In the tightly regulated chlorophyll biosynthesis pathway, the transcription factor Rice Phytochrome-Interacting Factor-Like1 promotes chlorophyll biosynthesis by up-regulating chlorophyll biosynthetic genes through feed-forward regulatory loops involving GOLDEN2-LIKE1 (OsGLK1) and OsGLK2.
Keywords: Chlorophyll biosynthesis, OsCAO1, OsGLK, OsPIL1, OsPORB, rice, transcriptional regulation
Abstract
In phototrophic plants, the highly conserved and tightly regulated process of chlorophyll (Chl) biosynthesis comprises multi-step reactions involving more than 15 enzymes. Since the efficiency of Chl biosynthesis strongly affects plant productivity, understanding the underlying regulatory mechanisms in crop plants can be useful for strategies to increase grain and biomass yields. Here, we show that rice (Oryza sativa) Phytochrome-Interacting Factor-Like1 (OsPIL1), a basic helix-loop-helix transcription factor, promotes Chl biosynthesis. The T-DNA insertion knockdown ospil1 mutant showed a pale-green phenotype when grown in a natural paddy field. Transcriptome analysis revealed that several genes responsible for Chl biosynthesis and photosynthesis were significantly down-regulated in ospil1 leaves. Using promoter binding and transactivation assays, we found that OsPIL1 binds to the promoters of two Chl biosynthetic genes, OsPORB and OsCAO1, and promotes their transcription. In addition, OsPIL1 directly up-regulates the expression of two transcription factor genes, GOLDEN2-LIKE1 (OsGLK1) and OsGLK2. OsGLK1 and OsGLK2 both bind to the promoters of OsPORB and OsCAO1, as well as some of genes encoding the light-harvesting complex of photosystems, probably promoting their transcription. Thus, OsPIL1 is involved in the promotion of Chl biosynthesis by up-regulating the transcription of OsPORB and OsCAO1 via trifurcate feed-forward regulatory loops involving two OsGLKs.
Introduction
Chlorophyll (Chl), a green pigment found in phototrophic organisms, harvests light and transfers the resulting excitation energy to other components of the electron transport chain. Land plants, green algae, and a few cyanobacteria synthesize and use two types of Chl, Chl a and Chl b (Melis, 1991; Blankenship, 1992). In addition to its light-harvesting role, Chl and its intermediates also act as strong photosensitizers and generate reactive oxygen species when they are present in excess and irradiated by light (Meskauskiene et al., 2001; Nagata et al., 2005; Skribanek et al., 2008; Hideg et al., 2010). To prevent adverse effects, Chl biosynthesis is a highly co-ordinated process, with multi-step reactions catalysed by various biosynthetic enzymes. In Arabidopsis thaliana, 15 enzymes associated with Chl biosynthesis have been identified to date via several biochemical and genetic approaches, as well as genomic analysis (Tanaka and Tanaka, 2007). Since mutations in some Chl biosynthetic genes negatively affect Chl biosynthesis and accumulation, arabidopsis mutants of some of these genes show a pale-green phenotype (Oster et al., 2000; Mochizuki et al., 2001; Nagata et al., 2005). Chl levels are largely controlled by the balance between anabolism and catabolism (Hörtensteiner and Kräutler, 2011; Czarnecki and Grimm, 2012), which has a direct effect on photosynthetic efficiency, a process that greatly affects productivity in cereal crops.
Rice (Oryza sativa), a major cereal crop worldwide, has been intensively studied as a model monocot species. The regulatory mechanism underlying Chl biosynthesis has been explored in rice using Chl-deficient mutants, such as pale-green, variegated, or albino mutants. Among Chl-deficient rice mutants, several are associated with Chl biosynthetic enzymes. For example, the chlorina1 (chl1) and chl9 mutants affect the genes encoding OsCHLD and OsCHLI, two of the three subunits of Mg-chelatase; the chl1 and chl9 mutants have yellowish-green leaves due to reduced Mg-protoporphyrin IX and total Chl contents (Zhang et al., 2006). Chlorophyllide a oxygenase (CAO) and 3,8-divinyl protochlorophyllide a 8-vinyl reductase (DVR) are key enzymes involved in Chl homeostasis (Tanaka et al., 1998; Nagata et al., 2005), and rice mutants of OsCAO1 and OsDVR also have a pale-green leaf phenotype (Lee et al., 2005; Wang et al., 2010). By contrast, the rice mutant faded green leaf (fgl), harboring a mutation in OsPORB (encoding protochlorophyllide a oxidoreductase B), has variegated leaves, especially under high-light conditions (Sakuraba et al., 2013).
In addition to mutants of Chl biosynthetic enzymes, other Chl-deficient rice mutants have also been reported. The young leaf chlorosis1 (ylc1) mutant, with a mutation in a DUF3353 superfamily gene, has pale-green leaves at the early seedling stage (Zhou et al., 2013). The yellow green leaf2 (ygl2) mutant, which is impaired in heme oxygenase 1 (HO1) production, also has pale-green leaves, indicating that HO1 indirectly affects Chl biosynthesis, as heme and chlorophyll share the same substrates prior to protoporphyrin IX formation (Chen et al., 2013).
GOLDEN2-LIKE (GLK) is another important factor that helps to regulate Chl homeostasis. GLK genes encode GARP-type transcription factors (TFs) that play regulatory roles in chloroplast development and Chl biosynthesis, and thus glk mutants in various plants, including arabidopsis, rice, tomato, and the moss Physcomitrella patens, have pale-green leaves (Fitter et al., 2002; Yasumura et al., 2005; Powell et al., 2012; Wang et al., 2013). In arabidopsis, two GLK proteins, AtGLK1 and AtGLK2, bind to the promoters of genes encoding components of the photosynthetic apparatus, including the light-harvesting complex of photosystem II (LHCII), as well as Chl biosynthetic genes, including CAO, PORB, and CHLH, and up-regulate their expression (Waters et al., 2009). However, it is still unknown whether two GLK proteins in rice, OsGLK1 and OsGLK2, also directly up-regulate the expression of genes for photosynthetic apparatus components and Chl biosynthesis, although the osglk1 osglk2 double-mutant has pale-green leaves due to reduced Chl accumulation throughout development (Wang et al., 2013).
Phytochrome-Interacting Factors (PIFs) are plant-specific basic helix-loop-helix (bHLH)-type TFs whose regulatory roles have been widely studied in arabidopsis (Castillon et al., 2007). PIF TFs regulate various biological processes in a red-light phytochrome (phy)-dependent manner, including seed germination (Oh et al., 2004), hypocotyl elongation (Nusinow et al., 2011), flowering (Kumar et al., 2012), leaf senescence (Sakuraba et al., 2014), Chl biosynthesis (Huq et al., 2004), and the biosynthesis or signaling pathways of phytohormones, including gibberellic acid (Feng et al., 2008), auxin (Franklin et al., 2011; Oh et al., 2014), and brassinosteroids (Shahnejat-Bushehri et al., 2016). However, to date, the regulatory roles of rice PIFs are largely unknown. Six rice PIF TFs, termed OsPIF-Like11 (OsPIL11), OsPIL12, OsPIL13 (also termed OsPIL1), OsPIL14, OsPIL15, and OsPIL16, are considered homologs of arabidopsis PIF TFs based on sequence similarity (Nakamura et al., 2007). OsPIL15-overexpressing (OX) transgenic rice plants exhibit shorter shoots and roots under dark conditions, indicating that OsPIL15 is involved in growth of etiolated seedlings (Zhou et al., 2014), similar to arabidopsis PIFs (Shin et al., 2009). Microarray analysis has shown that OsPIL1/OsPIL13 (hereafter referred to as OsPIL1) is a stress-responsive gene (Maruyama et al., 2012). Todaka et al. (2012) reported that overexpressing OsPIL1 promotes internode elongation by increasing internode cell size, especially under drought-stress conditions. Therefore, OsPIL1-OX plants are significantly taller than the wild-type, but transgenic rice plants expressing OsPIL1-RD (fused to a transcriptional repression domain) are shorter (Todaka et al., 2012).
In this study, we found that OsPIL1 is a key regulator of Chl biosynthesis. The T-DNA insertion ospil1 knockdown mutant exhibited a pale-green leaf phenotype, with significantly reduced levels of Chl and Chl-binding proteins compared to the wild-type. Microarray analysis revealed that the genes for Chl biosynthetic enzymes and the photosynthetic apparatus, as well as two OsGLK genes, were significantly down-regulated in ospil1 mutants. Furthermore, promoter binding and transactivation assays revealed that OsPIL1 binds to the promoters of OsPORB, OsCAO1, OsGLK1, and OsGLK2 and up-regulates their expression. Moreover, OsGLK1 and OsGLK2 bind to the promoters of OsPORB and OsCAO1. We propose a possible model for the regulation of Chl biosynthesis in rice via OsPIL1.
Materials and methods
Plant material and growth conditions
A T-DNA insertion knockdown mutant of OsPIL1 (LOC_Os03g56950; PFG_4A-03590.R; hereafter termed ospil1) was isolated in the Korean japonica rice cultivar ‘Dongjin’ (hereafter referred to as the wild-type; WT) using information obtained from the Salk Institute Genomic Analysis Laboratory (http://signal.salk.edu/cgi-bin/RiceGE) (Jeong et al., 2002). The plants were grown in a paddy field at the Seoul National University Experiment Farm under natural long-day (NLD) conditions (latitude 37° N, Suwon, Korea). The seeds were sown on seedbeds in a greenhouse and after 1 month the seedlings were transplanted to the paddy field. Rice plants were also grown in growth chambers under short-day (SD; 10 h light, 30 °C / 14 h dark, 24 °C) and long-day (LD; 14.5 h light, 30 °C / 9.5 h dark, 24 °C) conditions using light-emitting diodes at a photon flux density of approximately 300 μmol m–2 s–1 PAR, with 60% relative humidity.
Plasmid construction and plant transformation
The OsPIL1 cDNA was amplified by RT-PCR using the gene-specific primers OsPIL1-F and OsPIL1-R (Supplementary Table S1 at JXB online) and sub-cloned into the pCR8/GW/TOPO vector (Invitrogen). After verifying its sequence, the OsPIL1 cDNA was inserted into the pMDC32 Gateway binary vector containing the 35S promoter (Curtis and Grossniklaus, 2003) through LR recombination (Lambda integrase/excisionase, Elpisbio, Korea). The resulting plasmid was transformed into Agrobacterium tumefaciens strain EHA105, which was introduced into rice calli from mature ospil1 embryos using Agrobacterium-mediated transformation (Jeon et al., 1999; Lee et al., 2006). The transgenic rice plants were selected on 2N6 medium containing hygromycin (50 mg l–1) and confirmed by genomic PCR using specific primers (see Supplementary Table S1).
RNA extraction, reverse transcription (RT), and quantitative PCR (qPCR) analysis
Total RNA was extracted from leaf tissues using an MG Total RNA Extraction kit (Macrogen, Korea) according to the manufacturer’s instructions. First-strand cDNA for RT was synthesized from 2.5 µg total RNA using the oligo(dT)15 primer and M-MLV reverse transcriptase (Promega) and diluted with water to 100 µl. The relative expression levels of OsPIL1 and Chl biosynthetic genes were measured by RT-qPCR using gene-specific primers and either Ubiquitin5 (UBQ5; Os01g0328400) or GAPDH (glyceraldehyde-3-phosphate dehydrogenase; Os06g0666600) as an internal control (see Supplementary Table S1) (Jain et al., 2006), along with GoTaq qPCR Master Mix (Promega) in a total reaction volume of 20 μl. The expression level of each gene was measured by the relative quantification method using the LightCycler 480 real-time PCR system (Roche Applied Science) under the following cycling conditions: 95 °C for 2 min, followed by 45 cycles of 95 °C for 10 s, and 60 °C for 1 min.
Quantification of photosynthetic pigments
To measure total chlorophyll (Chl) and carotenoid (Car) contents, pigments were extracted from leaf tissues using 80% ice-cold acetone. Chl and Car concentrations were determined by spectrophotometry as described previously (Porra et al., 1989).
SDS-PAGE and immunoblot analysis
To extract total proteins, the middle parts of the first leaves in the main culms of 6-week-old rice plants grown under LD conditions (14 h light/10 h dark) were used. To extract total proteins, leaf tissues were ground in liquid nitrogen and 10-mg aliquots were homogenized with 100 µl of sample buffer [50 mM Tris, pH 6.8, 2 mM EDTA, 10% glycerol, 2% sodium dodecyl sulfate (SDS), and 6% 2-mercaptoethanol]. The homogenates were centrifuged at 10000 g for 3 min, and the supernatants were denatured at 80 °C for 5 min. A 4-µl aliquot of each sample was subjected to 12% (w/v) polyacrylamide SDS-polyacrylamide gel electrophoresis (PAGE), and the resolved proteins were electroblotted onto a Hybond-P membrane (GE Healthcare, USA). Antibodies against photosystem proteins Lhca1, Lhca2, Lhcb1, Lhcb2, Lhcb4, CP43, and PsaA (Agrisera, Sweden) were used for immunoblot analysis. The level of each protein was measured using the ECL system with WESTSAVE (AbFrontier, Korea) according to the manufacturer’s protocol.
Transmission electron microscopy
Transmission electron microscopy was performed using a previously described method (Inada et al., 1998) with some modifications. The middle part of the first leaf in the main culm was used for the experiments. Small leaf pieces were fixed in modified Karnovsky’s fixative (2% paraformaldehyde, 2% glutaraldehyde, and 50 mM sodium cacodylate buffer, pH 7.2), followed by three washes with 50 mM sodium cacodylate buffer, pH 7.2 at 4 °C for 10 min. The samples were post-fixed at 4 °C for 2 h with 1% osmium tetroxide in 50 mM sodium cacodylate buffer, pH 7.2, and washed twice with distilled water at room temperature. The samples were stained en bloc in 0.5% uranyl acetate at 4 °C overnight and dehydrated in an ethanol gradient solution with propylene oxide, followed by infiltration with Spurr’s resin. The samples were polymerized at 70 °C for 24 h and sectioned with an ultramicrotome (MT-X). The sections were mounted on copper grids and stained with 2% uranyl acetate for 7 min and with Reynolds’ lead citrate for 7 min. Micrographs were obtained with a LIBRA 120 transmission electron microscope.
Yeast one-hybrid assays
Yeast one-hybrid assays were performed according to the Yeast Protocols Handbook (Clontech). OsPIL1 cDNA was inserted into the pGAD424 vector (Clontech) as prey. DNA fragments corresponding to the promoters (1050 bp) of OsPORA, OsPORB, and OsCAO1 were cloned into the pLacZi vector (Clontech) as bait. For each gene, two DNA fragments (–2000 to –951, and –1050 to –1 from the start codon) were prepared. Primers used for cloning are listed in Supplementary Table S1. The yeast strain YM4271 was used for the bait and prey clones, and β-galactosidase activity was measured by liquid assay using chlorophenol red-β-D-galactopyranoside (CPRG; Roche Biochemicals).
Microarray analysis
Three-week-old WT and ospil1 plants grown under LD conditions were used for microarray analysis. Total RNA was extracted from the first leaves of WT and ospil1 plants using an MG Total RNA Extraction kit according to the manufacturer’s protocol (Macrogen, Korea). Total RNA quality was checked using a 2100 Bioanalyzer (Agilent Technologies). All microarray experiments, including data analysis, were performed according to the manufacturer’s manual (http://www.genomics.agilent.com/literature.jsp?crumbAction=push&tabId=AG-PR-1001&contentType=User+Manual). The arrays were air-dried and scanned using a high-resolution array scanner (Agilent) with the appropriate settings for two-color gene expression arrays. GeneSpring GX 7.3 (Agilent) was used to calculate the intensity ratio and fold-changes, and quantified with the Feature Extraction Software (Agilent). For evaluating the statistical significance and obtaining the P-value, one-sample t-tests were performed using GeneSpring GX 7.3 (Agilent). Microarray analysis was performed with two experimental replicates with two different biological replicates of WT and ospil1 samples. Information about phytohormone- and photosynthesis-associated genes was obtained from the Oryzabase (www.shigen.nig.ac.jp/rice/oryzabase).
Chromatin immunoprecipitation (ChIP) assay
For the ChIP assay, the 35S:OsPIL1-GFP, 35S:OsGLK1-GFP, and 35S:OsGLK2-GFP constructs in the pMDC43 binary vector (Curtis and Grossniklus, 2003) were transfected into rice protoplasts as previously described (Zhang et al., 2011). The protoplasts were then subjected to cross-linking for 20 min with 1% formaldehyde under vacuum. The chromatin complexes were isolated and sonicated as previously described (Saleh et al., 2008) with slight modifications. An anti-GFP antibody (Abcam) and Protein A agarose/salmon sperm DNA (Millipore) were used for immunoprecipitation. After reverse cross-linking and protein digestion, the DNA was purified using a QIAquick PCR Purification kit (Qiagen). The primer sequences for each gene are listed in Supplementary Table S1.
Results
Phenotypic characterization of the ospil1 knockdown mutant
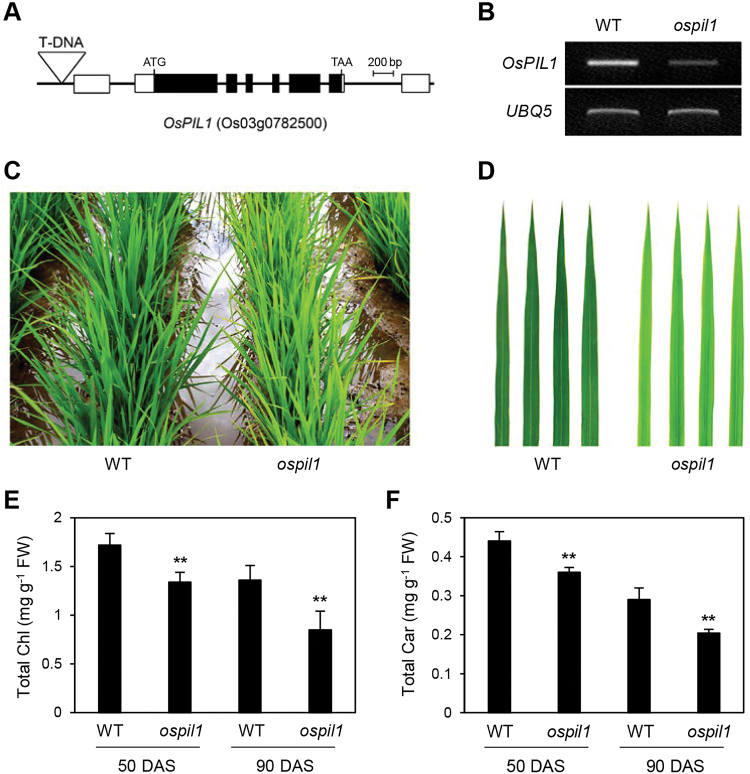
Among rice phytochrome-interacting factors (PIFs), PIF-LIKE1 (OsPIL1; also known as OsPIL13; Os03g0782500) has high similarity to the arabidopsis PIF4 and PIF5 TFs, which play regulatory roles in plant growth and development. OsPIL1 is involved in shoot elongation: OsPIL1-overexpressing (OsPIL1-OX) rice is taller than its parental cultivar (Todaka et al., 2012). To identify other possible function(s) of OsPIL1, we searched for mutant lines in the RiceGE database (http://signal.salk.edu/cgi-bin/RiceGE) and found one T-DNA insertion line (PFG_4A-03590.R), which harbors a T-DNA fragment in the promoter region of OsPIL1 (Fig. 1A). Using RT-qPCR analysis, we confirmed that this line has much lower levels of OsPIL1 transcript than its wild-type parental line, japonica cultivar ‘Dongjin’ (hereafter WT; Fig. 1B), indicating that this line is a knockdown mutant of OsPIL1 (hereafter ospil1).
Fig. 1.
Pale-green leaf phenotype of ospil1 mutants grown in a paddy field. (A) Gene structure and T-DNA insertion site (inverted triangle) in the 1000-bp upstream region of OsPIL1 (PFG_4A-03590.R). (B) Decrease in OsPIL1 transcript levels in the ospil1 mutant confirmed by RT-PCR. UBQ5 was used for an internal control. (C, D) Color difference in whole plants (C) and the first leaf of the main culm (D) between WT and ospil1 at 70 d after sowing (DAS). (E, F) Reduced levels of total Chl (E) and Car (F) in ospil1. The first leaves of the main culm at 50 and 90 DAS were used for analysis. Means and SD were obtained from 10 biological replicates. Significant differences between WT and ospil1 was determined by Student’s t-test (** P<0.01).
To examine the possible phenotypic effect of the ospil1 mutation, we grew ospil1 plants in a paddy field under natural long-day (NLD) conditions (>14 h light/day at 37° N latitude, Suwon, Korea). The ospil1 leaf blades exhibited a pale-green phenotype compared to the WT (Fig. 1C, D). To verify this phenotype, we measured the contents of photosynthetic pigments at two different developmental stages. At 50 d after sowing (DAS), the Chl contents in ospil1 leaves were reduced by 16.2% compared to the WT, while those of carotenoids (Car) were reduced by 10.9%. The phenotype was more severe at 90 DAS, with Chl and Car contents in ospil1 leaves reduced to 24.6% and 18.7% of the WT levels, respectively (Fig. 1E, F).
To confirm that the knockdown mutation in OsPIL1 is responsible for the pale-green leaf phenotype, we performed a complementation test of the ospil1 mutant. Using Agrobacterium-mediated transformation, we obtained three independent transgenic lines containing 35S:OsPIL1 cDNA, which had normal green leaves throughout development (see Supplementary Fig. S1A, B), and validated the overexpression of OsPIL1 transcripts in the leaves of transgenic lines by RT-qPCR (Fig. S1C). Consistent with the visible phenotype, total Chl levels in the transgenic lines were similar to those of WT (Supplementary Fig. S1D), confirming that the knockdown mutation in OsPIL1 results in the development of pale-green leaves.
To examine the pale-green phenotype of ospil1 leaves in more detail, we grew the plants in a growth chamber under LD conditions (14.5 h light, 30 °C / 9.5 h dark, 24 °C). Similar to the phenotype under NLD conditions, young leaves of 2-week-old ospil1 plants were pale green (see Supplementary Fig. S2A, B), with lower levels of Chl and Car compared to the WT (Fig. S2C, D). Immunoblot analysis showed that the levels of photosystem proteins were significantly reduced in ospil1 leaves, with up to a 20–30 % reduction in the levels of light-harvesting complex of photosystem II (LHC II) subunits (Lhcb1, Lhcb2, and Lhcb4), LHC I subunits (Lhca1 and Lhca2), and core subunits of PSII (PsbC) and PSI (PsaA) compared to the WT (Supplementary Fig. S2E). In addition, the ospil1 mutant displayed a slightly higher Chl a/b ratio than the WT (Fig. S2F). We also found by transmission electron microscopy analysis that the chloroplasts of the ospil1 mutant were not defective, but appeared to be slightly smaller and have a looser grana structure compared with the WT (Supplementary Fig. S3).
The chlorosis and/or necrosis observed in some leaf-color mutants in rice largely depend on the photoperiod (Kusumi et al., 2000; Han et al., 2012). Thus, we examined the leaf color of ospil1 plants grown under SD conditions (10 h light/14 h dark), finding that the leaves of this mutant were paler than those of the WT (see Supplementary Fig. S4A), with lower levels of photosynthetic pigments (Fig. S4B, C). This result indicates that the levels of photosynthetic pigments in ospil1 are reduced regardless of photoperiod.
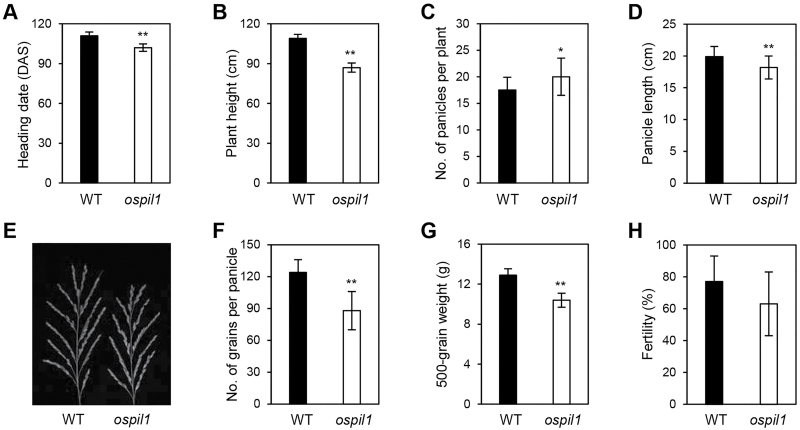
Mutation of OsPIL1 decreases agronomic performance in rice
Since reduced photosynthetic pigment levels and photosynthetic activity negatively affect plant production, many leaf-color-associated mutants show poor agronomic traits compared to the WT (Sakuraba et al., 2013). To examine the relationship between the mutation in ospil1 and crop production, we evaluated several agronomic traits in this mutant, including heading date, plant height, panicle length, number of panicles per plant, number of grains per panicle, spikelet fertility, and 500-grain weight under NLD conditions (Fig. 2). The ospil1 mutant [105 d to heading([DTH)] flowered earlier than the WT (115 DTH) (Fig. 2A). The height of ospil1 plants was significantly smaller than that of the WT (Fig. 2B), which corresponds to the previous finding (Todaka et al., 2012) that OsPIL1-OX plants have significantly increased height due to elongated internode cells. The number of panicles per plant (Fig. 2C) was higher in ospil1 than in the WT; however, the ospil1 mutant had significantly lower values for other agronomic traits compared to the WT, including panicle length (Fig. 2D, E), the number of grains per panicle (Fig. 2F) and 500-grain weight (Fig. 2G), without affecting seed fertility (Fig. 2H). These results indicate that the ospil1 mutation has negative effects on agronomic traits, ultimately reducing rice grain production.
Fig. 2.
Agronomic traits of ospil1 plants. (A) Heading date, (B) plant height, (C) number of panicles per plant, (D) panicle length, (E) panicle phenotype, (F) number of grains per panicle, (G) 500-grain weight, and (H) fertility in WT and ospil1. Means and SD were obtained from at least 10 biological replicates. Significant differences were determined by Student’s t-test (* P<0.05, ** P<0.01).
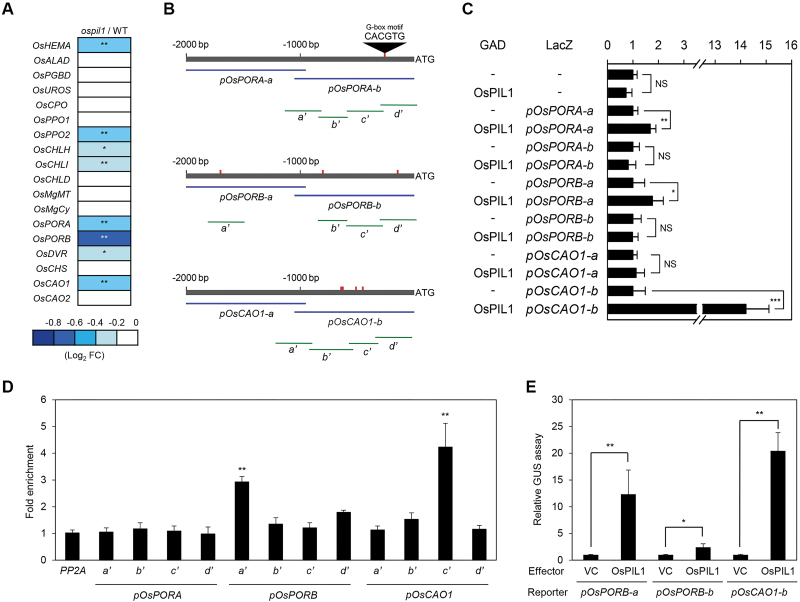
OsPIL1 directly activates the transcription of Chl biosynthetic genes
To examine the downstream regulatory cascade of OsPIL1, we conducted a genome-wide microarray analysis to identify differentially expressed genes between the WT and ospil1 in 1-month-old plants under LD conditions. We identified 725 genes that were significantly up-regulated (ospil1/WT, >2-fold) and 840 genes (including OsPIL1) that were significantly down-regulated (ospil1/WT, <2-fold) in ospil1 compared to the WT. To assess the quality of the microarray data, we investigated whether cell wall-related genes that are up-regulated in OsPIL1-OX plants (Todaka et al., 2012) are differentially expressed in ospil1. In contrast to their expression patterns in OsPIL1-OX, some cell wall-related genes, including expansin, cellulose synthase, and pectinesterase genes, were down-regulated in ospil1 (see Supplementary Fig. S5). In addition, several genes related to the biosynthesis and signaling pathways of growth-promoting phytohormones were also down-regulated (Supplementary Fig. S6), perhaps leading to the reduced plant height of the mutant (Fig. 2B).
Nearly 20 enzymes are involved in the biosynthesis of Chl from glutamic acid (Tanaka and Tanaka, 2006), and rice mutants of Chl anabolic enzymes exhibit leaf chlorosis or necrosis (Lee et al., 2005; Zhang et al., 2006; Wang et al., 2010; Sakuraba et al., 2013). Thus, we examined our microarray data to determine whether the genes for Chl biosynthetic enzymes are differentially expressed in ospil1. Among the 18 Chl biosynthetic genes, several genes such as OsHEMA, OsCHLH, OsPORA, OsPORB, OsDVR, and OsCAO1 were down-regulated in the ospil1 mutants (Fig. 3A); the down-regulation of these genes in ospil1 was further confirmed by RT-qPCR analysis (Supplementary Fig. S7). Furthermore, OsHEMA, OsPORA, OsPORB, and OsCAO1 were up-regulated in OsPIL1-OX plants, whereas the expression levels of OsCHLH and OsDVR were not significantly altered (Supplementary Fig. S8).
Fig. 3.
OsPIL1 directly up-regulates OsPORB and OsCAO1 transcription. (A) Relative expression (ospil1/WT) of Chl biosynthetic genes. Relative expression levels of genes in ospil1 were normalized to those of the WT. Asterisks indicate significant difference between WT and ospil1 plants (* P<0.05, ** P<0.01). (B) The positions of G-boxes in the promoters of OsPORA, OsPORB, and OsCAO1 (–2000 bp to ATG) and the promoter fragments used for the yeast one-hybrid assay (Y1H), transactivation assays (blue horizontal lines), and ChIP assays (green horizontal lines). (C) The binding activity of OsPIL1 to the promoter regions of OsPORA (pOsPORA-a and pOsPORA-b), OsPORB (pOsPORB-a and pOsPORB-b), and OsCAO1 (pOsCAO1-a and pOsCAO1-b) examined by Y1H assays. Empty bait and prey plasmids (-) were used for the negative controls. The relative β-galactosidase activity was obtained by normalizing to the level of each negative control. Means and SD were obtained from more than five independent colonies. (D) OsPIL1 binding affinity to the promoter regions of OsPORA, OsPORB, and OsCAO1 in planta examined by ChIP assays. OsPIL1-GFP was transiently expressed in protoplasts isolated from 10-d-old WT seedlings. Fold-enrichment of the promoter fragments was measured by immunoprecipitation with an anti-GFP antibody (see Methods). PP2A was used as a negative control. (E) Transactivation of OsPORB and OsCAO1 by OsPIL1. The protoplasts were co-transfected with 5 μl of effector plasmid containing 35S:OsPIL1-GFP and 3 μl of reporter plasmids containing pOsPORB-a::GUS, pOsPORB-b::GUS, and pOsCAO1-b::GUS. Empty vector was used as a vector control for the effector. Significant differences were determined by Student’s t-test (* P<0.05, ** P<0.01, *** P<0.001, NS, not significant).
Based on the microarray and RT-qPCR analyses described above, it appears that OsHEMA, OsPORA, OsPORB, and OsCAO1 might be direct targets of OsPIL1. Since OsPIL1, as well as other PIF TFs, specifically bind to the G-box motif (CACGTG) in the promoters of target genes (Todaka et al., 2012), we searched for G-box elements in 2000-bp upstream regions (–2000 bp) of the target genes and found that the promoter regions of OsPORA, OsPORB, and OsCAO1 each contain more than one G-box sequence (Fig. 3B). Therefore, we examined whether OsPIL1 directly binds to the promoter regions of these three candidate genes by yeast one-hybrid (Y1H) assays. OsPIL1 bound to the promoters of OsPORA-a (–2000 to –1000 bp), OsPORB-a (–2000 to –1000 bp), and OsCAO1-b (–1000 bp to 0 bp) in the Y1H assay (Fig. 3C), although OsPORA-a does not contain a G-box motif. To confirm these interactions in vivo, we performed chromatin immunoprecipitation (ChIP) assays using WT protoplasts in which OsPIL1-GFP was transiently expressed. Consistent with the results of the Y1H assay, OsPIL1 strongly bound to amplicon-a′ of the OsPORB promoter and amplicon-c′ of the OsCAO1 promoter containing the G-box motif (Fig. 3D). By contrast, OsPIL1 did not bind to the promoter of OsPORA in vivo.
To further investigate whether OsPIL1 acts as a transcriptional activator of OsPORB and OsCAO1, we performed transactivation assays using rice protoplasts. Protoplasts were isolated from the shoots of 10-d-old seedlings and transfected with a plasmid containing 35S:OsPIL1-GFP, together with plasmids containing the GUS reporter gene behind the promoter regions of OsPORB (–2000 bp to –1000 bp) and OsCAO1 (–1000 bp to –1 bp). OsCAO1 promoter-directed and OsPORB promoter-directed GUS activity were significantly enhanced in the cells expressing OsPIL1, compared with the vector control (Fig. 3E). Taken together, these results indicate that OsPORB and OsCAO1 are the direct target genes of OsPIL1 among genes encoding Chl biosynthetic enzymes.
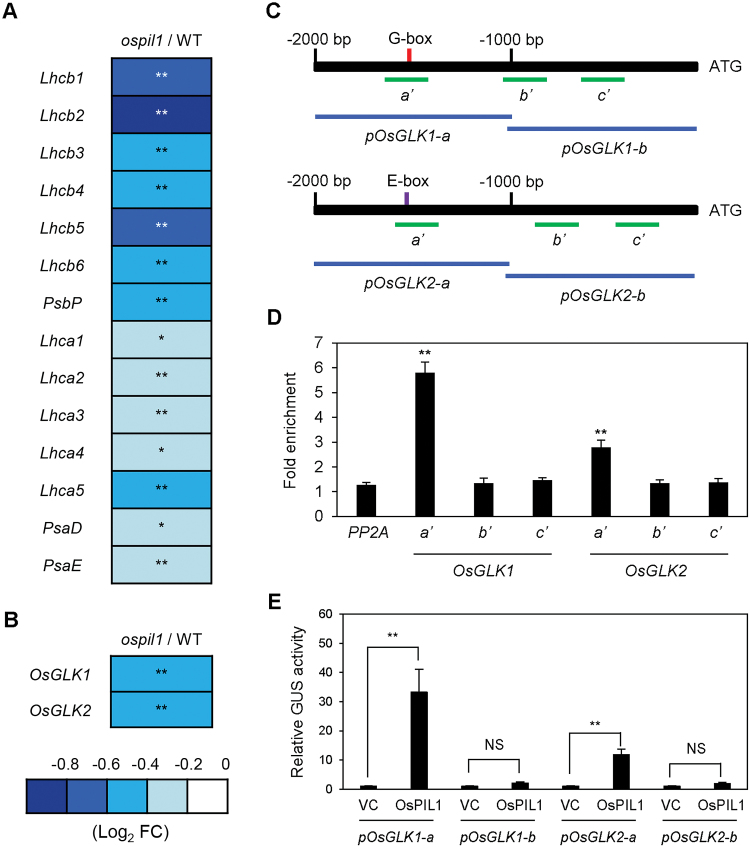
OsPIL1 directly up-regulates the expression of two OsGLK genes
In the microarray analysis, we also found that genes associated with photosynthesis, such as genes encoding light-harvesting complex subunits of photosystem I and II (Lhca and Lhcb) and the photosystem I core complex (PsaD, PsaE, and PsbP), were significantly down-regulated in ospil1 compared to the WT, and six Lhcb genes (Lhcb1–6) were severely down-regulated (Fig. 4A). In addition, OsGLK1 and OsGLK2, encoding a pair of GOLDEN2-LIKE (GLK) TFs, were down-regulated in ospil1 (Fig. 4B). The down-regulation of two OsGLKs was also confirmed by RT-qPCR analysis (see Supplementary Fig. S9A, B); this analysis also showed that the expression levels of these genes were higher in OsPIL1-OX (Fig. S9C, D). In arabidopsis, two GLK TFs (AtGLK1 and AtGLK2) directly up-regulate the expression of several genes encoding Chl-binding photosystem subunits and Chl biosynthetic enzymes (Waters et al., 2009), suggesting that the down-regulation of OsGLK genes in ospil1 contributes to its pale-green phenotype.
Fig. 4.
OsPIL1 directly promotes the expression of OsGLK1 and OsGLK2. (A, B) Relative expression levels (ospil1/WT) of photosynthetic apparatus genes (A) and two GLK genes (B). Relative expression levels of genes in ospil1 are normalized to those of the WT. Asterisks indicate significant difference between the WT and ospil1 plants (Student’s t-test, * P<0.05, ** P<0.01). (C) Positions of the G-box (red vertical bar) and E-box (purple vertical bar) in the OsGLK1 and OsGLK2 promoters and the promoter fragments (green horizontal bars) used for ChIP and transactivation assays. (D) The binding affinity of OsPIL1 to the promoter regions of OsGLK1 and OsGLK2 in planta examined by ChIP assay. OsPIL1-GFP was transiently expressed in protoplasts isolated from 10-d-old WT seedlings. Fold-enrichment of the promoter fragments was measured by immunoprecipitation with anti-GFP antibody (see Methods). PP2A was used as a negative control. (E) Transactivation of OsGLK1 and OsGLK2 by OsPIL1. The protoplasts were co-transfected with 5 μl of effector plasmid containing 35S:OsPIL1-GFP and 3 μl of reporter plasmids containing pOsGLK1-a::GUS, pOsGLK1-b::GUS, and pOsGLK2-a::GUS, and pOsGLK2-b::GUS. Empty vector was used as a vector control for the effector. Significant differences were determined by Student’s t-test (* P<0.05, ** P<0.01, NS, not significant).
To examine whether OsPIL1 directly activates the expression of the two OsGLK genes, we performed a ChIP assay. We searched for the G-box (CACGTG) motif in the promoter region (–2000 bp) of OsGLK1 and found a single motif near –1500 bp. Although the OsGLK2 promoter does not contain a G-box motif, we found an E-box motif (CACATG; another binding motif for PIF TFs) near –1500 bp of the promoter region (Fig. 4C). ChIP assays revealed that OsPIL1 strongly binds to the promoter fragments of both GLK genes containing the PIF binding motifs, G- and E-boxes (Fig. 4D). Furthermore, we performed a transactivation assay using rice protoplasts to confirm that OsPIL1 acts as a transcriptional activator of the OsGLK genes, finding that both OsGLK1 promoter-directed and OsGLK2 promoter-directed GUS activity increased in the presence of OsPIL1 expression (Fig. 4E). These results indicate that in addition to OsPORB and OsCAO1, OsGLK1 and OsGLK2 are also up-regulated by OsPIL1 in vivo.
OsGLK1 and OsGLK2 directly activate OsPORB, OsCAO1, and LHC genes
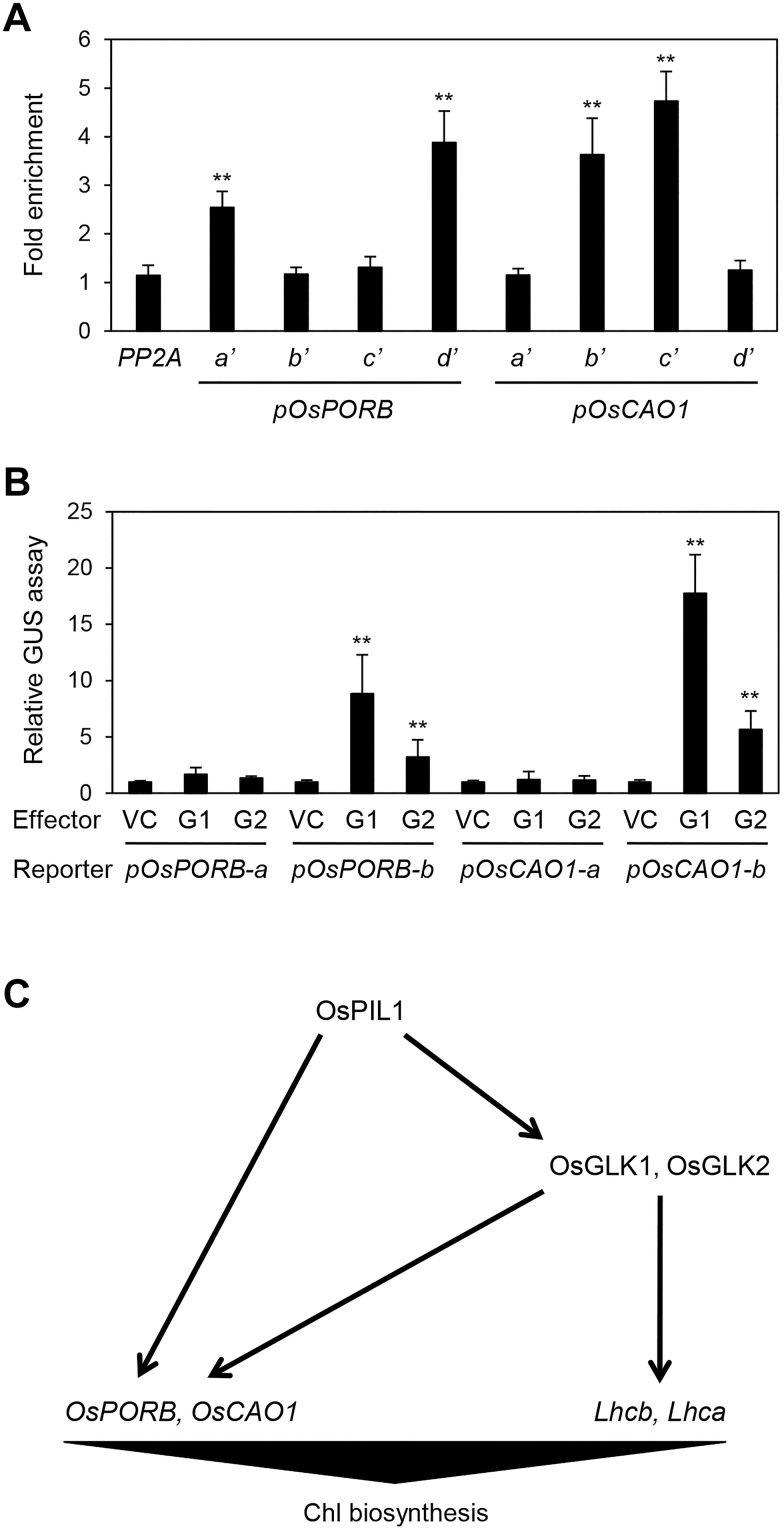
In arabidopsis, two GLK TFs (GLK1 and GLK2) bind to the promoter regions of various photosynthetic and Chl biosynthetic genes, including PORB and CAO1, and up-regulate their transcription (Waters et al., 2009). GLK TFs can bind to CCAATC as well as the G-box motif, CACGTG. However, these sequences are not present in the promoter regions (–2000 bp) of OsPORB or OsCAO1, although they contain a few G-box motifs (Fig. 3B). Therefore, we used ChIP assays to examine whether OsGLKs directly interact with the promoters of OsPORB and OsCAO1; these assays revealed that OsGLK1 and OsGLK2 bind to the promoter regions that contain the G-box motif (Fig. 5A). Next, we performed a transactivation assay using rice leaf protoplasts, and found that pOsPORB-b-directed and pOsCAO1-b-directed GUS activity was strongly enhanced in the presence of OsGLK1 and OsGLK2 expression compared with the vector control (Fig. 5B). These results indicate that OsPORB and OsCAO1 are up-regulated by both OsPIL1 and OsGLK, forming trifurcate feed-forward loops for the up-regulation of Chl biosynthesis (Fig. 5C).
Fig. 5.
OsGLK1 and OsGLK2 also up-regulate OsPORB and OsCAO1 transcription. (A) Binding of OsGLK1 and OsGLK2 to the promoter regions of OsPORB and OsCAO1 in planta examined by ChIP assays. OsGLK1-GFP or OsGLK2-GFP was transiently expressed in protoplasts isolated from 10-d-old WT seedlings. Fold-enrichment of the promoter fragments was measured by immunoprecipitation with an anti-GFP antibody (see Methods). PP2A was used as a negative control. (B) Transactivation of OsPORB and OsCAO1 by OsGLK1 and OsGLK2. The protoplasts were co-transfected with 5 μl of effector plasmid containing 35S:OsGLK1-GFP or 35S:OsGLK2-GFP and 3 μl of reporter plasmids containing pOsPORB-a::GUS, pOsPORB-b::GUS, pOsCAO1-a::GUS, and pOsCAO1-b::GUS. Empty vector was used as a vector control for the effector. Significant differences were determined by Student’s t-test (** P<0.01). (C) Working model of OsPIL1-mediated up-regulation of Chl biosynthetic genes. OsPIL1 directly up-regulates the expression of OsPORB and OsCAO1 by forming trifurcate feed-forward loops involving OsGLK1 and OsGLK2. Arrows indicate direct up-regulation.
We further examined whether the promoters of the rice Lhcb and Lhca genes contain the GLK binding motif (CACGTG or CCAATC). Our bioinformatic analysis revealed that the promoter regions of five Lhcb genes (Lhcb1, Lhcb2, Lhcb4, Lhcb5, and Lhcb6) and five Lhca genes (Lcha1, Lhca2, Lhca3, Lhca5, and Lhca6) contain one or both motifs (see Supplementary Fig. S10A). ChIP assays showed that both OsGLK1 and OsGLK2 bind to the promoters of the four Lhcb genes (Lhcb1, Lhcb2, Lhcb4, and Lhcb6) and three Lhca genes (Lhca1, Lhca2, and Lhca3) (Fig. S10B). These results indicate that like arabidopsis GLKs, these two OsGLK TFs activate the expression of genes for not only Chl synthesis enzymes (OsPORB and OsCAO1) but also Lhcb and Lhca genes responsible for the accumulation of Chl and the photosystem apparatus in developing leaves.
Discussion
OsPIL1 TF up-regulates the expression of Chl biosynthetic and photosynthetic genes
In rice, OsPIL1 is the closest homolog of arabidopsis PIF4 among the OsPILs (Nakamura et al., 2007). Arabidopsis PIF4 is involved in various biological processes, such as phytohormone synthesis, light responses, shoot elongation, flowering, circadian rhythms, stress responses, and leaf senescence (Leivar and Quail, 2011; Kumar et al., 2012; Sakuraba et al., 2014). Therefore, OsPIL1 probably acts as a pivotal regulator in various biological processes or signaling pathways. Todaka et al. (2012) described the phenotype of OsPIL1-OX in detail: OsPIL1-OX plants significantly increase in height throughout their development because of internode elongation. By transcriptome analysis, Todaka et al. (2012) found that OsPIL1-OX up-regulates cell wall synthesis-related genes, which probably promotes cell elongation of internodes. Furthermore, they found that OsPIL1 expression was severely down-regulated in water-deficit conditions, and the set of drought stress-responsive genes were differentially expressed in OsPIL1-OX, strongly suggesting that the reduction of plant height under drought stress conditions is closely associated with down-regulation of OsPIL1.
Here, we found that OsPIL1 functions to promote Chl synthesis in leaves. Yeast one-hybrid and ChIP assays revealed that the OsPIL1 directly binds to the promoters of OsPORB and OsCAO1, both of which contain the PIF binding G-box motif, CACGTG (Fig. 3C, D). Transactivation assays further revealed that OsPIL1 activates the expression of OsPORB and OsCAO1 (Fig. 3E). The physiological function of OsPORB has been determined by investigating its knockout mutant, faded green leaf (fgl); in both the paddy field and growth chamber conditions, the fgl mutant produced pale-green leaves with necrotic spots in the tip regions (Sakuraba et al., 2013). In addition, several Chl biosynthetic genes (OsHEMA, OsCHLH, and OsCAO1) and photosynthesis-associated genes (Lhcb1 and Lhcb4) are down-regulated in fgl (Sakuraba et al., 2013), probably through retrograde signaling from the chloroplast to the nucleus. Thus, it is highly possible that the down-regulation of OsPORB partially contributes to the reduced expression levels of other Chl biosynthetic and photosynthetic genes in the ospil1 leaves (Figs 3 and 4). We also found that OsPORA, another rice POR homolog, was significantly down-regulated in the ospil1 mutant (Fig. 3A and Supplementary Fig. S7C), although OsPIL1 does not bind to the promoter regions of OsPORA in vivo (Fig. 3D). We previously found that the OsPORA transcript level drastically decreased after illumination of etiolated seedlings, similar to arabidopsis and wheat PORA (Sakuraba et al., 2013); however, the OsPORB transcript level was not affected by illumination. In addition, the OsPORA transcript level was strongly affected by high light and leaf age, while the OsPORB transcript was less sensitive to those conditions. In addition, the overexpression of OsPORA in fgl mutants complemented the leaf chlorosis phenotype (Kwon et al., 2017). Thus, the down-regulation of OsPORA also contributes to the pale-green phenotype of the ospil1 mutant. Like fgl, the T-DNA insertional oscao1 knockout mutant also has a pale-green leaf phenotype (Lee et al., 2005). The arabidopsis cao mutants are deficient in Chl b, as the reaction from Chl a to Chl b via 7-hydroxymethyl Chl a is impaired by the loss of CAO catalytic activity. As a result, the Chl a/b ratios of cao mutants are considerably higher than the WT (Murray and Kohorn, 1991; Falbel et al., 1996; Tanaka et al., 1998). In this study, we found that the Chl a/b ratio of ospil1 was significantly higher than that of the WT (see Supplementary Fig. S2F), probably due to the down-regulation of OsCAO1. Rice has two CAO homologs, OsCAO1 and OsCAO2 (Lee et al., 2005). Unlike OsCAO1, however, the expression level of OsCAO2 was not altered in ospil1 (Fig. 3A). The expression patterns of OsCAO1 and OsCAO2 are quite different; OsCAO1 mRNA levels increase in the light, while OsCAO2 mRNA levels decrease (Lee et al., 2005). Thus, OsCAO1 plays a major role in Chl b biosynthesis and photosynthetic protein accumulation in rice, because both Chl levels and the expression of photosynthetic genes increase upon light exposure (Ilag et al., 1994; Teramoto et al., 2002).
In addition to OsPIL1, we found that two rice GLK TFs, OsGLK1 and OsGLK2, also directly up-regulate the expression of OsPORB and OsCAO1 (Fig. 5A, B). Because OsPIL1 is directly involved in up-regulating both OsGLK1 and OsGLK2, OsPIL1 and OsGLK form coherent trifurcate feed-forward loops to induce the transcription of OsPORB and OsCAO1 during Chl biosynthesis (Fig. 5C). The regulation of coherent feed-forward loops has been described previously (Kim et al., 2009; Sakuraba et al., 2015); this mechanism is thought to make pathways less prone to disruption by various environmental fluctuations. In arabidopsis, two GLK TFs, AtGLK1 and AtGLK2, directly up-regulate Chl biosynthetic genes, including AtPORB and AtCAO, which is similar to the role of OsGLK TFs. In addition, AtGLKs directly up-regulate photosynthetic genes such as Lhcb and Lhca (Waters et al., 2009). In this study, we also found by ChIP assay that both OsGLK1 and OsGLK2 bind to the promoters of several Lhcb and Lhca genes (Supplementary Fig. S10). Therefore, it is possible that the down-regulation of OsGLK genes directly contributes to the reduced expression of Lhc genes in ospil1.
Cytokinin enhances Chl synthesis during greening of barley cotyledons (Yaronskaya et al., 2006) and also delays the onset of leaf senescence (Romanko et al., 1969). By contrast, other phytohormones, such as abscisic acid (ABA), ethylene, jasmonic acid, and salicylic acid, promote the onset of leaf senescence and Chl catabolism (Kusaba et al., 2013). In our microarray analysis, several phytohormone biosynthesis- and signaling-associated genes were differentially expressed; for example, ABA synthesis genes (ABA2, NCED3, and NCED5) and signaling-associated genes (ABF1, ABI2, and ABI3) were up-regulated (see Supplementary Fig. S6E, F). Therefore, it is possible that differential expression of the phytohormone synthesis- and signaling-associated genes affect Chl accumulation in the ospil1 leaves.
Collectively, these findings suggest that OsPIL1 directly or indirectly enhances the expression of Chl biosynthetic and photosynthetic genes via various regulatory cascades.
The differences and similarities between OsPIL1 and arabidopsis PIFs
In this study, we found that ospil1 plants had pale-green leaves in both the paddy field (Fig. 1) and in the growth chambers (see Supplementary Figs S2 and S4). However, this color-defective phenotype has not been observed in arabidopsis pif mutants, including pif1, pif3, pif4, and pif5; at the vegetative stage, these mutants produce normal green leaves like those of the WT, although Chl biosynthesis is strongly inhibited in de-etiolated seedlings of pif1 upon light exposure (Huq et al., 2004). Thus, the physiological roles of PIFs in Chl biosynthesis in arabidopsis and rice (at least OsPIL1) are somehow different, although OsPIL1 is phylogenetically the closest homolog of arabidopsis PIF4 (Nakamura et al., 2007).
In arabidopsis, phyB interacts with and rapidly phosphorylates PIFs, leading to ubiquitination and degradation of PIFs by the 26S proteasome system (Al-Sady et al., 2006). Thus, the hypocotyl, petiole, flowering time, and leaf senescence phenotypes of arabidopsis phyB mutants are opposite to those of pif mutants (Huq and Quail, 2002; Nozue et al., 2007; Kumar et al., 2012; Sakuraba et al., 2014). Interestingly, OsPIL1 does not interact with OsphyB (Todaka et al., 2012), indicating that the stability of OsPIL1 is not regulated by OsphyB at the post-translational level. Indeed, osphyB knockout mutant plants are considerably shorter than the WT (Takano et al., 2005), with pale-green leaves (Inagaki et al., 2015), like those of the ospil1 mutant observed in the present study (Fig. 1, and Supplementary Figs S2 and S4).
It is currently unknown if OsphyB indirectly up-regulates or down-regulates the expression of OsPIL1. Arabidopsis PIF4 and PIF5 directly up-regulate another PIF gene, AtPIL1 (Hornitschek et al., 2012). Thus, AtPIL1 expression is up-regulated in the phyB mutant (Sakuraba et al., 2014). A transactivation assay showed that OsphyB does not affect OsPIL1 expression (Todaka et al., 2012). However, we previously found that OsPIL1 expression is down-regulated in osphyB mutants during dark-induced senescence, although this down-regulation is negligible under normal growth conditions (Piao et al., 2015). Therefore, it is possible that OsphyB indirectly regulates OsPIL1 expression positively or negatively under specific conditions, such as darkness. Further investigation of the molecular connection between OsphyB and OsPIL1 will be necessary to understand phyB-mediated red-light signaling in rice in more detail.
Supplementary Data
Supplementary data are available at JXB online.
Fig. S1. Complementation of the pale-green phenotype of ospil1.
Fig. S2. Characterization of the pale-green phenotype of ospil1.
Fig. S3. TEM images showing the structures of chloroplasts and thylakoid membranes in WT and ospil1 leaves.
Fig. S4. The ospil1 mutant has pale-green leaves under both LD and SD conditions.
Fig. S5. Cell wall-related genes are down-regulated in ospil1.
Fig. S6. Expression analysis of phytohormone biosynthesis- and signaling-associated genes in ospil1.
Fig. S7. Chlorophyll biosynthetic gene expression is reduced in ospil1.
Fig. S8. Expression of chlorophyll biosynthetic genes in OsPIL1-OX.
Fig. S9. The expression of OsGLK1 and OsGLK2 in ospil1 and OsPIL1-OX.
Fig. S10. OsGLK1 and OsGLK2 directly up-regulate genes encoding components of the photosystem apparatus.
Table S1. Primers used in this study.
Supplementary Material
Acknowledgements
This work was carried out with the support of the Cooperative Research Program for Agriculture & Technology Development (PJ011063), Rural Development, Administration, Republic of Korea. The authors declare that they have no conflicts of interest.
References
- Al-Sady B, Ni W, Kircher S, Schäfer E, Quail PH. 2006. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Molecular Cell 23, 439–446. [DOI] [PubMed] [Google Scholar]
- Blankenship RE. 1992. Origin and early evolution of photosynthesis. Photosynthesis Research 33, 91–111. [PubMed] [Google Scholar]
- Castillon A, Shen H, Huq E. 2007. Phytochrome interacting factors: central players in phytochrome-mediated light signaling networks. Trends in Plant Science 12, 514–521. [DOI] [PubMed] [Google Scholar]
- Chen H, Cheng Z, Ma X et al. . 2013. A knockdown mutation of YELLOW-GREEN LEAF2 blocks chlorophyll biosynthesis in rice. Plant Cell Reports 32, 1855–1867. [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. 2003. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology 133, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki O, Grimm B. 2012. Post-translational control of tetrapyrrole biosynthesis in plants, algae, and cyanobacteria. Journal of Experimental Botany 63, 1675–1687. [DOI] [PubMed] [Google Scholar]
- Falbel TG, Meehl JB, Staehelin LA. 1996. Severity of mutant phenotype in a series of chlorophyll-deficient wheat mutants depends on light intensity and the severity of the block in chlorophyll synthesis. Plant Physiology 112, 821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Martinez C, Gusmaroli G et al. . 2008. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451, 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitter DW, Martin DJ, Copley MJ, Scotland RW, Langdale JA. 2002. GLK gene pairs regulate chloroplast development in diverse plant species. The Plant Journal 31, 713–727. [DOI] [PubMed] [Google Scholar]
- Franklin KA, Lee SH, Patel D et al. . 2011. Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proceedings of the National Academy of Sciences, USA 108, 20231–20235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SH, Sakuraba Y, Koh HJ, Paek NC. 2012. Leaf variegation in the rice zebra2 mutant is caused by photoperiodic accumulation of tetra-cis-lycopene and singlet oxygen. Molecules and Cells 33, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideg E, Vitányi B, Kósa A, Solymosi K, Bóka K, Won S, Inoue Y, Ridge RW, Böddi B. 2010. Reactive oxygen species from type-I photosensitized reactions contribute to the light-induced wilting of dark-grown pea (Pisum sativum) epicotyls. Physiologia Plantarum 138, 485–492. [DOI] [PubMed] [Google Scholar]
- Hornitschek P, Kohnen MV, Lorrain S et al. . 2012. Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. The Plant Journal 71, 699–711. [DOI] [PubMed] [Google Scholar]
- Hörtensteiner S, Kräulter B. 2011. Chlorophyll breakdown in higher plants. Biochimica et Biophysica Acta - Bioenergetics 1807, 977–988. [DOI] [PubMed] [Google Scholar]
- Huq E, Al-Sady B, Hudson M, Kim C, Apel K, Quail PH. 2004. Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305, 1937–1941. [DOI] [PubMed] [Google Scholar]
- Huq E, Quail PH. 2002. PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. The EMBO Journal 21, 2441–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilag LL, Kumar AM, Söll D. 1994. Light regulation of chlorophyll biosynthesis at the level of 5-aminolevulinate formation in Arabidopsis. The Plant Cell 6, 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada N, Sakai A, Kuroiwa H, Kuroiwa T. 1998. Three-dimensional analysis of the senescence program in rice (Oryza sativa L.) coleoptiles. Investigations of tissues and cells by fluorescence microscopy. Planta 205, 153–164. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Kinoshita K, Kagawa T, Tanaka A, Ueno O, Shimada H, Takano M. 2015. Phytochrome B mediates the regulation of chlorophyll biosynthesis through transcriptional regulation of ChlH and GUN4 in rice seedlings. PLoS ONE 10, e0135408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Nijhawan A, Tyagi AK, Khurana JP. 2006. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochemical and Biophysical Research Communications 345, 646–651. [DOI] [PubMed] [Google Scholar]
- Jeon JS, Chung YY, Lee S, Yi GH, Oh BG, An G. 1999. Isolation and characterization of an anther-specific gene, RA8, from rice (Oryza sativa L.). Plant Molecular Biology 39, 35–44. [DOI] [PubMed] [Google Scholar]
- Jeong DH, An S, Kang HG, Moon S, Han JJ, Park S, Lee HS, An K, An G. 2002. T-DNA insertional mutagenesis for activation tagging in rice. Plant Physiology 130, 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Woo HR, Kim J, Lim PO, Lee IC, Choi SH, Hwang D, Nam HG. 2009. Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science 323, 1053–1057. [DOI] [PubMed] [Google Scholar]
- Kumar SV, Lucyshyn D, Jaeger KE, Alós E, Alvey E, Harberd NP, Wigge PA. 2012. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 484, 242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba M, Tanaka A, Tanaka R. 2013. Stay-green plants: what do they tell us about the molecular mechanism of leaf senescence. Photosynthesis Research 117, 221–234. [DOI] [PubMed] [Google Scholar]
- Kusumi K, Komori H, Satoh H, Iba K. 2000. Characterization of a zebra mutant of rice with increased susceptibility to light stress. Plant & Cell Physiology 41, 158–164. [DOI] [PubMed] [Google Scholar]
- Kwon CT, Kim SH, Song G, Kim D, Paek NC. 2017. Two NADPH: protochlorophyllide oxidoreductase (POR) isoforms play distinct roles in environmental adaptation in rice. Rice 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim JH, Yoo ES, Lee CH, Hirochika H, An G. 2005. Differential regulation of chlorophyll a oxygenase genes in rice. Plant Molecular Biology 57, 805–818. [DOI] [PubMed] [Google Scholar]
- Lee SM, Kang K, Chung H, Yoo SH, Xu XM, Lee SB, Cheong JJ, Daniell H, Kim M. 2006. Plastid transformation in the monocotyledonous cereal crop, rice (Oryza sativa) and transmission of transgenes to their progeny. Molecules and Cells 21, 401–410. [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Quail PH. 2011. PIFs: pivotal components in a cellular signaling hub. Trends in Plant Science 16, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Todaka D, Mizoi J et al. . 2012. Identification of cis-acting promoter elements in cold- and dehydration-induced transcriptional pathways in Arabidopsis, rice, and soybean. DNA Research 19, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A. 1991. Dynamics of photosynthetic membrane composition and function. Biochimica et Biophysica Acta 1058, 87–106. [Google Scholar]
- Meskauskiene R, Nater M, Goslings D, Kessler F, op den Camp R, Apel K. 2001. FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 98, 12826–12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J. 2001. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proceedings of the National Academy of Sciences, USA 98, 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray DL, Kohorn BD. 1991. Chloroplasts of Arabidopsis thaliana homozygous for the ch-1 locus lack chlorophyll b, lack stable LHCPII and have stacked thylakoids. Plant Molecular Biology 16, 71–79. [DOI] [PubMed] [Google Scholar]
- Nagata N, Tanaka R, Satoh S, Tanaka A. 2005. Identification of a vinyl reductase gene for chlorophyll synthesis in Arabidopsis thaliana and implications for the evolution of Prochlorococcus species. The Plant Cell 17, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Kato T, Yamashino T, Murakami M, Mizuno T. 2007. Characterization of a set of phytochrome-interacting factor-like bHLH proteins in Oryza sativa. Bioscience, Biotechnology, and Biochemistry 71, 1183–1191. [DOI] [PubMed] [Google Scholar]
- Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL, Maloof JN. 2007. Rhythmic growth explained by coincidence between internal and external cues. Nature 448, 358–361. [DOI] [PubMed] [Google Scholar]
- Nusinow DA, Helfer A, Hamilton EE, King JJ, Imaizumi T, Schultz TF, Farré EM, Kay SA. 2011. The ELF4–ELF3–LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 475, 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Kim J, Park E, Kim JI, Kang C, Choi G. 2004. PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. The Plant Cell 16, 3045–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Zhu JY, Bai MY, Arenhart RA, Sun Y, Wang ZY. 2014. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. eLIFE 3, e03031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster U, Tanaka R, Tanaka A, Rüdiger W. 2000. Cloning and functional expression of the gene encoding the key enzyme for chlorophyll b biosynthesis (CAO) from Arabidopsis thaliana. The Plant Journal 21, 305–310. [DOI] [PubMed] [Google Scholar]
- Piao W, Kim EY, Han SH, Sakuraba Y, Paek NC. 2015. Rice phytochrome B (OsPhyB) negatively regulates dark- and starvation-induced leaf senescence. Plants 4, 644–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. 1989. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectrometry. Biochimica et Biophysica Acta 975, 384–394. [Google Scholar]
- Powell AL, Nguyen CV, Hill T et al. . 2012. Uniform ripening encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science 336, 1711–1715. [DOI] [PubMed] [Google Scholar]
- Romanko EG, Hein HJ, Kulaeva ON, Nichiporovich AA. 1969. Effect of cytokinin on the physiological activity of chloroplasts. Progress Photosynthesis Research 1, 296–303. [Google Scholar]
- Sakuraba Y, Jeong J, Kang MY, Kim J, Paek NC, Choi G. 2014. Phytochrome-interacting transcription factors PIF4 and PIF5 induce leaf senescence in Arabidopsis. Nature Communications 5, 4636. [DOI] [PubMed] [Google Scholar]
- Sakuraba Y, Kim YS, Han SH, Lee BD, Paek NC. 2015. The Arabidopsis transcription factor NAC016 promotes drought stress responses by repressing AREB1 transcription through a trifurcate feed-forward regulatory loop involving NAP. The Plant Cell 27, 1771–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba Y, Rahman ML, Cho SH, Kim YS, Koh HJ, Yoo SC, Paek NC. 2013. The rice faded green leaf locus encodes protochlorophyllide oxidoreductase B and is essential for chlorophyll synthesis under high light conditions. The Plant Journal 74, 122–133. [DOI] [PubMed] [Google Scholar]
- Saleh A, Alvarez-Venegas R, Avramova Z. 2008. An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nature Protocols 3, 1018–1025. [DOI] [PubMed] [Google Scholar]
- Shahnejat-Bushehri S, Tarkowska D, Sakuraba Y, Balazadeh S. 2016. Arabidopsis NAC transcription factor JUB1 regulates GA/BR metabolism and signalling. Nature Plants 2, 16013. [DOI] [PubMed] [Google Scholar]
- Shin J, Kim K, Kang H, Zulfugarov IS, Bae G, Lee CH, Lee D, Choi G. 2009. Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proceedings of the National Academy of Sciences, USA 106, 7660–7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skribanek A, Solymosi K, Hideg E, Böddi B. 2008. Light and temperature regulation of greening in dark-grown ginkgo (Ginkgo biloba). Physiologia Plantarum 134, 649–659. [DOI] [PubMed] [Google Scholar]
- Takano M, Inagaki N, Xie X et al. . 2005. Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. The Plant Cell 17, 3311–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Ito H, Tanaka R, Tanaka NK, Yoshida K, Okada K. 1998. Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proceedings of the National Academy of Sciences, USA 95, 12719–12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Tanaka R. 2006. Chlorophyll metabolism. Current Opinion in Plant Biology 9, 248–255. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Tanaka A. 2007. Tetrapyrrole biosynthesis in higher plants. Annual Review of Plant Biology 58, 321–346. [DOI] [PubMed] [Google Scholar]
- Teramoto H, Nakamori A, Minagawa J, Ono TA. 2002. Light-intensity-dependent expression of Lhc gene family encoding light-harvesting chlorophyll-a/b proteins of photosystem II in Chlamydomonas reinhardtii. Plant Physiology 130, 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaka D, Nakashima K, Maruyama K et al. . 2012. Rice phytochrome-interacting factor-like protein OsPIL1 functions as a key regulator of internode elongation and induces a morphological response to drought stress. Proceedings of the National Academy of Sciences, USA 109, 15947–15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Fouracre J, Kelly S et al. . 2013. Evolution of GOLDEN2-LIKE gene function in C3 and C4 plants. Planta 237, 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Gao J, Wan C, Zhang F, Xu Z, Huang X, Sun X, Deng X. 2010. Divinyl chlorophyll(ide) a can be converted to monovinyl chlorophyll(ide) a by a divinyl reductase in rice. Plant Physiology 153, 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Wang P, Korkaric M, Capper RG, Saunders NJ, Langdale JA. 2009. GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. The Plant Cell 21, 1109–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaronskaya E, Vershilovskaya I, Poers Y, Alawady AE, Averina N, Grimm B. 2006. Cytokinin effects on tetrapyrrole biosynthesis and photosynthetic activity in barley seedlings. Planta 224, 700–709. [DOI] [PubMed] [Google Scholar]
- Yasumura Y, Moylan EC, Langdale JA. 2005. A conserved transcription factor mediates nuclear control of organelle biogenesis in anciently diverged land plants. The Plant Cell 17, 1894–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li J, Yoo JH, Yoo SC, Cho SH, Koh HJ, Seo HS, Paek NC. 2006. Rice Chlorina-1 and Chlorina-9 encode ChlD and ChlI subunits of Mg-chelatase, a key enzyme for chlorophyll synthesis and chloroplast development. Plant Molecular Biology 62, 325–337. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Su J, Duan S et al. . 2011. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Liu Q, Zhang F et al. . 2014. Overexpression of OsPIL15, a phytochrome-interacting factor-like protein gene, represses etiolated seedling growth in rice. Journal of Integrative Plant Biology 56, 373–387. [DOI] [PubMed] [Google Scholar]
- Zhou K, Ren Y, Lv J et al. . 2013. Young Leaf Chlorosis 1, a chloroplast-localized gene required for chlorophyll and lutein accumulation during early leaf development in rice. Planta 237, 279–292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.