Mathematical modeling suggests that rapid diagnostics that report antibiotic susceptibility have the potential to extend the usefulness of existing antibiotics for treatment of gonorrhea compared with the current guidelines for empiric 2-drug treatment.
Keywords: antibiotic resistance, gonorrhea, mathematical model, point-of-care test
Abstract
Background
Increasing antibiotic resistance limits treatment options for gonorrhea. We examined the impact of a hypothetical point-of-care (POC) test reporting antibiotic susceptibility profiles on slowing resistance spread.
Methods
A mathematical model describing gonorrhea transmission incorporated resistance emergence probabilities and fitness costs associated with resistance based on characteristics of ciprofloxacin (A), azithromycin (B), and ceftriaxone (C). We evaluated time to 1% and 5% prevalence of resistant strains among all isolates with the following: (1) empiric treatment (B and C), and treatment guided by POC tests determining susceptibility to (2) A only and (3) all 3 antibiotics.
Results
Continued empiric treatment without POC testing was projected to result in >5% of isolates being resistant to both B and C within 15 years. Use of either POC test in 10% of identified cases delayed this by 5 years. The 3 antibiotic POC test delayed the time to reach 1% prevalence of triply-resistant strains by 6 years, whereas the A-only test resulted in no delay. Results were less sensitive to assumptions about fitness costs and test characteristics with increasing test uptake.
Conclusions
Rapid diagnostics reporting antibiotic susceptibility may extend the usefulness of existing antibiotics for gonorrhea treatment, but ongoing monitoring of resistance patterns will be critical.
Increasing antibiotic resistance poses an immense challenge to the clinical and public health community [1] and underscores the importance of developing new strategies to control resistance. Among the most urgent threats to our ability to treat infections is antibiotic-resistant gonorrhea. Treatment of gonorrhea is almost always empiric, because diagnosis is most commonly made by nucleic acid amplification test, which provides no susceptibility data. Even when culture is available, clinical and public health principles demand rapid treatment of patients without waiting for antibiotic susceptibility results.
Gonorrhea treatment guidelines are based on population resistance surveys, with antibiotics no longer recommended once resistance prevalence exceeds 5%. Only ceftriaxone and azithromycin remain as first-line therapy [2], and increasing resistance to both has been observed [1]. With empiric treatment strategies, a majority of gonococcal infections may remain susceptible to antibiotics no longer recommended for use. For example, based on data from 2014, 81% of gonococcal infections in the United States were susceptible to fluoroquinolones [3].
Given the prevalence of susceptible isolates, one proposed strategy to control resistance is the use of rapid diagnostics that allow clinicians to both diagnose gonorrhea infections and tailor treatment to the antibiotic susceptibilities of individual infections [4–7]. Sequence-based diagnostics for fluoroquinolone susceptibility report high positive and negative predictive values [8, 9] and are moving toward use in clinical care [10]. The test characteristics of sequence-based diagnostics for susceptibility to other antibiotics are emerging from the sequencing and analysis of large collections of clinical isolates [11, 12].
Underlying the promise of rapidly determining antibiotic susceptibility is the hypothesis that tailored therapy will prolong the utility of antigonococcal agents and better control resistance than empiric treatment. Previous mathematical modeling studies have considered the impact of treatment on resistance, including the role that rapid diagnostics may have for curbing resistance spread and improving timeliness of resistance detection [13–18]. As we improve our understanding of the properties associated with resistance to different antigonococcal agents [19], we can incorporate this knowledge into our models, enabling the evaluation of the potential effectiveness of POC tests under more realistic and nuanced conditions. Here, we used a mathematical model of gonorrhea transmission to test the potential impact of tailored therapy on the prevalence of gonococcal infection and antibiotic resistance to commonly used drugs.
METHODS
Model Overview
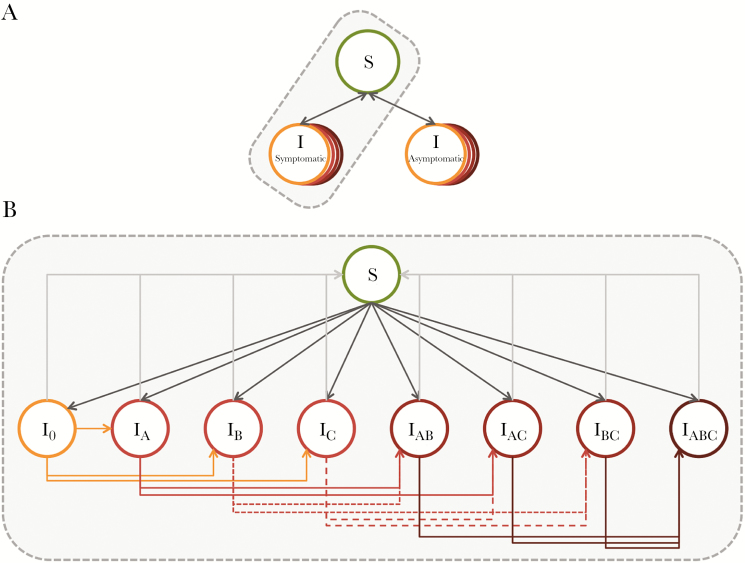
We developed a dynamic compartmental model that describes gonorrhea transmission in a single sex population stratified by sexual risk. This model represented a population of men who have sex with men (MSM), who experience a significant burden of gonorrhea in the United States and in whom emergence of resistance is of concern [3, 20]. The model population was divided into 3 groups based on levels of sexual activity (low, intermediate, and high), characterized by rates of partner change, as informed by data from the National HIV Behavioral Surveillance System [21]. We assumed that individuals remained in their assigned risk group for the duration of their time in the model. We used the approach of Garnett and Anderson [22] to describe mixing within and between risk groups. The natural history of gonorrhea infection was described by the following states: susceptible, symptomatic infection, and asymptomatic infection (Figure 1). Each of the infectious states was further subdivided to represent the resistance profile of the infecting strain. Model parameters are presented in Table 1, and additional model details are provided in the Technical Appendix.
Figure 1.
Overview of gonorrhea transmission model. (A) The model includes 3 states: susceptible, symptomatic infectious, and asymptomatic infectious. Infected individuals can return to the susceptible state via treatment or natural clearance of infection. (B) Expanded view of the different possible infected states, where subscripts indicate resistance to antibiotics A, B, and/or C. I0 indicates infection with a completely drug susceptible strain. Note that the same series of infectious states and transitions exist for symptomatic and asymptomatic infections. The model is further stratified by 3 sexual activity classes.
Table 1.
Model Population, Gonorrhea Natural History, and Treatment Parameters
| Parameter | Details | Symbol | Value | Source |
|---|---|---|---|---|
| Population size | - | N | 106 | Assumption |
| Gonorrhea prevalence at start (%) | Calibration target | 2.3 (1.2–2.8) | [15, 30, 31] | |
| Proportion of cases resistant to drug X at start of evaluation period | - | θ | - | [3] |
| A (ciprofloxacin) | θA | 0.189 | ||
| B (azithromycin) | θB | 0.023 | ||
| C (ceftriaxone) | θC | 0.0001 | ||
| A and B | θAB | 0.0022 | ||
| A and C | θAC | 0.0009 | ||
| B and C | θBC | 1/105 | Assumption | |
| A, B, and C | θABC | 1/106 | Assumption | |
| Sexual risk group distribution | - | n | - | Assumption |
| High | 0.1 | |||
| Intermediate | 0.6 | |||
| Low | 0.3 | |||
| Relative rate of partner change in risk groups | rp | [21] | ||
| High | 20 | |||
| Intermediate | 5 | |||
| Low | 1 | |||
| Rate of partner change in low risk group (per year) | - | cmin | 1.16 | Model fitting |
| Mixing parameter | - | ε | 0.23 | Model fitting |
| Rate of model entry/exit (years) | - | ρ | 1/20 | Assumption |
| Transmission probability per partnership | - | β | 0.44 | [32–38]; model fitting |
| Probability symptomatic infection | - | σ | 0.6 | Assumption; model fitting |
| Average duration of infection without treatment (years) | - | 1/δ | 0.5 | [33, 36, 38, 39]; model fitting |
| Average time to treatment (years) | Symptomatic | 1/τs | 0.026 | [40]; model fitting |
| Screening rate (per year) | Asymptomatic | 1/τm | 0.39 (0.20–1.56) | Assumption; model fitting |
| Probability of retreatment with effective drug, if initial treatment failure | - | - | - | Assumption |
| Symptomatic infection | πs | 0.9 | ||
| Asymptomatic infection | πm | 0 | ||
| Treatment rate if initial treatment failure (per year) | - | - | - | Assumption |
| Symptomatic infection | τsr | τs/3 | ||
| Asymptomatic infection | τmr | τm/3 |
Treatment
We modeled treatment with 3 different antibiotics, which could be used individually or in combination. Each antibiotic had a probability of resistance emergence on treatment and a fitness cost associated with resistance, where fitness refers to the capability of the pathogen to survive [23]. We modeled fitness cost in terms of transmissibility relative to the susceptible strain [24]. The properties of each of the antibiotics were selected to mirror the classes of antibiotics used to treat gonorrhea infection: fluoroquinolones (A), macrolides (B), and extended-spectrum cephalosporins (C) (Table 2) [19]. Details of the characterization of these properties are provided in the Technical Appendix. Given that these properties are not known with certainty, we opted to use the labels “A”, “B”, and “C” to emphasize that we were not modeling specific antibiotics, but rather those with properties similar to existing antibiotics. In the absence of a point-of-care (POC) test to determine strain susceptibility, antibiotic choice reflected US treatment guidelines of combination therapy with azithromycin and ceftriaxone (B and C in our model) [2].
Table 2.
Characteristics Associated With Point-of-Care Test and Drug-Resistant Neisseria gonorrhoeae Strains
| Parameter | Details | Symbol | Value (Base Case and Range) |
|---|---|---|---|
| Probability of resistance with treatment | |||
| Antibiotic A | ωA | 10–6 (10–9 to 10–3) | |
| Antibiotic B | ωB | 5 × 10–7 (10–9 to 10–3) | |
| Antibiotic C | ωC | 10–8 (10–9 to 10–3) | |
| Relative fitness of resistant strain | |||
| Susceptible | f0 | 1 | |
| Antibiotic A resistant | fA | 1 (0.85–1) | |
| Antibiotic B resistant | fB | 0.94 (0.85–1) | |
| Antibiotic C resistant | fC | 0.98 (0.85–1) | |
| Antibiotics AB resistant | fAB | 0.94 (0.72–1) | |
| Antibiotics AC resistant | fAC | 0.98 (0.72–1) | |
| Antibiotics BC resistant | fBC | 0.92 (0.72–1) | |
| Antibiotics ABC resistant | fABC | 0.92 (0.61–1) | |
| Point-of-care test sensitivity | |||
| Antibiotic A | κA | 1 (0.5–1) | |
| Antibiotic B | κB | 1 (0.5–1) | |
| Antibiotic C | κC | 1 (0.5–1) | |
| Point-of-care test specificity | |||
| Antibiotic A | ψA | 1 (0.5–1) | |
| Antibiotic B | ψB | 1 (0.5–1) | |
| Antibiotic C | ψC | 1 (0.5–1) |
Deployment of a Rapid Diagnostic to Determine Susceptibility
We compared empirical treatment to treatment guided by a hypothetical POC test that rapidly diagnoses gonorrhea infections and determines susceptibility to the following: (1) a single antibiotic (A) and (2) all 3 antibiotics. Based on a case’s resistance profile, antibiotic treatment was selected (Supplementary Table S1), with the probability that the chosen antibiotic effectively treated the infection dependent on the test characteristics.
Scenario I. Point-of-Care Test for Determining Resistance to Antibiotic A Only
Antibiotic A was used to treat A-susceptible infections, while A-resistant infections were treated with combination BC therapy.
Scenario II. Point-of-Care Test for Determining Resistance to Antibiotics A, B, and C
If multiple antibiotics would be effective at treating a case (ie, infected with a completely susceptible strain or a strain resistant to only one antibiotic), we treated with the antibiotic with the highest fitness cost associated with resistance. In scenario II, if an individual was identified as having a triply-resistant infection, we assumed that the infection was ultimately successfully treated, with an alternative agent or higher antibiotic doses [25–27].
We evaluated different levels of POC test uptake in the population. When susceptibility was not determined before treatment, we assumed treatment according to guidelines, as described above.
Model Fitting
We calibrated model parameters describing gonorrhea natural history and sexual behavior using maximum likelihood estimation. Details are provided in the Technical Appendix.
Model Outputs and Analysis
The model was initiated at the equilibrium prevalence determined through model fitting in the absence of resistant strains. The initial distribution of resistant isolates was based on surveillance data [3]. The major outcomes of interest were prevalence once equilibrium had been re-established and time to reach particular resistance thresholds in the population. We focused our threshold analyses on strains resistant to antibiotics B and C, but not A, and on strains resistant to all 3 antibiotics, because these are of clinical and public health importance. Time to reach 1% and 5% thresholds was calculated as time from model initiation to time at which strains displaying the resistance profile of interest represented n% or more of prevalent strains. The baseline comparator for all analyses was combination treatment of identified cases with antibiotics B and C. Simulations were run for 40 years, the point at which the system without a POC test had re-established equilibrium.
Sensitivity Analyses
We conducted sensitivity analyses for parameters describing the properties of the different resistant strains, test characteristics, test coverage, and frequency of asymptomatic screening (ranging from every 3 months to every 2 years). We varied the fitness cost associated with resistance to antibiotic B (the antibiotic associated with the highest fitness cost for resistance) from 0% to 15%. We varied the “relative” fitness cost for antibiotics A and C from 0 to 1, and we calculated the fitness cost for antibiotics A or C as follows: fitness cost for antibiotic B × relative fitness cost for antibiotic A or C. In addition, we allowed the probability of de novo resistance acquisition to range from 10–3 to 10–9 per treatment event with each antibiotic.
For simplicity, we assumed that the POC test detected resistance with perfect sensitivity and specificity in the main analysis. We then varied test sensitivity and specificity to reflect that a test with perfect sensitivity and specificity is unlikely to be achieved in practice and that deoxyribonucleic acid-based tests may miss unrecognized mechanisms of resistance [19]. We assumed that the test properties for detecting resistance to each antibiotic were independent.
For the single resistance test, we assessed the impact of a fitness cost for antibiotic A resistance. For the 3-resistance POC test, we evaluated the alternate antibiotic selection strategy of treating with the antibiotic with the greatest barrier to resistance emergence (lowest probability of resistance emergence) when multiple treatment options were available.
RESULTS
Expected Time Course of Resistance Spread Without a Point-of-Care Test
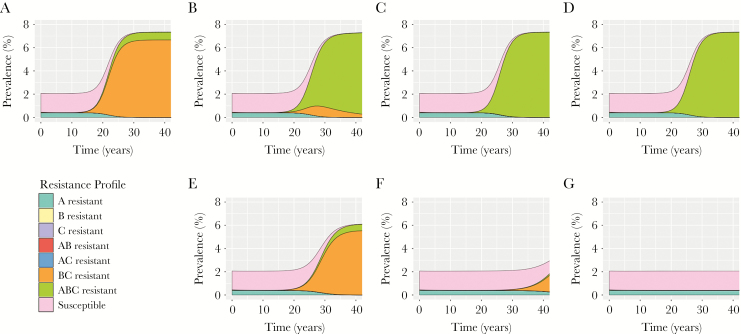
Under our baseline assumptions regarding fitness costs and probabilities of de novo resistance emergence, as well as current patterns of resistance in the US population, the continued use of dual antibiotic treatment in the population was projected to result in >1% of isolates being resistant to both of these antibiotics within 12 years (Figure 2). With continued use of dual therapy in the population, this threshold surpassed 5% after an additional 3 years (year 15). Strains resistant to all 3 antibiotics took longer to become established, comprising >1% of all cases within 16 years. As resistant strains comprised a greater proportion of circulating strains and resulted in more treatment failures, the average time to successful treatment increased. Consequently, equilibrium prevalence was projected to increase approximately 3.5-fold in the modeled population, increasing from 2.1% at model start to 7.3% once the system re-equilibrated.
Figure 2.
Projected impact of point-of-care (POC) tests on gonorrhea prevalence and resistance. Population prevalence and prevalence of different strains are shown in the face of (A) no POC testing, (B and E) 10%, (C and F) 25%, and (D and G) 50% of cases tested. B–D show the results for a POC test for resistance to antibiotic A only. E–G show results for a POC test for resistance to all 3 antibiotics. For the 3-resistance POC test, cases undergoing testing and displaying susceptibility to >1 antibiotic were treated with the antibiotic with the highest fitness cost associated with resistance acquisition. For both scenarios, all untested cases were treated in combination with antibiotics B and C. Results are shown for tests with perfect sensitivity and specificity.
Impact of a Point-of-Care Test for Resistance to a Single Antibiotic or Three Antibiotics
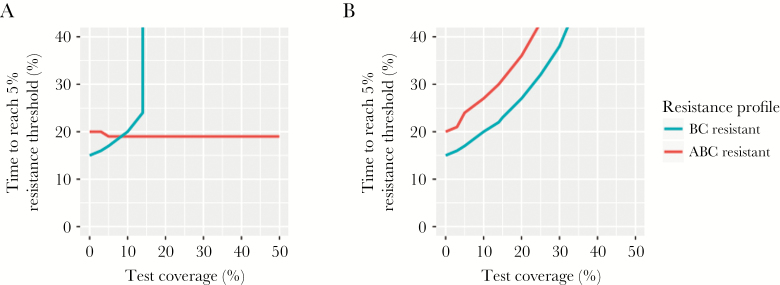
Under the baseline assumption of perfect test sensitivity and specificity, both POC test strategies had the identical probability that a BC-resistant infection would be effectively treated (supplementary figure S1). However, the single resistance POC test prompted a binary treatment decision based only on antibiotic A resistance status. Thus, all infections that tested resistant to A (including ABC-resistant infections) were assigned BC treatment, making the probability of treating a triple-resistant infection with an effective antibiotic zero, regardless of test coverage (Figure 3).
Figure 3.
Time to resistance emergence with varying use of point-of-care (POC) tests. Time for BC or ABC resistant strains to comprise 5% of prevalent gonorrhea isolates in the population with different POC test use in the population. Results are shown for POC tests that identify resistance to (A) antibiotic A only, or (B) all 3 antibiotics, with perfect sensitivity and specificity. Note that results are qualitatively similar for the 1% threshold, although times required to reach the threshold are reduced.
Unlike the base case, where BC resistance increased in the population, followed by ABC resistance, use of a single resistance POC delayed the spread of BC-resistant gonococcal isolates but not ABC-resistant isolates (Figure 2). Consequently, triple-resistant strains could cross the resistance thresholds without BC resistance serving as a forewarning. For example, when test use was >20%, BC-resistant isolates circulated in the population but did not reach 1% of prevalent infections. Despite the reduction in BC resistant isolate transmission, the test did not reduce overall gonorrhea prevalence at equilibrium, relative to model projections in the absence of such a test, and had no or detrimental impact on triple resistance. With use of the test in 5% or more of cases, the time for ABC-resistant strains to represent >5% of isolates was accelerated by 1 year, relative to no test use in the population.
By contrast, using a test that identifies resistance to all 3 antibiotics reduced overall equilibrium prevalence and delayed the spread of BC- and ABC-resistant strains in the population (Figure 2). With test use in >37% of identified cases, strains resistant to antibiotics B and C never reached 1% of prevalent cases within the 40-year time horizon. With higher test use, the rapid identification and treatment of BC and ABC-resistant isolates allowed for isolates resistant to antibiotic A to persist. This reflected the assumption of no fitness cost associated with A resistance.
Fitness Cost Associated With Resistance to Antibiotic A
Assuming a minor (1%) fitness cost for resistance to antibiotic A, triple-resistant isolates were not projected to reach the 5% threshold during the 40-year time horizon when combination therapy was used to treat all identified infections. By contrast, use of the single resistance POC test (scenario I) resulted in this threshold being crossed in approximately 20 years once the test was used in greater than 5% of identified cases (supplementary figure S2).
Resistant Strain Properties
In sensitivity analyses, when fitness costs were relatively high (>15% for strains resistant to antibiotic B), resistant strains were outcompeted by other strains and did not reach the resistance thresholds, even without a POC test to guide antibiotic choice. When fitness costs were minor, the impact of the POC tests was diminished, in terms of the amount of time gained before resistance thresholds were crossed (supplementary figure S3). As in our main analysis, the single resistance POC did not delay emergence of triple-resistant isolates. Our results were less sensitive to fitness assumptions in scenarios with higher test coverage in the population (supplementary figure S4 for triple resistance test, similar results for single resistance POC test).
Our findings were minimally sensitive to assumptions about the probability of resistance acquisition. Model projections changed only when probabilities were 10–3 per treatment event, larger values than would be considered biologically plausible [28]; even at this high level of resistance acquisition, the time to reach resistance thresholds was changed by 1–2 years compared with the base case estimates (supplementary figure S5).
Test Characteristics
Test specificity only affected the projected impact of the single resistance test (supplementary figure S6). For the triple resistance test, a false-positive result would limit treatment options, but cases would still receive an effective treatment. For example, a BC-resistant infection that was also falsely identified as A-resistant would receive an alternate treatment. In the case of the single resistance test, a BC-resistant infection falsely identified as A-resistant would be treated with BC, resulting in treatment failure.
Increased test sensitivity modestly increased the time until resistance thresholds were reached in the population (supplementary figure S6). For example, when the test was used for 10% of cases, time to the reach the 5% BC resistance threshold increased by 4 years, as test sensitivity increased from 50% to 100%.
For the triple resistance test, relaxing the assumption that test sensitivity was identical for all 3 antibiotic-resistant strains did not dramatically change our findings. As described above, with reduced test sensitivities for detecting antibiotic A, B, and/or C resistance, the utility of the test for reducing gonorrhea burden and delaying the time until resistance thresholds were crossed was diminished (supplementary figure S7).
Alternate Antibiotic Selection Approach With 3-Resistance Point-of-Care Test
Basing antibiotic choice on probability of resistance acquisition on treatment rather than fitness costs associated with resistance did not have an impact on time to resistance emergence, regardless of assumed test sensitivity and test coverage, under the time horizon considered here (supplementary figure S8).
Alternate Intensities of Asymptomatic Screening in the Population
Our base case assumed an annual screening rate of 39%. With more frequent screening (every 3 or 6 months) in the population to identify asymptomatic cases, we projected an initial decrease in population prevalence, followed by a rapid increase in prevalence of resistant isolates (supplementary figure S9). Reducing screening frequency to every 2 years resulted in a gradual increase in prevalence and a longer time for dual and triple-resistant strains to become established in the population, compared with the base-case analysis. Despite the different dynamics observed with different screening intensities, our qualitative findings on the impact of the POC tests remained unchanged: both tests delayed the time until BC-resistant strains crossed different resistance thresholds, whereas only the test for all 3 antibiotics was beneficial for preventing triple resistance. With more frequent screening in the population, the overall benefit of the POC tests, in terms of absolute number of years gained before an antibiotic would no longer be recommended for use, was diminished. For example, when screening occurred every 3 months, use of a POC test delayed the time until the 5% BC resistance threshold was crossed by only 1 year, whereas with biannual screening, there was 12–14 year delay.
DISCUSSION
Using a mathematical model, we have shown that rapid diagnostics that report antibiotic susceptibility have the potential to extend the usefulness of existing antibiotics for treatment of gonorrhea compared with the current guidelines for empiric 2-drug treatment. Although most impactful when used in a large proportion of cases, even modest levels of use in the population can delay the establishment of resistance and reduce overall infection burden in the population. Using an infection’s susceptibility profile to guide treatment also has the beneficial effect of allowing for the reintroduction of antimicrobials, such as fluoroquinolones, that are no longer recommended for general population use due to widespread resistance.
Although our model projected a net benefit of a POC test, we found that a test for determining resistance to a single antimicrobial is not expected to delay, and may accelerate, emergence of triply-resistant gonococcal isolates. The single antimicrobial test scenario was designed to replicate the potential effect of introduction of a rapid diagnostic for determining fluoroquinolone susceptibility. Although genomic and experimental analyses suggest there may not be a fitness cost associated with ciprofloxacin resistance [19, 29], our conclusions were unchanged even with a minor fitness cost. The failure of a single POC test to delay emergence of triply-resistant isolates arises in part because all tested cases are treated appropriately except for triply-resistant infections, thereby reducing the burden of all other isolates and clearing the way for triply-resistant isolates. This finding underscores the importance of robust surveillance systems for understanding the landscape of resistance as such tests begin to be integrated into clinical care [10]. Surveillance data will be essential to detect and react to changes in resistance that may occur as a result of adoption of novel test technologies.
A POC test would enable clinicians to select antibiotic treatment from the set of drugs to which the pathogen is susceptible. Given that resistance to antibiotics can incur fitness costs, and that those costs differ by antibiotic, we evaluated a treatment strategy in which an infection with a strain susceptible to more than 1 antibiotic was treated with the antibiotic associated with the highest fitness cost, as inferred from population genomic and experimental data [19, 29]. We based this strategy on the reasoning that, should resistance to the antibiotic develop, a strain carrying a higher fitness cost would be less likely to persist in the population. In addition, we evaluated the impact of guiding antibiotic selection by the probability of novel resistance emergence during the course of treatment. We note that both of these strategies delayed the emergence of resistance and rises in overall prevalence. Inferring the fitness costs of resistance, whether through population genomic data [19] or other means, would be an important parallel activity. Of note, the fitness costs need not be measured with precision. Instead, the relative ranking of costs per antibiotic guides selection.
Our study has several limitations. We used a deterministic model to determine how resistance would spread and assumed that the population was seeded with each of the resistant strains. Other modeling approaches that capture the stochastic nature of emergence and transmission are better suited to represent the inherent randomness in the emergence of resistance. However, use of a deterministic system allows us to draw initial inferences about the impact of POC test use, which can then be further explored using alternate modeling approaches. We did not explicitly model mixed infections (ie, infections with multiple gonorrhea strains with different drug-susceptibility profiles). However, imperfect test sensitivity in our model captures the impact of unrecognized resistant infections, whether they occur because a particular resistance marker is not included in the test, or because an individual has a mixed infection, with the resistant strain in low abundance. Actual treatment regimens used in the population are not 100% consistent with guidelines [3]. Similarly, although resistance is not a binary trait [3], in our model, isolates were assumed to be either susceptible or resistant. We did not model reduced susceptibility to antibiotics. This simplification of response to treatment likely resulted in shorter estimates of the lifespan of antibiotics, because more gradual development of resistance could allow for continued use of existing antibiotics at higher doses. More generally, as with all mathematical models, we made simplifying assumptions to describe gonorrhea transmission and resistance emergence and transmission. Given the early stages of POC test development, we sought to use this model to illustrate the potential impact of the use of such tests, and extensive sensitivity analyses demonstrate that the qualitative findings are robust under a range of assumptions and parameter values. We focused on a population experiencing a high burden of infection (MSM). As such, our analysis may be limited in generalizability to heterosexual populations, although trends in resistance in MSM often serve as harbingers of resistance in the broader population [11, 15]. The choice of an appropriate time horizon for this analysis was a compromise between allowing enough time for resistance to the current treatment regime to become established in the population and acknowledging that novel treatment options may be introduced in the near future, limiting the policy relevance of long-term projections. Finally, our model did not consider changing fitness costs associated with antibiotic resistance, or declining test sensitivities over time, as might be expected if novel resistance mechanisms emerge. These represent complex questions and are an important avenue of exploration for future modeling studies.
CONCLUSIONS
Despite these limitations, this mathematical model demonstrates both the promise and potential need for caution associated with future POC tests for determining antibiotic susceptibility of gonococcal infections. The use of such tests cannot be done in isolation; continued real-time surveillance will be critical for guiding decision-making and monitoring resistance emergence.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. All authors contributed to the study conception and design. A. R. T. constructed the model and performed the analyses. A. R. T. and Y. H. G. developed the scenarios, interpreted the results, and drafted the manuscript. T. L. G., H. W. C., K. H., and J. A. S. revised the manuscript.
Financial support. This work was supported by the US Centers for Disease Control and Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement (grant no. 5U38PS004642).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Material
References
- 1. Bolan GA, Sparling PF, Wasserheit JN. The emerging threat of untreatable gonococcal infection. N Engl J Med 2012; 366:485–7. [DOI] [PubMed] [Google Scholar]
- 2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 3. Kirkcaldy RD, Harvey A, Papp JR et al. Neisseria gonorrhoeae antimicrobial susceptibility surveillance - the Gonococcal Isolate Surveillance Project, 27 sites, United States, 2014. MMWR Surveill Summ 2016; 65:1–19. [DOI] [PubMed] [Google Scholar]
- 4. Trembizki E, Buckley C, Donovan B et al. Direct real-time PCR-based detection of Neisseria gonorrhoeae 23S rRNA mutations associated with azithromycin resistance. J Antimicrob Chemother 2015; 70:3244–9. [DOI] [PubMed] [Google Scholar]
- 5. Buckley C, Trembizki E, Baird RW et al. Multitarget PCR assay for direct detection of penicillinase-producing Neisseria gonorrhoeae for enhanced surveillance of gonococcal antimicrobial resistance. J Clin Microbiol 2015; 53:2706–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nicol M, Whiley D, Nulsen M, Bromhead C. Direct detection of markers associated with Neisseria gonorrhoeae antimicrobial resistance in New Zealand using residual DNA from the Cobas 4800 CT/NG NAAT assay. Sex Transm Infect 2015; 91:91–3. [DOI] [PubMed] [Google Scholar]
- 7. Trembizki E, Guy R, Donovan B et al. Further evidence to support the individualised treatment of gonorrhoea with ciprofloxacin. Lancet Infect Dis 2016; 16:1005–6. [DOI] [PubMed] [Google Scholar]
- 8. Siedner MJ, Pandori M, Leon SR et al. Detection of quinolone-resistant Neisseria gonorrhoeae in urogenital specimens with the use of real-time polymerase chain reaction. Int J STD AIDS 2008; 19:69–71. [DOI] [PubMed] [Google Scholar]
- 9. Siedner MJ, Pandori M, Castro L et al. Real-time PCR assay for detection of quinolone-resistant Neisseria gonorrhoeae in urine samples. J Clin Microbiol 2007; 45:1250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allan-Blitz LT, Humphries RM, Hemarajata P et al. Implementation of a rapid genotypic assay to promote targeted ciprofloxacin therapy of Neisseria gonorrhoeae in a large health system. Clin Infect Dis 2016; 64:1268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grad YH, Kirkcaldy RD, Trees D et al. Genomic epidemiology of Neisseria gonorrhoeae with reduced susceptibility to cefixime in the USA: a retrospective observational study. Lancet Infect Dis 2014; 14:220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demczuk W, Martin I, Peterson S et al. Genomic epidemiology and molecular resistance mechanisms of azithromycin resistant Neisseria gonorrhoeae in Canada from 1997 to 2014. J Clin Microbiol 2016; 54:1304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan CH, McCabe CJ, Fisman DN. Core groups, antimicrobial resistance and rebound in gonorrhoea in North America. Sex Transm Infect 2012; 88:200–4. [DOI] [PubMed] [Google Scholar]
- 14. Xiridou M, Soetens LC, Koedijk FD et al. Public health measures to control the spread of ntimicrobial resistance in Neisseria gonorrhoeae in men who have sex with men. Epidemiol Infect 2015; 143:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fingerhuth SM, Bonhoeffer S, Low N, Althaus CL. Antibiotic-resistant Neisseria gonorrhoeae spread faster with more treatment, not more sexual partners. PLoS Pathog 2016; 12:e1005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hui BB, Ryder N, Su JY et al. Exploring the benefits of molecular testing for gonorrhoea antibiotic resistance surveillance in remote settings. PLoS One 2015; 10:e0133202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trecker MA, Hogan DJ, Waldner CL et al. Revised simulation model does not predict rebound in gonorrhoea prevalence where core groups are treated in the presence of antimicrobial resistance. Sex Transm Infect 2015; 91:300–2. [DOI] [PubMed] [Google Scholar]
- 18. Saddler CA, Wu Y, Valckenborgh F, Tanaka MM. Epidemiological control of drug resistance and compensatory mutation under resistance testing and second-line therapy. Epidemics 2013; 5:164–73. [DOI] [PubMed] [Google Scholar]
- 19. Grad YH, Harris SR, Kirkcaldy RD et al. Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013. J Infect Dis 2016; 214:1579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirkcaldy RD, Zaidi A, Hook EW 3rd et al. Neisseria gonorrhoeae antimicrobial resistance among men who have sex with men and men who have sex exclusively with women: the Gonococcal Isolate Surveillance Project, 2005–2010. Ann Intern Med 2013; 158:321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention. HIV infection risk, prevention, and testing behaviors among men who have sex with men - National HIV Behavioral Surveillance, 20 U.S. Cities, 2014. HIV Surveillance Special Report 15 Availble at: http://www.cdc.gov/hiv/library/reports/surveillance. 2016. Accessed 7 October 2016.
- 22. Garnett GP, Anderson RM. Balancing sexual partnerships in an age and activity stratified model of HIV transmission in heterosexual populations. IMA J Math Appl Med Biol 1994; 11:161–92. [DOI] [PubMed] [Google Scholar]
- 23. Andersson DI, Hughes D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat Rev Microbiol 2010; 8:260–71. [DOI] [PubMed] [Google Scholar]
- 24. Schulz zur Wiesch P, Engelstädter J, Bonhoeffer S. Compensation of fitness costs and reversibility of antibiotic resistance mutations. Antimicrob Agents Chemother 2010; 54:2085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kirkcaldy RD, Weinstock HS, Moore PC et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis 2014; 59:1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hook EW 3rd, Golden M, Jamieson BD et al. A phase 2 trial of oral solithromycin 1200 mg or 1000 mg as single-dose oral therapy for uncomplicated gonorrhea. Clin Infect Dis 2015; 61:1043–8. [DOI] [PubMed] [Google Scholar]
- 27. Katz A, Komeya A, Tomas J et al. Cluster of Neisseria gonorrhoeae isolates with high-level azithromycin resistance and decreased ceftriaxone susceptibility Available at: https://cdc.confex.com/cdc/std2016/webprogram/Paper38102.html. In: 2016 STD Prevention Conference, Altanta, GA, 2016. Accessed 15 Febuary 2017. [Google Scholar]
- 28. Didelot X, Dordel J, Whittles LK et al. Genomic analysis and comparison of two gonorrhea outbreaks. MBio 2016; 7:e00525–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kunz AN, Begum AA, Wu H et al. Impact of fluoroquinolone resistance mutations on gonococcal fitness and in vivo selection for compensatory mutations. J Infect Dis 2012; 205:1821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jin F, Prestage GP, Mao L et al. Incidence and risk factors for urethral and anal gonorrhoea and chlamydia in a cohort of HIV-negative homosexual men: the Health in Men Study. Sex Transm Infect 2007; 83:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grov C, Cain D, Rendina HJ et al. Characteristics associated with urethral and rectal gonorrhea and chlamydia diagnoses in a US national sample of gay and bisexual men: results from the one thousand strong panel. Sex Transm Dis 2016; 43:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hooper RR, Reynolds GH, Jones OG et al. Cohort study of venereal disease. I: the risk of gonorrhea transmission from infected women to men. Am J Epidemiol 1978; 108:136–44. [DOI] [PubMed] [Google Scholar]
- 33. Wiesner PJ, Thompson SE 3rd. Gonococcal diseases. Dis Mon 1980; 26:1–44. [DOI] [PubMed] [Google Scholar]
- 34. Holmes KK, Johnson DW, Trostle HJ. An estimate of the risk of men acquiring gonorrhea by sexual contact with infected females. Am J Epidemiol 1970; 91:170–4. [DOI] [PubMed] [Google Scholar]
- 35. Platt R, Rice PA, McCormack WM. Risk of acquiring gonorrhea and prevalence of abnormal adnexal findings among women recently exposed to gonorrhea. JAMA 1983; 250:3205–9. [PubMed] [Google Scholar]
- 36. Holmes KK. Sexually Transmitted Diseases. 4th ed. New York: McGraw-Hill Medical, 2008. [Google Scholar]
- 37. Hethcote HW, Yorke JA.. Gonorrhea Transmission Dynamics and Control Vol. 56. New York: Springer-Verlag, 1984. (Levin S, ed. Lect Notes Biomath). [Google Scholar]
- 38. Garnett GP, Mertz KJ, Finelli L et al. The transmission dynamics of gonorrhoea: modelling the reported behaviour of infected patients from Newark, New Jersey. Philos Trans R Soc Lond B Biol Sci 1999; 354:787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kretzschmar M, van Duynhoven YT, Severijnen AJ. Modeling prevention strategies for gonorrhea and Chlamydia using stochastic network simulations. Am J Epidemiol 1996; 144:306–17. [DOI] [PubMed] [Google Scholar]
- 40. Newman LM, Dowell D, Bernstein K et al. A tale of two gonorrhea epidemics: results from the STD surveillance network. Public Health Rep 2012; 127:282–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.