The role of T cells within breast milk is poorly understood. This report suggests that, instead of local human cytomegalovirus control, the presence of virus serves as a means to recruit human cytomegalovirus–specific T cells into the breast milk to increase their numbers.
Keywords: breast milk, CD8 T cells, human cytomegalovirus (HCMV), cell-mediated immunity, mucosal immunity
Abstract
The role of human cytomegalovirus (HCMV)–specific T-cell responses in breast milk of HCMV-seropositive mothers is not well defined. In these studies, we demonstrate that the frequency of cytomegalovirus (CMV)-pp65–specific T-cell responses in peripheral blood mononuclear cells (PBMCs) and breast milk cells (BMCs) is increased for CD8+ T cells in both sample sources when compared with CD4+ T cells. The frequency of pp55-specific CD8 T cells producing interferon γ (IFN-γ) alone or dual IFN-γ/granzyme rB producers is increased in breast milk compared with PBMCs. Lastly, we observed a positive correlation between breast milk viral load and the CD8 pp65-specific response, suggesting that local virus replication drives antigen-specific CD8 T cells into the breast.
Human cytomegalovirus (HCMV) is the most frequent cause of congenital infection and an important cause of invasive disease in immunocompromised individuals [1]. Although the development of vaccines to prevent or reduce disabilities related to this intrauterine infection is a high priority, no vaccine is available currently, and early clinical trials have had only limited success [2]. A major hurdle in HCMV vaccine development is an incomplete understanding of adaptive immune responses that limit virus transmission.
There is a large gap in our understanding of the role of cell-mediated immunity in controlling infections within mucosal tissues and how local T-cell immunity is established after infection. Studies in the murine cytomegalovirus (MCMV) model have provided evidence that primary MCMV infection is controlled by T cells (CD4+ and CD8+) [3, 4]. However, the role T-cell immunity plays in the protection and control of HCMV infection in humans has been much harder to determine. Nevertheless, virus control has been demonstrated after adoptive transfer of HCMV-specific T cells to patients undergoing stem cell transplantation [5, 6]. Moreover, adoptive transfer has also been shown to reduce the risk of HCMV infection and to restore HCMV immunity in individuals undergoing solid organ transplants [7]. A recent study of HCMV in tissues from human organ donors demonstrated that the responses in peripheral blood mononuclear cells (PBMCs) did not reflect those of tissue, suggesting the need to study immunity in the tissues [8]. Very few reports have studied HCMV-specific T-cell immunity in breast milk or the mammary gland. A recent study in humans using an interferon γ (IFN-γ) ELISpot was unable to demonstrate a relationship between breast milk T-cell immunity and the viral load in breast milk in preterm infants with very low birth weight [9].
In this report, we determined the association among the number of mononuclear cells, the frequency of HCMV-specific CD8+ T cells, and the level of HCMV load in breast milk, suggesting that the local HCMV infection drives the establishment of HCMV-specific T cells in the breast.
METHODS
Subjects
Human cytomegalovirus–seropositive mothers at the time of delivery were enrolled and monitored prospectively for HCMV shedding and HCMV-specific T-cell responses in breast milk during the first 6 months postpartum. Breast milk was collected monthly, and breast milk collected at 1, 2, and 3 months postpartum was analyzed. In addition, peripheral blood samples were collected from a subset of participants. Written informed consent was obtained from all women who participated in this study. The Institutional Review Board of the University of Alabama at Birmingham approved the study.
Isolation of Breast Milk Cells
Breast milk and peripheral blood specimens were processed to separate mononuclear cells as described previously [10, 11]. Briefly, milk samples were centrifuged at 400 × g for 15 minutes and washed 2 times with phosphate buffered saline.
Antigens
Human cytomegalovirus–specific T-cell responses were measured using HCMV-pp65 (15-mers overlapping by 11; National Institutes of Health AIDS repository). Phorbol-12,13 dibutyrate/ionomycin (Sigma-Aldrich) was used as a positive control, and unstimulated cells were used as a negative control.
Intracellular Cytokine Staining
Intracellular cytokine staining was performed as previously described [12]. Briefly, isolated blood mononuclear cells (BMCs) and (PBMCs) were added to each tube along with costimulatory monoclonal antibodies (anti-CD28 and anti-CD49d; each at 1μg/mL) and 50U/mL of Benzonase (Novagen) and pulsed with the appropriate antigen followed by the addition of 10 μg/mL of Monensin (Golgistop, BD Biosciences). Cells were then incubated overnight at 37°C, 5% carbon dioxide for 6 hours, and placed at 4°C ON. The following day cells were labeled with a fluorescent LIVE/DEAD fixable Dead Cell Stain (Molecular probes, Invitrogen), and the cell-surface markers CD3-Alexa780 (eBioscience), CD8-V500, CD45-Pecy7, CD4-Qdot655 (ThermoFisher) were added. All antibodies were obtained from BD Biosciences unless otherwise noted. Cells were permeabilized with the Cytofix/cytoperm reagent (BD) for 20 minutes followed by intracellular cytokine staining using anti-IFN-γ–Alexa700 and anti-GranzymeB–V450 conjugated antibodies. At least 100000 lymphocytes were acquired from each sample using a BD LSR II flow cytometer. Data were analyzed using FlowJo version 9.9 software (TreeStar). Lymphocytes were analyzed based on forward and side scatter profiles for PBMCs and by gating on CD45 for BMCs, followed by the exclusion of dead cells. Cytokines produced were measured using the CD3+CD4+ or CD3+CD8+ gates relative to the media control values, and these gates were applied to all samples with different antigens from the same individual. We used Fisher’s exact test to compare the number of cytokine-producing cells between the antigen-stimulated and unstimulated (media alone) samples to determine whether a response is considered positive. P < .001 was considered positive for CD8 T-cell responses, and P < .05 was considered positive for CD4 T-cell responses. To be considered a positive response the frequency had to be >0.05%.
Detection of Human Cytomegalovirus
Breast milk supernatant specimens were subjected to DNA extraction using commercial column kids (Qiagen, Inc). A real-time polymerase chain reaction (PCR) protocol developed in our laboratory for testing dried blood spots and newborn saliva specimens was adapted to test DNA specimens from breast milk supernatant specimens for HCMV [13, 14]. Briefly, to determine the performance characteristics of the PCR assay, breast milk specimens from 10 CMV-seronegative women were included as controls. In addition, plasmid standards incorporating target sequences in 10-fold dilutions ranging 10–100000 copies prereaction to generate a standard curve for each run. Each PCR run also included a no-target control and DNA from CMV-negative and CMV-positive breast milk DNA as negative and positive controls, respectively. Each specimen was run in duplicate using 25 µL of reaction mixture and 5 µL of test specimen. Specimen was considered positive if >1 copies per reaction were detected in both wells. The detection limit of our real-time PCR assay was 50–100 copies/mL.
Statistics
Statistical analyses were performed using the nonparametric Wilcoxon signed rank test for paired samples; otherwise the Mann-Whitney U test was used. Correlations were determined using Spearman correlation. Analyses where done with Graphpad Prism software 5.0 for Mac. Differences were considered to be significant on the basis of 95% confidence intervals (P < .05).
RESULTS
Study Population
Human cytomegalovirus shedding in breast milk from 94 seropositive mothers was examined. First appearance of HCMV in breast milk, duration of shedding, and viral load were determined. Most women (82%) had HCMV in breast milk at least once during the study. Human cytomegalovirus first appeared in breast milk at 32.2 ± 32.7 days postpartum, and the mean duration of HCMV shedding was 1.8 ± 1.3 months. The mean peak breast milk HCMV load was 3.01 × 104 ± 3.5 × 104 copies/mL.
Mononuclear Cells in Breast Milk and Human Cytomegalovirus Viral Load
The number of mononuclear cells in breast milk varied significantly between samples. Therefore we wanted to determine whether there was a relationship between the numbers of mononuclear cells and the breast milk viral load. Our data demonstrated that the numbers of cells present in breast milk is increased in samples with detectable HCMV loads (supplementary figure 1A).
Human Cytomegalovirus-pp65–Specific T Cells in Breast Milk
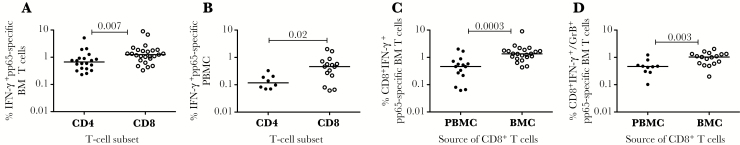
We next determined the frequency of HCMV-pp65-specific CD4+ and CD8+ T cells present in both breast milk and PBMCs from HCMV-seropositive mothers using intracellular cytokine staining assays on cells isolated from both samples (BMC and PBMC). A sample flow plot (supplementary figure 1B) depicts our gating strategy for IFN-γ (supplementary figure 1C) for both CD3+CD4+ and CD3+CD8+ T cells from breast milk and dual production of IFN-γ/granzyme B (GrB) (supplementary figure 1D) in response to HCMV-pp65 peptide pools for CD3+CD8+ T cells isolated from breast milk. Unstimulated cells (media) were used as the negative control and stimulation with the polyclonal stimulator phorbol-12,13 dibutyrate/ionomycin was used as the positive control. Using this data, we compared the frequency of IFN-γ–producing pp65-specific CD4+ and CD8+ T cells from breast milk and PBMCs. Our data demonstrated that for both sample sources (BMCs and PBMCs), the frequency is higher for CD8+ T cells when compared with CD4+ T cells (Figure 1A and B). We then proceeded to compare the frequency of pp65-specific T cells from breast milk to the frequency of cells from PBMCs. We found that the frequency of the response was highest in breast milk irrespective of whether the cells were only IFN-γ producers (Figure 1C) or dual IFN-γ/GrB producers (Figure 1D). In addition, an increased frequency was also observed for IFN-γ–producing CD4+ T cells isolated from breast milk when compared with PBMCs (data not shown).
Figure 1.
Human cytomegalovirus (HCMV)-specific T cells present in breast milk from HCMV infected mothers. (A) Comparison between HCMV-pp65-specific interferon γ (IFN-γ) producing CD4 and CD8 T cells isolated from breast milk or (B) PBMC. (C) Comparison between HCMV-pp65-specific IFN-γ or (D) IFN-γ/granzyme B producing CD8 T cells isolated from PBMC and breast milk, that have a positive response only. Horizontal line represents median value. Statistically significant differences (P < .05) were obtained using Mann Whitney test.
Association Between Human Cytomegalovirus-Specific T-Cell Responses and Viral Load
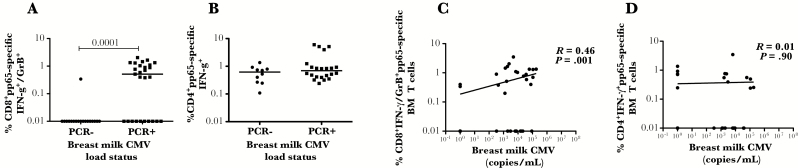
Given the positive association between the number of mononuclear cells present in breast milk and breast milk HCMV load (supplementary figure 1A), we wanted to determine whether there was also an association between the frequency of pp65-specific responses in breast milk with the local viral load. When we compared the frequency of pp65-specific CD8 T cells producing IFN-γ (not shown) or both IFN-γ/GrB (Figure 2A) in breast milk with and without detectable breast milk viral loads, we found a significant difference, with most of the responses seen in samples with detectable breast milk viral loads. This difference was not seen with pp65-specific CD4 T cells producing IFN-γ (Figure 2B). We further correlated the frequency of pp65-specific CD8 T cells in breast milk, whether they produced IFN-γ (not shown) or both IFN-γ/GrB (Figure 2C), to the viral load in breast milk and found a significant positive association that was not seen with CD4+ T cells (Figure 2D).
Figure 2.
Human cytomegalovirus (HCMV)-pp65-specific T cell responses in breast milk from HCMV infected mothers with and without a detectable viral load in breast milk. (A) HCMV-pp65-specific CD3+CD8+ T cells producing interferon γ (IFN-γ)/granzyme B (GrB) or (B) CD3+CD4+ T cells producing IFN-γ from breast milk with and without a detectable viral load. (C) Correlation between HCMV-pp65-specific CD3+CD8+ T cells dual producing IFN-γ/GrB and HCMV breast milk viral load and (D) the correlation between HCMV-pp65-specific CD3+CD4+ T cells producing IFN-γ and HCMV breast milk viral. Horizontal line represents median value. Statistically significant differences (P < .05) were obtained using Mann Whitney test. Spearman correlation was used to determine statistical significance (P < .05).
Discussion
In these studies, we have demonstrated that in HCMV-seropositive mothers the number of mononuclear cells and the frequency of pp65-specific CD8+ T cells in the breast milk are influenced by the local HCMV load. In addition, as demonstrated in previous studies, most seropositive women shed HCMV in breast milk. Moreover, our data support a predominance of CD8+ HCMV-specific T cells over CD4+ T cells in both breast milk and PBMCs.
Our data are in contrast with the findings from a recent study [9] but consistent with our data from human immunodeficiency virus–seropositive mothers [10], in whom a higher frequency of HCMV-specific responses is present in BMCs when compared with PBMC. The difference between our findings and the previous study can be attributed to the fact that we used intracellular cytokine staining and therefore could measure CD8+ and CD4+ T-cell responses, whereas Ehlinger et al used an IFN-γ ELISPOT assay where the responding T-cell population cannot be characterized and therefore most likely included both CD4 and CD8 responding cells. Our data also demonstrated a positive correlation between the frequency of HCMV-specific CD8+ T cells and breast milk viral load. Unlike our results, in the report by Ehlinger et al, a correlation between viral load and a HCMV-specific response was not found. These differences can be explained by the differences in the assays used. Again the IFN-γ ELISpot assay included CD4+ T cells, which could negate the correlation seen with CD8 because we don’t see a correlation with CD4+ T cells.
The fact that breast milk contains a large number of viable T cells raises the question as to their role in breast and infant immunity. The presence of HCMV-specific CD8 T cells in breast milk suggests that BMCs should have the ability to control pathogens within the breast. However, the data presented in this report strongly suggest that instead of local HCMV control, the presence of virus serves as a means to recruit HCMV-specific T cells into the breast milk. These data then raise the interesting possibility that these cells may have some function in the infant and warrant further investigation.
Our study shows that HCMV-specific responses consist predominantly of CD8 T cells and the presence of virus in the breast milk is associated with an increased frequency of these cells. Further studies are needed to clarify the role of cell-mediated immunity in breast milk in the control of virus replication and the forward transmission of HCMV to the infant.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. All flow cytometry experiments were performed by the University of Alabama at Birmingham, Center for AIDS Research (UAB CFAR) Flow Cytometry Core/Joint UAB Flow Cytometry Facility, which are funded in part by NIH/NIAID P30 AI27767. We thank Leslie Roop and Misty Purser for procuring breast milk samples and Karan Singh for statistics.
Funding. This work was supported by National Institutes of Health grant 1R01 AI109001-01A1 and AMC21 grants to S. B. B.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 6th International Congenital CMV Conference, Noordwijkerhout, the Netherlands, 3 May 2017. Abstract 135.
References
- 1. Morton CC, Nance WE. Newborn hearing screening—a silent revolution. N Engl J Med 2006; 354:2151–64. [DOI] [PubMed] [Google Scholar]
- 2. Krause PR, Bialek SR, Boppana SB et al. Priorities for CMV vaccine development. Vaccine 2013; 32:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jonjić S, Pavić I, Polić B, Crnković I, Lucin P, Koszinowski UH. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J Exp Med 1994; 179:1713–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reddehase MJ, Mutter W, Münch K, Bühring HJ, Koszinowski UH. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol 1987; 61:3102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Einsele H, Roosnek E, Rufer N et al. Infusion of cytomegalovirus (CMV)–specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 2002; 99:3916–22. [DOI] [PubMed] [Google Scholar]
- 6. Walter EA, Greenberg PD, Gilbert MJ et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med 1995; 333:1038–44. [DOI] [PubMed] [Google Scholar]
- 7. Macesic N, Langsford D, Nicholls K et al. Adoptive T cell immunotherapy for treatment of ganciclovir-resistant cytomegalovirus disease in a renal transplant recipient. Am J Transplant 2015; 15:827–32. [DOI] [PubMed] [Google Scholar]
- 8. Gordon CL, Miron M, Thome JJ et al. Tissue reservoirs of antiviral T cell immunity in persistent human CMV infection. J Exp Med 2017; 214:651–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ehlinger EP, Webster EM, Kang HH et al. Maternal cytomegalovirus-specific immune responses and symptomatic postnatal cytomegalovirus transmission in very low-birth-weight preterm infants. J Infect Dis 2011; 204:1672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sabbaj S, Edwards BH, Ghosh MK et al. Human immunodeficiency virus-specific CD8(+) T cells in human breast milk. J Virol 2002; 76:7365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sabbaj S, Ghosh MK, Edwards BH et al. Breast milk-derived antigen-specific CD8+ T cells: an extralymphoid effector memory cell population in humans. J Immunol 2005; 174:2951–6. [DOI] [PubMed] [Google Scholar]
- 12. Sabbaj S, Pass RF, Goepfert PA, Pichon S. Glycoprotein B vaccine is capable of boosting both antibody and CD4 T-cell responses to cytomegalovirus in chronically infected women. J Infect Dis 2011; 203:1534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boppana SB, Ross SA, Novak Z et al. ; National Institute on Deafness and Other Communication Disorders CMV and Hearing Multicenter Screening (CHIMES) Study Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA 2010; 303:1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boppana SB, Ross SA, Shimamura M et al. ; National Institute on Deafness and Other Communication Disorders CHIMES Study Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N Engl J Med 2011; 364:2111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.