Map-based cloning provides evidence that the restorer gene Rfn for nap CMS in Brassica napus encodes a pentatricopeptide repeat protein and shares many properties with previously cloned restorer genes.
Keywords: Brassica napus, cytoplasmic male sterility, map-based cloning, mitochondria, pentatricopeptide repeat protein, restorer of fertility (Rf)
Abstract
Two forms of male-sterile cytoplasm, designated nap and pol, are found in the oilseed rape species, Brassica napus. The nap cytoplasm is observed in most B. napus varieties, and it confers male sterility on a limited number of cultivars that lack the corresponding restorer gene, Rfn. In the present study, using linkage analysis in combination with 5652 BC1 progeny derived from a cross between a nap cytoplasmic male sterility (CMS) line 181A and a restorer line H5, we delimited the Rfn gene to a 10.5 kb region on chromosome A09, which contained three putative ORFs. Complementation by transformation rescue revealed that the introduction of ORF2, which encodes a pentatricopeptide repeat (PPR) protein, resulted in the recovery of fertility of nap CMS plants. Expression analysis suggested that the Rfn was highly expressed in flower buds and it was preferentially expressed in the tapetum and meiocytes during anther development. Further RNA gel blots and immunodetection suggested that the Rfn gene may play a complicated role in restoring the nap CMS. Our work laid the foundation for dissecting the molecular basis of CMS fertility restoration and the nuclear–mitochondrial interactions in CMS/Rf systems.
Introduction
Cytoplasmic male sterility (CMS), a maternally inherited trait that fails to produce functional pollen, has been observed in >150 higher plant species. At the molecular level, CMS is typically associated with an aberrant chimeric gene in the mitochondria (Hanson and Bentolila, 2004). In most cases, the male sterility phenotype can be recovered by dominant nuclear genes, termed restorer of fertility (Rf) genes, which can specifically reduce the accumulation of CMS-associated RNAs or proteins in F1 hybrids. Thus, CMS/Rf systems provide both an ideal model for investigating nuclear–cytoplasmic interaction and a practical tool for facilitating hybrid seed production.
In oilseed rape species, Brassica napus, there are two different endogenous male-sterile cytoplasms, nap and pol. The nap cytoplasm is the most prevalent cytoplasm in B. napus germplasm, but it confers male sterility only in a limited number of cultivars that lack the corresponding fertility restorer gene, Rfn. In the absence of the nuclear restorer gene Rfn, plants carrying the nap cytoplasm display male sterility when grown under relatively low temperature (22/16 °C; 16/8 h), while the sterility can be gradually repaired or even completely reversed with an increase of temperature (Fan and Stefansson, 1986). Because flowering occurs in spring or summer and the environmental temperature gradually goes up, this specific temperature-sensitive male sterility is hardly used for hybrid production. In contrast, the pol CMS is highly stable in most cases; thus it can be widely used for commercial hybrid seed production (Fan and Stefansson, 1986; Zhou and Fu, 2007).
Previous studies indicated that pol CMS is associated with expression of the novel ORF, orf224, which is located upstream of a normal mitochondrial gene, atp6 (Singh and Brown, 1991, 1993), while nap CMS is associated with a different but related ORF, orf222, that is co-transcribed with an exon of a trans-spliced gene, nad5c, and another short ORF, orf139 (L’Homme et al., 1997). The nuclear fertility restorer genes for nap and pol CMS, Rfn and Rfp, represent different alleles or haplotypes of a single nuclear locus (Li et al., 1998). For both the nap and pol CMS systems, the nuclear restorer genes may take the role of down-regulating the expression of their corresponding mitochondrial CMS-associated transcripts (Singh and Brown, 1991; L’Homme et al., 1997; Liu et al., 2016). To validate the allelism of Rfn and Rfp and better understand how Rfn regulates CMS-associated transcripts, it is necessary to clone Rfn.
To date, except for Rf2 (encoding a mitochondrial aldehyde dehydrogenase) for T-CMS in maize (Cui et al., 1996), Rf17 (encoding an acyl-carrier protein synthase-like protein) for CW-CMS in rice (Fujii and Toriyama, 2009), Rf2 (encoding a mitochondrial glycine-rich protein) for LD-CMS in rice (Itabashi et al., 2011), and Rf1 (encoding a putative peptidase of the M48 family) for Owen-CMS in sugar beet (Matsuhira et al., 2012), the rest of the cloned Rf genes all encode pentatricopeptide repeat (PPR) proteins (Bentolila et al., 2002; Brown et al., 2003; Kazama and Toriyama, 2003; Koizuka et al., 2003; Komori et al., 2004; Klein et al., 2005; Wang et al., 2006, 2013; Hu et al., 2012; Tang et al., 2014; Huang et al., 2015; Igarashi et al., 2016; Liu et al., 2016). Such proteins are characterized by the succession of tandem degenerate motifs of ~35 amino acids. Based on the structure of the PPR motifs, PPR proteins can be divided into P and PLS subfamilies. P-type PPR proteins contain only canonical 35 amino acid repeats (P), whereas PLS PPR proteins consist of sequential repeats of P, long (L), and short (S) PPR motifs (Lurin et al., 2004). P-type PPR proteins were shown to participate in multiple aspects of organelle RNA processing steps such as cleavage, splicing, stabilization, and/or translation of their target transcripts, while PLS PPR proteins have been almost exclusively associated with C-to-U RNA editing (Barkan and Small, 2014). It has been verified that PPR proteins have the ability to recognize ssRNA following a one-motif to one-base rule (Barkan et al., 2012; Okuda et al., 2014). Recent computational, biochemical, and structural studies indicated that the PPR motif first produces an antiparallel helix–turn–helix fold whose repetition then forms a solenoid-like structure (Barkan et al., 2012; Yagi et al., 2013; Yin et al., 2013). Within each PPR repeat, the combinations of two amino acids, known as the ‘PPR code’ at two key positions (the 5th and the 35th), confer recognized RNA specificity (Barkan et al., 2012;Ban et al., 2013; Ke et al., 2013; Yagi et al., 2013; Yin et al., 2013; Gully et al., 2015; Shen et al., 2016).
In the present study, we finely mapped and cloned the nuclear restorer gene Rfn for nap CMS in B. napus by using a map-based cloning strategy and preliminarily investigated how it regulates the fertility of nap CMS. The Rfn encodes a mitochondria-targeted PPR protein, and it may play a much more complicated role in restoring the nap CMS.
Materials and methods
Genetic analysis of nap CMS restoration
A nap CMS male-sterile line 181A, a male-fertile line H5, and three pol CMS lines (1141A, 245A, and 7492A) were used in this study. The cross between 181A as female parent and H5 as male parent yielded male-fertile F1 plants. Self-pollination of F1 plants yielded an F2 segregating population, which was used to analyze the genetic control of nap CMS restoration. The pol CMS lines 1141A, 245A, and 7492A as female were also each used to cross with H5 for genetic analysis. The individual fertility of each cross combination and self-pollination was assessed as previously described (Liu et al., 2012) during the early flowering period.
Fine mapping of the Rfn gene
The nap CMS line 181A as female was crossed with H5 to create the F1 plants, and the CMS plants were backcrossed with F1 plants as pollen donor to produce a backcross segregation population containing 5652 individuals. Polymorphic markers around the Rfn locus were developed based on the synteny region of B. napus and other Brassica species, which were then used for fine mapping of the Rfn gene.
BAC screening, sequencing, and candidate gene prediction
A bacterial artificial chromosome (BAC) clone library described by Li et al. (2015) was screened with co-segregated markers through a two-stage PCR screening method (Crooijmans et al., 2000). The target BAC clone was then sequenced by Illumina Hiseq 2000 according to the manufacture’s standard protocol. The online-based tool FGENESH (http://www.softberry.com) was used to predict putative ORFs in the delimited gene region. The genomic or coding sequences (CDSs) of predicted genes were submitted to BRAD (http://brassicadb.org;Cheng et al., 2011) for homology search and basic functional annotation.
Complementation by transformation rescue
The genomic sequence of candidate gene (including the putative promoter, CDS, and 3'-downstream region) was PCR amplified with H5 as a template using specific primers (see Supplementary Table S1 at JXB online). The PCR product was inserted into the EcoRI and BamHI sites of the binary vector pFGC5941 and then introduced into the CMS line 181A by Agrobacterium-mediated transformation. Individual transgenic plants were raised to maturity and visually assessed for male fertility/sterility under relatively low temperature during flowering time. Self-pollination of the fertility-recovered T0 plants produced T1 plants, and the CMS parent line as female was backcrossed with T0 plants to produce test cross progeny plants. Pollen grains from the anthers of the transgenic plants were collected before anthesis and dyed in 1% aceto-carmine staining solution. The stained pollen was photographed under a microscope.
RNA extraction and quantitative reverse transcription–PCR
Total RNA was extracted from various rapeseed tissues using an RNeasy plant mini kit (Qiagen, http://www.qiagen.com/). RNA samples of ~2 μg were converted into cDNA with GoScript™ Reverse Transcriptase following the manufacturer’s instructions (Promega, USA), and the products were diluted 10-fold with distilled water for template. Quantitative reverse transcription–PCR (qRT–PCR) was performed with the Bio-Rad CFX96 Real-time system (Bio-Rad) and the GoTaq qPCR Master Mix (Promega, USA). Relative expression levels were calculated by the 2–ΔΔCt method (Livak and Schmittgen, 2001). The relative amount of PCR product that was amplified using the designed primer sets (listed in Supplementary Table S1) was normalized to the internal control gene BnActin (GenBank: AF111812.1). The data are expressed as the mean ±SD (n=3 biological replicates).
GUS staining assay
An ~2000 bp upstream region of the Rfn gene was amplified from the restorer line H5 (Primers Pro-F and Pro-R; Supplementary Table S1) and inserted into the cloning vector pMDC162. The ProRfn–GUS (β-glucuronidase) construct was then introduced into wild-type Arabidopsis plants by floral-dip transformation (Clough and Bent, 1998). GUS activity was visualized by staining various tissues from the heterozygous transgenic plants and wild-type plants overnight at 37 °C in X-Gluc solution containing 50 mM sodium phosphate buffer of pH 7.0, 10 mM EDTA, 1 mM X-Gluc (5-bromo-4-chloro-3-indolyl-glucuronide), and 20% methanol (w/v). The stained samples were cleared in 75% (v/v) ethanol and then photographed under a stereomicroscope (Olympus SZX16, Japan).
RNA in situ hybridization analysis
Floral buds at different developmental stages from the restorer line H5 were freshly collected and fixed in FAA (50% ethanol, 5.0% acetic acid, and 3.7% formaldehyde). Fixed floral buds were dehydrated with a 50–100% ethanol series, cleaned by a series of xylenes from 25% to 100%, and embedded in Paraplast. Anther sections (8 μm thickness) were cut using a Leica RM2235 microtome and placed on RNase-free polylysine-coated slides. A 124 bp CDS fragment from the Rfn gene was amplified using the insitu-F and insitu-R primers (Supplementary Table S1) and inserted into the pGEM-T Easy vector (Promega, USA) for sequencing. The probes were labeled with digoxigenin (DIG) using an RNA labeling kit (Roche Applied Science). The subsequent hybridization and immunological detection procedures were performed as previously described by Wan et al. (2010).
Transient expression and subcellular localization of RFN
The coding sequence of the putative N-terminal 44 residue signal peptide was PCR amplified using primers Rfn-Xba-F and Rfn-Xba-R (Supplementary Table S1) and then inserted into the pM999-GFP vector to generate a green fluorescent protein (GFP) fusion product. The fusion construct was introduced into Arabidopsis protoplasts by polyethylene glycol (PEG)/calcium-mediated transformation (Yoo et al., 2007). The mitochondria of protoplasts were marked using MitoTracker Red CMX-Ros (Molecular Probes, Invitrogen) staining solution after incubation at 23 °C for 14–18 h. GFP expression and co-localization of GFP fusion protein to mitochondria were imaged using a confocal scanning microscope system (Olympus FV1200, Japan) with 488 nm laser light for fluorescence excitation of GFP and 580 nm laser light for excitation of MitoTracker Red CMX-Ros.
RNA gel blot analysis
For RNA gel blot analysis, total RNA was isolated from young flower buds using Trizol reagent (Invitrogen, http://www.invitrogen.com/) as described by the manufacturer. Approximately 20 μg of total RNA from each sample was size fractionated on 1.2% agarose/2.2 M formaldehyde horizontal gels and transferred to Hybond-N+ nylon membranes (Amersham) by capillary blotting with 3 M NaCl/0.3 M sodium citrate for 16–20 h. The orf222 and orf139 fragments were amplified from 181A using the primers listed in Supplementary Table S1. The PCR products were inserted into the pGEM-T Easy vector (Promega, USA) for sequencing. Antisense DIG-labeled RNA probes were made with the RNA labeling kit (Roche Applied Science). The hybridization and detection with DIG Easy Hyb and the DIG Nucleic Acid Detection Kit (Roche Applied Science) were performed following the manufacturer’s instructions.
Antibody preparation and immunodetection
A peptide antigen (QSKLKLGGKDRTSK) corresponding to 14 residues of ORF222 was synthesized by a chemical synthesis method (GenScript, Nanjing, China) and used to immunize rabbits for antibody production. Total proteins were extracted from the young flower buds of CMS line, restorer line, and transgenic fertility-restored plants. The proteins were separated by 12% SDS–PAGE and transferred onto a membrane (PVDF type, Millipore). The following procedures were performed as previously described by Jing et al. (2012).
Results
Male fertility restoration is controlled by one dominant gene
To investigate the genetic control of fertility restoration of nap CMS, we prepared an F2 population derived from the cross of a nap CMS line 181A as female and a male fertile line H5 (Supplementary Fig. S1). Fertility assessment showed that 715 of 940 plants had a fertile phenotype and the remaining plants had a sterile phenotype, fitting the 3:1 segregation ratio (χ2=0.51, P<0.05) of a single locus, whereas all the F1 plants derived from the crosses between three pol CMS lines and H5 remained male sterile (Table 1). These results indicated that the male-fertile line H5 can specifically restore the nap CMS line 181A in a sporophytic manner and possesses only one dominant Rfn gene.
Table 1.
Genetic analysis of the fertility restorer gene in H5The male-sterile lines 1141A, 245A, and 7492A are pol cytoplasm; the male-sterile line 181A is nap cytoplasm
| Cross combination | Progeny | Total plants | Fertile plants | Sterile plants | Expected ratio | χ2 valuea |
|---|---|---|---|---|---|---|
| 1141A (pol)×H5 | F1 | 115 | 0 | 115 | – | – |
| 245A (pol)×H5 | F1 | 67 | 0 | 67 | – | – |
| 7492A (pol)×H5 | F1 | 69 | 0 | 69 | – | – |
| 181A (nap)×H5 | F1 | 83 | 83 | 0 | – | – |
| F2 | 940 | 715 | 225 | 3:1 | 0.51 |
aχ2 (0.05, 1)=3.84.
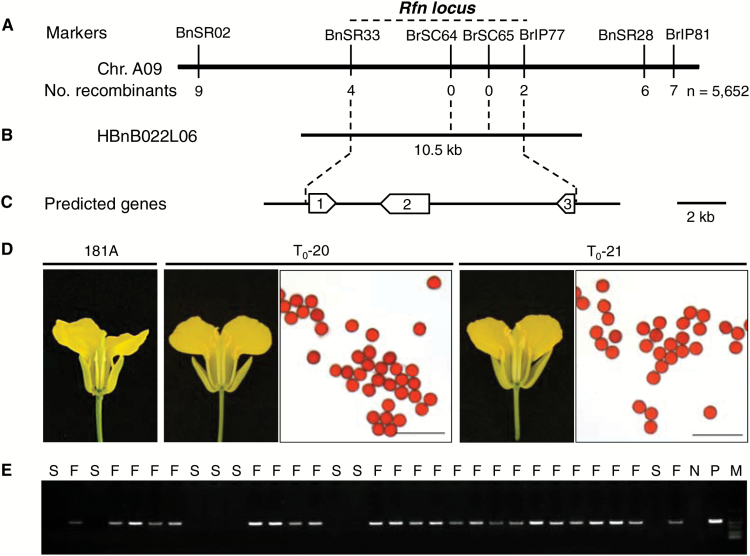
The Rfn locus was limited to a 10.5 kb region
A previous study indicated that the Rfn and Rfp genes represent different alleles or haplotypes of a single nuclear locus (Li et al., 1998). To map the Rfn locus precisely, sequence information from the collinear region around the Rfp/Rfn locus was used for development of simple sequence repeat (SSR) or sequence characterized amplified region (SCAR) markers. Subsequently, 16 markers tightly linked to the Rfn gene were successfully obtained. These markers were further used to assay each individual of the backcross segregation population. Among these plants, four and two recombinant individuals were identified using the closest flanking markers BnSR33 and BrIP77, respectively, and two markers (BrSC64 and BrSC65) co-segregated with the Rfn locus (Fig. 1A). To characterize precisely the genomic sequence around the Rfn locus in B. napus, we further screened a BAC clone library using the two co-segregated markers; a BAC clone (HBnB022L06) was also successfully identified. After sequencing the target BAC clone and sequence alignment of the closest flanking markers, the Rfn locus was narrowed down to a 10.5 kb physical region on chromosome A09 of B. napus (Fig. 1B). Gene prediction showed that this region contained two complete CDSs (ORF1 and ORF2) and a truncated CDS (ORF3) (Fig. 1C). Homologous gene annotation in Arabidopsis showed that ORF1 and ORF2 encode a zinc finger (AN1-like) family protein and a PPR-containing protein, respectively, while ORF3 encodes a serine-type endopeptidase. Considering most Rf genes encoding PPR proteins, we took ORF2 as the Rfn candidate.
Fig. 1.
Map-based cloning of the Rfn gene for nap CMS in Brassica napus. (A) Molecular mapping of the Rfn locus using a BC1 population containing 5652 individuals. The numbers of recombinants between the markers and Rfn are shown. (B) Physical map based on a sequenced BAC. The Rfn locus was narrowed down to a 10.5 kb region. (C) The predicted genes from http://www.softberry.com. It contained two complete CDSs (ORF1 and ORF2) and a partial CDS (ORF3). (D) Morphology of flowers and pollen grains of a nap CMS plant and two hemizygous Rfn candidate transgenic plants. Dissected flowers were photographed with a digital camera. Pollen grains were stained with 1% aceto-carmine staining solution. Scale bar=100 μm. (E) Co-segregation analysis of the introduced DNA and fertile plants among the T1-20 progeny. F, fertile plant; S, sterile plant; N, negative control; P, positive control; M, DL2000 molecular markers. (This figure is available in colour at JXB online.)
Validation of the target gene of Rfn for nap CMS
To validate ORF2 as the target of Rfn, we isolated the genomic sequence (including the putative promoter, ORF, and 3'-downstream region) of ORF2 from the restorer line H5 and cloned it into binary vector pFGC5941. The construct was then introduced into the nap CMS line 181A by Agrobacterium-mediated transformation. Nine independent T0 transgenic plants displayed a normal male fertile phenotype when grown to maturity under relatively low temperature (Fig. 1D). The recovered fertility of T1 progeny plants could be stably co-transmitted and co-segregated with the introduced DNA (Fig. 1E). Meanwhile, a 3:1 or 15:1 genetic segregation ratio was observed in the five independent transgenic T1 progeny of the self-cross and a 1:1 or 3:1 genetic segregation ratio was observed in the corresponding test-crossed progeny (Supplementary Table S2). These results suggested that a single site or two independently heritable T-DNA insertions was introduced into 181A in the T0 transgenic plants. Thus, our data proved that ORF2 possesses the ability to restore the nap CMS in a sporophytic manner and it is the causal gene of Rfn.
Sequence analysis of the Rfn gene and its recessive allele
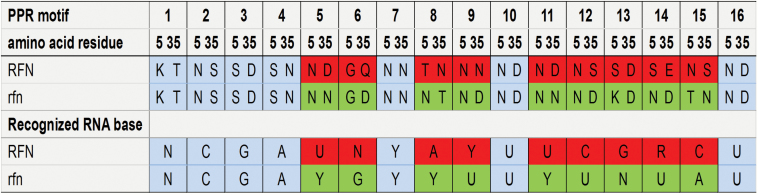
To characterize the recessive allele of Rfn existing in the CMS line, ORF2 was amplified from the 181A genome and sequenced. Comparing the putative promoter region (~2.0 kb) and the full-length CDS of Rfn from H5 with that of the rfn allele from 181A, we found that only five single nucleotide polymorphisms (SNPs) were detected in the putative promoter region, while rich sequence variations including 184 SNPs were identified in the coding sequence. These SNPs have no effect on the deduced protein length, but result in 99 amino acids mutations (Supplementary Figs S2, S3). Sequence analysis indicated that both Rfn and rfn encode novel PPR proteins (RFN and rfn, respectively) of 629 amino acids with 16 PPR motifs (Supplementary Fig. S4), which contain a predicted mitochondrial transit signal at the N-terminus (https://ihg.gsf.de/ihg/mitoprot.html;Claros and Vincens, 1996). Further analysis of the PPR motifs between RFN and rfn indicated that nine of the ‘code’ combinations in each PPR motif are different from each other (Fig. 2). This difference suggested that the recognized sequence or the binding ability of CMS-associated RNA target sequence may be different, and thus cause the functional divergence between RFN and rfn.
Fig. 2.
Combinations of amino acid residues at positions 5 and 35 of PPR motifs and the RNA sequence predicted to be recognized by these combinations for RFN and rfn proteins. ‘N’ represents that the recognized RNA base is unknown; ‘R’ represents that the recognized RNA base is A or G; ‘Y’ represents that the recognized RNA base is U or C. The same or different amino acid residue combinations in each PPR motif between RFN and rfn and their recognized RNA bases are indicated by different shading. (This figure is available in colour at JXB online.)
Expression pattern of the Rfn gene
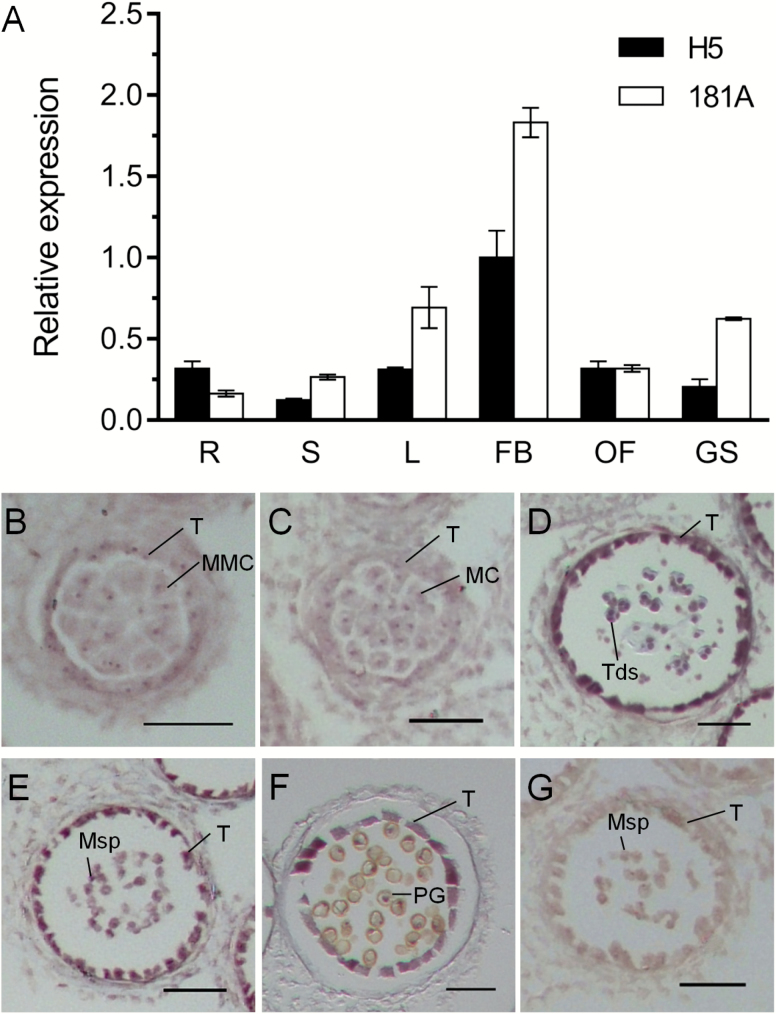
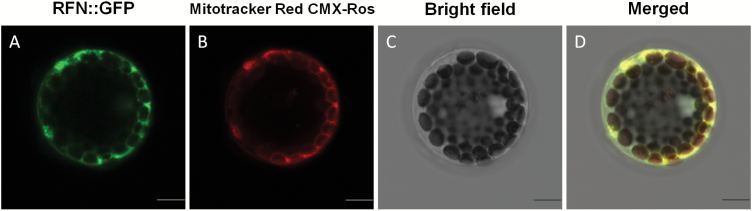
qRT–PCR analysis showed that the Rfn and rfn alleles are constitutively expressed in the selected tissues, and both maintained relatively high-level expression in the flower buds (Fig. 3A). Similar results were obtained by GUS staining assay in the Rfn promoter–GUS fusion transgenic Arabidopsis plants (Supplementary Fig. S5). To determine precisely the spatial and temporal expression pattern of Rfn during different anther development stages, we performed RNA in situ hybridization using an Rfn-derived probe. As shown in Fig. 3, faint hybridization signals were visible at the microspore mother cell (MMC) and meiotic stages (Fig. 3B, C). At the tetrad stage, the expression of the Rfn gene was detected in the tapetum and tetrads at its maximal level (Fig. 3D). The hybridization signals were slightly weakened in the subsequent stages and remained detectable in the tapetum but sharply decreased in the pollen grains (Fig. 3E, F). Subcellular localization analysis using a construct expressing a GFP fused with the N-terminal 44 residue signal peptide of RFN confirmed that this protein targets to mitochondria (Fig. 4), consistent with its function in restoring CMS.
Fig. 3.
The expression patterns of Rfn (rfn) by qRT–PCR and RNA in situ hybridization. (A) qRT–PCR analysis of Rfn and rfn expression in the selective tissues of parent lines H5 and 181A. R, root; S, stem; L, leaves; FB, flower buds; OF, opening flowers; GS, green siliques. The expression levels were normalized to BnActin. The values are presented as the means ±SD (n=3 biological replicates). (B–G) The expression patterns of Rfn by RNA in situ hybridization in the anther of the fertile parent H5 at different developmental stages detected by the antisense probe (B–F) and sense probe (G). MC, meiotic cell; MMC, microspore mother cell; Msp, microspores; PG, pollen grains; T, tapetum; Tds, tetrads. Scale bars=50 μm. (This figure is available in colour at JXB online.)
Fig. 4.
Subcellular localization of the N-terminal 44 residue signal peptide of RFN fused with green fluorescent protein (GFP). (A) The protoplast showed a green fluorescent signal at 488 nm. (B) The same protoplast showed a red fluorescent signal (stained by MitoTracker Red CMX-Ros) at 580 nm. (C) The bright-field image. (D) The merged image of (A), (B), and (C). Scale bars=10 µm. (This figure is available in colour at JXB online.)
Effects of Rfn on processing of nap CMS-associated transcripts or protein
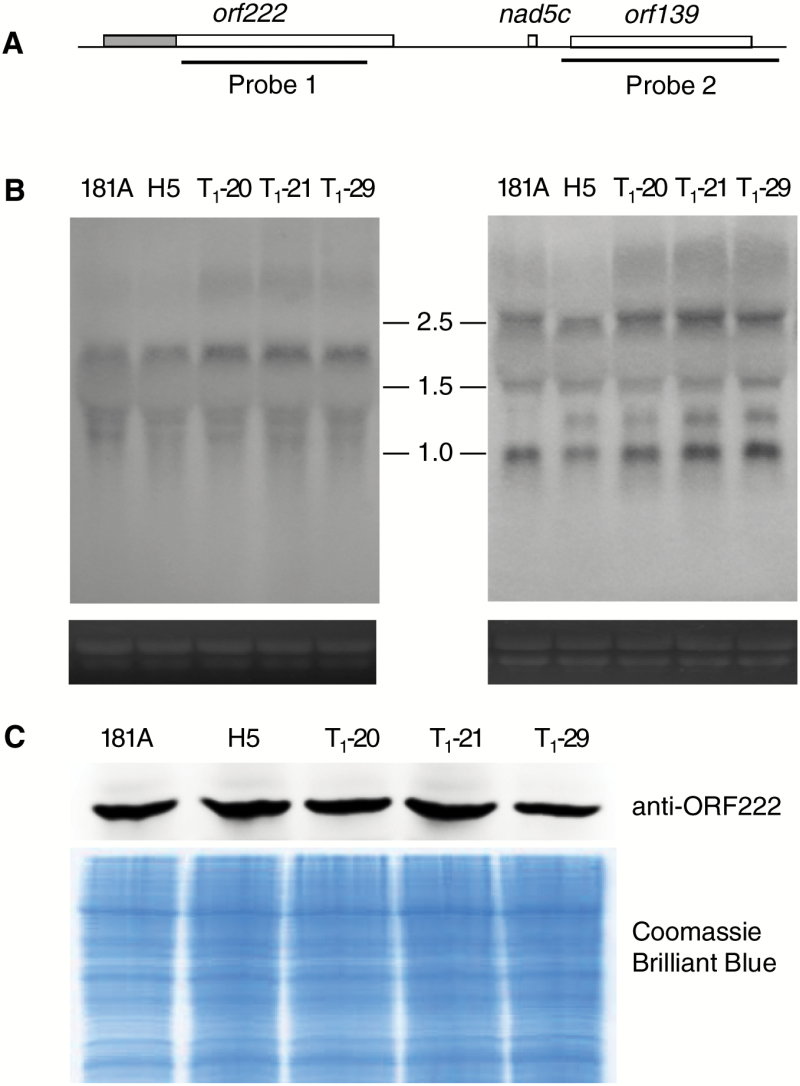
In a previous study, northern blot analysis suggested that expression of the orf222/nad5c/orf139 region may be associated with the nap CMS (L’Homme et al., 1997). To examine the mechanism through which RFN affects this CMS-associated transcript, RNA gel blot analysis was performed with the probes orf222 and orf139, respectively. As shown in Fig. 5, the orf222-containing transcript levels in the restorer and transgenic fertility-restored lines were not significantly changed when compared with those in the nap CMS line (Fig. 5B). In contrast, an ~1.2 kb transcript was specifically observed in the restorer and transgenic fertility-restored lines when using orf139 as a probe (Fig. 5B). Further immunodetection by the antibody against the orf222-encoded protein (ORF222) showed that the accumulation of ORF222 was not obviously changed in the restorer line and transgenic fertility-restored lines compared with the CMS line (Fig. 5C). These results indicate that the Rfn gene may not responsible for processing the orf222 transcripts but rather the orf139 transcripts.
Fig. 5.
RNA gel blot analysis of the orf222/nad5c/orf139 transcripts with probes orf222 and orf139, respectively, and immunodetection of ORF222 from CMS, restorer line, and transgenic fertility-restored plants. (A) The structure of nap CMS-associated transcripts orf222/nad5c/orf139. The shaded portion indicated the orfB homologous region. (B) RNA gel blots analysis with probes orf222 (left panel) and orf139 (right panel), respectively. The estimated sizes of the different transcripts are shown in kilobases. Ethidium bromide stain of the gel confirms equal RNA loading. (C) Immunodetection of CMS protein expression in T1 progeny with an anti-ORF222 antibody. Equal amounts of total protein were loaded in each lane stained by Coomassie Brilliant Blue. (This figure is available in colour at JXB online.)
Discussion
Rfn and Rfp are tightly linked independent genes and highly homologous
A previous study indicated that the restorer genes Rfn for nap cytoplasm and Rfp for pol cytoplasm represent different alleles or haplotypes of a single nuclear genetic locus (Li et al., 1998). Very recent research confirmed that Rfn indeed localizes to the same genomic region as Rfp (Gaborieau and Brown, 2016). In this study, through a map-based cloning strategy, we finely mapped the Rfn gene on the end of chromosome A09 of B. napus and successfully isolated it; and it was shown to encode a novel mitochondria-localized PPR protein. According to the B. napus reference genome of Darmor-bzh (Chalhoub et al., 2014), we found that the physical distance between of Rfn and Rfp (Liu et al., 2016) is ~125 kb. Sequence comparison showed that RFN and RFP are highly conserved, due to their sequence identity of 70% at the amino acid level (Supplementary Fig. S4). BlastP showed that both RFN and RFP have the highest similarity to RFL2 (At1g12300) in Arabidopsis thaliana (Supplementary Fig. S4). However, the gene Rfp which can restore the pol CMS does not possess the function in recovering the nap CMS, because some pol CMS restorer lines failed to restore the nap CMS of 181A in F1 plants, and all the positive T0 transformants remained male sterile phenotype when we introduced Rfp into 181A (data not shown). So, our results strongly suggested that Rfn and Rfp are tightly linked independent genes and they are likely to be evolutionarily homologous genes, but underwent a diversifying selection for adapting to newly emerging sterility-inducing genes (Geddy and Brown, 2007).
The possibility of functional divergence between RFN and rfn
PPR proteins constitute a large group of RNA-binding proteins in terrestrial plants, and function as sequence-specific ssRNA-binding proteins mainly in chloroplasts and mitochondria, where they are involved in various steps of organelle gene expression (Barkan and Small, 2014). Recent structural studies of PPR proteins indicated that one PPR motif has the ability to recognize a single nucleotide, and the amino acid combinations at the 5th and 35th residues of each PPR motif are very important for recognizing the specific RNA sequence (Yin et al., 2013; Shen et al., 2016). In A. thaliana, an amino acid mutation at position 35 of the third PPR motif results in RFL9 losing its function of processing the rps3 and orf240 transcript in the mitochondria (Arnal et al., 2014). The comparison of coded amino acids combinations between RFN and rfn showed that nine of them are different. Furthermore, we found that Rfn and rfn are constitutively expressed in various tissues, and the expression level of rfn is even higher than that of Rfn in flower buds. So, it is suggested that the functional divergence between RFN and rfn may not due to their few sequence variations in promoter regions, but have resulted from the changes in nine amino acids residing in the code positions.
The possible role of Rfn
The restoration of fertility in CMS/Rf systems may be achieved by various mechanisms at different molecular levels, such as the genomic level, post-transcriptional level, translational or post-translational level, and metabolic level (Chen and Liu, 2014). Most CMS-associated transcripts or proteins in various CMS crops are cleaved or degraded by the corresponding RF proteins. In BT-CMS rice, the CMS-associated transcripts B-atp6/orf79 were cleaved by RF1A but degraded by RF1B (Wang et al., 2006). In Ogu-CMS rapeseed, the RFO protein may impede orf138 mRNA translation by preventing either association or progression of mitochondrial ribosomes with the orf138 mRNA (Uyttewaal et al., 2008). A previous study indicated that the nap CMS-associated transcripts orf222/nad5c/orf139 were affected by the action of Rfn at the post-transcriptional level (L’Homme et al., 1997). In the current work, we detected the CMS-associated transcripts in the nap CMS line, restorer line, and transgenic fertility-restored lines using the orf222 and orf139 probe, respectively. We found that the CMS-associated transcripts were not significantly changed when using orf222 as a probe, but an ~1.2 kb transcript appeared in the restorer line and transgenic fertility-restored lines when orf139 was used as a probe. The accumulation of ORF222 was also not obviously changed in the restorer line and transgenic fertility-restored lines compared with the nap CMS line. These findings suggested two possibilities: first, there is another CMS-associated gene but not orf222; secondly, RFN may process the nap CMS-associated orf222 in a unique stage. Further investigation is needed to identify the target RNA of RFN or RNA blotting with a specific stage of the anther and thus illustrate the molecular mechanism of how RFN recovers the nap CMS in B. napus.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Opening flower morphology of the parent plants 181A and H5 under relatively low temperature.
Fig. S2. The putative promoter region and coding sequence of Rfn and its deduced amino acids from the restorer line H5.
Fig. S3. Nucleotide sequence of recessive rfn allele from 181A and the deduced amino acids.
Fig. S4. Alignment of the amino acid sequences of RFN, RFP, and their homologous protein RFL2 in Arabidopsis.
Fig. S5. GUS assay from different tissues of ProRfn:GUS-introduced Arabidopsis plants.
Table S1. The primers used in this study.
Table S2. Fertility of T1 progeny derived from five independent T0 transgenic plants by selfing and test cross.
Supplementary Material
Acknowledgements
We thank Qinghua Zhang and Liang Liu for sequencing and assembling the BAC clone. We are grateful to the anonymous reviewers for their constructive comments and suggestions. This work was supported by the National Natural Science Foundation of China (no. 31300160), National Key Research and Development Plan of China (2016YFD0101300), Technological innovation project of Hubei (2016ABA084), and Modern Agro-Industry Technology Research System (CARS-13).
Glossary
Abbreviations:
- BAC
bacterial artificial chromosome
- CDS
coding sequence
- CMS
cytoplasmic male sterility
- DIG
digoxigenin
- GFP
green fluorescent protein
- PPR
pentatricopeptide repeat
- qRT–PCR
quantitative reverse transcription–PCR
- Rf
restorer of fertility.
References
- Arnal N, Quadrado M, Simon M, Mireau H. 2014. A restorer-of-fertility like pentatricopeptide repeat gene directs ribonucleolytic processing within the coding sequence of rps3-rpl16 and orf240a mitochondrial transcripts in Arabidipsis thaliana. The Plant Journal 78, 134–145. [DOI] [PubMed] [Google Scholar]
- Ban T, Ke J, Chen R et al. 2013. Structure of a PLS-class pentatricopeptide repeat protein provides insights into mechanism of RNA recognition. Journal of Biological Chemistry 288, 31540–31548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A, Rojas M, Fujii S, Yap A, Chong YS, Bond CS, Small I. 2012. A combinatorial amino acid code for RNA recognition by pentatricopeptide repeat proteins. PLoS Genetics 8, e1002910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A, Small I. 2014. Pentatricopeptide repeat proteins in plants. Annual Review of Plant Biology 65, 415–442. [DOI] [PubMed] [Google Scholar]
- Bentolila S, Alfonso AA, Hanson MR. 2002. A pentatricopeptide repeat-containing gene restores fertility to cytoplasmic male sterile plants. Proceedings of the National Academy of Sciences, USA 99, 10887–10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GG, Formanová N, Jin H et al. 2003. The radish Rfo restorer gene of Ogura cytoplasmic male sterility encodes a protein with multiple pentatricopeptide repeats. The Plant Journal 35, 262–272. [DOI] [PubMed] [Google Scholar]
- Chalhoub B, Denoeud F, Liu S et al. 2014. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 345, 950–953. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu YG. 2014. Male sterility and fertility restoration in crops. Annual Review of Plant Biology 65, 579–606. [DOI] [PubMed] [Google Scholar]
- Cheng F, Liu S, Wu J, Fang L, Sun S, Liu B, Li P, Hua W, Wang X. 2011. BRAD, the genetics and genomics database for Brassica plants. BMC Plant Biology 11, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claros MG, Vincens P. 1996. Computational method to predict mitochondrially imported proteins and their targeting sequences. European Journal of Biochemistry 241, 779–786. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Crooijmans RP, Vrebalov J, Dijkhof RJ, van der Poel JJ, Groenen MA. 2000. Two-dimensional screening of the Wageningen chicken BAC library. Mammalian Genome 11, 360–363. [DOI] [PubMed] [Google Scholar]
- Cui X, Wise RP, Schnable PS. 1996. The rf2 nuclear restorer gene of male-sterile T-cytoplasm maize. Science 272, 1334–1336. [DOI] [PubMed] [Google Scholar]
- Fan Z, Stefansson BR. 1986. Influence of temperature on sterility of two cytoplasmic male-sterility systems in rape (Brassica napus L.). Canadian Journal of Plant Science 66, 221–227. [Google Scholar]
- Fujii S, Toriyama K. 2009. Suppressed expression of RETROGRADE- REGULATED MALE STERILITY restores pollen fertility in cytoplasmic male sterile rice plants. Proceedings of the National Academy of Sciences, USA 106, 9513–9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaborieau L, Brown GG. 2016. Comparative genomic analysis of the compound Brassica napus Rf locus. BMC Genomics 17, 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddy R, Brown GG. 2007. Genes encoding pentatricopeptide repeat (PPR) proteins are not conserved in location in plant genomes and may be subject to diversifying selection. BMC Genomics 8, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gully BS, Cowieson N, Stanley WA, Shearston K, Small ID, Barkan A, Bond CS. 2015. The solution structure of the pentatricopeptide repeat protein PPR10 upon binding atpH RNA. Nucleic Acids Research 43, 1918–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MR, Bentolila S. 2004. Interactions of mitochondrial and nuclear genes that affect male gametophyte development. The Plant Cell 16 (Suppl), S154–S169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Wang K, Huang W et al. 2012. The rice pentatricopeptide repeat protein RF5 restores fertility in Hong-Lian cytoplasmic male-sterile lines via a complex with the glycine-rich protein GRP162. The Plant Cell 24, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Yu C, Hu J et al. 2015. Pentatricopeptide-repeat family protein RF6 functions with hexokinase 6 to rescue rice cytoplasmic male sterility. Proceedings of the National Academy of Sciences, USA 112, 14984–14989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K, Kazama T, Toriyama K. 2016. A gene encoding pentatricopeptide repeat protein partially restores fertility in RT98-type cytoplasmic male-sterile rice. Plant and Cell Physiology 57, 2187–2193. [DOI] [PubMed] [Google Scholar]
- Itabashi E, Iwata N, Fujii S, Kazama T, Toriyama K. 2011. The fertility restorer gene, Rf2, for Lead Rice-type cytoplasmic male sterility of rice encodes a mitochondrial glycine-rich protein. The Plant Journal 65, 359–367. [DOI] [PubMed] [Google Scholar]
- Jing B, Heng S, Tong D et al. 2012. A male sterility-associated cytotoxic protein ORF288 in Brassica juncea causes aborted pollen development. Journal of Experimental Botany 63, 1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama T, Toriyama K. 2003. A pentatricopeptide repeat-containing gene that promotes the processing of aberrant atp6 RNA of cytoplasmic male-sterile rice. FEBS Letters 544, 99–102. [DOI] [PubMed] [Google Scholar]
- Ke J, Chen RZ, Ban T et al. 2013. Structural basis for RNA recognition by a dimeric PPR-protein complex. Nature Structural and Molecular Biology 20, 1377–1382. [DOI] [PubMed] [Google Scholar]
- Klein RR, Klein PE, Mullet JE, Minx P, Rooney WL, Schertz KF. 2005. Fertility restorer locus Rf1 [corrected] of sorghum (Sorghum bicolor L.) encodes a pentatricopeptide repeat protein not present in the colinear region of rice chromosome 12. Theoretical and Applied Genetics 111, 994–1012. [DOI] [PubMed] [Google Scholar]
- Koizuka N, Imai R, Fujimoto H, Hayakawa T, Kimura Y, Kohno-Murase J, Sakai T, Kawasaki S, Imamura J. 2003. Genetic characterization of a pentatricopeptide repeat protein gene, orf687, that restores fertility in the cytoplasmic male-sterile Kosena radish. The Plant Journal 34, 407–415. [DOI] [PubMed] [Google Scholar]
- Komori T, Ohta S, Murai N, Takakura Y, Kuraya Y, Suzuki S, Hiei Y, Imaseki H, Nitta N. 2004. Map-based cloning of a fertility restorer gene, Rf-1, in rice (Oryza sativa L.). The Plant Journal 37, 315–325. [DOI] [PubMed] [Google Scholar]
- L’Homme Y, Stahl RJ, Li XQ, Hameed A, Brown GG. 1997. Brassica nap cytoplasmic male sterility is associated with expression of a mtDNA region containing a chimeric gene similar to the pol CMS-associated orf224 gene. Current Genetics 31, 325–335. [DOI] [PubMed] [Google Scholar]
- Li S, Chen L, Zhang L et al. 2015. BnaC9.SMG7b functions as a positive regulator of the number of seeds per silique in Brassica napus by regulating the formation of functional female gametophytes. Plant Physiology 169, 2744–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XQ, Jean M, Landry BS, Brown GG. 1998. Restorer genes for different forms of Brassica cytoplasmic male sterility map to a single nuclear locus that modifies transcripts of several mitochondrial genes. Proceedings of the National Academy of Sciences, USA 95, 10032–10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Liu P, Long F, Hong D, He Q, Yang G. 2012. Fine mapping and candidate gene analysis of the nuclear restorer gene Rfp for pol CMS in rapeseed (Brassica napus L.). Theoretical and Applied Genetics 125, 773–779. [DOI] [PubMed] [Google Scholar]
- Liu Z, Yang Z, Wang X et al. 2016. A mitochondria-targeted PPR protein restores pol cytoplasmic male sterility by reducing orf224 transcript levels in oilseed rape. Molecular Plant 9, 1082–1084. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lurin C, Andrés C, Aubourg S et al. 2004. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. The Plant Cell 16, 2089–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhira H, Kagami H, Kurata M et al. 2012. Unusual and typical features of a novel restorer-of-fertility gene of sugar beet (Beta vulgaris L.). Genetics 192, 1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K, Shoki H, Arai M, Shikanai T, Small I, Nakamura T. 2014. Quantitative analysis of motifs contributing to the interaction between PLS-subfamily members and their target RNA sequences in plastid RNA editing. The Plant Journal 80, 870–882. [DOI] [PubMed] [Google Scholar]
- Schmitz-Linneweber C, Small I. 2008. Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends in Plant Science 13, 663–670. [DOI] [PubMed] [Google Scholar]
- Shen C, Zhang D, Guan Z et al. 2016. Structural basis for specific single-stranded RNA recognition by designer pentatricopeptide repeat proteins. Nature Communications 7, 11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Brown GG. 1991. Suppression of cytoplasmic male sterility by nuclear genes alters expression of a novel mitochondrial gene region. The Plant Cell 3, 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Brown GG. 1993. Characterization of expression of a mitochondrial gene region associated with the Brassica ‘Polima’ CMS: developmental influences. Current Genetics 24, 316–322. [DOI] [PubMed] [Google Scholar]
- Tang H, Luo D, Zhou D, Zhang Q, Tian D, Zheng X, Chen L, Liu YG. 2014. The rice restorer Rf4 for wild-abortive cytoplasmic male sterility encodes a mitochondrial-localized PPR protein that functions in reduction of WA352 transcripts. Molecular Plant 7, 1497–1500. [DOI] [PubMed] [Google Scholar]
- Uyttewaal M, Arnal N, Quadrado M et al. 2008. Characterization of Raphanus sativus pentatricopeptide repeat proteins encoded by the fertility restorer locus for Ogura cytoplasmic male sterility. The Plant Cell 20, 3331–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Xia X, Hong D, Yang G. 2010. Molecular analysis and expression of a floral organ-specific polygalacturonase gene isolated from rapeseed (Brassica napus L.). Molecular Biology Reports 37, 3851–3862. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zou Y, Li X et al. 2006. Cytoplasmic male sterility of rice with boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. The Plant Cell 18, 676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZW, Wang C, Wang C, Gao L, Mei SY, Zhou Y, Xiang CP, Wang T. 2013. Heterozygous alleles restore male fertility to cytoplasmic male-sterile radish (Raphanus sativus L.): a case of overdominance. Journal of Experimental Botany 64, 2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y, Hayashi S, Kobayashi K, Hirayama T, Nakamura T. 2013. Elucidation of the RNA recognition code for pentatricopeptide repeat proteins involved in organelle RNA editing in plants. PLoS One 8, e57286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P, Li Q, Yan C et al. 2013. Structural basis for the modular recognition of single-stranded RNA by PPR proteins. Nature 504, 168–171. [DOI] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. 2007. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nature Protocols 2, 1565–1572. [DOI] [PubMed] [Google Scholar]
- Zhou YM, Fu TD. 2007. Genetic improvement of rapeseed in China. In: Proceedings of the 12th International Rapeseed Congress Wuhan, China, 26–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.