors and antiviral therapy reduce HIV antibody responses by limiting viral replication. Inexpensive, high-throughput quantitative antibody assays—originally developed as HIV recency assays for surveillance—could have important new uses in antiretroviral adherence monitoring and could facilitate research into HIV cure interventions.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This work was supported by the Bill and Melinda Gates Foundation (OPP1017716 to the Consortium for the Evaluation and Performance of Incidence Assays, of or with which all authors are members or collaborators, respectively), the amfAR Institute for HIV Cure Research (108545 to S. K. P.), the National Institutes of Health (NIH; R21 AI108503 to S. K. P., P30 AI027763 to the University of California–San Francisco [UCSF] and Gladstone Institute of Virology and Immunology Center for AIDS Research [CFAR], and KL2TR000143 [via the UCSF Clinical and Translational Science Institute] to V. J.), the National Institutes of Health and DARE (U19 AI0961090), and Ortho Clinical Diagnostics (HIV testing kits). The SCOPE cohort was supported by the Delaney AIDS Research Enterprise (AI096109 and A127966), the National Institute of Allergy and Infectious Diseases (K24 AI069994), the UCSF and Gladstone Institute of Virology and Immunology CFAR (P30 AI027763), and the CFAR Network of Integrated Systems (R24 AI067039).
Potential conflicts of interest. M. P. B. receives ongoing funding from Ortho Clinical Diagnostics, provided to Blood Systems Research Institute, to enable ongoing evaluations of their respective assays. V. J. has received grant support from Gilead Sciences for work unrelated to this study. Ortho Clinical Diagnostics provided the kits free of charge. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med 1995; 1:1284–90. [DOI] [PubMed] [Google Scholar]
- 2. Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 1997; 387:183–8. [DOI] [PubMed] [Google Scholar]
- 3. Damouche A, Lazure T, Avettand-Fènoël V, et al. Adipose tissue is a neglected viral reservoir and an inflammatory site during chronic HIV and SIV infection. PLoS Pathog 2015; 11:e1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kramer-Hämmerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res 2005; 111:194–213. [DOI] [PubMed] [Google Scholar]
- 5. Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antivir Ther 2011; 16:1149–67. [DOI] [PubMed] [Google Scholar]
- 6. Fukazawa Y, Lum R, Okoye AA, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 2015; 21:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diaz RS, Kallas EG, Castelo A, Rawal BD, Busch MP. Use of a new ‘less-sensitive enzyme immunoassay’ testing strategy to identify recently infected persons in a Brazilian prison: estimation of incidence and epidemiological tracing. AIDS 1999; 13:1417–8. [DOI] [PubMed] [Google Scholar]
- 8. Keating SM, Hanson D, Lebedeva M, et al. Lower-sensitivity and avidity modifications of the vitros anti-HIV 1 + 2 assay for detection of recent HIV infections and incidence estimation. J Clin Microbiol 2012; 50:3968–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kassanjee R, Pilcher CD, Keating SM, et al. ; Consortium for the Evaluation and Performance of HIV Incidence Assays (CEPHIA). Independent assessment of candidate HIV incidence assays on specimens in the CEPHIA repository. AIDS 2014; 28:2439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wei X, Liu X, Dobbs T, et al. Development of two avidity-based assays to detect recent HIV type 1 seroconversion using a multisubtype gp41 recombinant protein. AIDS Res Hum Retroviruses 2010; 26:61–71. [DOI] [PubMed] [Google Scholar]
- 11. Chaillon A, Le Vu S, Brunet S, et al. Decreased specificity of an assay for recent infection in HIV-1-infected patients on highly active antiretroviral treatment: implications for incidence estimates. Clin Vaccine Immunol 2012; 19:1248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wendel SK, Longosz AF, Eshleman SH, et al. Short communication: the impact of viral suppression and viral breakthrough on limited-antigen avidity assay results in individuals with clade B HIV infection. AIDS Res Hum Retroviruses 2017; 33:325–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Longosz AF, Mehta SH, Kirk GD, et al. Incorrect identification of recent HIV infection in adults in the United States using a limiting-antigen avidity assay. AIDS 2014; 28:1227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wendel SK, Mullis CE, Eshleman SH, et al. Effect of natural and ARV-induced viral suppression and viral breakthrough on anti-HIV antibody proportion and avidity in patients with HIV-1 subtype B infection. PLoS One 2013; 8:e55525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laeyendecker O, Brookmeyer R, Oliver AE, et al. ; Multicenter Aids Cohort Study Macs. Factors associated with incorrect identification of recent HIV infection using the BED capture immunoassay. AIDS Res Hum Retroviruses 2012; 28:816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ostrowski M, Benko E, Yue FY, et al. Intensifying antiretroviral therapy with raltegravir and maraviroc during early human immunodeficiency virus (HIV) infection does not accelerate HIV reservoir reduction. Open forum infect dis 2015; 2:ofv138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aono Y, Imai J, Tominaga K, Orita S, Sato A, Igarashi H. Rapid, sensitive, specific, and quantitative detection of human T-cell leukemia virus type 1 sequence in peripheral blood mononuclear cells by an improved polymerase chain reaction method with nested primers. Virus Genes 1992; 6:159–71. [DOI] [PubMed] [Google Scholar]
- 18. Manns A, Miley WJ, Wilks RJ, et al. Quantitative proviral DNA and antibody levels in the natural history of HTLV-I infection. J Infect Dis 1999; 180:1487–93. [DOI] [PubMed] [Google Scholar]
- 19. Burbelo PD, Bayat A, Rhodes CS, et al. HIV antibody characterization as a method to quantify reservoir size during curative interventions. J Infect Dis 2014; 209:1613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hecht FM, Busch MP, Rawal B, et al. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS 2002; 16:1119–29. [DOI] [PubMed] [Google Scholar]
- 21. Jain V, Hartogensis W, Bacchetti P, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis 2013; 208:1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abdel-Mohsen M, Wang C, Strain MC, et al. Select host restriction factors are associated with HIV persistence during antiretroviral therapy. AIDS 2015; 29:411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abdel-Mohsen M, Chavez L, Tandon R, et al. Human Galectin-9 is a potent mediator of HIV transcription and reactivation. PLoS Pathog 2016; 12:e1005677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janssen RS, Satten GA, Stramer SL, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA 1998; 280:42–8. [DOI] [PubMed] [Google Scholar]
- 25. Hecht FM, Wellman R, Busch MP, et al. ; Acute Infection Early Disease Research Program. Identifying the early post-HIV antibody seroconversion period. J Infect Dis 2011; 204:526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kothe D, Byers RH, Caudill SP, et al. Performance characteristics of a new less sensitive HIV-1 enzyme immunoassay for use in estimating HIV seroincidence. J Acquir Immune Defic Syndr 2003; 33:625–34. [DOI] [PubMed] [Google Scholar]
- 27. Duong YT, Qiu M, De AK, et al. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies. PLoS One 2012; 7:e33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002; 3:RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee TH, el-Amad Z, Reis M, et al. Absence of HIV-1 DNA in high-risk seronegative individuals using high-input polymerase chain reaction. AIDS 1991; 5:1201–7. [DOI] [PubMed] [Google Scholar]
- 30. Lee TH, Sunzeri FJ, Tobler LH, Williams BG, Busch MP. Quantitative assessment of HIV-1 DNA load by coamplification of HIV-1 gag and HLA-DQ-alpha genes. AIDS 1991; 5:683–91. [DOI] [PubMed] [Google Scholar]
- 31. Sunzeri FJ, Lee TH, Brownlee RG, Busch MP. Rapid simultaneous detection of multiple retroviral DNA sequences using the polymerase chain reaction and capillary DNA chromatography. Blood 1991; 77:879–86. [PubMed] [Google Scholar]
- 32. Hatano H, Jain V, Hunt PW, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis 2013; 208:50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bernardin F, Tobler L, Walsh I, Williams JD, Busch M, Delwart E. Clearance of hepatitis C virus RNA from the peripheral blood mononuclear cells of blood donors who spontaneously or therapeutically control their plasma viremia. Hepatology 2008; 47:1446–52. [DOI] [PubMed] [Google Scholar]
- 34. Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol 2009; 83:329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benjamini Y. Discovering the false discovery rate. J R Stat Soc Series B Stat Methodol 2010; 72:405–16. [Google Scholar]
- 36. Ananworanich J, Sacdalan CP, Pinyakorn S, et al. Virological and immunological characteristics of HIV-infected individuals at the earliest stage of infection. J Virus Erad 2016; 2:43–8. [PMC free article] [PubMed] [Google Scholar]
- 37. Henrich TJ, Hu Z, Li JZ, et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis 2013; 207:1694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Keating SM, Pilcher CD, Busch MP. Editorial commentary: timing is everything: shortcomings of current HIV diagnostics in the early treatment era. Clin Infect Dis 2016; 63:562–4. [DOI] [PubMed] [Google Scholar]
- 39. Ananworanich J, Schuetz A, Vandergeeten C, et al. ; RV254/SEARCH 010 Study Group. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 2012; 7:e33948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med 2012; 209:1241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cubas RA, Mudd JC, Savoye AL, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 2013; 19:494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang Y, Gandhi M, Greenblatt RM, Gee W, Lin ET, Messenkoff N. Sensitive analysis of anti-HIV drugs, efavirenz, lopinavir and ritonavir, in human hair by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun Mass Spectrom 2008; 22:3401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Else L, Watson V, Tjia J, et al. Validation of a rapid and sensitive high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) assay for the simultaneous determination of existing and new antiretroviral compounds. J Chromatogr B Analyt Technol Biomed Life Sci 2010; 878:1455–65. [DOI] [PubMed] [Google Scholar]
- 44. Li JZ, Etemad B, Ahmed H, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS 2016; 30:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hill AL, Rosenbloom DI, Goldstein E, et al. Real-time predictions of reservoir size and rebound time during antiretroviral therapy interruption trials for HIV. PLoS Pathog 2016; 12:e1005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Laird GM, Rosenbloom DI, Lai J, Siliciano RF, Siliciano JD. Measuring the frequency of latent HIV-1 in resting CD4⁺ T cells using a limiting dilution coculture assay. Methods Mol Biol 2016; 1354:239–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.