Prototype hepatitis B virus (HBV)-derived synthetic long peptide (SLP) cross-presented by autologous dendritic cells boosted HBV-specific (CD4,CD8) T-cell responses in chronic HBV (CHB) patients ex vivo. Often, PD-L1 blockade improved SLP-responses. This supports therapeutic SLPbased vaccine development for CHB treatment.
Keywords: cross-presentation, synthetic long peptide vaccine, HBV, T cell
Abstract
Background
Vaccination with synthetic long peptides (SLP) is a promising new treatment strategy for chronic hepatitis B virus (CHB). SLP can induce broad T-cell responses for all HLA types. Here we investigated the ability of a prototype HBV-core (HBc)-sequence-derived SLP to boost HBV-specific T cells in CHB patients ex vivo.
Methods
HBc-SLP was used to assess cross-presentation by monocyte-derived dendritic cells (moDC) and BDCA1+ blood myeloid DC (mDC) to engineered HBV-specific CD8+ T cells. Autologous SLP-loaded and toll-like receptor (TLR)-stimulated DC were used to activate patient HBc-specific CD8+ and CD4+ T cells.
Results
HBV-SLP was cross-presented by moDC, which was further enhanced by adjuvants. Patient-derived SLP-loaded moDC significantly increased autologous HBcAg18-27-specific CD8+ T cells and CD4+ T cells ex vivo. HBV-specific T cells were functional as they synthesized tumor necrosis factor-alpha and interferon-gamma. In 6/7 of patients blockade of PD-L1 further increased SLP effects. Also, importantly, patient-derived BDCA1+ mDC cross-presented and activated autologous T-cell responses ex vivo.
Conclusions
As a proof of concept, we showed a prototype HBc-SLP can boost T-cell responses in patients ex vivo. These results pave the way for the development of a therapeutic SLP-based vaccine to induce effective HBV-specific adaptive immune responses in CHB patients.
About one-third of the world’s population is exposed to hepatitis B virus (HBV) during their lifetime. Although most people effectively clear the virus, chronic HBV (CHB) still affects about 250 million people worldwide [1]. Because effective therapy is lacking, yearly around 1 million people die of HBV-related liver failure, liver cirrhosis, and liver cancer [2]. Therefore, there is a high need for more effective therapy to treat CHB.
CD8+ cytotoxic T lymphocytes play a crucial role in viral defense by directly killing virus-infected cells and by producing antiviral cytokines. CD4+ helper T cells facilitate induction of optimal and long-lasting CD8+ T-cell responses and exert antiviral effector function themselves [3, 4]. In contrast to HBV-resolvers, chronic patients have very few functional HBV-specific T cells, and this is thought to be responsible for viral persistence [5, 6]. Previous studies on bone marrow and liver transplantation demonstrated that CHB infection can be cleared by a fully functional immune system, which supports the concept that therapeutic restoration of antiviral immunity in chronic patients could lead to disease resolution.
Dendritic cells (DC) play a crucial role in inducing T-cell responses. DC have the unique capacity to present exogenous antigens not only in MHC class II to activate CD4+ T cells, but also to digest these antigens into short peptides and present them in MHC class I to activate CD8+ T cells, a process called cross-presentation [7]. Synthetic long peptides (SLP) are designed to make use of these unique capacities of DC to boost/ induce T-cell responses, and have already been very effective in patients that suffer from HPV-induced (pre)malignant lesions [8], metastatic colorectal cancer [9], or ovarian cancer [10]. SLP are typically, and most optimally, 20–45 amino acids in length and contain epitopes for both CD8+ and CD4+ T cells, the latter facilitating optimal, long-lasting CD8+ T-cell responses [11]. For SLP, in contrast to short peptides or DNA, presentation of viral peptides by nonprofessional antigen presenting cells is excluded. Importantly, in vivo presentation of epitopes from SLP by DC was more efficient and longer lived than from whole protein [11]. Adjuvants can further improve SLP vaccines by enhancing the immune response [12] and importantly are required to prevent the induction of tolerance upon naive T-cell priming. Toll-like receptor 2 (TLR2) ligands boosted the effectiveness of ovalbumin- and murine leukemia virus-based SLP in mice and HPV-based SLP in humans [12, 13]. Also, TLR3 ligands have particular potential to aid antiviral immune responses because TLR3 is highly expressed in myeloid DC subsets with high cross-presenting capacities [14].
Finally, for immunotherapy development it is essential to consider the suppressive immune context in CHB patients. The immune system in these patients is worn out by chronic infection and continuous antigen exposure. As a result, DC function can be impaired [14, 15] and HBV-directed T cells may be exhausted [16, 17]. Animal models for chronic viral infections (including HBV) demonstrated that blocking PD-1/ PD-L1, as typical molecules related to exhaustion, can enhance vaccine success [18].
We hypothesize that SLP vaccination, with or without additional immune modulation, can ultimately induce HBV-specific immune responses leading to full viral elimination, thereby preventing fatal liver disease. Therefore, we designed a prototype SLP, based on HBV core protein (HBcAg). Using this SLP, we investigated the ability of CHB patient DC to (cross-)present and to stimulate autologous HBV-specific T cells ex vivo. Additionally, the effect of adjuvants and PD-L1/PD-1 blockade were examined. Our results demonstrate that the prototype HBc-SLP can boost HBV-directed T-cell responses ex vivo, putting forward SLP-based vaccination as a promising treatment strategy for CHB.
MATERIALS AND METHODS
All detailed information about material and methods is provided in the section Supplementary Methods.
Prototype hepatitis B virus (HBV)-derived synthetic long peptide (SLP) cross-presented by autologous dendritic cells boosted HBV-specific (CD4,CD8) T-cell responses in chronic HBV (CHB) patients ex vivo. Often, PD-L1 blockade improved SLP-responses. This supports therapeutic SLP-based vaccine development for CHB treatment.
RESULTS
Efficient Cross-Presentation by Human moDC Loaded With SLP
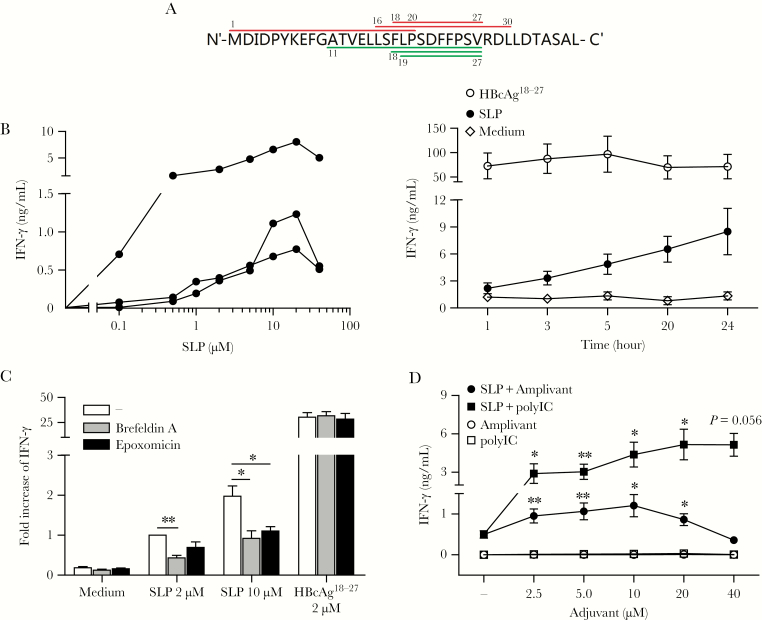
We designed a prototype HBc-SLP of 37 amino acids long, a sequence derived from the conserved region of HBV genotype A, including the immunodominant HLA-A*02 restricted CD8+ T-cell epitope HBcAg18-27, and 2 other published CD8+ T-cell epitopes and 3 CD4+ epitopes (Figure 1A).
Figure 1.
Cross-presentation by human monocyte-derived dendritic cells (moDC). A, Sequence of amino acids constituting the hepatitis B virus core–synthetic long peptides (HBc-SLP1-37). Upper (red online) lines indicate reported CD4+ T-cell epitopes, which are the HLA-DR restricted HBcAg1-20 epitope, the HLA-DR restricted HBcAg16-30 epitope, and the HLA-DP restricted HBcAg18-27 epitope. Lower (green online) lines indicate reported CD8+ T-cell epitopes, which are the HLA-A*02 restricted HBcAg18-27 epitope, the HLA-B*51 restricted HBcAg19-27 epitope, and the HLA-A*02 restricted HBcAg11-27 epitope. B, SLP dose titration and loading kinetics on moDC, read out by HBcAg18-27 CD8+ T-cell activation. Left panel: interferon-gamma (IFN-γ) production (by enzyme-linked immunosorbent assay, ELISA) upon coculture of in vitro engineered HBcAg18-27 CD8+ T cells with moDC of 3 different donors exposed to increasing concentrations of HBc-SLP. Each line represent data from a different donor. Right panel: IFN-γ production upon 20-hour coculture of HBcAg18-27-specific T cells with moDC pulsed for indicated time points with 2 µM HBc-SLP or HBcAg18-27 short peptide. Mean ± SEM. N = 4 donors. C, IFN-γ production upon 20-hour coculture of engineered HBcAg18-27 specific T cells with moDC pulsed with indicated concentration of antigens in the presence or absence of epoxomicin or Brefeldin A. moDC were fixed with 0.2% paraformaldehyde prior to the start of coculture. Mean + SEM. N = 4 donors. IFN-γ levels were normalized to the amount induced by SLP only (2 µM) in each experiment. Statistical analysis based on log transformed raw data. D, IFN-γ production upon 20-hour cocultured engineered HBcAg18-27-specific T cells with moDC pulsed for 20 hours with HBc-SLP (10 µM) either in the absence or presence of increasing concentrations of Amplivant or PolyI:C. Mean ± SEM. N = 4 donors. C, D *P < .05, **P < .01 by 1-tailed paired t tests.
To visualize antigen presentation by DC, we generated a novel HBcAg18-27-specific CD8+ T-cell readout system by retroviral transduction of the HBcAg18-27 cognate T-cell receptor (TCR), described by Gehring et al, into a CMV-pp65495-503-specific CD8+ T-cell clone with high expansion capacity and functionality (Supplementary Figure 1A, B) [19]. Having confirmed the sensitivity of the generated HBcAg18-27recognizing CD8+ T cells (Supplementary Figure 1C), we tested the ability of SLP-loaded DC to present the HBcAg18-27 epitope. SLP-loaded moDC induced interferon-gamma (IFN-γ) production by HBcAg18-27-specific CD8+ T cells in all donors, indicating the epitope was readily processed and cross-presented (Figure 1B). Dose titration revealed that IFN-γ production increased with higher SLP concentrations. Optimal cross-presentation was reached at a concentration of 10—20 µM HBc-SLP (Figure 1B). At higher SLP concentrations, T-cell activation again decreased, likely by a negative effect of the solvent dimethyl sulfoxide (DMSO) on DC function (not shown). Presentation of HBcAg18-27 by SLP-loaded DC increased with time, whereas presentation of short HBcAg18-27 peptide did not (Figure 1B).
To demonstrate that release of the HBcAg18-27 epitope from HBc-SLP depended on intracellular processing by moDC, we inhibited intracellular protein transport or the proteasome. Blocking transport of peptide/MHC-I complexes from endoplasmic reticulum to the cell surface with Brefeldin A resulted in a significant reduction in SLP cross-presentation (Figure 1C). Also a significant reduction was observed by the proteasome inhibitor epoxomicin (Figure 1C). As expected, presentation of HBcAg18-27 short peptide, which does not require internalization or proteasomal processing, was unchanged by these inhibitors. Together these findings confirm that processed and subsequent cross-presentation of HBcAg18-27 epitope from SLP by DC required proteasome activity and intracellular transport. To obtain a maximum response while minimizing negative effects of DMSO, we continued with 10 µM SLP and 20 hours of peptide loading in following experiments.
Subsequently, we assessed whether TLR2-ligand Amplivant or TLR3-ligand PolyI:C enhanced cross-presentation of the SLP-contained HBcAg18-27 epitope. Both adjuvants induced upregulation of costimulatory markers CD83, CD86 (Supplementary Figure 2A), and cytokine production by DC (Supplementary Figure 2B). Concordantly, both adjuvants significantly enhanced SLP-induced activation of HBcAg18-27-specific CD8+ T cells in a dose-dependent manner (Figure 1D).
These data show that SLP are efficiently cross-presented by moDC and that both Amplivant and PolyI:C further enhance cross-presentation and activation of antigen-specific T cells.
SLP-Induced Patient-Derived HBV-Specific CD8+ T-Cell Proliferation Ex Vivo
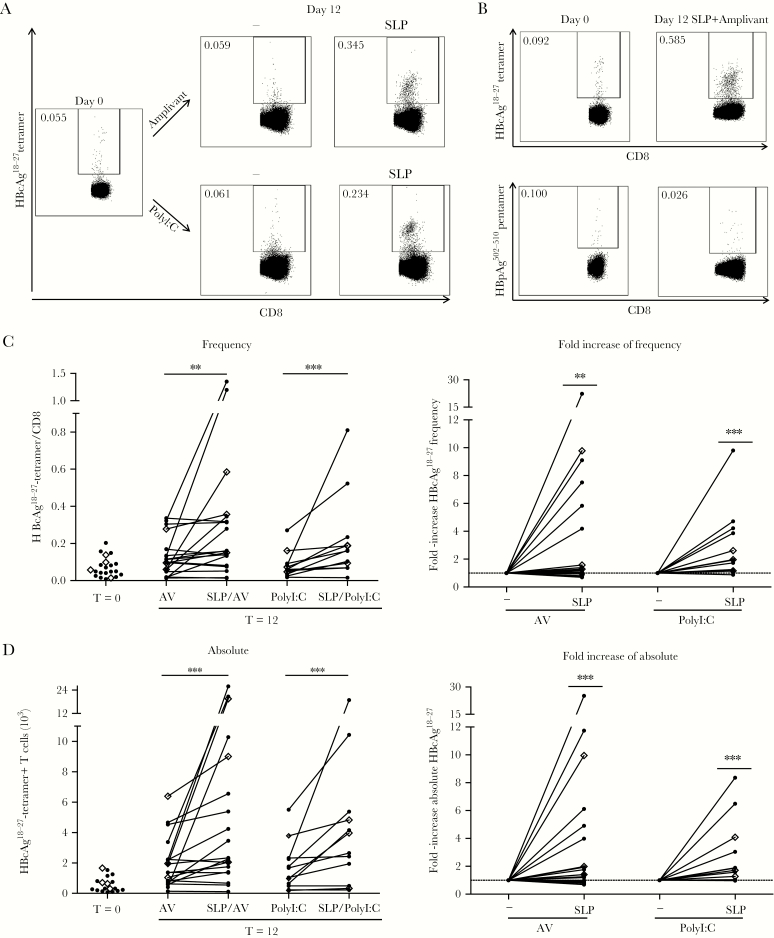
To assess the potential of our SLP to boost T-cell responses in CHB patients, we analyzed the capacity of HBc-SLP-loaded patient-derived moDC to activate autologous HBV-specific T cells ex vivo. After coculturing patient PBLs (monocytes and B cell-depleted-PBMC, hereinafter referred to as PBLs. See Supplementary Methods) for 12 days with SLP-loaded moDC in the presence of Amplivant or PolyI:C, both the frequency (Figure 2A–C; 3.6 ± 5.3 and 2.9 ± 2.5-fold, respectively) and absolute numbers (Figure 2D; 4.0 ± 5.9 and 2.8 ± 2.4-fold, respectively) of HBcAg18-27-specific CD8+ T cells significantly increased compared to day 0 (Figure 2) and also compared to a 12-day coculture with adjuvants alone (Figure 2C, D). In some patients only absolute numbers, but not frequencies, of HBcAg18-27-specific T cells were augmented, suggesting additional proliferation of HBV-specific CD8+ T cells recognizing other SLP-contained epitopes. Importantly, irrelevant HBpol502-510-specific CD8+ T cells did not increase (Figure 2B).
Figure 2.
Patient-derived HBcAg18-27-specific CD8+ T-cell induction by hepatitis B virus–synthetic long peptides (HBV-SLP) stimulation ex vivo. A, Representative tetramer staining of HBcAg18-27-specific CD8+ T cells in peripheral blood lymphocytes from a chronic hepatitis B virus patient before (day 0) and after 12-day culture with autologous monocyte-derived dendritic cells (moDC) loaded with Amplivant ± SLP (upper panels) or PolyI:C ± SLP (lower panels). B, Patient cultures as in (A) for indicated conditions, now comparing multimer staining for HBcAg18-27-specific CD8+ T cells and irrelevant HBpAg502-510-specific CD8+ T cells. Representative of N = 7 donors. C, Left panel: frequency of HBcAg18-27-specific CD8+ T cells among total CD8+ T cells before and after 12-day cultures performed as described in (A). Right panel: relative change caused by SLP in the frequency of HBcAg18-27-specific CD8+ T cells compared to stimulation with adjuvant alone. n = 19 for Amplivant and n = 12 for PolyI:C. D, Data as in (C) but for absolute HBcAg18-27-specific CD8+ T-cells numbers. Samples from 1 individual are connected by a line (C, D). Diamonds (◊) represent treatment-naive patients. * P < .05, **P < .01, ***P < .001 by Wilcoxon signed rank test (1-tailed) on raw data. Abbreviation: AV, Amplivant.
Of note, obvious differences in response rate/level between naive patients or those treated with nucleoside analogs were not found (Figure 2). Taken together, we demonstrate that SLP-loaded patient-derived moDC can effectively expand autologous HBV-specific CD8+ T cells ex vivo.
Functionality of SLP-Expanded HBcAg18-27-Specific CD8+ T Cells
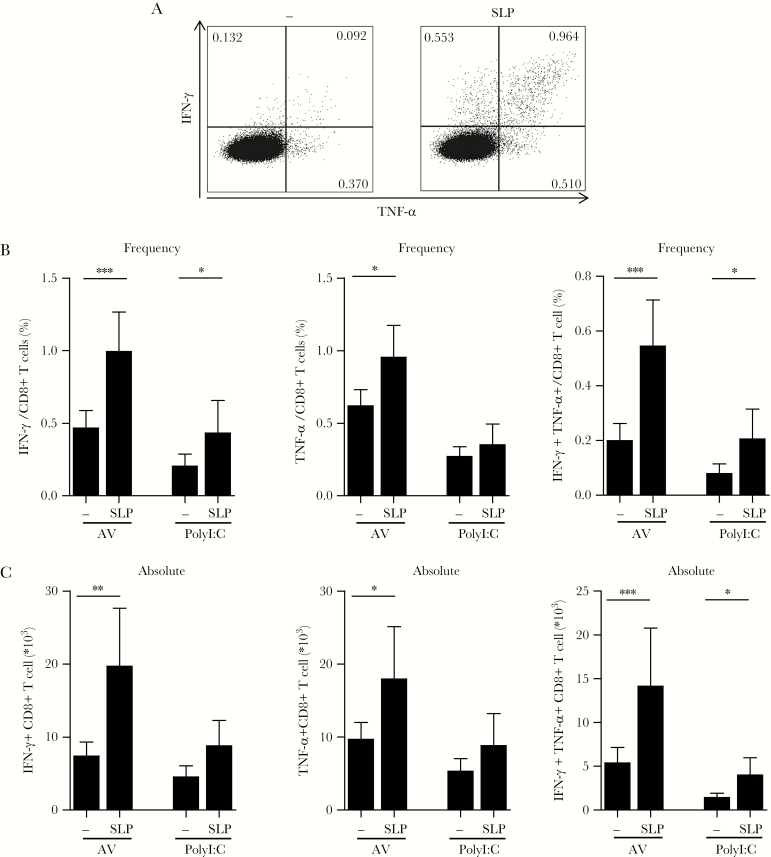
To determine the functionality of SLP-induced HBcAg18-27-specific CD8+ T cells, 12-day expanded PBLs were restimulated with HBcAg18-27 short peptide. We identified a clear population of IFN-γ and tumor necrosis factor-alpha (TNF-α)- producing CD8+ T cells. Importantly, both frequencies and absolute numbers of functional T cells were significantly larger in cultures where SLP had been present during the expansion phase and this was most pronounced for multifunctional T cells expressing both IFN-γ and TNF-α (Figure 3A–C). The SLP effect was most significant for cultures with Amplivant (Figure 3). In a subset of experiments the effect of SLP without adjuvants was also tested (Supplementary Figure 3). Unexpectedly, in patient cell-derived cultures the effect of adjuvant was not clear (Supplementary Figure 3), which may be due to the long cytokine supplemented culture protocol, the readout used, and/ or the relative high concentration of SLP (10 µM) used. Nonetheless, our experiments clearly indicate SLP can augment HBV-specific functional CD8+ T cells ex vivo.
Figure 3.
Patient-derived functional hepatitis B virus (HBV)-specific CD8+ T-cell induction by HBV-synthetic long peptides (SLP) stimulation ex vivo. A, T cells were restimulated with HBcAg18-27 peptide after an initial 12-day expansion culture by Amplivant ± SLP. Representative dot plots from an intracellular cytokine staining for interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) production in CD8+ T cells are shown. B, C, Summary of the mean relative (B) and absolute cell numbers (C) of cytokine-producing CD8+ T cells upon restimulation with HBcAg18-27 peptide after an initial 12-day expansion culture by Amplivant ± SLP and by PolyI:C ± SLP. Depicted are mean + SEM for 12 (Amplivant) and 6 (PolyI:C) different CHB patients. * P < .05, **P < .01, ***P < .001 by 1-tailed Wilcoxon signed rank test. Abbreviation: AV, Amplivant.
HBc-SLP-Loaded moDC Induce Functional CD4+ T Cells
Key to SLP is that they can contain multiple CD4+ and CD8+ T-cell epitopes [11]. We selected patients based on HLA-A*02, but not on HLA class II genotype. The absence of HLA class II typing makes searching for specific CD4+ T-cell responses difficult. Nonetheless, our data strongly indicated HBV-specific CD4+ T-cell responses were also boosted. First, total CD4+ T-cell numbers increased upon HBV-SLP exposure, especially in the presence of Amplivant (Supplementary Figure 4). More importantly, upon restimulation of the 12-day culture that was stimulated by SLP-loaded moDC with either adjuvant in the expansion phase, a significant increase in cytokine-producing CD4+ T cells was observed, in both frequency and absolute number, compared to adjuvant alone (Figure 4). Of note, increase in functional CD4+ T-cell numbers were also observed when T cells were stimulated with SLP alone (Supplementary Figure 4). Again, we found no indication of differences in HBV-specific CD4+ T responses between untreated and treated patients (Figure 4).
Figure 4.
Patient-derived functional HBV-specific CD4+ T-cell induction by hepatitis B virus-synthetic long peptides (HBV-SLP) stimulation ex vivo. A, After an initial 12-day expansion culture with either SLP + Amplivant (SLP) or Amplivant-exposed (−) moDC, cells were restimulated with SLP-loaded autologous moDC. Shown are representative dot plots of cytokine production by intracellular cytokine staining for interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) production in CD4+ T cells. B, Summary of the mean + SEM frequency (upper row) and absolute cell numbers (lower row) of cytokine-producing CD4+ T cells from CHB patients upon restimulation of T cells with SLP-loaded autologous monocyte-derived dendritic cells (moDC) after an initial 12-day expansion culture by Amplivant ± SLP (n = 11) or by PolyI:C ± SLP (n = 5). C, Relative change in frequency and (D) absolute cell count of CD4+ cytokine producing (ie, IFN-γ + TNF-α) T cells after restimulation with SLP-loaded moDC. SLP plus adjuvant was compared to adjuvant alone during initial culture phase. Samples from 1 individual are connected by a line. Diamonds (◊) represent treatment-naive patients. * P < .05, **P < .01, ***P < .001 by Wilcoxon signed rank test (1-tailed) on raw data. Abbreviation: AV, Amplivant.
Blockade of PD-1/PD-L1 can Further Increase the Response to SLP
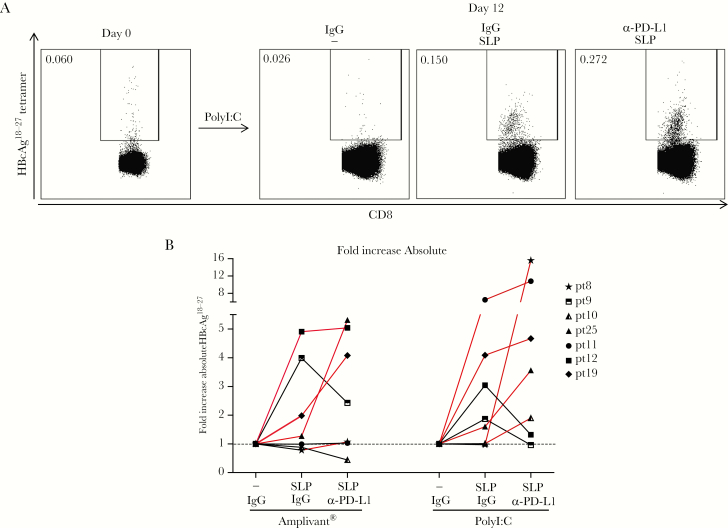
Previous studies showed that antiviral responses of peripheral blood and intrahepatic T cells can be improved by blocking PD-1/PD-L1 interaction [5, 16, 20–22]. To investigate whether this also improved SLP effect, patient PBLs were expanded by SLP-loaded autologous moDC for 12 days as before, but now in the presence of PD-L1 blocking or isotype control antibodies. PD-L1 blockade improved CD8+ T-cell responses in both Amplivant and polyI:C stimulated cultures in 4 and 5 out of 7 patients, respectively, up to 16-fold (Figure 5). We found no relationship between the effect of PD-L1 blockade and patient characteristics (ethnicity, age, gender), viral parameters (viral load, alanine aminotransferase [ALT], HBsAg), or T-cell PD-1 expression (not shown). Overall, these data demonstrate the effect of PD-L1 blockade was heterogeneous between patients, but indicate that for some patients PD-1/PD-L1 blockade may improve SLP vaccine efficacy.
Figure 5.
PD-L1 blockade further boosts expansion of synthetic long peptides (SLP)-induced hepatitis B virus (HBV)-specific T cells. A, Representative dot plots of flow cytometric analyses of HBcAg18-27-specific CD8+ T cells from a chronic HBV (CHB) patient before (day 0) and after a 12-day expansion culture by PolyI:C ± SLP, either in the presence of PD-L1 blocking antibodies or isotype control antibodies. B, Summary of absolute numbers of HBcAg18-27-specific CD8+ T cells in 7 CHB patient samples upon SLP-driven expansion in the presence or absence of PD-L1 blocking or isotype control antibodies relative to adjuvant alone (set to 1). Data without blocking antibody are also included in Figure 2. Samples from 1 individual are connected by a line. Patients for which a further increase of tetramer-specific CD8+ T cells was observed by PD-L1 blockade are emphasized in red (online).
BDCA1+ mDC Derived From HBV Patients and Healthy Controls Cross-Present HBV-SLP to a Similar Extent and Stimulate Autologous HBcAg-Specific T Cells Ex Vivo
Having established that patient-derived T cells can be boosted by HBc-SLP-loaded in vitro generated moDC, we next assessed whether also circulating myeloid DC (mDC) are able to effectively present the SLP, because the SLP vaccine will rely on these cells in vivo. So far, no conclusive data exist on the effect of CHB infection on the cross-presenting capacity of primary mDC. Gehring et al demonstrated a remaining ability of patient-derived mDC to cross-present HBsAg protein [23], but data directly comparing the cross-presentation capacity of patient mDC to healthy control-derived mDC were not shown. Therefore, we assessed the cross-presenting ability of primary BDCA1+ mDC from CHB patients, and compared them to cells from gender-matched healthy subjects (Supplementary Table 1). We selected CHB patients with low viral load and low serum ALT levels (Table 1) as we reasoned this patient population will be most suitable for initial clinical testing of SLP vaccine considering safety and immune fitness.
Table 1.
Characteristics of Chronic HBV Patients Included in the Study
| Patient number | Viral load (IU/ mL) | ALT (IU/L) | HBeAg | Fibrosis | Genotype | Ethnicity | Current treatment | Gender | Age (y) | |
|---|---|---|---|---|---|---|---|---|---|---|
| T-cell proliferation assay in Figures 2–5 | pt8 | 2.0E1 | 17 | neg | F0-F1 | C | Asian | Entecavir | M | 53 |
| pt9 | 4.8E3 | 47 | neg | F0 | A | African | Tenofovir | M | 29 | |
| pt10 | 4.0E1 | 54 | neg | F2 | D | Caucasian | Tenofovir | M | 52 | |
| pt11 | 2.0E1 | 19 | neg | F0-F1 | B | Asian | Entecavir | M | 46 | |
| pt12 | 2.0E1 | 8 | neg | F0 | D | Turkey | Entecavir | F | 48 | |
| pt13 | 2.0E1 | 49 | neg | F0-F1 | A | African | Tenofovir | M | 39 | |
| pt14 | 2.0E1 | 16 | neg | F1-F2 | B | Asian | Entecavir | F | 31 | |
| pt15 | 2.0E1 | 17 | neg | F0 | C | Asian | Entecavir | M | 56 | |
| pt16 | 2.0E1 | 14 | neg | F0 | A&G | Caucasian | Entecavir | M | 59 | |
| pt17 | 2.0E1 | 36 | neg | F0-F1 | A | Caucasian | Tenofovir | M | 26 | |
| pt18 | 2.0E1 | 11 | neg | F0-F1 | n.d. | African | Tenofovir | M | 56 | |
| pt19 | 1.0E1 | 49 | neg | F0-F1 | n.d. | African | Naive | M | 54 | |
| pt20 | 3.1E2 | 21 | neg | F1 | n.d. | Asian | Naive | F | 49 | |
| pt21 | 4.2E0 | 59 | neg | F0-F1 | n.d. | Turkey | Lamivudine | M | 31 | |
| pt22 | 3.1E2 | 26 | neg | F0-F1 | D | Caucasian | Naive | F | 59 | |
| pt23 | 1.4E2 | 33 | neg | F0-F1 | E | African | Naive | M | 37 | |
| pt24 | 2.5E2 | 44 | neg | F1 | n.d. | Asian | Naive | F | 41 | |
| pt25 | 2.0E1 | 26 | pos | F0-F1 | G | Asian | Tenofovir | M | 40 | |
| pt26 | 2.0E1 | 24 | pos | F0-F1 | D | Asian | Tenofovir | M | 34 | |
| pt27 | 8.0E3 | 25 | neg | F0-F1 | A | Caucasian | Naive | F | 46 | |
| pt28 | 2.0E1 | 22 | neg | F2 | C | Asian | Entecavir | F | 48 | |
| pt29 | 5.5E2 | 29 | neg | F0-F1 | n.d. | Caucasian | Treated before | M | 48 | |
| pt30 | 1.5E2 | 30 | neg | F1 | n.d. | Asian | Treated before | F | 63 | |
| pt31 | 2.0E1 | 17 | neg | F0-F1 | n.d. | Asian | Entecavir | M | 47 | |
| pt32 | 2.0E1 | 22 | neg | F2 | D | Caucasian | Entecavir | F | 62 | |
| pt33 | 2.0E1 | 25 | neg | F0-F1 | n.d. | Asian | Tenofovir | F | 48 | |
| pt34 | 2.0E1 | 17 | neg | F0-F1 | A | Caucasian | Tenofovir | F | 55 | |
| pt35 | 4.0E1 | 21 | neg | F2 | n.d. | Asian | Entecavir | M | 61 | |
| BDCA1+mDC cross-presentation experiment in Figure 6A | pt1 | 4.1E4 | 55 | neg | F1-F2 | n.d. | African | Tenofovir | M | 29 |
| pt2 | 2.0E1 | 19 | neg | F0-F1 | B | Asian | Entecavir | M | 45 | |
| pt3 | 2.0E1 | 27 | neg | F0-F1 | D | Asian | Entecavir | M | 54 | |
| pt4 | 2.0E1 | 16 | neg | F0-F1 | n.d. | African | Naive | F | 28 | |
| pt5 | 2.9E2 | 17 | neg | F0-F1 | A | African | Entecavir | M | 51 | |
| pt6 | 2.6E3 | 26 | pos | F0 | B | Asian | Tenofovir | F | 30 | |
| pt7 | 1.9E2 | 100 | pos | F1-F2 | D | Asian | Tenofovir | M | 43 | |
| T-cell proliferation assay in Figure 6B, C | pt36 | 1.8E3 | 19 | neg | F0-F1 | n.d. | Asian | Treated before | F | 36 |
| pt37 | 2.5E1 | 25 | neg | F0 | D | Asian | Treated before | M | 33 | |
| pt38 | 2.8E4 | 46 | neg | F2 | n.d. | Asian | Treated before | M | 35 | |
| pt39 | 2.0E1 | 17 | neg | F0-F1 | n.d. | African | Tenofovir | M | 58 |
M, male; F, female; n.d., not determined; ALT, alanine aminotransferase. Typically the range for normal ALT is between 7 and 56 units per liter.
Naive = never treated.
BDCA1+ mDC were loaded with SLP with or without adjuvants and analyzed for their capacity to cross-present and activate HBcAg18-27-specific CD8+ T cells. Exogenous binding of HBcAg18-27 short peptide to HLA-A*02 resulted in similar activation of HBcAg18-27-specific T cells by patient and healthy control mDC (Supplementary Figure 5). To correct for variation between donors, T-cell responses were normalized to those observed after incubation of T cells with HBcAg18-27-loaded BDCA1+ mDC of each donor in the same experiment. Importantly, patient-derived BDCA1+ mDC cross-presented HBcAg18-27 epitope from HBc-SLP equally well as those from healthy controls (Figure 6A). Both adjuvants significantly improved T-cell activation in healthy controls and patients to a similar extent.
Figure 6.
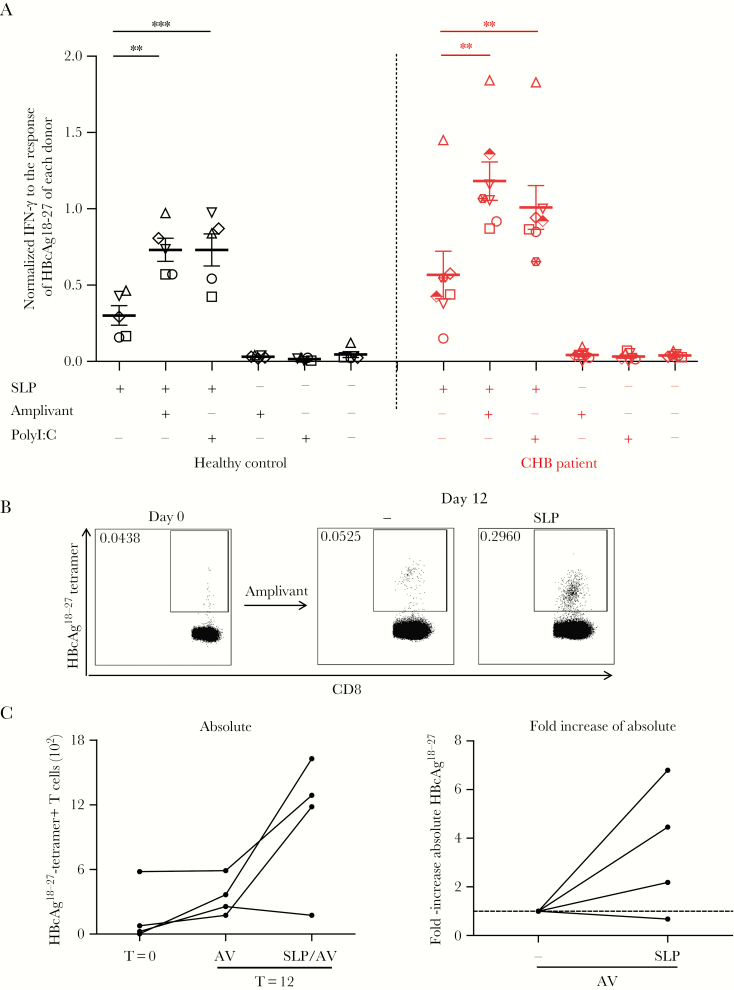
Patient- and healthy control-derived BDCA1+ myeloid DC (mDC) cross-present hepatitis B virus-synthetic long peptides (HBV-SLP) and stimulate autologous HBcAg-specific T cells. A, Interferon-gamma (IFN-γ) production (by enzyme-linked immunosorbent assay, ELISA) upon 20-hour coculture of engineered HBcAg18-27-specific CD8+ T cells with BDCA1+ mDC from chronic hepatitis B virus (CHB) patients (n = 7) or healthy controls (n = 5) pulsed with SLP ± adjuvant or adjuvant alone for 20 hours. For each donor, data were normalized to IFN-γ levels produced in cocultures with BDCA1+ mDC loaded with short HBcAg18-27 peptides (see also Supplementary Figure 5). Each symbol represents a donor. Mean ± SEM. *P < .05, **P < .01, ***P < .001 by 1-tailed paired t tests on log transformed raw data. B, Representative tetramer staining of HBcAg18-27-specific CD8+ T cells in peripheral blood lymphocytes from a CHB patient before and after 12-day culture with autologous BDCA1+mDC loaded with SLP + Amplivant (AV), or Amplivant only. C, Left panel: absolute numbers of HBcAg18-27-specific CD8+ T cells before and after 12-day cultures as described in (B). Each line represents a different patient (N = 4). Right panel: SLP-induced relative change in absolute numbers of HBcAg18-27-specific CD8+ T cells for each patient compared to adjuvant alone. Samples from 1 individual are connected by a line.
Next, we tested whether SLP-loaded patient BDCA1+mDC are also able to activate autologous T cells. As observed for moDC, BDCA1+mDC loaded with SLP in the presence of Amplivant, induced a clear increase in HBcAg18-27-specific T cells as compared to day 0, and day 12 with Amplivant alone in 3 out of 4 CHB patients (Figure 6B, C; 3.5 ± 2.7-fold).
These data demonstrate that CHB-patient DC are able to cross-present HBc-SLP and activate endogenous T-cell responses. Therefore patient mDC can be targeted by an SLP-based therapeutic vaccine to activate HBV-specific T cells in vivo.
DISCUSSION
Antigen-specific immunotherapy represents an attractive treatment option for CHB [24]. Vaccines aiming to treat established disease require long-lived presentation of MHC-epitopes by appropriately activated DC and should induce both virus-specific CD8+ and CD4+ T-cell responses. The latter can strengthen and prolong CD8+ T-cell responses and also facilitate antibody responses [25]. One high-potential form of clinically validated immunotherapy is SLP-based vaccination [11]. To obtain proof of principle, we designed an SLP based on a stretch of HBcAg protein containing the HBcAg18-27 HLA-A*02 epitope and additional CD8+ and CD4+ T-cell epitopes naturally flanking the HBcAg18-27 epitope. The present study provides proof of concept and demonstrates that SLP can be effectively cross-presented by patient-derived BDCA1+ mDC and that adjuvants such as Amplivant and PolyI:C can further enhance this. Furthermore, the HBc-SLP boosted functional patient HBV-directed CD4+ and CD8+ T-cell responses in multiple patients ex vivo, which could be further enhanced by PD-L1 blockade.
Apart from providing proof-of-concept for SLP-based therapeutic vaccination in CHB, we generated a tool, that is dual specific HBcAg18-27-TCR transduced CMV-specific CD8+ T cells, to easily visualize (cross-)presentation by DC. Advantages of this novel read-out system include the high production of IFN-γ by these cells upon cognate antigen recognition, and the possibility to expand them, which is extremely difficult with CHB patient-derived T-cell clones, which are often exhausted. Responses of these engineered T cells to endogenous CMV presented by antigen presenting cells can be a risk theoretically; however, cultures without exogenous antigen resulted in only a few experiments in detectable IFN-γ production, which was always clearly below conditions with HBV-antigens. Therefore, we can exclude a significant influence of endogenously presented CMV on our conclusions. This system can aid HBV research and also the study of immune responses against other infectious diseases and cancer.
In contrast to short peptides that can directly bind to HLA molecules, SLP require intracellular processing. Hence, presentation of SLP-derived epitopes is restricted to professional antigen presenting cells, such as DC. Bystander cells that are not equipped to deliver the necessary signals for proper T-cell activation and therefore can induce tolerance to presented antigens, cannot process SLP and extract the contained epitopes [11, 25]. Furthermore, SLP are able to induce polyclonal CD8+ T-cell responses because they can harbor several epitopes. Moreover, as opposed to short peptides, SLP can contain epitopes for multiple HLA alleles. Therefore SLP vaccines can be designed to accommodate most of the population.
Studies using HBcAg18-27 short peptide to stimulate peripheral cognate CD8+ T cells from CHB patients with low viral load and ALT, have shown variable responses after 10–14 days expansion in vitro, ranging from 0.01% to 14.9% of total CD8+ T cells [5, 20, 21, 26, 27]. Effects on absolute T-cell numbers were barely reported. In our study, SLP-induced frequencies varied between 0.02% and 6.56% of total CD8+ T cells. The lower HBcAg18-27-specific CD8+ T-cell frequency may be at least in part explained by the fact that SLP-loaded moDC probably present a variety of epitopes, resulting in competition for the presentation of HBcAg18-27 epitope, but allowing also other HBc-specific T cells to respond. We observed no differences between Amplivant and PolyI:C in CD8+ T-cell induction. This is not surprising because we also observed that addition of an adjuvant does not improve the in vitro response (Supplementary Figure 3). Furthermore, although the HBcAg-derived SLP successfully expanded autologous HBcAg18-27-specific CD8+ T cells in most patients, it should be noted that the HBcAg18-27 epitope does not bind all HLA-A*02 subtypes equally well [28, 29]. It binds best to HLA-A*0201 but poorly to HLA-A*0203 [30]. Because we selected our patients by using pan-HLA-A*02 antibodies, the response in some patients may have been subject to a suboptimal HLA-A*02 subtype.
Aside from CD8 epitopes, SLP can also cover a wide range of CD4 epitopes for multiple HLA alleles. The simultaneous induction of CD4+ T cells facilitates generation of powerful and long lasting CD8+ T-cell responses [3, 4]. Despite the fact that we did not include HLA class II typing of our CHB patients, CD4+ epitopes for multiple HLA II alleles were present in the SLP. Concordantly, we detected SLP-driven proliferation of CD4+ T cells (ie, an increase in absolute numbers) and cytokine production in most patients.
Optimal maturation of DC is important for an effective (SLP)vaccine and to exclude the induction of tolerance. TLR ligands are generally well known for their capacity to boost T-cell responses in vivo [31, 32]. Each TLR ligand triggers a unique DC maturation program, resulting in a specific combination of molecules that delivers signals for antigen presentation, costimulation, and T-cell function. Therefore, it is essential to offer SLP with the right adjuvant to steer the immune response in the right direction and to prevent induction of peripheral tolerance. In mice, TLR2 ligands were effective in inducing both DC maturation and CD8+ T-cell activation [13, 33]. Also, in human SLP studies, TLR2 ligation was very effective in facilitating both CD4+ and CD8+ T-cell responses [12]. PolyI:C is an interesting alternative because of its reported effectiveness in activating myeloid DC subsets with cross-presenting abilities [14, 34]. In our study, both adjuvants enhanced cross-presentation of moDC and BDCA1+mDC to activate engineered T cells. Unexpectedly, addition of Amplivant to SLP did not further increase the number of HBV-specific T cells in patient-derived culture compared to SLP alone and also not much more functional T cells were observed. To study the additive effect of adjuvants, we should have used a different experimental set-up with additional lower/suboptimal concentrations of SLP. Most importantly, however, regardless of adjuvants and fundamental to our proof-of-concept, SLP consistently augmented HBV-specific T-cell numbers as well as functional CD4+ and CD8+ T cells. In particular, the production of TNF-α and IFN-γ is highly relevant in the context of CHB, because noncytolytic mechanisms are considered to be the most important mechanism by which CD8+ cells can suppress HBV and these cytokines are known to suppress HBV replication [35–38]. With respect to the coapplication of SLP with adjuvants, we would finally like to note that these in vitro experiments likely favor amplification of existing memory T-cell responses, which will respond much more rapidly and outcompete naive responses. In vivo, however, the vaccine may also prime naive T-cell response for which adjuvants will be crucial. As said, at the risk of inducing tolerance, SLP in the setting of HBV would never be applied without adjuvant. Our experiments so far do not argue in favor of one of the adjuvants tested, but it will be interesting to further investigate the additive effect of adjuvants on T-cell function and also on naive T-cell priming to identify clearly the optimal adjuvant for SLP vaccination. Strategies such as combining adjuvants in one regimen and conjugation of adjuvants to SLP should also be considered in future study to enhance SLP-triggered immune responses, especially in vivo [12].
Patient selection in the present study was performed on mostly HBeAg negative patients under antiviral treatment because low viral load and serum ALT levels may favor the CD8+ T-cell response, DC function as well as the function of other immune cells [14, 15, 26, 39–42]. These patients likely suffer least from the suppressive immune context found in CHB patients and thus would be the most interesting patient group for therapeutic vaccination. As demonstrated here, BDCA1+ mDC isolated from these patients are as effective as mDC isolated from healthy controls with respect to cross-presentation and induction of T-cell responses.
Nevertheless, despite the selection as described above, the immune system may still suffer from chronic infection and continuous antigen exposure such as T-cell exhaustion [27, 43]. We demonstrate that PD-1/PD-L1 blockade synergized in some patients with SLP stimulation. Due to the limited number of available samples, we could not identify any factors that could predict benefit of checkpoint blockade. Our results add to previous reports that PD-1/PD-L1 blockade can partly restore intrahepatic HBV-specific T-cell responses in vitro in CHB patients [20]. Targeting the PD-1 pathway may thus be an additional component in a multifactorial approach, combining immune modulation, vaccination, and antiviral treatment, to restore the HBV directed T-cell response [44].
To summarize, we demonstrated that SLP vaccination represents an attractive treatment modality to trigger broad multifunctional HBV-specific T-cell responses in many HBV patients. This proof of concept paves the way for further development of an SLP-based vaccine to treat CHB patients. Careful SLP design to include CD4 and CD8 T-cell epitopes for the most prevalent HLA types and combination of several SLP, covering multiple viral proteins in one vaccine, should warrant the induction of polyclonal and long lasting T-cell responses in many individuals.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Authors’ contributions: Y. D., N. M., G. G. Z., W. J. K., C. J. M., S. I. B., and A. M. W. designed the study. Y. D. and A. B. performed the experiments and analyses. Y. D., S. I. B., and A. M. W. wrote the manuscript. N. M., S. I. B., and A. M. W. supervised the study. N. M, R. A. M., G. G. Z., W. J. K., C. J. M., S. I. B., and A. M. W. critically reviewed the manuscript. G. G. Z., W. J. K., and C. J. M. provided materials.
Acknowledgments. We thank all patients and research nurses that participated to this study. We thank R. S. Hagedoorn and M. H. Heemskerk (LUMC, Leiden, NL), A. J. Gehring and A. Bertoletti (Duke-Nus Medical School, Singapore) for their kind help on HBcAg18-27-specific CD8+ T-cell generation.
Financial support. This work was supported by VIDI grant (grant number 016.126.329) from the Netherlands Organisation for Scientific Research (NWO) to A. M. W.
Potential conflicts of interest. W. J. K., C. J. M. M., and G. G. Z. are employed by biotech company ISA (Immune System Activation), which is aiming to develop synthetic peptide-based cancer vaccines. W. J. K., C. J. M. M., and G. G. Z. receive a salary from ISA; C. J. M. M. is in possession of stock appreciation rights. In addition, C. J. M. M. and W. J. K. are inventors on numerous patents that are licensed to be owned by ISA, concerning synthetic long peptide vaccines or the proprietary TLR ligand used in this paper. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Previous presentation: Data have been partially presented at the International HBV Meeting, Washington DC, USA, September, 2017.
References
- 1. Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012; 30:2212–9. [DOI] [PubMed] [Google Scholar]
- 2. Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet 2014; 384:2053–63. [DOI] [PubMed] [Google Scholar]
- 3. Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res 2010; 70:8368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schietinger A, Philip M, Liu RB, Schreiber K, Schreiber H. Bystander killing of cancer requires the cooperation of CD4(+) and CD8(+) T cells during the effector phase. J Exp Med 2010; 207:2469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boni C, Fisicaro P, Valdatta C et al. . Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol 2007; 81:4215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopes AR, Kellam P, Das A et al. . Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J Clin Invest 2008; 118:1835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Montfoort N, van der Aa E, Woltman AM. Understanding MHC class I presentation of viral antigens by human dendritic cells as a basis for rational design of therapeutic vaccines. Front Immunol 2014; 5:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kenter GG, Welters MJ, Valentijn AR et al. . Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med 2009; 361:1838–47. [DOI] [PubMed] [Google Scholar]
- 9. Speetjens FM, Kuppen PJ, Welters MJ et al. . Induction of p53-specific immunity by a p53 synthetic long peptide vaccine in patients treated for metastatic colorectal cancer. Clin Cancer Res 2009; 15:1086–95. [DOI] [PubMed] [Google Scholar]
- 10. Sabbatini P, Tsuji T, Ferran L et al. . Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res 2012; 18:6497–508. [DOI] [PubMed] [Google Scholar]
- 11. Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer 2008; 8:351–60. [DOI] [PubMed] [Google Scholar]
- 12. Zom GG, Welters MJ, Loof NM et al. . TLR2 ligand-synthetic long peptide conjugates effectively stimulate tumor-draining lymph node T cells of cervical cancer patients. Oncotarget 2016; 7:67087–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zom GG, Khan S, Britten CM et al. . Efficient induction of antitumor immunity by synthetic toll-like receptor ligand-peptide conjugates. Cancer Immunol Res 2014; 2:756–64. [DOI] [PubMed] [Google Scholar]
- 14. Tjwa ET, van Oord GW, Biesta PJ, Boonstra A, Janssen HL, Woltman AM. Restoration of TLR3-activated myeloid dendritic cell activity leads to improved natural killer cell function in chronic hepatitis B virus infection. J Virol 2012; 86:4102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Woltman AM, Boonstra A, Janssen HL. Dendritic cells in chronic viral hepatitis B and C: victims or guardian angels?Gut 2010; 59:115–25. [DOI] [PubMed] [Google Scholar]
- 16. Bengsch B, Martin B, Thimme R. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J Hepatol 2014; 61:1212–9. [DOI] [PubMed] [Google Scholar]
- 17. Phillips S, Chokshi S, Riva A, Evans A, Williams R, Naoumov NV. CD8(+) T cell control of hepatitis B virus replication: direct comparison between cytolytic and noncytolytic functions. J Immunol 2010; 184:287–95. [DOI] [PubMed] [Google Scholar]
- 18. Liu J, Zhang E, Ma Z et al. . Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog 2014; 10:e1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gehring AJ, Xue SA, Ho ZZ et al. . Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J Hepatol 2011; 55:103–10. [DOI] [PubMed] [Google Scholar]
- 20. Fisicaro P, Valdatta C, Massari M et al. . Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology 2010; 138:682–93, 93.e1–4. [DOI] [PubMed] [Google Scholar]
- 21. Fisicaro P, Valdatta C, Massari M et al. . Combined blockade of programmed death-1 and activation of CD137 increase responses of human liver T cells against HBV, but not HCV. Gastroenterology 2012; 143:1576–85.e4. [DOI] [PubMed] [Google Scholar]
- 22. Maier H, Isogawa M, Freeman GJ, Chisari FV. PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol 2007; 178:2714–20. [DOI] [PubMed] [Google Scholar]
- 23. Gehring AJ, Haniffa M, Kennedy PT et al. . Mobilizing monocytes to cross-present circulating viral antigen in chronic infection. J Clin Invest 2013; 123:3766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Michel ML, Deng Q, Mancini-Bourgine M. Therapeutic vaccines and immune-based therapies for the treatment of chronic hepatitis B: perspectives and challenges. J Hepatol 2011; 54:1286–96. [DOI] [PubMed] [Google Scholar]
- 25. Melief CJ. Cancer immunotherapy by dendritic cells. Immunity 2008; 29:372–83. [DOI] [PubMed] [Google Scholar]
- 26. Maini MK, Boni C, Lee CK et al. . The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med 2000; 191:1269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Niet A, Stelma F, Jansen L et al. . Restoration of T cell function in chronic hepatitis B patients upon treatment with interferon based combination therapy. J Hepatol 2016; 64:539–46. [DOI] [PubMed] [Google Scholar]
- 28. Tan AT, Koh S, Goh V, Bertoletti A. Understanding the immunopathogenesis of chronic hepatitis B virus: an Asian prospective. J Gastroenterol Hepatol 2008; 23:833–43. [DOI] [PubMed] [Google Scholar]
- 29. Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol 2016; 64:71–83. [DOI] [PubMed] [Google Scholar]
- 30. Tan AT, Loggi E, Boni C et al. . Host ethnicity and virus genotype shape the hepatitis B virus-specific T-cell repertoire. J Virol 2008; 82:10986–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol 2006; 27:49–55. [DOI] [PubMed] [Google Scholar]
- 32. Zhu Q, Egelston C, Vivekanandhan A et al. . Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: implications for vaccines. Proc Natl Acad Sci U S A 2008; 105:16260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khan S, Bijker MS, Weterings JJ et al. . Distinct uptake mechanisms but similar intracellular processing of two different toll-like receptor ligand-peptide conjugates in dendritic cells. J Biol Chem 2007; 282:21145–59. [DOI] [PubMed] [Google Scholar]
- 34. van der Aa E, Buschow SI, Biesta PJ, Janssen HL, Woltman AM. The effect of chronic hepatitis B virus infection on BDCA3+ dendritic cell frequency and function. PLoS One 2016; 11:e0161235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guidotti LG, Eggers CM, Raney AK et al. . In vivo regulation of hepatitis B virus replication by peroxisome proliferators. J Virol 1999; 73:10377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science 1999; 284:825–9. [DOI] [PubMed] [Google Scholar]
- 37. Biermer M, Puro R, Schneider RJ. Tumor necrosis factor alpha inhibition of hepatitis B virus replication involves disruption of capsid Integrity through activation of NF-kappaB. J Virol 2003; 77:4033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Visvanathan K, Skinner NA, Thompson AJ et al. . Regulation of Toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatology 2007; 45:102–10. [DOI] [PubMed] [Google Scholar]
- 39. Das A, Hoare M, Davies N et al. . Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J Exp Med 2008; 205:2111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tjwa ET, van Oord GW, Hegmans JP, Janssen HL, Woltman AM. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. J Hepatol 2011; 54:209–18. [DOI] [PubMed] [Google Scholar]
- 41. Woltman AM, Op den Brouw ML, Biesta PJ, Shi CC, Janssen HL. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLoS One 2011; 6:e15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boni C, Laccabue D, Lampertico P et al. . Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology 2012; 143:963–73.e9. [DOI] [PubMed] [Google Scholar]
- 43. Park JJ, Wong DK, Wahed AS et al. . Hepatitis B Virus--Specific and Global T-Cell Dysfunction in Chronic Hepatitis B. Gastroenterology 2016; 150:684–95.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Watanabe T, Bertoletti A, Tanoto TA. PD-1/PD-L1 pathway and T-cell exhaustion in chronic hepatitis virus infection. J Viral Hepat 2010; 17:453–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.