Expression of CAU1 (encoding a histone methylase) decreases under drought, leading to de-repression of the NAC transcription factor ANAC055 and its genetically downstream proline synthetase gene P5CS1, and this results in proline accumulation and a consequent increase in drought tolerance.
Keywords: ANAC055, CAU1, drought tolerance, histone methylase, P5CS1, proline
Abstract
Proline plays a crucial role in the drought stress response in plants. However, there are still gaps in our knowledge about the molecular mechanisms that regulate proline metabolism under drought stress. Here, we report that the histone methylase encoded by CAU1, which is genetically upstream of P5CS1 (encoding the proline biosynthetic enzyme Δ1-pyrroline-5-carboxylate synthetase 1), plays a crucial role in proline-mediated drought tolerance. We determined that the transcript level of CAU1 decreased while that of ANAC055 (encoding a transcription factor) increased in wild-type Arabidopsis under drought stress. Further analyses showed that CAU1 bound to the promoter of ANAC055 and suppressed its expression via H4R3sme2-type histone methylation in the promoter region. Thus, under drought stress, a decreased level of CAU1 led to an increased transcript level of ANAC055, which induced the expression of P5CS1 and increased proline level independently of CAS. Drought tolerance and the level of proline were found to be decreased in the cau1 anac055 double-mutant, while proline supplementation restored drought sensitivity in the anac055 mutant. Our results reveal the details of a novel pathway leading to drought tolerance mediated by CAU1.
Introduction
In plants under osmotic stress, proline is mainly synthesized by P5CS (Δ1-pyrroline-5-carboxylate synthetase) (Savouré et al., 1995; Yoshiba et al., 1995; Székely et al., 2008) and P5CR (P5C reductase) from glutamate in chloroplasts (Szoke et al., 1992; Verbruggen et al., 1993). Proline catabolism is controlled by PDH (proline dehydrogenase) (Kiyosue et al., 1996; Verbruggen et al., 1996) and P5CDH (P5C dehydrogenase) in Arabidopsis (Deuschle et al., 2001). As well as functioning as a compatible osmolyte, proline may act as a metabolic signal that regulates the stabilization of proteins and antioxidant enzymes, the direct scavenging of ROS (reactive oxygen species), and the balance of intracellular redox homeostasis, such as the ratios of NADP+/NADPH and GSH/GSSG (Hamilton and Heckathorn, 2001; Kavi Kishor et al., 2005; Miller et al., 2009; Szabados and Savouré, 2010; Alves et al., 2011).
Several studies have shown that the transcription of P5CS1 is activated by H2O2-derived signals, the calcium signal, PLC (phospholipase C), PLD (phospholipase D), and by the ABA-dependent pathway (Yoshiba et al., 1995; Savouré et al., 1997; Strizhov et al., 1997; Parre et al., 2007; Verslues et al., 2007; Ghars et al., 2008). ABI1 (ABA-INSENSITIVE 1) and the CaM4 calmodulin-MYB2 regulatory pathway are involved in the control of P5CS1 transcription (Knight et al., 1997; Strizhov et al., 1997; Yoo et al., 2005; Parre et al., 2007). Although much is known about the biological functions of proline in stress tolerance, its regulation needs further investigation.
In plants, histone modifications have been implicated in the response to drought stress (Sokol et al., 2007; van Dijk et al., 2010; Kim et al., 2012; Sani et al., 2013; Zong et al., 2013). The dehydration-stress response gene ATX1 encodes a protein that trimethylates histone H3 at lysine 4 (H3K4me3) in NCED3 (NINE-CIS-EPOXYCAROTENOID DIOXYGENASE 3) (Ding et al., 2009, 2011). The H3K4 demethylase homolog HvPKDM7-1 may be involved in drought tolerance in barley (Papaefthimiou and Tsaftaris, 2012). The histone acetylation levels increase in RD20 (RESPONSIVE TO DEHYDRATION 20), RD29A (RESPONSIVE TO DESICCATION 29A), and RD29B (RESPONSIVE TO DESICCATION 29B) in Arabidopsis, and in four HATs (Histone acetyltransferases) genes OsHAC703, OsHAG703, OsHAF701, and OsHAM701 in rice under drought stress (Kim et al., 2008; Fang et al., 2014). Histone deacetylase 2, encoded by AtHD2C, is involved in tolerance to drought stress in Arabidopsis (Sridha and Wu, 2006). Histone H4 deacetylation is also involved in ABA-induced stomatal closing (Sridha and Wu, 2006; Zhu et al., 2008). However, there is still much to learn about the mechanisms of histone modification under drought stress.
Previously, we showed that the H4R3sme2-type histone methylase CAU1/PRMT5/SKB1 mediates stomatal closure by repressing the expression of CAS (CALCIUM SENSOR, mediating the sensing of extracellular Ca2+ in guard cells) in response to extracellular calcium (Han et al., 2003; Fu et al., 2013). However, the cas-1 mutant showed a partly restored water-loss rate and the same rate of stomatal closure as that of the wild-type (Fu et al., 2013), suggesting that other components may function in the drought tolerance pathway mediated by CAU1.
In this study, we show that the transcription factor encoded by ANAC055 acts as a downstream component of CAU1 independently of CAS. Our results show that drought stress represses the levels of CAU1 RNA and CAU1 protein, which lead to decreased H4R3sme2 methylation levels of chromatin in the ANAC055 promoter. The subsequent increase in ANAC055 expression leads to increased expression of its genetically downstream gene, P5CS1, resulting in proline accumulation and drought tolerance.
Materials and methods
Plant material, growth conditions, and physiological analyses
Plants of Arabidopsis thaliana were grown in soil at 22 °C with 16-h light/8-h dark cycles. At 14 d after emergence, drought stress was induced by withholding watering for 14 d, and the survival rate was scored at 7 d after watering recommenced. Rosette leaves of 22-d-old sample plants were collected and used to determine rates of water loss by time-course measurements of their fresh weights (Vartanian et al., 1994; Pei et al., 1998). Stomatal assays were performed as previously described (Pei et al., 2000; Fu et al., 2013). Stomatal apertures were determined by measuring the pore widths and lengths with a digital ruler in Image-Pro Plus 6.0 (MediaCybernetics).
Alternatively, plants were grown in quarter-strength hydroponics as previously described (Arteca and Arteca, 2000; Gong et al., 2003). At 4 weeks of age, plants were treated with 10%, 20%, 30%, or 40% PEG-6000 and/or 50 mM proline for 12 h, or with 10% PEG-6000 for 0, 1, 3, 6, 12 h. Shoots were sampled and subjected to further analyses as indicated. Plants were weighed at the start of treatment (initial weight) and then reweighed at the end of treatment (final weight). Plants were then dried to a constant weight at 80 °C and reweighed to obtain dry weight. RWC was calculated as: (final weight – dry weight)/(initial weight – dry weight) ×100%.
DNA constructs and plant transformation
The ANAC055 cDNA was amplified by RT-PCR. The two restriction sites for BamHI and SacI were introduced using ANAC055-1 primers and for XhoI and EcoRI using CAU1-1 (see Table S1 available at the Dryad Digital Repository, https://doi.org/10.5061/dryad.hc4bj). The resulting fragments were confirmed by sequencing and then sub-cloned into the binary vector pBI121 (pre-digested with BamHI and SacI) or 35S:EYFP/pMON530 (pre-digested with XhoI and EcoRI). The ANAC055 promoter was amplified by RT-PCR. The two restriction sites for PstI and BamHI were introduced using ANAC055-2 primers (see Table S1 at Dryad). The resulting fragments were confirmed by sequencing and then sub-cloned into the binary vector GUS/pCAMBIA1300 (pre-digested with PstI and BamHI). The generated constructs 35S:ANAC055/pBI121, 35S:EYFP-CAU1/pMON530 and pANAC055:GUS/pCAMBIA1300 were transformed into Col-0 or cau1 using the floral dip method (Clough and Bent, 1998). Transgenic lines with a segregation rate of 3:1 grown on kanamycin or hygromycin plates were used for further homozygote and strong allele screenings.
RT-PCR/quantitative RT-PCR
Total RNA from plants was isolated using the TRIzol reagent (Invitrogen). First-strand cDNA synthesis, RT-PCR, and quantitative RT-PCR (qPCR) were performed as previously described (Aggarwal et al., 2010). ANAC055-QP, CAU1-QP, P5CS1-QP, SAND-QP, and drought-related gene primers were used in quantitative RT-PCR (see Table S1 at Dryad).
Histochemical analysis
Transgenic plants of the pANAC055:GUS/cau1 mutant were subjected to histochemical analysis as previously described (Aggarwal et al., 2010).
Isolation of anac055 and cau1 anac055 mutant plants
The T-DNA insertion line SALK_014331 obtained from the Arabidopsis Biological Resource Center (https://abrc.osu.edu/) was screened for the homozygous knockout mutant anac055 as previously described (Weinl et al., 2008). To generate the cau1 anac055 double-mutant, cau1 was crossed to anac055 to make an F2 population; cau1-like plants were further analysed to isolate the genotype anac055/anac055 using the PCR primers ANAC055-SALK and LBA1 as previously described (Krysan et al., 1999).
Determination of proline levels
Proline concentrations were determined as described by Bates et al. (1973). Leaves were freeze-dried and then homogenized in 3% sulfosalicylic acid, and were then centrifuged at 3000 g for 20 min. The sample supernatant, acetic acid, and 2.5% acid ninhydrin solution were boiled for 30 min, and the absorbance was measured at 520 nm.
Chromatin immunoprecipitation (ChIP) assay
For the ChIP assay, 21-d-old Col-0, cau1, and 35S:EYFP-CAU1/cau1 plants grown under long-day conditions were harvested. Approximately 4 g of plant material was cross-linked for 20 min in 1% formaldehyde. ChIP assays were performed as previously described (Vartanian et al., 1994; Ascenzi and Gantt, 1999). The sonicated chromatin extractions were immunoprecipitated overnight with antisymmetric dimethyl-H4R3 antibody (Abcam) for plants of Col-0 and cau1, with an anti-GFP (green fluorescent protein) antibody (Invitrogen) for plants of 35S:EYFP-CAU1/cau1, or without antibody. Incubation of chromatin with mouse IgG (Abcam) served as a mock immunoprecipitation control. Protein A beads (Millipore) were used to capture the immunocomplexes. After reverse cross-linking and proteinase-K digestion, the DNA was extracted with phenol-chloroform and then precipitated with ethanol. The immunoprecipitated DNA was subsequently used for qPCR. The sequences were amplified from –1388 to 646 bp of the ANAC055 gene and each DNA fragment was approximately 120 bp in length. Primers used for ChIP-qPCR were as follows: Region A (ANAC055-1), region B (ANAC055-2), region C (ANAC055-3), region D (ANAC055-8), region E (ANAC055-9), region F (ANAC055-10), region G (ANAC055-11), and the primer sequences are given in Table S1 at Dryad. TUB8 was used as a control (Mathieu et al., 2005).
Protein gel blotting analysis
Transgenic 35S:EYFP-CAU1/cau1 plants were grown in hydroponics to 24 days of age, and then exposed to 10% PEG treatments. Total proteins were extracted from leaf samples using buffer E [125 mMTris-HCl pH 8.0; 1% (w/v) SDS, 10% (v/v) glycerol, 50mM NaS2O5]. From each sample 30 µg total proteins were separated on 12% SDS-PAGE (Beyotime) gel and analysed by protein gel blotting according to the manufacturer’s instructions. Mouse anti-Actin2 and anti-GFP (Abmart; at 1:5000 dilution) were used as primary antibodies. The membranes were visualized using a Super-Signal West Pico Chemiluminescent Substrate Kit (Thermo Scientific) according to the manufacturer’s instructions.
Statistical analysis
Data were statistically analysed using one-way ANOVA with LSD tests (for multiple comparisons) or two-tailed Student’s t-tests (for comparisons of two sets of data).
Results
ANAC055 expression is enhanced in the cau1 mutant
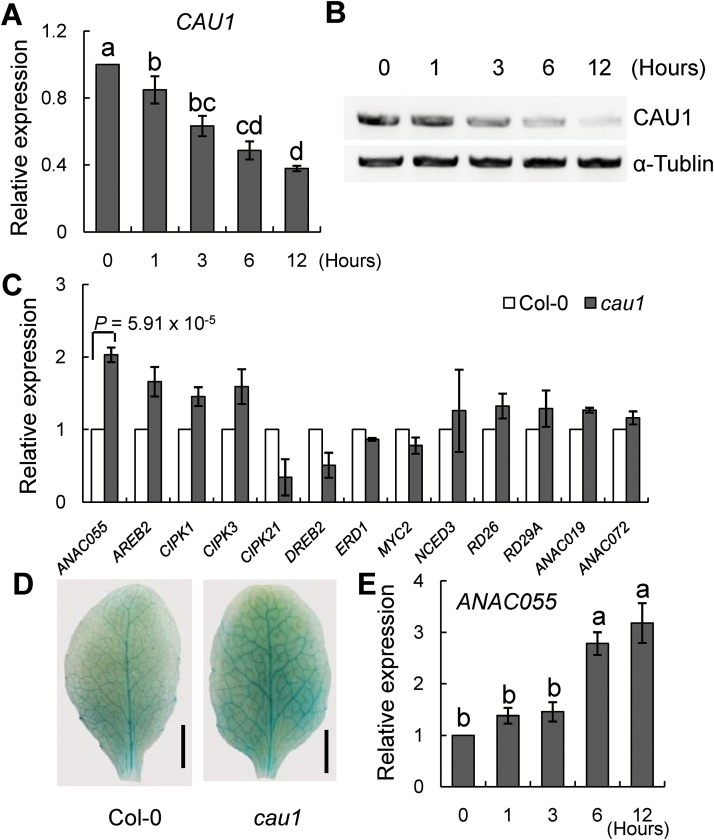
Previously, we showed that the cau1 mutant is resistant to drought stress (Fu et al., 2013). To investigate the role of CAU1 in drought tolerance, the levels of CAU1 transcripts and CAU1 protein were determined in the wild-type (Col-0) and the cau1 mutant under drought stress conditions. The CAU1 transcript levels (Fig. 1A) and CAU1 protein levels (Fig. 1B) in Col-0 decreased under drought stress. Next, we analysed the transcript levels of drought tolerance-related genes including ANAC055, AREB2, CIPK1, CIPK3, CIPK21, DREB2, ERD1, MYC2, NCED3, RD26, RD29A, ANAC019, and ANAC072 by quantitative RT-PCR. The transcript level of ANAC055, which encodes a transcription factor, was enhanced in the cau1mutant (Fig. 1C), compared with that in Col-0. Histochemical analyses showed that the activity of GUS driven by the ANAC055 promoter was higher in cau1 leaves than in Col-0 leaves (Fig. 1D). The transcript level of ANAC055 in Col-0 was enhanced under drought stress (Fig. 1E). These results indicated that the ANAC055 level was enhanced in cau1, and that drought stress suppressed CAU1 expression but increased ANAC055 expression.
Fig. 1.
Enhanced expression of ANAC055 in the cau1 mutant compared with Col-0. (A) Decrease in CAU1 transcript levels in Col-0 under drought stress. Transcript levels of CAU1 in 4-week-old hydroponically grown plants treated with 10% PEG-6000 for 0, 1, 3, 6, and 12 h. SAND (AT2G28390) was used as an internal control. Values are means ±SE, n=3. (B) Decrease in CAU1 protein levels in Col-0 under drought stress. CAU1 detected by immunoblotting in 4-week-old plants exposed to 10% PEG-6000 treatment for 0, 1, 3, 6, and 12 h. (C) Relative transcript levels of genes functioning in the drought tolerance pathway as determined by RT-PCR. For each gene, the transcript level was set to 1 in Col-0. (D) Expression of ANAC055 promoter–GUS fusions in leaves of Col-0 and the cau1 mutant. Scale bars =0.2 cm. (E) Increase in ANAC055 transcript levels in Col-0 under drought stress. Transcript levels of ANAC055 in 4-week-old plants treated with 10% PEG-6000 for 0, 1, 3, 6, and 12 h. (This figure is available in colour at JXB online.)
CAU1 regulates ANAC055 expression by H4R3sme2 methylation in its promoter region
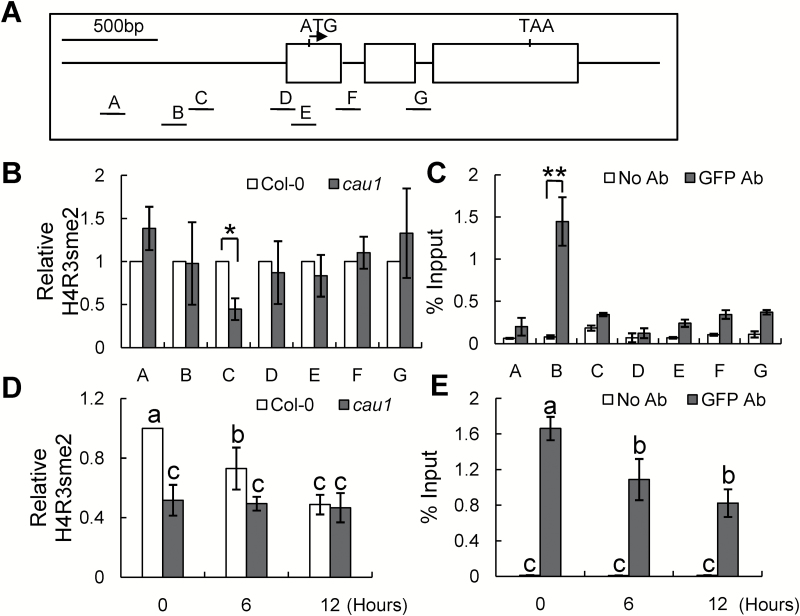
To explore the mechanism by which CAU1 regulates ANAC055 expression, we performed a ChIP-qPCR assay to analyse the H4R3sme2 level in the ANAC055 promoter region, using an H4R3sme2 antibody (Fig. 2A, B). The H4R3me2 level in region C of the ANAC055 promoter was significantly reduced in cau1 (Fig. 2A, B). This result suggested that CAU1 regulated ANAC055 transcription through histone methylation.
Fig. 2.
Suppression of CAU1-mediated histone methylation in the ANAC055 promoter region under drought stress. (A) Diagram of the ANAC055 gene. The genomic regions A to G used in the ChIP assays are indicated. (B, C) ChIP assays with antibodies against H4R3sme2 (B) using Col-0 and cau1 plants, and GFP (C) using 35S:EYFP-CAU1/cau1 plants. TUB8 was used as an internal control in (C). Three independent experiments were performed, and values are means ±SE. *P<0.05 and **P<0.01. (D, E) ChIP assays with antibodies against H4R3sme2 within the C region (D) or GFP within the B region (E) of ANAC055. Plants were treated with 10% PEG-6000 for the period indicated. Three independent experiments were performed. TUB8 was used as an internal control in (D). Values are means ±SE. Different letters above each bar indicate significant differences (ANOVA tests).
We also conducted a ChIP-qPCR assay using a GFP antibody to determine whether CAU1 binds to the ANAC055 chromatin (Fig. 2A, C). CAU1 strongly associated with region B of the ANAC055 promoter, whereas a similar CAU1–ANAC055 interaction was not detected in regions A or C–G. These results confirmed that CAU1 bound directly to the ANAC055 chromatin in region B, and mediated the level of histone methylation in region C (Fig. 2A–C).
Given that ANAC055 expression was up-regulated in response to drought stress (Fig. 1E), we analysed the correlation between drought stress and the level of H4R3sme2 in the ANAC055 promoter. As shown in Fig. 2D, drought stress significantly decreased the H4R3sme2 level in region C of the ANAC055 promoter in Col-0, while no change was observed in the cau1 mutant. Further analyses showed that there were significant decreases in CAU1 binding to the ANAC055 promoter (Fig. 2E) as well as significant decreases in CAU1 mRNA and CAU1 protein levels in Col-0 under drought stress (Fig. 1A, B). These data indicated that drought stress decreased the CAU1 mRNA and CAU1 protein levels and decreased CAU1 binding to the ANAC055 promoter, thus decreasing H4R3sme2 methylation of the ANAC055 chromatin and enhancing ANAC055 expression.
CAU1 acts with ANAC055 in response to drought stress
A previous study showed that the NAC gene family member ANAC055 was up-regulated by drought stress, and its over-expression increased drought tolerance (Tran et al., 2004). Given that cau1 was shown to be insensitive to drought stress (Fu et al., 2013) and showed enhanced ANAC055 expression (Fig. 1C), we sought to determine whether these phenotypes were genetically correlated with ANAC055. A T-DNA insertion line for ANAC055 was isolated (see Fig. S1A, B at Dryad). RT-PCR analysis confirmed that ANAC055 mRNA was not detectable in the anac055 mutant (see Fig. S1C at Dryad).
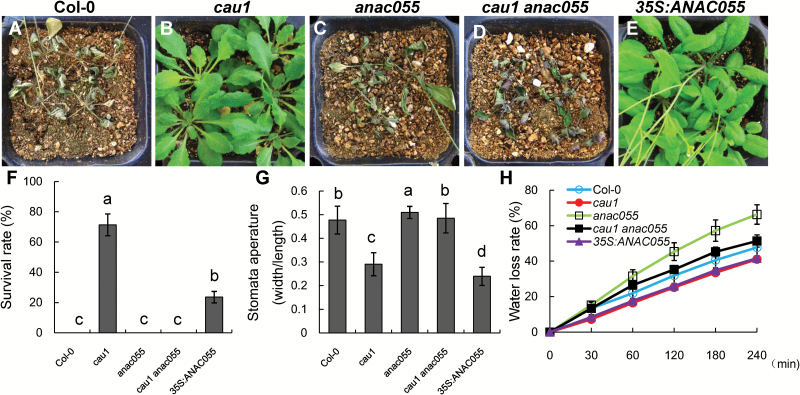
Further analyses showed that Col-0 (Fig. 3A, F) and anac055, the loss-of-function mutant of ANAC055 (Fig. 3C, F), were sensitive to drought stress, while cau1 plants were drought tolerant (Fig. 3B, F). However, the drought tolerance conferred by the cau1 mutation was abolished in the double mutant cau1 anac055 (Fig. 3D, F), even though the visible developmental phenotypes of cau1 anac055 were similar to those of cau1. A drought-tolerant phenotype was also observed in 35S:ANAC055 (Fig. 3E, F). These results indicated that ANAC055 is downstream of CAU1, which functions in drought tolerance.
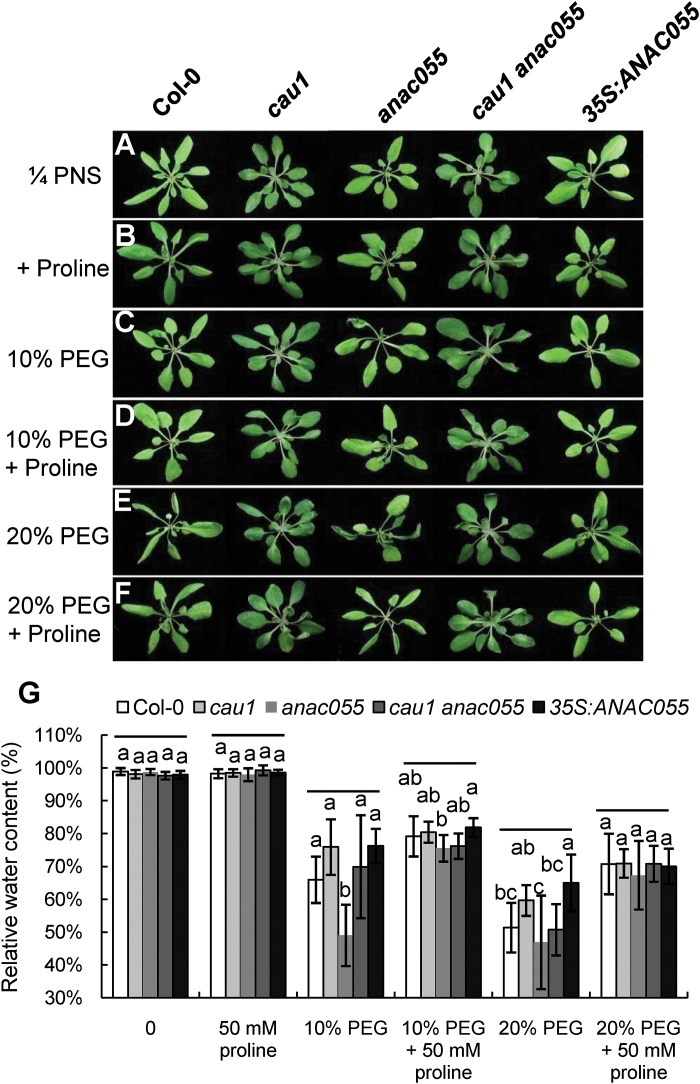
Fig. 3.
CAU1–ANAC055 acts in the drought tolerance pathway. (A–E) Drought tolerance of Col-0 (A), cau1 (B), anac055 (C), cau1 anac055 (D), and 35S:ANAC055 (E). When plants were 14 d old watering was withheld for 14 d, and then watering recommenced for 7 d. (F) Survival rates of plants in (A–E) under drought stress. Values are means ±SD from three independent experiments. (G) Stomatal apertures on rosette leaves. Values are means ±SD (n≥30). (H) Water loss rates from leaves of Col-0, cau1, anac055, cau1 anac055, and 35S:ANAC055. Values are means ±SD from three independent experiments (n=6 leaves per treatment). Different letters above each bar indicate significant differences (ANOVA tests).
Next, we evaluated differences in stomatal apertures among the mutants and Col-0. As shown in Fig. 3G, the stomatal aperture was smaller in cau1 than in Col-0, while that of anac055 was larger than that of Col-0. In the double-mutant cau1 anac055, stomatal aperture was restored to a level between those of Col-0 and anac055 (Fig. 3G). The rate of water loss from the leaves was decreased in cau1 and 35S:ANAC055, and increased in anac055 compared with that of Col-0 (Fig. 3H). These results suggested that ANAC055 functions downstream of CAU1, and plays an important role in stomatal closure and consequently in drought tolerance.
CAU1 affects proline accumulation via its effects on P5CS1
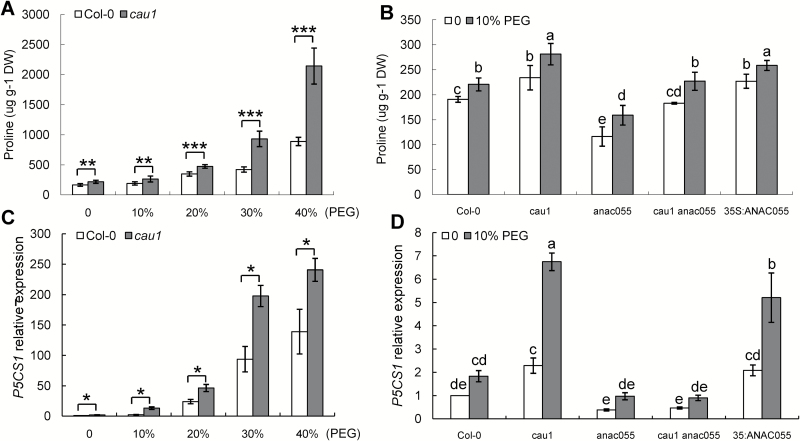
To elucidate the molecular mechanism of CAU1 in the drought response, we measured the proline levels in Col-0 and cau1 under drought stress imposed by PEG-6000. Proline accumulated in both Col-0 and cau1 under drought stress with a clear dose-dependent effect, and to higher levels in cau1 than in Col-0 (Fig. 4A). In the absence of drought stress, there were higher proline contents in cau1and 35S:ANAC055 than in Col-0 and cau1 anac055, but lower proline content in anac055 than in Col-0 (Fig. 4B). The transcript levels of P5CS1 in Col-0 and cau1 also increased under drought stress with a dose-dependent effect, and to higher levels in cau1 than in Col-0 (Fig. 4C). In the absence of drought stress, the P5CS1 transcript levels were higher in cau1 and 35S:ANAC055 than in Col-0, but lower in anac055 and cau1 anac055 than in Col-0 (Fig. 4D). The proline level and P5CS1 transcript levels increased in plants in response to PEG-6000. Higher proline contents and P5CS1 transcript levels existed in cau1 and 35S:ANAC055 than in Col-0 and cau1 anac055, and lower proline content and P5CS1 transcript levels existed in anac055 than in Col-0 (Fig. 4B, D).
Fig. 4.
CAU1 regulates proline metabolism via P5CS1. (A, B) Proline levels in shoots. n=6 in (A), 3–6 in (B). (C, D) Transcript levels of P5CS1 in shoots as determined by RT-PCR. SAND (AT2G28390) was used as an internal control. Values are means ±SE, n=3. *P<0.05, **P<0.01, and ***P<0.001. Different letters above each bar indicate significant differences (ANOVA tests).
To identify the role of CAU1–ANAC055 in drought tolerance via the regulation of P5CS1, we tested whether proline could restore the sensitivity to osmotic stress imposed by PEG-6000 in anac055 plants. When treated with 10% (Fig. 5C, G) or 20% PEG (Fig. 5E, G) for 12 h, Col-0 and cau1 anac055 showed wilting symptoms and decreased RWC in a dose-dependent manner compared with their respective untreated controls (Fig. 5A, G) or with proline-treated plants (Fig. 5B, G). cau1 and 35S:ANAC055 plants showed higher RWC under 10% or 20% PEG treatment compared with those of Col-0 and cau1 anac055 (Fig. 5C, E, G). anac055 plants showed lower RWC under 10% or 20% PEG treatment compared with those of Col-0 and cau1 anac055 (Fig. 5C, E, G).
Fig. 5.
Phenotypic analysis of plants exposed to drought stress (PEG-6000) and proline. Hydroponically grown 4-week-old plants of Col-0, cau1, anac055, cau1 anac055, and 35S:ANAC055 are shown. (A) Control plants grown in 1/4 plant nutrient solution (PNS). (B) Plants treated with 50 mM proline for 12 h. (C) Plants treated with 10% PEG-6000 for 12 h. (D) Plants treated with 10% PEG-6000 and 50 mM proline for 12 h. (E) Plants treated with 20% PEG-6000 for 12 h. (F) Plants treated with 20% PEG-6000 and 50 mM proline for 12 h. (G) Relative water content from plants of Col-0, cau1, anac055, cau1 anac055, and 35S:ANAC055. Values are means ±SD from three independent experiments (n=6–7 per treatment). Different letters above each bar indicate significant differences (ANOVA tests) among the five samples in one treatment.
Proline restored the RWC of Col-0, cau1, anac055, cau1 anac055, and 35S:ANAC055 plants treated with 10% or 20% PEG (Fig. 5G). Plants showed mild wilting in a dose-dependent manner when treated with 10% PEG and proline and 20% PEG and proline (Fig. 5D, F, G) compared to those treated with 10% and 20% PEG without proline (Fig. 5C, E, G). Under the same treatments, cau1 anac055 showed similar phenotypes to those of Col-0, and 35S:ANAC055 showed similar phenotypes to those of cau1 (Fig. 5). These results indicated that CAU1–ANAC055 acts in drought tolerance by regulating the expression of P5CS1 and consequently the proline level.
The CAU1–ANAC055 pathway acts independently of the CAU1–CAS pathway in drought tolerance
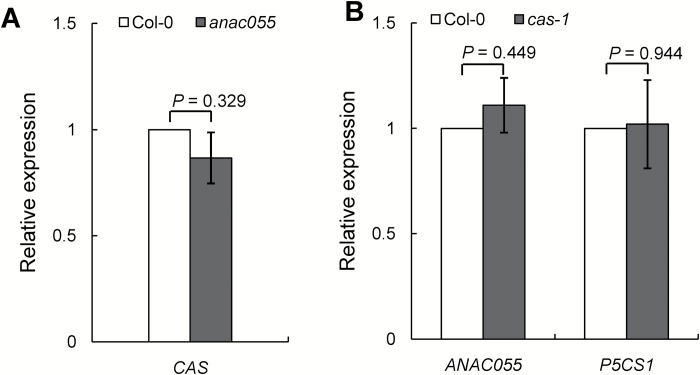
Previously, we showed that the CAU1–CAS pathway regulates stomatal closure (Fu et al., 2013). To investigate whether ANAC055 is involved in the CAU1–CAS pathway, we analysed the transcript levels of CAS in the anac055 mutant and the transcript levels of ANAC055 and P5CS1 in the cas-1 mutant. The transcript levels of CAS in the anac055 mutant (Fig. 6A) and of ANAC055 and P5CS1 in the cas-1 mutant (Fig. 6B) were similar to those in Col-0. These results suggested that CAU regulates ANAC055 independently of CAS in drought tolerance (Fig. 7).
Fig. 6.
Analyses of transcript levels of CAS in the anac055 mutant, and ANAC055 and P5CS1 in the cas-1 mutant. (A) RT-PCR amplification of CAS in anac055. (B) RT-PCR amplification of ANAC055 and P5CS1 in cas-1. SAND (AT2G28390) was used as an internal control. Values are means ±SE, n=3. Statistical significance was determined by t-tests.
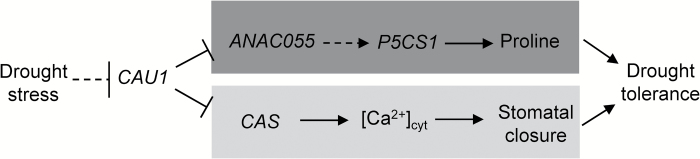
Fig. 7.
A model of drought tolerance mediated by CAU1–ANAC055 and proline. Drought stress reduces CAU1 mRNA and CAU1 protein levels, and decreases the binding of CAU1 to the promoter of ANAC055 (dark grey box). This decreases histone methylation of the ANAC055 chromatin, leading to increased ANAC055 expression. The subsequent increase in P5CS1 expression leads to increased proline levels and enhanced drought tolerance. The CAU1–ANAC055 pathway (dark grey box) may play a redundant role with the CAU1–CAS pathway (light grey box) to mediate plant adaptation to drought. Dashed lines represent possible unidentified steps, or steps that have been identified but are not shown here.
Discussion
Proline acts as an osmolyte and a signaling molecule to modulate responses to abiotic and biotic stresses (Szabados and Savouré, 2010), but the regulation of proline synthesis is not completely understood. In this study, CAU1, an H4R3sme2-type histone methylase, was shown to bind to the promoter region of ANAC055 and repress its expression. The decrease in CAU1 under drought stress led to higher expressions of ANAC055 and its genetically downstream gene P5CS1.
CAU1 regulates expressions of ANAC055 and P5CS1 in response to drought stress
In plants, proline accumulates under abiotic stress (Saradhi et al., 1995; Yoshiba et al., 1995; Schat et al., 1997; Choudhary et al., 2005; Yang et al., 2009) and biotic stress (Fabro et al., 2004; Haudecoeur et al., 2009). Proline synthetase (P5CS1) is regulated by calcium signals, H2O2-derived signals, and an ABA-dependent pathway (Yoshiba et al., 1995; Savouré et al., 1997; Strizhov et al., 1997; Parre et al., 2007; Verslues et al., 2007). The known regulators in plants include CaM4-MYB2 (Yoo et al., 2005), PLC (Knight et al., 1997; Parre et al., 2007), PLD (Thiery et al., 2004; Ghars et al., 2008), ABI (Strizhov et al., 1997), LOS5/ABA3 (Xiong et al., 2001), LcMYB1 (Cheng et al., 2013), SpERD15 (Ziaf et al., 2011), GmbZIP132 (Liao et al., 2008), GsZFP1 (Luo et al., 2012), and TaABC1 (Wang et al., 2011).
Our results show that CAU1 represses the expression of ANAC055; thus, the decrease in CAU1 under drought stress leads to higher transcript levels of ANAC055 and the genetically downstream P5CS1, resulting in increased proline synthesis. However, the complete NACRS (NAC recognition sequence, TCNNNNNNNACACGCATGT) was not determined in the P5CS1 region (Tran et al., 2004). P5CS1 might act genetically downstream of ANAC055 while not being directly bound in the upstream region with the ANAC055 protein.
CAU1–ANAC055 affects the gene expression involved in proline metabolism. The expression of P5CR, involved in the biosynthesis of proline, was higher in cau1 and 35S:ANAC055 than in Col-0 and cau1 anac055, and lower in anac055 than in Col-0 (see Fig. S2 at Dryad). In terms of the higher level of P5CS1, GSA/P5C might be the major factor that enhanced the level of P5CR in cau1 and 35S:ANAC055. The expression of PDH1 and P5CDH, involved in the catabolism of proline, tended to be lower in cau1 and 35S:ANAC055 than in Col-0 and cau1 anac055, and higher in anac055 than in Col-0 (see Fig. S2 at Dryad). These observations might be the result of changed levels of proline in the mutants. The results support a model wherein CAU1 functions upstream of a P5CS1–proline cascade (Fig. 7).
Proline functions as an osmolyte and also as a signaling molecule to regulate metabolite pools and the redox balance, to control gene expression, and ultimately to control drought tolerance (Szabados and Savouré, 2010). The transcript levels of P5CS1 were enhanced in the cau1 mutant and suppressed in the anac055 and cau1 anac055 mutants (Fig. 4D). The proline level in cau1 anac055 was partly restored to a level similar to that in Col-0, but the level in the anac055 mutant was very low (Fig. 4B). These results indicate that there must be other components besides P5CS1 that function in CAU1-mediated proline synthesis.
A previous study showed that the transcript levels of three NAC transcription factors, ANAC055, ANAC019, and ANAC072, were increased under drought stress, and transgenic plants overexpressing these factors showed increased drought tolerance (Tran et al., 2004). In the present study, however, the transcript level of ANAC055 was up-regulated in cau1 while the transcript levels of ANAC019 and ANAC072 were similar to those in the wild-type (Fig. 1C). This result indicated that ANAC055, but not ANAC019 and ANAC072, is suppressed by CAU1 in drought tolerance. ANAC019 and ANAC072 may have redundant functions with ANAC055, and may be controlled by different regulators.
CAU1– ANAC055 plays a major role in drought response redundantly with CAU1–CAS
CAU1 may function as a signal junction. Previous studies have shown that CAU1 suppresses FLC to mediate flowering time (Pei et al., 2007; Wang et al., 2007; Schmitz et al., 2008; Deng et al., 2010), LSM4 to mediate salt tolerance (Zhang et al., 2011), bHLH to mediate iron homeostasis (Fan et al., 2014), CAS to mediate the [Ca2+]o signal (Fu et al., 2013), CRN to maintain the shoot apical meristem (Yue et al., 2013), PRR7/PRR9/GI to mediate circadian rhythms (Deng et al., 2016; Li et al., 2016), PRP8 to mediate diverse developmental processes (Deng et al., 2016), and SHR to maintain root stem cells after DNA damage (Li et al., 2016). These results indicate that CAU1 may serve as a signal junction to regulate different downstream genes in diverse biological and developmental processes.
Our results show that CAU1 acts in response to drought, and regulates two genetically downstream pathways to modulate tolerance to drought. One downstream pathway is CAS-[Ca2+]cyt, which might involve ROS or IP3 signals (Fu et al., 2013). The other downstream pathway is ANAC055–P5CS1, which leads to proline accumulation (Fig. 7). CAU1–ANAC055 may function redundantly with CAU1–CAS in drought tolerance. The expression of CAS was unaffected in the anac055 mutant (Fig. 6A), and the expressions of ANAC055 and P5CS1 were unaffected in the cas-1 mutant (Fig. 6B). The stomatal closure and water loss phenotypes in cau1 were partially restored in the cas-1 mutant (Fu et al., 2013) and restored in the anac055 mutant (Fig. 3). These results indicate that ANAC055 and CAS are independent genetically downstream genes of CAU1, and that CAU1–ANAC055 plays a major role in response to drought stress.
CAU1 suppresses ANAC055 expression via histone modification
CAU1/SKB1/AtPRMT5 is an arginine methyltransferase 5 that regulates target genes by histone methylation or pre-mRNA splicing (Pei et al., 2007; Wang et al., 2007; Zhang et al., 2011; Fu et al., 2013; Yue et al., 2013; Fan et al., 2014; Deng et al., 2016; Li et al., 2016). The results of this study show that CAU1 suppresses the expression of ANAC055 by histone methylation. The transcript levels of ANAC055 and P5CS1 were significantly increased in cau1 (Figs 1C and 4D). Under PEG treatments, CAU1 mRNA and CAU1 protein levels significantly decreased (Fig. 1A, B), and the histone methylation level decreased in ANAC055 chromatin (Fig. 2B, D). Alternatively, spliced transcripts of ANAC055 and P5CS1 were not detected in cau1 (see Fig. S3 at Dryad).
In summary, these functional analyses reveal that CAU1 serves as an epigenetic suppressor of ANAC055, which regulates the expression of P5CS1, and hence proline accumulation in response to drought stress. The results also show that the CAU1–ANAC055 pathway is redundant with the CAU1–CAS pathway.
Data deposition
The following figures and table are available at the Dryad Data Repository: https://doi.org/10.5061/dryad.hc4bj
Fig. S1. Isolation of T-DNA insertion lines for anac055.
Fig. S2. Transcript levels of P5CR, PDH1 and P5CDH.
Fig. S3. Analysis of alternative splicing of ANAC055 and P5CS1.
Table S1. List of primer sequences.
Acknowledgements
We thank Lin Xu (Shanghai Institutes for Biological Sciences, CAS) for helpful discussions and Shanghai OE Biotech. Co. Ltd for conducting the ChIP assay. This work was supported by the National Science Foundation of China (31371227), and the Sanofi-Aventis-Shanghai Institutes for Biological Sciences Scholarship Program to Yanlei Fu. We thank Jennifer Smith, PhD, from LiwenBianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of this manuscript.
Glossary
Abbreviations:
- ChIP-qPCR
chromatin immunoprecipitation quantitative PCR
- H3K4me3
trimethylates histone H3 at lysine 4
- P5CDH
P5C dehydrogenase
- P5CR
P5C reductase
- P5CS
Δ1-pyrroline-5-carboxylate synthetase
- PDH
proline dehydrogenase
- PLC
phospholipase C
- PLD
phospholipase D
- ROS
reactive oxygen species.
References
- Aggarwal P, Vaites LP, Kim JK, et al. . 2010. Nuclear cyclin D1/CDK4 kinase regulates CUL4 expression and triggers neoplastic growth via activation of the PRMT5 methyltransferase. Cancer Cell 18, 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves M, Chicau P, Matias H, Passarinho J, Pinheiro C, Ricardo CP. 2011. Metabolic analysis revealed altered amino acid profiles in Lupinus albus organs as a result of boron deficiency. Physiologia Plantarum 142, 224–232. [DOI] [PubMed] [Google Scholar]
- Arteca RN, Arteca JM. 2000. A novel method for growing Arabidopsis thaliana plants hydroponically. Physiologia Plantarum 108, 188–193. [Google Scholar]
- Ascenzi R, Gantt JS. 1999. Subnuclear distribution of the entire complement of linker histone variants in Arabidopsis thaliana. Chromosoma 108, 345–355. [DOI] [PubMed] [Google Scholar]
- Bates L, Waldren R, Teare I. 1973. Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. [Google Scholar]
- Cheng L, Li X, Huang X, et al. . 2013. Overexpression of sheepgrass R1-MYB transcription factor LcMYB1 confers salt tolerance in transgenic Arabidopsis. Plant Physiology and Biochemistry 70, 252–260. [DOI] [PubMed] [Google Scholar]
- Choudhary NL, Sairam RK, Tyagi A. 2005. Expression of Δ1-pyrroline-5-carboxylate synthetase gene during drought in rice (Oryza sativa L.). Indian Journal of Biochemistry & Biophysics 42, 366–370. [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Deng X, Gu L, Liu C, et al. . 2010. Arginine methylation mediated by the Arabidopsis homolog of PRMT5 is essential for proper pre-mRNA splicing. Proceedings of the National Academy of Sciences, USA 107, 19114–19119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Lu T, Wang L, Gu L, Sun J, Kong X, Liu C, Cao X. 2016. Recruitment of the NineTeen Complex to the activated spliceosome requires AtPRMT5. Proceedings of the National Academy of Sciences, USA 113, 5447–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle K, Funck D, Hellmann H, Däschner K, Binder S, Frommer WB. 2001. A nuclear gene encoding mitochondrial Δ1-pyrroline-5-carboxylate dehydrogenase and its potential role in protection from proline toxicity. The Plant Journal 27, 345–356. [DOI] [PubMed] [Google Scholar]
- Ding Y, Avramova Z, Fromm M. 2011. The Arabidopsis trithorax-like factor ATX1 functions in dehydration stress responses via ABA-dependent and ABA-independent pathways. The Plant Journal 66, 735–744. [DOI] [PubMed] [Google Scholar]
- Ding Y, Lapko H, Ndamukong I, et al. . 2009. The Arabidopsis chromatin modifier ATX1, the myotubularin-like AtMTM and the response to drought. Plant Signaling & Behavior 4, 1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabro G, Kovács I, Pavet V, Szabados L, Alvarez ME. 2004. Proline accumulation and AtP5CS2 gene activation are induced by plant–pathogen incompatible interactions in Arabidopsis. Molecular Plant-Microbe Interactions 17, 343–350. [DOI] [PubMed] [Google Scholar]
- Fan H, Zhang Z, Wang N, et al. . 2014. SKB1/PRMT5-mediated histone H4R3 dimethylation of Ib subgroup bHLH genes negatively regulates iron homeostasis in Arabidopsis thaliana. The Plant Journal 77, 209–221. [DOI] [PubMed] [Google Scholar]
- Fang H, Liu X, Thorn G, Duan J, Tian L. 2014. Expression analysis of histone acetyltransferases in rice under drought stress. Biochemical and Biophysical Research Communications 443, 400–405. [DOI] [PubMed] [Google Scholar]
- Fu YL, Zhang GB, Lv XF, Guan Y, Yi HY, Gong JM. 2013. Arabidopsis histone methylase CAU1/PRMT5/SKB1 acts as an epigenetic suppressor of the calcium signaling gene CAS to mediate stomatal closure in response to extracellular calcium. The Plant Cell 25, 2878–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Ma H, Chen S, Gu T, Gong J. 2017. Data from: Control of proline accumulation under drought via a novel pathway comprising the histone methylase CAU1 and the transcription factor ANAC055. Dryad Data Repository. https://doi.org/10.5061/dryad.hc4bj [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghars MA, Parre E, Leprince AS, Bordenave M, Vos L, Richard L, Abdelly C, Savouré A. 2008. Opposite lipid signalling pathways tightly control proline accumulation in Arabidopsis thaliana and Thellungiella halophila. Biosaline Agriculture and High Salinity Tolerance 317–324. [Google Scholar]
- Gong JM, Lee DA, Schroeder JI. 2003. Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proceedings of the National Academy of Sciences, USA 100, 10118–10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton EW III, Heckathorn SA. 2001. Mitochondrial adaptations to NaCl. Complex I is protected by anti-oxidants and small heat shock proteins, whereas complex II is protected by proline and betaine. Plant Physiology 126, 1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tang R, Anderson LK, Woerner TE, Pei ZM. 2003. A cell surface receptor mediates extracellular Ca2+ sensing in guard cells. Nature 425, 196–200. [DOI] [PubMed] [Google Scholar]
- Haudecoeur E, Planamente S, Cirou A, Tannieres M, Shelp BJ, Morera S, Faure D. 2009. Proline antagonizes GABA-induced quenching of quorum-sensing in Agrobacterium tumefaciens. Proceedings of the National Academy of Sciences, USA 106, 14587–14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavi Kishor PB, Sangam S, Amrutha RN, et al. . 2005. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Current Science 88, 424–438. [Google Scholar]
- Kim JM, To TK, Ishida J, Matsui A, Kimura H, Seki M. 2012. Transition of chromatin status during the process of recovery from drought stress in Arabidopsis thaliana. Plant & Cell Physiology 53, 847–856. [DOI] [PubMed] [Google Scholar]
- Kim JM, To TK, Ishida J, et al. . 2008. Alterations of lysine modifications on the histone H3 N-tail under drought stress conditions in Arabidopsis thaliana. Plant & Cell Physiology 49, 1580–1588. [DOI] [PubMed] [Google Scholar]
- Kiyosue T, Yoshiba Y, Yamaguchi-Shinozaki K, Shinozaki K. 1996. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. The Plant Cell 8, 1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. 1997. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. The Plant Journal 12, 1067–1078. [DOI] [PubMed] [Google Scholar]
- Krysan PJ, Young JC, Sussman MR. 1999. T-DNA as an insertional mutagen in Arabidopsis. The Plant Cell 11, 2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhao Y, Yue M, Xue Y, Bao S. 2016. The Protein Arginine Methylase 5 (PRMT5/SKB1) gene is required for the maintenance of root stem cells in response to DNA damage. Journal of Genetics and Genomics 43, 187–197. [DOI] [PubMed] [Google Scholar]
- Liao Y, Zhang JS, Chen SY, Zhang WK. 2008. Role of soybean GmbZIP132 under abscisic acid and salt stresses. Journal of Integrative Plant Biology 50, 221–230. [DOI] [PubMed] [Google Scholar]
- Luo X, Bai X, Zhu D, Li Y, Ji W, Cai H, Wu J, Liu B, Zhu Y. 2012. GsZFP1, a new Cys2/His2-type zinc-finger protein, is a positive regulator of plant tolerance to cold and drought stress. Planta 235, 1141–1155. [DOI] [PubMed] [Google Scholar]
- Mathieu O, Probst AV, Paszkowski J. 2005. Distinct regulation of histone H3 methylation at lysines 27 and 9 by CpG methylation in Arabidopsis. The EMBO Journal 24, 2783–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Honig A, Stein H, Suzuki N, Mittler R, Zilberstein A. 2009. Unraveling Δ1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes. The Journal of Biological Chemistry 284, 26482–26492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaefthimiou D, Tsaftaris AS. 2012. Significant induction by drought of HvPKDM7-1, a gene encoding a jumonji-like histone demethylase homologue in barley (H. vulgare). Acta Physiologiae Plantarum 34, 1187–1198. [Google Scholar]
- Parre E, Ghars MA, Leprince AS, et al. . 2007. Calcium signaling via phospholipase C is essential for proline accumulation upon ionic but not nonionic hyperosmotic stresses in Arabidopsis. Plant Physiology 144, 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Niu L, Lu F, Liu C, Zhai J, Kong X, Cao X. 2007. Mutations in the Type II protein arginine methyltransferase AtPRMT5 result in pleiotropic developmental defects in Arabidopsis. Plant Physiology 144, 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schroeder JI. 1998. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282, 287–290. [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. 2000. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Sani E, Herzyk P, Perrella G, Colot V, Amtmann A. 2013. Hyperosmotic priming of Arabidopsis seedlings establishes a long-term somatic memory accompanied by specific changes of the epigenome. Genome Biology 14, R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saradhi PP, Alia, Arora S, Prasad KV. 1995. Proline accumulates in plants exposed to UV radiation and protects them against UV induced peroxidation. Biochemical and Biophysical Research Communications 209, 1–5. [DOI] [PubMed] [Google Scholar]
- Savouré A, Hua XJ, Bertauche N, Van Montagu M, Verbruggen N. 1997. Abscisic acid-independent and abscisic acid-dependent regulation of proline biosynthesis following cold and osmotic stresses in Arabidopsis thaliana. Molecular & General Genetics 254, 104–109. [DOI] [PubMed] [Google Scholar]
- Savouré A, Jaoua S, Hua XJ, Ardiles W, Van Montagu M, Verbruggen N. 1995. Isolation, characterization, and chromosomal location of a gene encoding the Δ 1-pyrroline-5-carboxylate synthetase in Arabidopsis thaliana. FEBS Letters 372, 13–19. [DOI] [PubMed] [Google Scholar]
- Schat H, Sharma SS, Vooijs R. 1997. Heavy metal-induced accumulation of free proline in a metal-tolerant and a nontolerant ecotype of Silene vulgaris. Physiologia Plantarum 101, 477–482. [Google Scholar]
- Schmitz RJ, Sung S, Amasino RM. 2008. Histone arginine methylation is required for vernalization-induced epigenetic silencing of FLC in winter-annual Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 105, 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol A, Kwiatkowska A, Jerzmanowski A, Prymakowska-Bosak M. 2007. Up-regulation of stress-inducible genes in tobacco and Arabidopsis cells in response to abiotic stresses and ABA treatment correlates with dynamic changes in histone H3 and H4 modifications. Planta 227, 245–254. [DOI] [PubMed] [Google Scholar]
- Sridha S, Wu K. 2006. Identification of AtHD2C as a novel regulator of abscisic acid responses in Arabidopsis. The Plant Journal 46, 124–133. [DOI] [PubMed] [Google Scholar]
- Strizhov N, Abrahám E, Okrész L, Blickling S, Zilberstein A, Schell J, Koncz C, Szabados L. 1997. Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. The Plant Journal 12, 557–569. [DOI] [PubMed] [Google Scholar]
- Szabados L, Savouré A. 2010. Proline: a multifunctional amino acid. Trends in Plant Science 15, 89–97. [DOI] [PubMed] [Google Scholar]
- Székely G, Abrahám E, Cséplo A, et al. . 2008. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. The Plant Journal 53, 11–28. [DOI] [PubMed] [Google Scholar]
- Szoke A, Miao GH, Hong Z, Verma DP. 1992. Subcellular location of Δ1-pyrroline-5-carboxylate reductase in root/nodule and leaf of soybean. Plant Physiology 99, 1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery L, Leprince AS, Lefebvre D, Ghars MA, Debarbieux E, Savouré A. 2004. Phospholipase D is a negative regulator of proline biosynthesis in Arabidopsis thaliana. The Journal of Biological Chemistry 279, 14812–14818. [DOI] [PubMed] [Google Scholar]
- Tran LS, Nakashima K, Sakuma Y, et al. . 2004. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. The Plant Cell 16, 2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk K, Ding Y, Malkaram S, et al. . 2010. Dynamic changes in genome-wide histone H3 lysine 4 methylation patterns in response to dehydration stress in Arabidopsis thaliana. BMC Plant Biology 10, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian N, Marcotte L, Giraudat J. 1994. Drought rhizogenesis in Arabidopsis thaliana (differential responses of hormonal mutants). Plant Physiology 104, 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen N, Hua XJ, May M, Van Montagu M. 1996. Environmental and developmental signals modulate proline homeostasis: evidence for a negative transcriptional regulator. Proceedings of the National Academy of Sciences, USA 93, 8787–8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen N, Villarroel R, Van Montagu M. 1993. Osmoregulation of a pyrroline-5-carboxylate reductase gene in Arabidopsis thaliana. Plant Physiology 103, 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE, Kim YS, Zhu JK. 2007. Altered ABA, proline and hydrogen peroxide in an Arabidopsis glutamate:glyoxylate aminotransferase mutant. Plant Molecular Biology 64, 205–217. [DOI] [PubMed] [Google Scholar]
- Wang C, Jing R, Mao X, Chang X, Li A. 2011. TaABC1, a member of the activity of bc1 complex protein kinase family from common wheat, confers enhanced tolerance to abiotic stresses in Arabidopsis. Journal of Experimental Botany 62, 1299–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang Y, Ma Q, Zhang Z, Xue Y, Bao S, Chong K. 2007. SKB1-mediated symmetric dimethylation of histone H4R3 controls flowering time in Arabidopsis. The EMBO Journal 26, 1934–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinl S, Held K, Schlücking K, Steinhorst L, Kuhlgert S, Hippler M, Kudla J. 2008. A plastid protein crucial for Ca2+-regulated stomatal responses. New Phytologist 179, 675–686. [DOI] [PubMed] [Google Scholar]
- Xiong L, Ishitani M, Lee H, Zhu JK. 2001. The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. The Plant Cell 13, 2063–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SL, Lan SS, Gong M. 2009. Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. Journal of Plant Physiology 166, 1694–1699. [DOI] [PubMed] [Google Scholar]
- Yoo JH, Park CY, Kim JC, et al. . 2005. Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in arabidopsis. The Journal of Biological Chemistry 280, 3697–3706. [DOI] [PubMed] [Google Scholar]
- Yoshiba Y, Kiyosue T, Katagiri T, Ueda H, Mizoguchi T, Yamaguchi-Shinozaki K, Wada K, Harada Y, Shinozaki K. 1995. Correlation between the induction of a gene for Δ1-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. The Plant Journal 7, 751–760. [DOI] [PubMed] [Google Scholar]
- Yue M, Li Q, Zhang Y, Zhao Y, Zhang Z, Bao S. 2013. Histone H4R3 methylation catalyzed by SKB1/PRMT5 is required for maintaining shoot apical meristem. PLoS ONE 8, e83258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang S, Zhang Y, et al. . 2011. Arabidopsis floral initiator SKB1 confers high salt tolerance by regulating transcription and pre-mRNA splicing through altering histone H4R3 and small nuclear ribonucleoprotein LSM4 methylation. The Plant Cell 23, 396–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Jeong JC, Zhu Y, et al. . 2008. Involvement of Arabidopsis HOS15 in histone deacetylation and cold tolerance. Proceedings of the National Academy of Sciences, USA 105, 4945–4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaf K, Loukehaich R, Gong P, Liu H, Han Q, Wang T, Li H, Ye Z. 2011. A multiple stress-responsive gene ERD15 from Solanum pennellii confers stress tolerance in tobacco. Plant & Cell Physiology 52, 1055–1067. [DOI] [PubMed] [Google Scholar]
- Zong W, Zhong X, You J, Xiong L. 2013. Genome-wide profiling of histone H3K4-tri-methylation and gene expression in rice under drought stress. Plant Molecular Biology 81, 175–188. [DOI] [PubMed] [Google Scholar]