In 198 selective sweeps we found functional genes or loci, such as FT and resistance, associated with environmental adaptability and yield-related traits.
Keywords: Brassica napus, domestication, ecotype, linkage disequilibrium, plant breeding, selective sweep
Abstract
Rapeseed (Brassica napus L.) is an important oilseed crop. Despite a short period of domestication and breeding, rapeseed has formed three diverse ecotype groups, namely spring, winter, and semi-winter. However, the genetic changes among the three ecotype groups have remained largely unknown. To detect selective signals, a set of 327 accessions from a worldwide collection were genotyped using a Brassica array, producing 33 186 high-quality single nucleotide polymorphisms (SNPs). Linkage disequilibrium (LD) was unevenly distributed across the genome. A total of 705 (78.2%) weak LD regions were found in the A subgenome, whereas 445 (72.6%) strong LD regions were in the C subgenome. By calculating the nucleotide diversity and population differentiation indices, a total of 198 selective sweeps were identified across ecotype groups, spanning 5.91% (37.9 Mb) of the genome. Within these genome regions, a few known functional genes or loci were found to be in association with environmental adaptability and yield-related traits. In particular, all 12 SNPs detected in significant association with flowering time among accessions were in the selection regions between ecotype groups. These findings provide new insights into the structure of the B. napus genome and uncover the footprints of domestication and breeding.
Introduction
Crop domestication and improvement is a selection process to adapt to different growth conditions and satisfy human preferences. A domestication allele and its neighboring neutral alleles suffer from strong positive selection pressure and produce selective sweeps to fix the domestication allele by reducing or eliminating variance among the nucleotides in neighboring DNA of a domestication locus (Purugganan and Fuller, 2009; Meyer and Purugganan, 2013). Recently, a few domesticated genes with large genetic effects have been identified in various crops, enhancing our understanding of domestication and improvement. For example, sh4 is responsible for the reduction of grain shattering in rice (Li et al., 2006; Zhang et al., 2009), Q controls shattering and free-threshing in wheat (Simons et al., 2006), DEP1 enhances grain yield in rice (Huang et al., 2009), KRN4 controls kernel row number in maize (Liu et al., 2015), fw2.2 influences fruit weight in tomato (Lin et al., 2014), and CsaBCH1 enhances nutritional value in cucumber (Qi et al., 2013).
The majority of domestication traits are controlled by many genes distributed over the whole genome (Cavanagh et al., 2013; Lin et al., 2014). Next-generation DNA sequencing technologies and SNP (single nucleotide polymorphism) arrays offer new and powerful tools to detect genomic footprints for those complex domestication traits. By genomic resequencing of diverse varieties, Mace et al. (2013) found that the genes in selection regions were enriched in functional categories in response to auxin and biotic and abiotic stress in sorghum. Xie et al. (2015) found that the genes in the regions under selection pressure are enriched in the regulatory pathways associated with flowering time (FT; heading time), nitrogen assimilation, and hormones in rice. More recently, Cheng et al. (2016) found that the genes related to four phytohormones (cytokinin, auxin, gibberellins, and jasmonic acid) were significantly enriched in selective sweeps of the leaf-heading morphotypes of both Brassica rapa and B. oleracea. Wang et al. (2014) identified genetic changes during modern breeding of rapeseed using a Brassica SNP array. Another study conducted by Wang et al. (2017) showed asymmetric subgenome selection and cis-regulatory divergence during cotton domestication.
Rapeseed (Brassica napus L., AACC) was derived from an interspecific hybridization between B. rapa (AA) and B. oleracea (CC), ~7500 years ago (Chalhoub et al., 2014). As compared with its parental species, the domestication history of rapeseed is relatively short. Rapeseed was documented as a winter crop in Europe 400 years ago, which has a biennial life cycle and strong vernalization requirement, and as a spring crop without vernalization ~300 years ago (Gómez-Campo and Prakash, 1999). After introduction and improvement to adapt to the local environment, rapeseed was widely grown in China as a semi-winter crop, which has a biennial life cycle and a moderate vernalization requirement (Liu, 2000). In the 1960s and 1970s, the crop was introduced into Canada and Australia as a spring crop (Chen et al., 2008). Presently, rapeseed is one of the most important oilseed crops in the world. Despite a short history of domestication and breeding, substantial genetic diversity was found among the three ecotype groups (Diers and Osborn, 1994; Becker et al., 1995; Wang et al., 2014). However, those loci and their genomic regions under selection remained poorly understood. The aim of this study is to understand genomic footprints of domestication and breeding among the three ecotype groups. We genotyped 327 accessions from a worldwide collection with a whole-genome SNP array, and found that the linkage disequilibrium (LD) decay was not evenly distributed throughout the genome of rapeseed. A few genes associated with yield-related and environmental adaptability traits, including FT and resistance were harbored in selective sweeps identified across different ecotype groups. Our investigation offers new opportunities to improve rapeseed by targeting those selected loci.
Materials and methods
Plant materials and phenotypic evaluation
The experimental set of 327 B. napus accessions was comprised of 71 winter ecotype lines from Europe, 60 spring ecotype lines from Europe (37), Canada (7), North Korea (3), Australia (4), and unknown regions (9) randomly selected from the ERANET-ASSYST B. napus diversity set (Bus et al., 2011), and 196 Chinese semi-winter accessions (Qian et al., 2014) (Supplementary Table S1 at JXB online). The accessions were sown in the middle of September in the experimental field of the Southwest University, Chongqing, China (29°33'N, 106°34'E), in four consecutive years from 2012 to 2015. A randomized complete block design was performed with two replications. Each plot consisted of 24 plants, with 30 cm between rows and 25 cm within rows. Field management followed essentially the normal agricultural practice. FT was recorded as days from sowing to flowering when 50% of the plants in a plot had reached the flowering stage.
SNP genotyping
Genomic DNA was extracted from the bulked young leaves of 24 plants of each genotype using the TIANGEN® plant genomic extraction kit (DP305-03) (Beijing, China). Accessions were genotyped using the Brassica 60K Illumina Infinium® SNP array (Edwards et al., 2013), according to the Infinium® HD Assay Ultra Protocol Guide. Using a local BLASTn search, the SNPs of the array were aligned to the B. napus reference genome assembly v4.1 (Chalhoub et al., 2014). Only the top BLAST hits against the reference genome were considered, allowing no less than 50 bp overlap, 90% sequence identity, and no gaps (Clarke et al., 2016).
Analysis of phylogenetic relationships
The population structure of rapeseed was analyzed with 5700 SNPs [minor allele frequency (MAF) >0.05] evenly distributed across 19 chromosomes by using STRUCTURE v2.3.4 (Pritchard et al., 2000). Five independent simulations having 1 × 105 MCMC (Markov chain Monte Carlo) replications and 1 × 105 burn-ins were performed, with the number of subpopulations (k) ranging from 1 to 10. The optimal k-value was determined by the log probability of data [LnP(D)] and an ad hoc statistic Δk based on the rate of change of LnP(D) between successive k as described by Evanno et al. (2005). Principal component analysis (PCA) was performed by TASSEL v5.2 software (Bradbury et al., 2007). The Neighbor–Joining tree was generated by TASSEL’s Cladogram function and visualized using Figtree v1.4.0 (http://tree.bio.ed.ac.uk/software/figtree/).
Linkage disequilibrium analysis
LD was estimated by measuring the r2 value via the software package TASSEL v5.2. Considering that crossovers and recombination events mainly occur within the 10 kb region (Paape et al., 2012), the highest or lowest 5% of r2 within the 10 kb region (P<0.01) was defined as strong or weak LD, respectively. The homoelogous regions were detected for the LD region between the A and C subgenomes using the LAST web service (Kielbasa et al., 2011) with the default parameter, match/mismatch=+1/–1, gap exist/extend= –7/–1 (Frith and Noé, 2014).
Identification of selective sweep
During crop domestication and breeding, both natural and artificial selection result in the reduction or elimination of variance in the selective sweeps. In order to detect those domestication footprints, using a sliding window approach (100 kb windows sliding in 10 kb steps), we calculated the nucleotide diversity (π) indicating the genomic signals of diversity within ecotype groups and the π ratio between ecotype groups with powermarker v3.25 (Liu and Muse, 2005), the selection statistics index (Tajima’s D) showing the allele frequency distribution relative to neutral expectations in ecotype groups by using TASSEL v5.2 software, and the population differentiation index (FST) quantifying levels of differentiation between ecotype groups using Genepop v4.2 software with the default parameter (Rousset, 2008). Since each statistical approach has its own strengths and limitations, combining multiple tests may increase the power and resolution to identify selection signals. According to an empirical procedure described by Li et al. (2013), the intersection regions with the top low or high π ratios (corresponding to the 5% left and right tails of the empirical π ratio distribution) and the top high FST values between ecotype groups were identified as selective sweeps in the genome. The genes harbored in these selective sweeps should have been under selection during rapeseed domestication and breeding.
To test whether the population structure influenced the identification of selective sweeps, a permutation test was performed using a Java script written in house by randomly shifting individuals across groups, and calculating FST between groups. We replicated this process 2000 times to assess the significance of an FST value.
To test whether some homoeologous genes with a similar function were possibly involved in ecotype differentiation in various plant species, the genes in the top 5% FST regions were isolated in our SNP data, while differentially expressed genes (DEGs) between winter and spring ecotypes were identified with the rapeseed transcriptome sequencing data set (Lu et al., 2014) through DEGseq of the R package (Wang et al., 2010), and with the Arabidopsis expression microarray data set (Des Marais et al., 2012) using a custom R script with Wilcox rank-sum test (P<0.01). Further, we searched for the overlapping homoeologous genes among the three data sets by aligning B. napus genes to the Arabidopsis genome with use of BLAST.
Genome-wide association analysis for flowering time
FT is a very important trait in association with crop domestication and adaptation breeding. In order to verify that loci associated with FT are located in the regions with strong selective signal, we investigated FT in accessions across 4 years. The best linear unbiased prediction (BLUP) for FT was estimated by using an R script based on a linear model as described by Merk et al. (2012). Association analysis for FT was carried out using a mixed model (MLM) with PC adjustment, which reduces the biased effects from population structure (Aulchenko et al., 2007). For the MLM analysis, we used the equation: y=Xα+Pβ+Kµ+e, where y represents phenotype, X represents genotype, P is the PCA matrix instead of the Q matrix, and K is the relative kinship matrix. Xα and Pβ represent fixed effects, and Kμ and e represent random effects (Yu et al., 2006; Zhang et al., 2010). The threshold of the genome-wide association study (GWAS) was set to P-value <4.86 × 10–5 (1/total SNPs used). The region of interest was defined using the LD decay around the most significantly associated SNP markers, which extended until r2 decayed to <0.5 on both sides or 200 kb on each side of the SNP peak (Zhang et al., 2015).
Enrichment analysis
The function of selected genes was analyzed by using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.kegg.jp/blastkoala/), and pathway analysis was determined by KOBAS version 2.0 (http://kobas.cbi.pku.edu.cn/). Gene Ontology (GO) for selected genes was studied with the web toolkit agriGO (http://bioinfo.cau.edu.cn/agriGO/) (Du et al., 2010) with the Fisher’s exact test method (P<0.05) and Hochberg multitest adjustment method [false discovery rate (FDR) <0.05].
Results
Genome structure of different rapeseed ecotypes
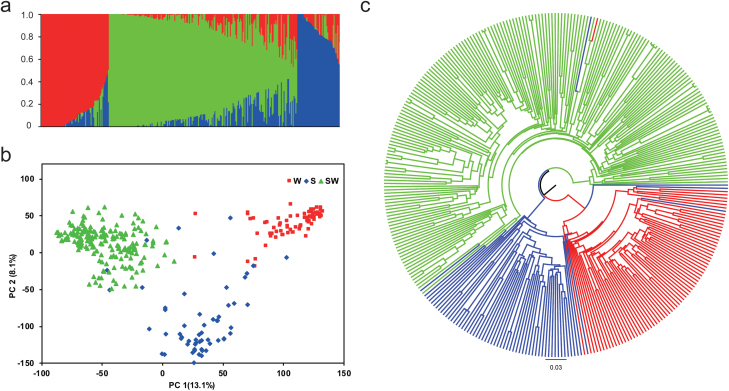
Of the 52 157 SNPs from the Brassica SNP array, 36 241 SNPs turned out to be informative among 327 accessions (Supplementary Tables S1, S2). Of these, 33 186 high-quality SNPs with an MAF >0.05 were used for genetic structure analysis. The SNP densities across the genome averaged 8 and 4 SNPs per 100 kb in the A and C subgenomes, respectively, ranging from 2 SNPs on C09 to 10 SNPs on A10 per 100 kb (Supplementary Table S3). Using a set of 5 700 SNPs (MAF >0.05) evenly distributed across 19 chromosomes, the accessions were classified into three major genetic groups by the Bayesian clustering program with the highest score of Δk at k=3 (Fig. 1a), almost in accordance with the classification of growth habit of the accessions. The result of classification was verified by PCA and a Neighbor–Joining tree based on all the SNPs in the collection (Fig. 1b, c). Moreover, we found clear evidence for genetic introgression from other gene pools. Some of the semi-winter accessions shared alleles with winter and spring accessions, while only a few of the winter and spring accessions possessed genome introgressions from the semi-winter accessions (Fig. 1a).
Fig. 1.
Phylogenetic relationships among 327 Brassica napus accessions revealed with a Brassica 60K SNP array. (a) Bayesian clustering. (b) Principal component analysis. (c) A Neighbor–Joining phylogenetic tree. Winter (W), semi-winter (SW), and spring (S) ecotypes are drawn in red, green, and blue colors, respectively.
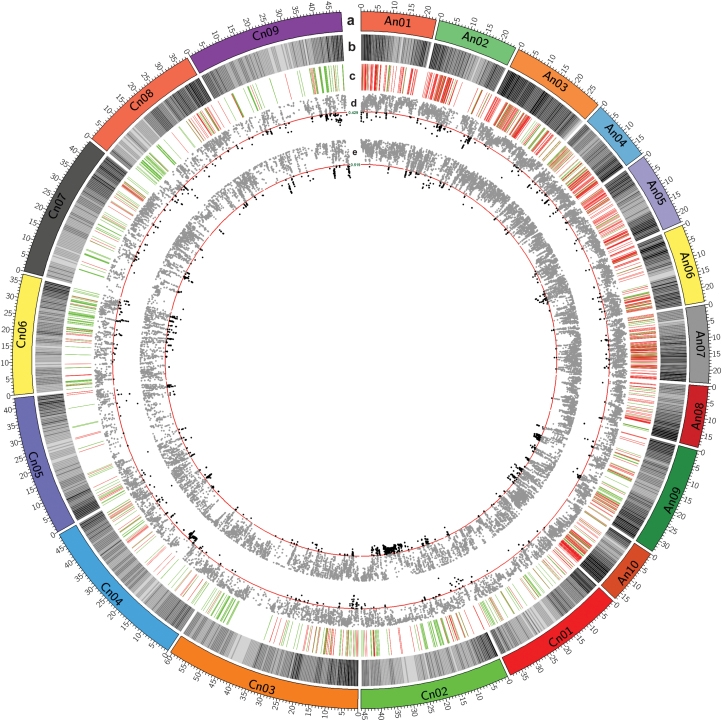
We also analyzed the LD throughout the whole genome. The average distance over which LD decayed to half of its maximum value was 150–200 kb. However, the size of LD decay varied greatly across chromosomes (Supplementary Fig. S1). The average distance of LD decay in the C subgenome was 10-fold longer (3.5–4 Mb) than that in the A subgenome (Supplementary Fig. S2). Within each 10 kb window of adjacent SNPs, we detected 902 weak LD regions at the bottom 5% of r2 (covering 0.61% of the genome) and 613 strong LD regions at the top 5% of r2 (covering 0.32% of the genome) (Supplementary Table S4). Among these, 705 (78.16%) weak LD regions are located in the A subgenome, whereas 445 (72.59%) strong LD regions are located in the C subgenome (Fig. 2). We also found that 101 (51.3%) weak LD regions in the C subgenome were homoeologous to 267 (37.9%) weak LD regions in the A subgenome, and that 205 (46.1%) strong LD regions in the C subgenome were homoeologous to 78 (46.4%) strong LD regions in the A subgenome (Supplementary Table S5). We found that weak LD regions had higher gene density and GC content whereas the regions with strong LD were enriched with retroelements and DNA transposons (Supplementary Table S6).
Fig. 2.
Concentric circles of population differentiation among ecotype groups of Brassica napus. (a) Chromosome; (b) heat map view of genes; (c) distribution of weak LD regions (red) and strong LD regions (green) in 10 kb window regions across the whole genome; (d) scatter spots distribution of FST in semi-winter versus winter/spring ecotype groups; (e) scatter spots distribution of FST in winter versus spring ecotype groups. The top 5% of FST values above the threshold line (red) were emphasized with a dark spot.
Selection signals and population differentiation between semi-winter and winter/spring ecotypes in rapeseed
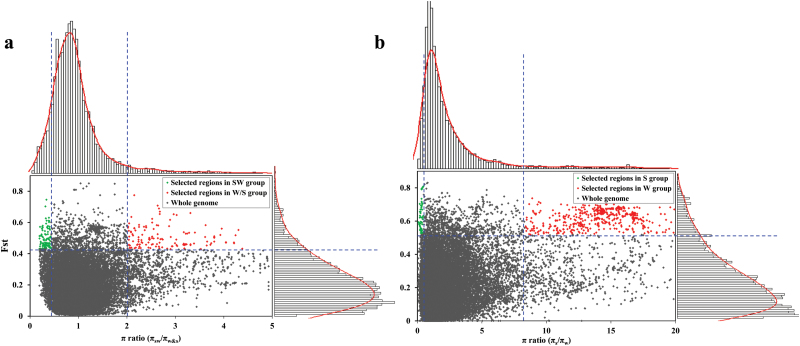
Semi-winter ecotype rapeseed was derived from winter and spring rapeseed no more than 100 years ago, but its genetic structure was diverged from the founder ecotypes as a consequence of breeder selection (Liu, 2000). To elucidate the genomic footprints left by selection, we searched for selection signals between semi-winter and winter/spring ecotypes. Setting a threshold of the 5% top FST, we identified 313 genome regions with strong population differentiation between semi-winter and winter/spring ecotypes. Our result was further supported by a permutation test (FDR <0.01) (Fig. 2). Among those genome regions, 66 and 51 regions were indicative of selective sweeps in winter/spring and semi-winter rapeseed, respectively (Fig. 3a). All together, these regions cover 1.52% (9.76 Mb) and 1.41% (9.04 Mb) of the assembled genome, and harbor 1202 and 1045 genes in winter/spring and semi-winter rapeseed, respectively. Interestingly, selective sweeps of winter/spring rapeseed are evenly distributed across the A (45%) and C (55%) subgenomes, while the majority of selective sweeps in semi-winter rapeseed lie in the C subgenome (82%) (Supplementary Tables S7, S8).
Fig. 3.
Selective signals in the whole genome between different rapeseed ecotypes. (a) Comparison between semi-winter (SW) and winter/spring (W/S) ecotypes. (b) Comparison between spring (S) and winter (W) ecotypes. The π ratios (πsw/πw&s and πs/πw) and FST values were calculated in a sliding window approach with 10 kb steps in a 100 kb window. The dashed blue lines correspond to the 5% left and right tails of the empirical π ratio distribution, where the π ratios are 0.43 and 2.02 for semi-winter and winter/spring groups (a) and 0.48 and 8.35 for spring and winter groups (b), respectively. Data points located to the left and right of the vertical dashed blue lines and above the horizontal dashed blue line [the 5% right tail of the empirical FST distribution, where FST is 0.429 in (a) and 0.519 in (b)] are indicative of selected regions for the SW group (green dots) and W/S group (red dots) in (a), and for the S group (green dots) and W group (red dots) in (b).
We further examined if the genes under selective pressure belong to specific functional categories. The annotated genes within the selective sweeps were subjected to GO analysis. We reasoned that genes responding to environmental cues are well represented within these regions of the genome, because changes of allele frequencies drive adaptive evolution and shape phenotypic variation between ecotypes with a divergent life cycle under different environmental conditions (semi-winter versus spring/winter ecotypes). GO analysis indicates an over-representation of genes involved in environmental adaptation (e.g. response to cold, vernalization response, and response to temperature) and development (e.g. cellular component assembly, morphogenesis, cell growth, and multicellular organismal development) (FDR <0.05) (Supplementary Table S9).
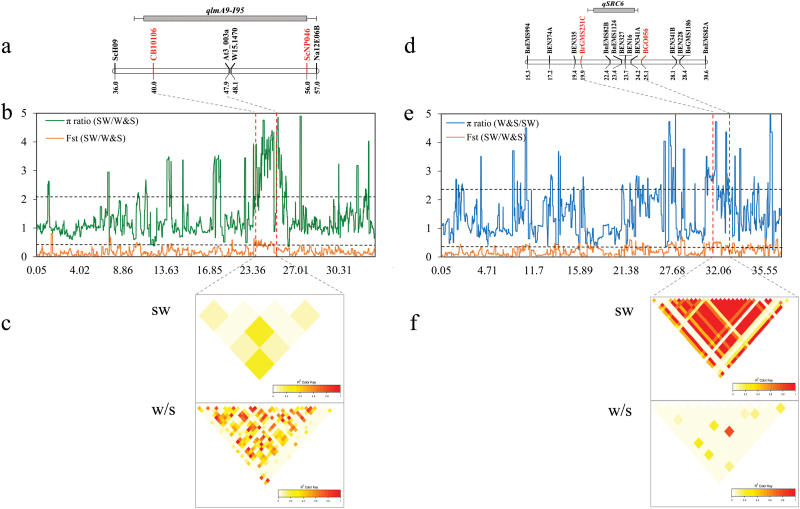
Here we present two examples to show diverse selection between ecotype groups. Blackleg (Leptosphaeria maculans) is the most devastating disease in the regions where winter and spring rapeseed are found, whereas white mold (Sclerotinia sclerotiorum) is a predominant disease in semi-winter rapeseed (Delourme et al., 2008; Sharma et al., 2015). We found that three linked selective sweeps on chromosome A09 (W&S-29, W&S-30, and W&S-31; from 23.6 Mb to 25.4 Mb) are located with a known blackleg resistance quantitative trait locus (QTL) region (qLmA9-I95) explaining 4.8–15.2% of the phenotypic variation (Delourme et al., 2008) (Fig. 4a, b). The region displayed strong and extensive LD, a significant negative Tajima’s D value (D= –0.427), and low genetic diversity (π=0.098) in winter and spring rapeseed group, but not in the semi-winter ecotype group (Fig. 4a–c; Supplementary Table S7). Likewise, a major locus (SRC6) explaining 29.01–32.61% of the phenotypic variation for Sclerotinia resistance across multiple environments (Wu et al., 2013; Wei et al., 2016) was located within a 1.5 Mb (from 30.85 Mb to 32.28 Mb) selective sweep region (SW-27) on chromosome C06 of the semi-winter group (Fig. 4d, e). This region is flanked by the markers Bn-scaff_23957_1-p270744 and Bn-scaff_16397_1-p622895, where a high genetic differentiation value (FST=0.534) and π ratio (πw&s/πsw=4.72) were observed between semi-winter and winter/spring groups (Figs.4d–f; Supplementary Table S7). To conclude, here we present two genome regions where divergent selection for pathogen resistance has left footprints of breeding for adaptation to certain geographical environments.
Fig. 4.
Selective sweep for the blackleg resistance quantitative trait loci (QTLs) on chromosome A09 and the Sclerotinia resistance QTLs on chromosome C06. (a and d) Map positions of the blackleg resistance QTL qLmA9-I95 (Delourme et al., 2008) and the Sclerotinia resistance QTL qSRC6 (Wu et al., 2013). (b and e) Empirical π ratio and FST distributions within the QTL region; the black dotted line indicates the threshold value of the top 5% π ratio and FST. (c and f) Linkage disequilibrium pattern of 196 semi-winter (SW) and 131 winter/spring (W/S) ecotypes in the region of the selective sweep; the color key indicates the r2 values.
Selection signals and population differentiation between winter and spring ecotypes in rapeseed
Historically, spring and winter rapeseed diverged ~300 years ago (Gómez-Campo and Prakash, 1999; Prakash et al., 2011). We detected 27 and 54 selective regions in spring and winter ecotypes, respectively (Fig. 3b; Supplementary Table S10). These regions cover 4.39 Mb and 14.69 Mb, and harbor 482 and 1 184 genes in spring and winter ecotypes, respectively (Supplementary Table S11). Genes controlling environmental adaptation should have undergone strong selection during domestication and breeding. As expected, GO enrichment analysis revealed strong bias towards genes involved in environmental adaptation and yield-related traits (e.g. response to abiotic and biotic stress or endogenous stimuli), other external factors (e.g. light, jasmonic acid, and nitrogen metabolism), development, carbohydrate biosynthesis, and post-embryonic development (FDR <0.05) (Supplementary Table S12).
We compared our data with the map positions of 61 QTLs for yield-related traits which were earlier identified in a population derived from a winter by spring rapeseed cross (Quijada et al., 2006; Udall et al., 2006). We found that 21 QTLs (34.4%) for seed yield, test weight, seed weight, plant height, days to flowering, lodging, and oil content are located within 33 selective sweep regions (Supplementary Table S13), which demonstrates how breeders and farmers have altered allele frequencies in the past centuries.
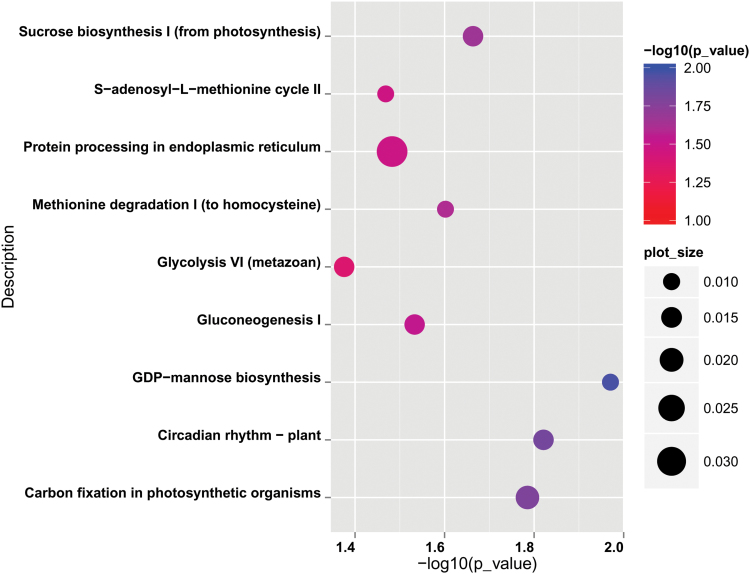
The annual and biennial life cycles widely exist in various plant species. We hypothesized that some homologous genes accounting for population differentiation should exhibit a similar differential expression pattern between spring and winter ecotypes in various plants. To test this hypothesis, we performed an in silico study between rapeseed and Arabidopsis. We aligned 4 304 genes (3 457 analogous Arabidopsis genes) from 228 genome regions differing between winter and spring rapeseed. These genes were then compared with 4 247 DEGs (Arabidopsis homologous genes) of rapeseed detected by transcriptome sequencing (Lu et al., 2014), and 5 500 DEGs from Arabidopsis detected by expression microarray analysis (Des Marais et al., 2012) (Supplementary Tables S14–S16). We detected a subset of 202 overlapping genes in the three data sets (Supplementary Fig. S3; Supplementary Table S17), which were allocated to nine functional categories (P-value <0.05) (Fig. 5). Most of the functional categories were found to be involved in energy metabolism, such as sucrose biosynthesis, protein processing in the endoplasmic reticulum, gluconeogenesis 1, GDP-mannose biosynthesis, and carbon fixation in photosynthetic organisms. Interestingly, the pathway of circadian clock regulation was enriched, which was reported in association with vigor and fitness (Dodd et al., 2005; Xie et al., 2015).
Fig. 5.
KEGG enrichment of 202 overlapping differential genes between spring and winter ecotypes in rapeseed and Arabidopsis. The enriched pathways are listed on the left. Colors denotes the –log10(P-value). The size of each bubble reflects the ratio of genes enriched in each pathway to 202 genes.
Genome-wide association studies for flowering time
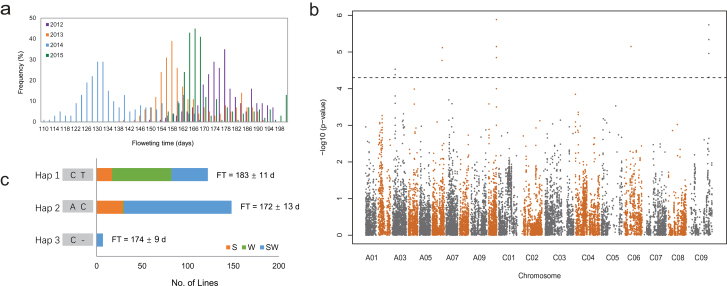
Flowering time is an important regulatory factor of plant adaptability. We performed GWASs for FT among 327 accessions of rapeseed across four consecutive years. A wide variation in FT was detected among accessions, ranging from 107 d to 204 d (Fig. 6a). As expected, the latest flowering genotypes belong to the winter rapeseed group.
Fig. 6.
Genome-wide association study (GWAS) and haplotype analysis for flowering time (FT). (a) The frequency distribution of FT across 4 years. (b) Manhattan plots of GWAS for FT. The black dotted line represents the significant level, −log10(P-value) >4.3. (c) Haplotype analysis for FT in two SNPs (6 450 533 and 6 450 763) on chromosome A03, linked with BnaA03g13630D (homologous to Arabidopsis Ath.FLC). S, W, and SW indicate the spring, winter, and semi-winter rapeseed group, respectively.
In total, 12 SNPs forming five haplotype blocks on chromosomes A03, A06, A10, C06, and C09 were significantly associated with FT, explaining 4.8–11.4% of the phenotypic variance per SNP (Fig. 6b; Supplementary Table S18). We found strong selection signals in the regions harboring these SNPs loci, which exhibited a high π ratio and/or FST between the three ecotype groups (Supplementary Table S18). Among these, three haplotype blocks were closely linked with the known homologous genes of FT, BnaA03g13630D, BnaA06g28900D, and BnaA10g24140D which are homologous to Ath.FLC (AT5G10140), Ath.FRI (AT5G27230), and Ath.ADC2 (AT4G34710), respectively (Supplementary Table S18) (Alcázar et al., 2005; Mendez-Vigo et al., 2011). Significant differences in FT were detected among haplotypes in the five haplotype blocks (Fig. 6b; Supplementary Table S18). For example, two SNPs in A03 (6 450 533 and 6 450 763), linked with BnaA03g13630D (homologous to Arabidopsis Ath.FLC), formed three haplotypes with significantly different FT (Hap 1–Hap 3) (Fig. 6c; Supplementary Table S18). Most of winter lines (65 out of 71) carried the Hap 1 haplotype (average FT 183 d), while 118 semi-winter accession ecotypes (72%) were of type Hap 2 (average FT 172 d) (Fig. 6c).
Discussion
Crop breeding has significantly improved crop adaptation to different growth conditions and human preferences. Although rapeseed was introduced from Europe to other parts of the world ~100 years ago, its genome has been diversified into three ecotype groups with diverse genetic basis (Gómez-Campo and Prakash, 1999). The recent availability of reference genome sequences from Brassica crops (Wang et al., 2011; Chalhoub et al., 2014; Liu et al., 2014; Yang et al., 2016) allows detailed dissection of the genomic alterations during domestication and breeding. In the present study, we identified 198 selective sweeps by analyzing the whole genome with a Brassica DNA microarray, where a number of known genes or loci associated with environmental adaptability and yield-related traits are harbored.
The knowledge about selective sweeps provides insights and targets for utilization of exotic germplasm. Rapeseed breeding suffers from low genetic diversity (Seyis et al., 2003). Exotic germplasm can serve as a valuable source to broaden the genetic basis since it harbors favorable alleles, which are not present in the current rapeseed pool (Quijada et al., 2006; Udall et al., 2006). QTLs for environmental adaptability and yield-related traits were identified in biparental mapping populations from diverse ecotypes in rapeseed (Quijada et al., 2006; Udall et al., 2006; Chen et al., 2007; Long et al., 2007; Radoev et al., 2008; Basunanda et al., 2010; Shi et al., 2011; Raman et al., 2014; Wei et al., 2014). Moreover, new genetic variation is needed to increase the heterotic potential of rapeseed hybrids. Several studies have demonstrated that hybrids between different rapeseed ecotypes exhibit high heterosis (Lefortbuson et al., 1987; Butruille et al., 1999; Qian et al., 2006, 2009). In our study, we detected numerous diverse selective sweeps among ecotype groups in association with environmental adaptability and yield-related traits by analyzing genome structure diversifications between the three ecotype groups. The information of selective sweeps and markers linked with domestication genes will be helpful for molecular breeding utilizing exotic germplasm in rapeseed.
Traditional QTL mapping using biparental populations only detects those QTLs which are polymorphic between the two parents. Here, our study verified that the comparison of genomic structure among diverse subpopulations is an efficient strategy for exploring adaptable genes or loci on a large scale. Similar studies reported in maize and rice also suggested that selective regions are associated with agronomic performance (van Heerwaarden et al., 2012; Xie et al., 2015).
An allopolyploid species contains two or more sets of chromosomes (subgenomes) from related ancestral species. We may ask the question of whether these subgenomes of an allotetraploid could ‘communicate’ with each other during domestication and breeding. Rapeseed (AACC) derived from an interspecific hybrid between B. rapa (AA) and B. oleracea (CC) is a good model to investigate the evolution of allotetraploid species. High homoeology between A and C genomes (Delourme et al., 2013; Chalhoub et al., 2014) ensures possible communication between A and C subgenomes of rapeseed. By analyzing genome structure among three diverse ecotype groups formed during domestication and breeding, we found an uneven distribution of LD within the two subgenomes. The majority of regions with weak and strong LD were located in the A and C subgenomes, respectively. This indicates higher diversity within the A subgenome than the C subgenome. A possible reason lies in the higher ability of B. napus to form a cross with B. rapa than with B. oleracea (Qian et al., 2006). As a consequence, the gene introgression rate from B. rapa into B. napus is higher. The introgression of genes from Asian B. rapa has further elevated the genetic divergence between semi-winter rapeseed and winter/spring rapeseed (Qian et al., 2006; Mei et al., 2011). Interestingly, we found a high degree of homoeology within weak or strong LD regions between A and C subgenomes. This indicates a high frequency of ‘communication’ between two subgenomes of rapeseed during domestication and breeding. This is in accordance with previous studies where QTLs were localized within regions of homoeologous exchanges (HEs) between A and C subgenomes, as in the case of Sclerotinia resistance, FT, seed quality, seed weight, and silique length (Zhao et al., 2006; Harper et al., 2012; Qian et al., 2014; Wei et al., 2014; Fu et al., 2015). Therefore, we propose a possible mechanism of rapeseed evolution, the introgression of genome regions from B. rapa, followed by HEs between subgenomes, and artificial selection as well as natural selection leading to fixation of elite loci within the two subgenomes.
Conclusions
We detected 198 selective sweep regions across ecotype groups. Of these, a number of known functional genes or loci were associated with environmental adaptability and yield-related traits, suggesting that these domestication loci in selective sweeps might have undergone independent and additional selection within ecotype groups for adaptation to the local environment and improvement of yield-related traits. Our findings provide new insights into the structure of the rapeseed genome and uncover the footprints of domestication and breeding.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Genome-wide patterns of linkage disequilibrium (LD) across chromosomes in the A (top) and C subgenome (bottom) of Brassica napus measured with 33 186 SNPs.
Fig. S2. The average distance of LD decay in the A and C subgenomes of B. napus.
Fig. S3. Venny plot of overlapping genes among three data sets in rapeseed and Arabidopsis.
Table S1. List of rapeseed accessions used in this study.
Table S2. Genotype data for 327 accessions in this study (http://pan.baidu.com/s/1kVFPflP).
Table S3. The distribution of SNPs across the 19 chromosomes of the rapeseed genome.
Table S4. Physical position of the weak and strong LDs.
Table S5. Homeologous exchanges between the A and C subgenomes in regions for weak and strong LD.
Table S6. Sequence features of genome regions with weak and strong LD.
Table S7. Regions of selective sweep between the winter/spring and semi-winter groups in rapeseed.
Table S8. Genes within selective sweep regions between semi-winter and winter/spring groups in rapeseed.
Table S9. Functional categories of the genes in the regions of selective sweep between winter/spring and semi-winter groups in rapeseed.
Table S10. Regions of selective sweep between spring and winter groups in rapeseed.
Table S11. Genes within selective sweep regions between spring and winter groups in rapeseed.
Table S12. Functional categories of the genes in the region of selective sweep between spring and winter groups in rapeseed.
Table S13. QTLs were aligned to selective sweeps within the segregation populations derived from a hybrid between spring and winter rapeseed reported by Udall et al. (2006) and Quijada et al. (2006).
Table S14. A list of 4 304 genes from genome regions differentiating between winter and spring groups in rapeseed.
Table S15. A list of 4 247 differentially expressed genes between winter and spring groups in rapeseed as detected by Lu et al. (2014).
Table S16. A list of 5 500 differentially expressed genes between summer and winter ecotypes in Arabidopsis thaliana as detected by Des Marais et al. (2012).
Table S17. A list of 202 genes overlapping between the three data sets from rapeseed and Arabidopsis.
Table S18. The significant SNPs associated with flowering time across 4 years.
Supplementary Material
Acknowledgements
We thank Dr Jinling Meng and Dr Jianbing Yan from Huazhong Agricultural University for critical discussion. Funding was provided by National Key Research and Development Program (2016YFD0100202), National Program on Key Basic Research Project of China (2015CB150201), and National Nature Science Foundation of China (1302266, 31471529, and 31601333).
Glossary
Abbreviations:
- DEG
differentially expressed gene
- FDR
false discovery rate
- FST
population differentiation index
- FT
flowering time
- GO
Gene Ontology
- GWAS
genome-wide association study
- HE
homoeologous exchange
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LD
linkage disequilibrium
- MAF
minor allele frequency
- π
nucleotide diversity
- PCA
principal component analysis
- QTL
quantitative trait locus
- SNP
single nucleotide polymorphism.
References
- Alcázar R, García-Martínez JL, Cuevas JC, Tiburcio AF, Altabella T. 2005. Overexpression of ADC2 in Arabidopsis induces dwarfism and late-flowering through GA deficiency. The Plant Journal 43, 425–436. [DOI] [PubMed] [Google Scholar]
- Aulchenko YS, Ripke S, Isaacs A, Van-Duijn CM. 2007. GenABEL: an R library for genome-wide association analysis. Bioinformatics 23, 1294–1296. [DOI] [PubMed] [Google Scholar]
- Basunanda P, Radoev M, Ecke W, Friedt W, Becker HC, Snowdon RJ. 2010. Comparative mapping of quantitative trait loci involved in heterosis for seedling and yield traits in oilseed rape (Brassica napus L.). Theoretical and Applied Genetics 120, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Engqvist GM, Karlsson B. 1995. Comparison of rapeseed cultivars and resynthesized lines based on allozyme and RFLP markers. Theoretical and Applied Genetics 91, 62–67. [DOI] [PubMed] [Google Scholar]
- Bradbury PJ, Zhang Z, Kroon DE et al. . 2007. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23, 2633–2635. [DOI] [PubMed] [Google Scholar]
- Bus A, Korber N, Snowdon RJ, Stich B. 2011. Patterns of molecular variation in a species-wide germplasm set of Brassica napus. Theoretical and Applied Genetics 123, 1413–1423. [DOI] [PubMed] [Google Scholar]
- Butruille DV, Guries RP, Osborn TC. 1999. Increasing yield of spring oilseed rape hybrids through introgression of winter germplasm. Crop Science 39, 1491–1496. [Google Scholar]
- Cavanagh CR, Chao S, Wang S et al. . 2013. Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proceedings of the National Academy of Sciences, USA 110, 8057–8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalhoub B, Denoeud F, Liu S et al. . 2014. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 345, 950–953. [DOI] [PubMed] [Google Scholar]
- Chen S, Nelson MN, Ghamkhar K, Fu T, Cowling WA. 2008. Divergent patterns of allelic diversity from similar origins: the case of oilseed rape (Brassica napus L.) in China and Australia. Genome 51, 1–10. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhang Y, Liu XP et al. . 2007. Detection of QTL for six yield-related traits in oilseed rape (Brassica napus) using DH and immortalized F2 populations. Theoretical and Applied Genetics 115, 849–858. [DOI] [PubMed] [Google Scholar]
- Cheng F, Sun R, Hou X et al. . 2016. Subgenome parallel selection is associated with morphotype diversification and convergent crop domestication in Brassica rapa and Brassica oleracea. Nature Genetics 48, 1218–1224. [DOI] [PubMed] [Google Scholar]
- Clarke WE, Higgins EE, Plieske J et al. . 2016. A high-density SNP genotyping array for Brassica napus and its ancestral diploid species based on optimised selection of single-locus markers in the allotetraploid genome. Theoretical and Applied Genetics 129, 1887–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delourme R, Falentin C, Fomeju BF et al. . 2013. High-density SNP-based genetic map development and linkage disequilibrium assessment in Brassica napus L. BMC Genomics 14, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delourme R, Piel N, Horvais R, Pouilly N, Domin C, Vallée P, Falentin C, Manzanares-Dauleux MJ, Renard M. 2008. Molecular and phenotypic characterization of near isogenic lines at QTL for quantitative resistance to Leptosphaeria maculans in oilseed rape (Brassica napus L.). Theoretical and Applied Genetics 117, 1055–1067. [DOI] [PubMed] [Google Scholar]
- Des Marais DL, McKay JK, Richards JH et al. . 2012. Physiological genomics of response to soil drying in diverse Arabidopsis accessions. The Plant Cell 24, 893–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diers BW, Osborn TC. 1994. Genetic diversity of oilseed Brassica napus germplasm based on restriction fragment length polymorphisms. Theoretical and Applied Genetics 88, 662–668. [DOI] [PubMed] [Google Scholar]
- Dodd AN, Salathia N, Hall A et al. . 2005. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309, 630–633. [DOI] [PubMed] [Google Scholar]
- Du Z, Zhou X, Ling Y, Zhang Z, Su Z. 2010. agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Research 38, W64–W70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D, Batley J, Snowdon RJ. 2013. Accessing complex crop genomes with next-generation sequencing. Theoretical and Applied Genetics 126, 1–11. [DOI] [PubMed] [Google Scholar]
- Evanno G, Regnaut S, Goudet J. 2005. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology 14, 2611–2620. [DOI] [PubMed] [Google Scholar]
- Frith MC, Noé L. 2014. Improved search heuristics find 20,000 new alignments between human and mouse genomes. Nucleic Acids Research 42, e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Wei D, Dong H et al. . 2015. Comparative quantitative trait loci for silique length and seed weight in Brassica napus. Scientific Reports 5, 14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Campo C, Prakash S. 1999. Origin and domestication. Biology of Brassica coenospecies. Amsterdam: Elsevier Science, 33–58. [Google Scholar]
- Harper AL, Trick M, Higgins J et al. . 2012. Associative transcriptomics of traits in the polyploid crop species Brassica napus. Nature Biotechnology 30, 798–802. [DOI] [PubMed] [Google Scholar]
- Huang X, Qian Q, Liu Z et al. . 2009. Natural variation at the DEP1 locus enhances grain yield in rice. Nature Genetics 41, 494–497. [DOI] [PubMed] [Google Scholar]
- Kiełbasa SM, Wan R, Sato K, Horton P, Frith MC. 2011. Adaptive seeds tame genomic sequence comparison. Genome Research 21, 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefortbuson M, Guillotlemoine B, Dattee Y. 1987. Heterosis and genetic distance in rapeseed (Brassica napus L.): crosses between European and Asiatic selfed lines. Genome 29, 413–418. [Google Scholar]
- Li C, Zhou A, Sang T. 2006. Rice domestication by reducing shattering. Science 311, 1936–1939. [DOI] [PubMed] [Google Scholar]
- Li M, Tian S, Jin L et al. . 2013. Genomic analyses identify distinct patterns of selection in domesticated pigs and Tibetan wild boars. Nature Genetics 45, 1431–1438. [DOI] [PubMed] [Google Scholar]
- Lin T, Zhu G, Zhang J et al. . 2014. Genomic analyses provide insights into the history of tomato breeding. Nature Genetics 46, 1220–1226. [DOI] [PubMed] [Google Scholar]
- Liu HL. 2000. Genetics and breeding in rapeseed. Beijing, China: Chinese Agricultural Universities Press. [Google Scholar]
- Liu K, Muse SV. 2005. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21, 2128–2129. [DOI] [PubMed] [Google Scholar]
- Liu L, Du Y, Shen X et al. . 2015. KRN4 controls quantitative variation in maize kernel row number. PLoS Genetics 11, e1005670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Liu Y, Yang X et al. . 2014. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nature Communications 5, 3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y, Shi J, Qiu D et al. . 2007. Flowering time quantitative trait loci analysis of oilseed brassica in multiple environments and genomewide alignment with Arabidopsis. Genetics 177, 2433–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Harper AL, Trick M et al. . 2014. Associative transcriptomics study dissects the genetic architecture of seed glucosinolate content in Brassica napus. DNA Research 30, 798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace ES, Tai S, Gilding EK et al. . 2013. Whole-genome sequencing reveals untapped genetic potential in Africa’s indigenous cereal crop sorghum. Nature Communications 4, 2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei J, Fu Y, Qian L et al. . 2011. Chinese semi-winter Brassica rapa can effectively widen the genetic basis of oilseed rape as revealed with virtual allopolyploid. Plant Breeding 130, 333–337. [Google Scholar]
- Méndez-Vigo B, Picó FX, Ramiro M, Martínez-Zapater JM, Alonso-Blanco C. 2011. Altitudinal and climatic adaptation is mediated by flowering traits and FRI, FLC, and PHYC genes in Arabidopsis. Plant Physiology 157, 1942–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merk HL, Yarnes SC, Van Deynze A et al. . 2012. Trait diversity and potential for selection indices based on variation among regionally adapted processing tomato germplasm. Journal of the American Society for Horticultural Science 137, 427–437. [Google Scholar]
- Meyer RS, Purugganan MD. 2013. Evolution of crop species: genetics of domestication and diversification. Nature Reviews. Genetics 14, 840–852. [DOI] [PubMed] [Google Scholar]
- Paape T, Zhou P, Branca A et al. . 2012. Fine-scale population recombination rates, hotspots, and correlates of recombination in the Medicago truncatula genome. Genome Biology and Evolution 4, 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S, Wu XM, Bhat SR. 2011. History, evolution, and domestication of Brassica crops. Plant Breeding Reviews 35, 19–84. [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155, 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purugganan MD, Fuller DQ. 2009. The nature of selection during plant domestication. Nature 457, 843–848. [DOI] [PubMed] [Google Scholar]
- Qi J, Liu X, Shen D et al. . 2013. A genomic variation map provides insights into the genetic basis of cucumber domestication and diversity. Nature Genetics 45, 1510–1515. [DOI] [PubMed] [Google Scholar]
- Qian L, Qian W, Snowdon RJ. 2014. Sub-genomic selection patterns as a signature of breeding in the allopolyploid Brassica napus genome. BMC Genomics 15, 1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W, Li Q, Noack J et al. . 2009. Heterotic patterns in rapeseed (Brassica napus L.): II. Crosses between European winter and Chinese semi-winter lines. Plant Breeding 128, 466–470. [Google Scholar]
- Qian W, Meng J, Li M, Frauen M, Sass O, Noack J, Jung C. 2006. Introgression of genomic components from Chinese Brassica rapa contributes to widening the genetic diversity in rapeseed (B. napus L.), with emphasis on the evolution of Chinese rapeseed. Theoretical and Applied Genetics 113, 49–54. [DOI] [PubMed] [Google Scholar]
- Quijada PA, Udall JA, Lambert B, Osborn TC. 2006. Quantitative trait analysis of seed yield and other complex traits in hybrid spring rapeseed (Brassica napus L.): 1. Identification of genomic regions from winter germplasm. Theoretical and Applied Genetics 113, 549–561. [DOI] [PubMed] [Google Scholar]
- Radoev M, Becker HC, Ecke W. 2008. Genetic analysis of heterosis for yield and yield components in rapeseed (Brassica napus L.) by quantitative trait locus mapping. Genetics 179, 1547–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman H, Dalton-Morgan J, Diffey S, Raman R, Alamery S, Edwards D, Batley J. 2014. SNP markers-based map construction and genome-wide linkage analysis in Brassica napus. Plant Biotechnology Journal 12, 851–860. [DOI] [PubMed] [Google Scholar]
- Rousset F. 2008. genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Molecular Ecology Resources 8, 103–106. [DOI] [PubMed] [Google Scholar]
- Seyis F, Snowdon RJ, Luhs W, Friedt W. 2003. Molecular characterization of novel resynthesized rapeseed (Brassica napus) lines and analysis of their genetic diversity in comparison with spring rapeseed cultivars. Plant Breeding 122, 473–478. [Google Scholar]
- Sharma P, Meena PD, Verma PR et al. . 2015. Sclerotinia sclerotiorum (Lib.) de Bary causing Sclerotinia rot in oilseed Brassicas. Journal of Oilseed Brassica 6, 1–44. [Google Scholar]
- Shi J, Li R, Zou J, Long Y, Meng J. 2011. A dynamic and complex network regulates the heterosis of yield-correlated traits in rapeseed (Brassica napus L.). PLoS One 6, e21645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons KJ, Fellers JP, Trick HN, Zhang Z, Tai YS, Gill BS, Faris JD. 2006. Molecular characterization of the major wheat domestication gene Q. Genetics 172, 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udall JA, Quijada PA, Lambert B, Osborn TC. 2006. Quantitative trait analysis of seed yield and other complex traits in hybrid spring rapeseed (Brassica napus L.): 2. Identification of alleles from unadapted germplasm. Theoretical and Applied Genetics 113, 597–609. [DOI] [PubMed] [Google Scholar]
- Van Heerwaarden J, Hufford MB, Ross-Ibarra J. 2012. Historical genomics of North American maize. Proceedings of the National Academy of Sciences, USA 109, 12420–12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LK, Feng ZX, Wang X, Wang XW, Zhang XG. 2010. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26, 136–138. [DOI] [PubMed] [Google Scholar]
- Wang M, Tu L, Lin M et al. . 2017. Asymmetric subgenome selection and cis-regulatory divergence during cotton domestication. Nature Genetics 49, 579–587. [DOI] [PubMed] [Google Scholar]
- Wang N, Li F, Chen B et al. . 2014. Genome-wide investigation of genetic changes during modern breeding of Brassica napus. Theoretical and Applied Genetics 127, 1817–1829. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang H, Wang J et al. . 2011. The genome of the mesopolyploid crop species Brassica rapa. Nature Genetics 43, 1035–1039. [DOI] [PubMed] [Google Scholar]
- Wei DY, Mei JQ, Fu Y et al. . 2014. Quantitative trait loci analyses for resistance to Sclerotinia sclerotiorum and flowering time in Brassica napus. Molecular Breeding 34, 1797–1804. [Google Scholar]
- Wei L, Jian H, Lu K, Filardo F, Yin N, Liu L, Qu C, Li W, Du H, Li J. 2016. Genome-wide association analysis and differential expression analysis of resistance to Sclerotinia stem rot in Brassica napus. Plant Biotechnology Journal 14, 1368–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cai G, Tu J, Li L, Liu S, Luo X, Zhou L, Fan C, Zhou Y. 2013. Identification of QTLs for resistance to sclerotinia stem rot and BnaC.IGMT5.a as a candidate gene of the major resistant QTL SRC6 in Brassica napus. PLoS One 8, e67740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie WB, Wang GW, Yuan M et al. . 2015. Breeding signatures of rice improvement revealed by a genomic variation map from a large germplasm collection. Proceedings of the National Academy of Sciences, USA 112, E5411–E5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu D, Wang X et al. . 2016. The genome sequence of allopolyploid Brassica juncea and analysis of differential homoeolog gene expression influencing selection. Nature Genetics 48, 1225–1232. [DOI] [PubMed] [Google Scholar]
- Yu J, Pressoir G, Briggs WH et al. . 2006. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nature Genetics 38, 203–208. [DOI] [PubMed] [Google Scholar]
- Zhang JP, Singh A, Mueller DS, Singh AK. 2015. Genome-wide association and epistasis studies unravel the genetic architecture of sudden death syndrome resistance in soybean. The Plant Journal 84, 1124–1136. [DOI] [PubMed] [Google Scholar]
- Zhang LB, Zhu Q, Wu ZQ et al. . 2009. Selection on grain shattering genes and rates of rice domestication. New Phytologist 184, 708–720. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Ersoz E, Lai CQ et al. . 2010. Mixed linear model approach adapted for genome-wide association studies. Nature Genetics 42, 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Udall JA, Quijada PA, Grau CR, Meng J, Osborn TC. 2006. Quantitative trait loci for resistance to Sclerotinia sclerotiorum and its association with a homeologous non-reciprocal transposition in Brassica napus L. Theoretical and Applied Genetics 112, 509–516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.