Abstract
Zika virus (ZIKV) (Flaviviridae, Flavivirus) has become one of the most medically important mosquito-borne viruses because of its ability to cause microcephaly in utero and Guillain-Barré syndrome in adults. This virus emerged from its sylvatic cycle in Africa to cause an outbreak in Yap, Federated States of Micronesia in 2007, French Polynesia in 2014, and most recently South America in 2015. The rapid expansion of ZIKV in the Americas largely has been due to the biology and behavior of its vector, Aedes aegypti. Other arboviruses transmitted by Ae. aegypti include the 2 flaviviruses dengue virus and yellow fever virus and the alphavirus chikungunya virus, which are also (re)emerging viruses in the Americas. This mosquito vector is highly domesticated, living in close association with humans in urban households. Its eggs are desiccation resistant, and the larvae develop rapidly in subtropical and tropical environments. Climate warming is facilitating range expansion of Ae. aegypti, adding to the threat this mosquito poses to human health, especially in light of the difficulty controlling it. Aedes albopictus, another highly invasive arbovirus vector that has only been implicated in one country (Gabon), is an important vector of ZIKV, but because of its wide geographic distribution may become a more important vector in the future. This article discusses the historical background of ZIKV and the biology and ecology of these 2 vectors.
Keywords: Zika, Flavivirus, Aedes aegypti, Aedes albopictus, vector competence
Zika virus (ZIKV) (Flaviviridae; Flavivirus) was considered a generally mild disease until it emerged in French Polynesia in 2013 and more dramatically in the Americas in 2015. It is almost exclusively transmitted by Aedes species mosquitoes, and it is neurotropic. These combined characteristics make this virus unusual; biologically it falls between the Aedes-transmitted hemorrhagic disease flaviviruses, such as dengue and yellow fever viruses that have nonhuman primates as their vertebrate hosts, and Culex-transmitted encephalitic flaviviruses, such as West Nile and St Louis encephalitis viruses, with birds as the amplifying hosts [1]. Therefore, ZIKV does not adhere to the classical separation of flaviviruses by disease association and epidemiology, generally correlated with the phylogenetic relationships among the flaviviruses. Another unusual aspect of ZIKV is its pathogenicity to fetuses, especially during the first trimester of pregnancy. While there are a number of teratogenic viruses, flaviviruses generally do not cross the placenta and cause disease in the developing human fetus.
ZIKV was initially isolated in 1947 from a rhesus macaque monkey caged on a tree platform in the canopy as a sentinel to detect yellow fever virus (YFV) in the Zika forest, Uganda [2]. The virus subsequently was isolated in 1948 from Aedes africanus, also from the Zika forest [2]. Isolation of virus from other Aedes species, specifically of the Aedimorphus, Diceromyia, and Stegomyia subgenera, in forested habitats include Ae. africanus and Aedes apicoargenteus (Uganda) [3, 4], Aedes luteocephalus (Nigeria) [5], and Aedes furcifer and Aedes vittatus (Senegal) [6], and several species have been demonstrated in the laboratory to be competent vectors [7–9].
Outside of Africa, Aedes aegypti (Linnaeus) is considered the predominant vector of ZIKV. In 1956, the first experimental studies indicated successful transmission of ZIKV by laboratory-infected Ae. aegypti to mice and monkeys [8], demonstrating that this virus could be transmitted in an urban as well as sylvatic cycle. And indeed, in 1966, ZIKV was isolated from this species in Malaysia [10]. In the laboratory, the extrinsic incubation period was estimated to be approximately 10 days, although virus titers remained high in the mosquito through 60 days.
Zika virus was reported to have emerged from its sylvatic cycle to a rural habitat in 2007 when disease was recognized on Yap Island, Federated States of Micronesia, in 2007. The vector is presumed to have been Aedes hensilli, the most abundant and widespread Aedes species mosquito in Yap State [11]. Although virus was not isolated from any mosquito on the island in spite of attempts made, Ae. hensilli has been demonstrated to be an efficient vector [12].
Also in 2007, ZIKV was detected for the first time in Aedes albopictus (Skuse), in Gabon, Africa, in an urban environment [13]. This species was first introduced into Africa in 1991, and found for the first time in Gabon in 2007; the same year, not only ZIKV, but also chikungunya virus (CHIKV) and dengue virus (DENV) were detected in the country. This finding was especially concerning because of the highly invasive nature of this mosquito species as evidenced by its geographic global expansion in Africa, Europe, and the Americas.
In 2013–2014, ZIKV was introduced into French Polynesia in the South Pacific, leading to an urban outbreak outside Africa for the first time [14]. The origin of this outbreak remains unknown, and the virus spread to other Pacific Islands from there, most likely from infected travelers. The 2 mosquito species thought to possibly be involved in this new location are Ae. aegypti, present on Pacific Islands, and the endemic species, Aedes polynesiensis [15]. Both species were found to be poorly competent, lending suspicion that other Aedes vectors may have been involved. The relatively low viral loads in patients infected with ZIKV with an order of magnitude of 1 × 105 copies/mL compared with 1 × 107 to 1 × 109 copies/mL for CHIKV [16, 17] suggest that vector competence may be critical—that is, the mosquito must be highly susceptible to infection to establish a human–mosquito transmission cycle.
ZIKV IN THE AMERICAS
The focus of attention on ZIKV vectors in the Americas has been on Ae. aegypti, the vector of DENV, CHIKV, and urban YFV. This species occurs in 2 distinct forms in its native country, Africa: the feral form, subspecies formosus, and the domesticated form, subspecies aegypti [18]. It is the latter form that has been inadvertently spread throughout the world, becoming established in receptive environments, specifically tropical and subtropical regions, not only as a consequence of accommodating temperatures in those regions, but also as a result of its highly domesticated nature leading to close association with urban households, and manmade perturbations on the environment. It was first introduced into the Americas in the 16th century. This species arguably poses the greatest threat for transmission of arboviral infections to mankind.
A potential secondary vector and even more invasive species is Ae. albopictus, which, like Ae. aegypti, is in the subgenus Stegomyia and is ecologically similar to Ae. aegypti, albeit with significant differences. Until recently, Ae. albopictus was found only in the Eastern Hemisphere, but became established in the United States in the mid-1980s [19]. Aedes albopictus distribution has expanded dramatically into temperate regions of Europe and North America, currently inhabiting 28 countries beyond its native tropical range in Southeast Asia, and is now found on every continent except Antarctica [20, 21]. A review of the vector status of Ae. albopictus (to 2004) examines its role in dengue and other arboviral outbreaks [22]. Aedes albopictus was the vector of the first CHIKV outbreak reported in Europe in 2007 when there were an estimated 254 human cases occurring in Italy [23]. CHIKV infection of Ae. albopictus was associated with a mutation in the envelope protein gene (E1-A226V), making this species competent. In addition, Ae. albopictus has been incriminated as likely the significant vector of ZIKV in Gabon [13] but also may play a secondary role in viral transmission in Mexico in the state of San Luis Potosi, as reported by the Pan American Health Organization/World Health Organization (WHO) in April 2016 [24] and elsewhere. But there is no definitive evidence it is playing a significant role in transmission in the current outbreak (since 2015 to July 2017) in other countries in the Americas. Whether this is due to behavioral differences, life table characteristics, vector competence, or lack of effective surveillance for this species is under study. Understanding this species’ biology is critical, as Ae. albopictus may become significant vectors of ZIKV in the future if the virus were to adapt to them through genome microevolution as occurred with CHIKV in La Réunion 2005–2006 during the Indian Ocean outbreak [25].
Aedes aegypti and Ae. albopictus are holometabolous. Eggs withstand some degree of drying depending on the species, with Ae. aegypti eggs more resistant to drying than temperate Ae. albopictus [26, 27], giving the former an advantage under dry conditions. Aedes aegypti eggs actually need to dry as a stimulus for embryonation. In Florida, Ae. aegypti are regaining terrain previously lost to the more competitive Ae. albopictus (see below), moving north as temperatures have increased and environments have become drier, favoring their survival. An Australian study demonstrated 2%–15% Ae. aegypti egg viability following 1 year of desiccation and viability remaining >88% through 56 days of varying levels of dryness. Intraspecific variations in egg survival times were recorded, suggesting local adaptation [28]. In the laboratory, –3°C for 24 hours was identified as a threshold for viability of Ae. albopictus eggs [29].
The eggs hatch when submerged under water. The larvae pass through 4 instars followed by pupation and emergence of the adult. The 2 species share larval habitats where they coexist and experience interspecific resource competition. Larvae of Ae. albopictus appear to be more competitive under such conditions [30], giving them an advantage when both species are developing in the same container of water. It has been hypothesized that the increased success of Ae. albopictus is due to its ability to feed very quickly and not to stop feeding even in the presence of a predator, thereby selecting for mosquitoes ovipositing in predator-free containers. This seems to be true for both temperate and tropical forms of Ae. albopictus [31]. They also seem to be refractory to apparently toxic compounds, possibly because of their association with tires that have noxious leachates [32]. Aedes albopictus adults may have an advantage possibly as a result of satyrization, or asymmetric mating interference with Ae. aegypti [33]; furthermore, evolved resistance to this phenomenon results in reduced fitness, but the phenomenon appears to be rare in the field [34, 35]. For all these reasons, Ae. albopictus appears to be displacing Ae. aegypti except under dry conditions where superior egg survival of Ae. aegypti gives this species a distinct advantage.
Additional differences in the biology of Ae. aegypti and Ae. albopictus include life table characteristics, preferred habitat, and feeding and breeding differences. Aedes aegypti prefer urban habitats, and breed in any container that holds water around homes, taking blood meals from the inhabitants of these houses after emergence as adults, and often resting indoors. Aedes aegypti feeding habits are highly focused on humans, and the females take blood meals frequently (ie, more than once in each gonotrophic cycle), potentially transmitting virus with each blood meal [18, 36, 37]. Aedes albopictus, on the other hand, prefer suburban back yards and green parks rather than urban city centers. In addition, their feeding preferences are more catholic—they will feed on domestic animals and such mammals as squirrels and chipmunks as readily as on humans. Faraji and colleagues [38] found that in the northeastern United States, Ae. albopictus fed exclusively on mammalian hosts with >90% of their blood meals derived from humans and domesticated pets, and they fed from humans significantly more often in suburban than in urban areas. In southern Thailand, 100% of the Ae. albopictus blood meals were human, with a very low proportion of double blood meals [37]. In general, 6%–10% of Ae. albopictus blood meals have been reported to be double blood meals from multiple vertebrate hosts [39, 40].
Vectorial capacity, VC = [ma2 (I*T)pn]/−ln(p), incorporates biology of the mosquito and its ability to become infected and transmit virus, including m, the vector density in relation to the host; a, the probability that a vector feeds on a host in 1 day (ie, the host preference index multiplied by the feeding frequency); p, the probability that a vector survives 1 day; n, the duration of the extrinsic incubation period (EIP) in days; I (infection rate) * T (transmission rate) is equal to vector competence (b) or the proportion of vectors ingesting an infective meal that are later able to transmit the infection; and 1/−ln(p) is the duration of the vector’s life in days after surviving the EIP. Frequency of feeding on the targeted host (host feeding [a]) is one of the most important components of vectorial capacity. Aedes aegypti’s focused feeding habits on humans partially explain the effectiveness of this species as a vector. While the ability of Ae. aegypti to become infected with and transmit YFV in Nigeria was demonstrated to be low, they sustained the outbreak due to both frequent blood feeding and high population density [41]. As will be discussed later, Ae. aegypti similarly appears to not be a highly efficient vector of ZIKV.
The current distribution of Ae. aegypti and Ae. albopictus in the continental United States is limited to the selected Atlantic and Gulf states (Figures 1 and 2) [42]. If Ae. albopictus were to become a significant vector of ZIKV in the United States through viral adaptation/mutation, as was the case for CHIKV in La Reunion [23, 43], Italy [44], France [45], and Asia [46], the United States and other temperate locations would be at greater risk of ZIKV transmission because the range of this species extends further north than that of Ae. aegypti, for example, north to New England and the lower Great Lakes in the United States (Figures 1 and 2). Aedes albopictus is increasing its range not only in the Americas, but also in Europe [47]. There have been multiple introductions of this species to the United States, first at the Port of Houston in 1985, and later in the Port of Los Angeles, and differences in genetics of distinct populations can be seen. (D. M. Fonseca, unpublished data). Used tires and “lucky bamboo” are 2 common culprits containing eggs [48]. The invasive success of both Ae. aegypti and Ae. albopictus [49] has been attributed to the “anthropogenically induced adaptation to invade” hypothesis of Hufbauer and colleagues [50].
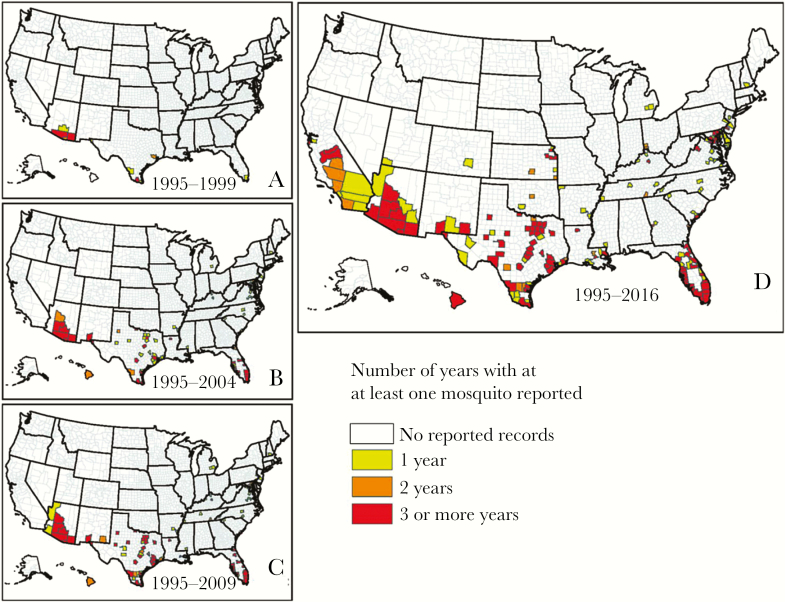
Figure 1.
Maps showing the reported occurrence of Aedes aegypti by county between 1 January 1995 and March 2016 in the United States. Reported occurrence from 1 January 1995 through 1999 (A), from 1 January 1995 through 2004 (B), from 1 January 1995 through 2009 (C), and from 1 January 1995 through March 2016 (D), representing the best knowledge of the current distribution of this mosquito based on collection records. Counties shown in white had no reported Ae. aegypti presence records within the specified time period. Counties shown in yellow had Ae. aegypti presence records for 1 year within the specified time period, those shown in orange had 2 years of presence records within the specified time period, and those shown in red had ≥3 years of presence records within the specified time period. Adapted from Hahn et al [42].
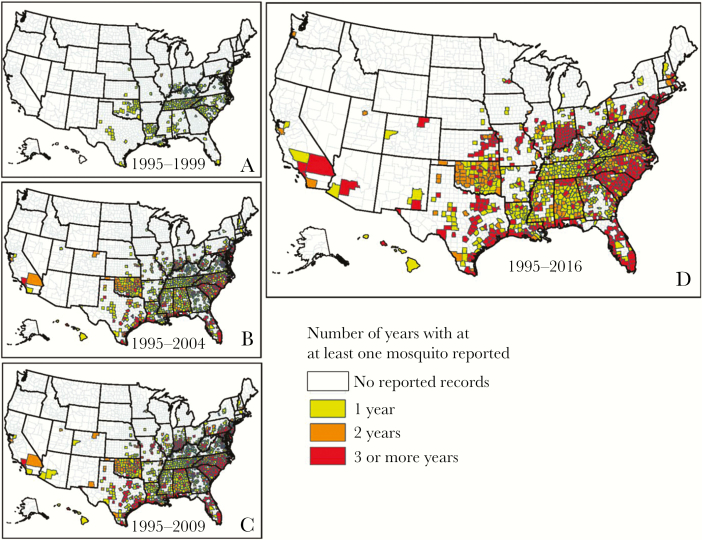
Figure 2.
Maps showing the reported occurrence of Aedes albopictus by county between 1 January 1995 and March 2016 in the United States. Reported occurrence from 1 January 1995 through 1999 (A), from 1 January 1995 through 2004 (B), from 1 January 1995 through 2009 (C), and from 1 January 1995 through March 2016 (D), representing the best knowledge of the current distribution of this mosquito based on collection records. Counties shown in white had no reported Ae. albopictus presence records within the specified time period. Counties shown in yellow had Ae. albopictus presence records for 1 year within the specified time period, those shown in orange had 2 years of presence records within the specified time period, and those shown in red had ≥3 years of presence records within the specified time period. Adapted from Hahn et al [42].
Aedes species survive adverse climatic periods in the egg stage; thus, the ability of the eggs to withstand cold and desiccation (discussed above) is critical to perpetuation of the species and continued transmission of viruses such as ZIKV in temperate environments. Both temperate and tropical populations of Ae. albopictus have become established in the United States, with the temperate ones being more cold adapted, allowing them to become established in cooler environments. Aedes aegypti and possibly Ae. albopictus egg survivorship/hatching success is important in understanding and predicting future ZIKV transmission in the temperate environments of North and South America. It is also likely that Aedes can gradually adapt to cooler temperatures, which will affect the limits of their ability to expand north in North America, and south, in the Southern Hemisphere, as well as increase their distribution vertically in elevation. One study conducted with a population of Ae. aegypti at the limits of its distribution in Argentina, demonstrated larvae completed development during a simulated cold season, with a trend toward increased survival of late-hatching cohorts. Survival was 30% at 13.2°C and >90% at 20°C; development time was 49.4 days at 13.2°C and 17.7 days at 20°C. These levels of success are only meaningful if the emerged adults are able to mate and take blood meals successfully, but the greater success at development under cool conditions than those seen in other studies suggests adaptation to the cooler climate in the country [51].
VECTOR COMPETENCE
The many studies evaluating vector competence of Aedes species for ZIKV have been difficult to compare with each other as different populations of Ae. aegypti have been used, including both colonized and field populations, different strains of ZIKV, various doses in the infectious blood meal (unnaturally high and lower doses), blood meal presentation, and other differences (Table 1). In general, geographic origin of the virus and vector make a difference; the African strains of virus have been found to be more infectious for Ae. aegypti than American strains [52].
Table 1.
Studies on Vector Competence for Zika virusa
| Reference | Mosquito vector | Zika virus strain | Vector competence study | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Origin | History | Strain | Origin | Lineage | Infection Route | Competence | Summary of results | |
| Aliota et al., 2016 [54] | Ae. aegypti | Black eyed Liverpool | Lab colony | PRV ABC59 | Human, Puerto Rico, 2015 | Asian | Murine 106.8 PFU 14 di | IR 100, DR 71, TR 24 | Ae. aegypti and albopictus highly competent, Ae. triseriatus and Cx. pipiens incompetent. |
| Ae. albopictus | Missouri 2002 | Lab colony | IR 100, DR 67, TR 22 | ||||||
| Ae. triseriatus | Iowa 2002 | Lab colony | IR 31, DR 0 | ||||||
| Cx. pipiens | Iowa 2002 | Lab colony | IR 0, DR 0 | ||||||
| Amraoui et al., 2016 [57] | Cx. pipiens | Tabarka, Tunisia 2010 | Lab colony | NC-2014–5132 | Human, New Caledonia, 2014 | Asian | BM; 107.2 PFU 3 - 21 dpi (d 14 shown) | IR 0, DR 0, TR 0 | Culex spp. incompetent |
| Cx. quinq. | San Joaquin Valley, CA 1950 | Lab colony | IR 17, DR 2, TR 0 | ||||||
| Boccolini et al., 2016 [58] | Cx. pipiens | Rome, 2015 | Lab colony | H/PF/2013 | Human, French Polynesia, 2013 | Asian | BM: 106.46 PFU 3–24 dpi (d14 shown) | IR 0, DR 0, TR 0 | Cx. pipiens incompetent. Ae. aegypti moderately competent. |
| Ae. aegypti | Reynosa, Mexico,1998 | Lab colony | IR 50, DR 50, TR 38 | ||||||
| Boorman and Porterfield,1956 [8] | Ae. aegypti | Nigeria 1948 | Lab colony | MR 766 | Monkey, Uganda, 1947 | E African | BM, 106.7 LD50 60 dpi | IR 100 TR 50 | Transmission to mice |
| Chouin-Carneiro et al., 2016 [113] | Ae aegypti | French Guiana | F1 | NC-2014–5132 | Human, New Caledonia, 2014 | Asian | BM 107 TCID50 per 0.03 ml 7 & 14 dpi | 7 dpi: IR 100, TR 0 | At 7 dpi high infection (low dissemination – not shown), no transmission by all vectors tested. At 14 dpi, Ae. albopictus and Ae. aegypti exhibited similar low transmission efficiency. |
| Ae aegypti | Guadeloupe | F2 | 7 dpi: IR 87, TR 0 | ||||||
| Ae aegypti | Martinique | F1 | 7 dpi I: IR 90, TR 0 | ||||||
| Ae aegypti | Orlando, Florida | >F10 | 7 dpi: IR 93, TR nd | ||||||
| Ae aegypti | Tubiacanga, Brazil | F1 | 7 DPI: IR 83, TR nd 14 dpi: IR 77, TR 8 |
||||||
| Ae. albopictus | Jurujuba, Brazil | F1 | 7 dpi: IR 23, TR nd | ||||||
| Ae. albopictus | Vero Beach, Florida | F7 | 7 dpi: IR 60, TR 0 14 dpi: IR 50, TR 2 | ||||||
| Ciota et al., 2017 [114] | Ae. albopictus | Suffolk, New York | F5-F7 | HND 2016–19563 | Human, Honduras, 2016, p2 | Asian | BM, fresh, 10 6.6-7.7 PFU 21 dpi | HND: IR 93 TR 20 CAM: IR 40 TR 10 |
Ae. albopictus IR≥ Ae. aegypti, but transmission efficiency higher for Ae. aegypti, indicating a transmission barrier in Ae. albopictus |
| Ae aegypti | Poza Rica, Mexico | F5-F7 | CAM FSS130325 | Human serum, Cambodia, 2010, p4 |
Asian | HND:IR 47, TR36 CAM:IR 44, TR33 |
|||
| Cornet et al., 1979 [9] | Ae. aegypti | Senegal 1971 | Lab colony | ArD 24280 | Ae. luteocephalus, 1976, Senegal | W African | IT dose unknown 7- 28 dpi | TR 91 | Ae. aegypti highly competent following inoculation |
| Diagne et al., 2015 [7] | Ae. aegypti | Dakar, Senegal | Domestic F1 | ArD 128000 | Ae. luteocephalus, 1997, Kedougou, | W African | BM 6.4–7.6 log10 PFU 5–15 dpi (results shown for 15 dpi) |
Ae. aegypti, Dakar:IR+, DR+ (4 strains), TR 0 |
All vectors tested were infected by all virus strains and exhibited dissemination with ≥2 strains. Only two vectors were able transmit virus (Ae. vittatus with HD 78788 and Ae. luteocephalus with MR766). |
| Ae. aegypti | Kedougou, Senegal | Sylvatic F1 | ArD 132912 | Ae. dalzieli,1998 Kedougou, p4 | W African | Ae. aegypti Kedougou: IR+ DR+ (4 strains), TR 0 | |||
| Ae. unilineatus | Kedougou, Senegal | Sylvatic F1 | ArD 157995 | Ae. dalzieli 2001 Kedougou, p6 | W African | Ae. unilineatus: IR+, DR+ (2 strains), TR 0 | |||
| Ae. vittatus | Kedougou, Senegal | Sylvatic F1 | ArD 165522 | Ae. Vittatus, 2002 Kedougou, p5 | W African | Ae. vittatus: IR+, DR+ (3 strains), TR 20 (HD787888) | |||
| Ae. luteocephalus | Kedougou, Senegal | Sylvatic F1 | HD 78788 | Human, Dakar, 1991 | W African | Ae. luteocephalus: IR+, DR+ (5 strains), TR 10 (MR 766) | |||
| MR 766 | Monkey, Uganda, 1947 | E African | |||||||
| Di Luca et al., 2016 [115] | Ae. aegypti | Mexico | Lab colony | H/PF/2013 | Human, French Polynesia | Asian | BM 106.4 PFU 3–21 dpi (14 dpi shown) | IR 40, DR 40, TR 40 | Ae. aegypti more competent than Ae. albopictus |
| Ae. albopictus | Italy 2015 | Lab colony | IR 20, DR 10, TR 10 | ||||||
| Dodson, et al., 2017 [63] | An. gambiae | NIH | Lab colony | MR 766 | Monkey, Uganda, 1947 | E African | BM, 10 4.3-7.7 PFU 7–14 dpi (14 dpi shown) | IR 0 | Anopheles gambiae, Anopheles stephensi, and Cx. quinq mosquitoes refractory to Zika virus infection. |
| An. stephensi | Johns Hopkins | Lab colony | IR 0 | ||||||
| Cx.quinq. | Wadsworth | Lab colony | MR 766 | Monkey, Uganda, 1947 | E African | IR 0 | |||
| PRV ABC59 | Human, Puerto Rico, 2015 | Asian | IR 0 | ||||||
| Dutra et al., 2016 [116] | Ae.aegypti | Urca, Rio de Janeiro, Brazil | Lab colony, wMel neg | BRPE 243/ 2015 | Human, Brazil, 2015 | Asian | BM, fresh 5x106 PFU 14 dpi | IR 100, DR 100, TR 100 | Dramatically reduced infection, dissemination and transmission rates were observed in Ae.aegypti mosquitoes naturally infected with Wolbachia bacteria. |
| Lab colony, wMel pos | IR 35, DR 10, TR 45 | ||||||||
| Lab colony, wMel neg | SPH/ 2015 | Human, Brazil, 2015 | Asian | BM, fresh 8.7x103 PFU 14 dpi | IR 95, DR 95, TR ND | ||||
| Lab colony, wMel pos | IR 30, DR 25, TR ND | ||||||||
| Fernandes et al., 2017 [69] | Cx. quinq. | Recife, NE Brazil | F1 | ZIKVPE243 | Human; Recife 2015 | Asian | BM 106.36 PFU | IR 0 |
Cx. quinq. from areas of SE Brazil were refractory to infection by ZIKV isolates from Brazil. Ae. aegypti were highly infected. 100% tested had positive salivary glands. Virus detected in salivary expectorates at level similar to Ae. aegypti |
| ZIKVSPH2014 | Human Sumare 2015 | Asian | BM 107.23 PFU | IR 0 | |||||
| ZIKU1/2015 | Human Rio de Janeiro | Asian | BM 106.56 PFU 7,14 dpi | 7d: IR 5 DR 0 14d: IR 0 DR 0 | |||||
| Cx. quinq. | Campina Grande, NE Brazil | F1 | ZIKVPE243 | Human; Recife | Asian | BM 106.36 PFU | IR 0 | ||
| ZIKVSPH2014 | Human Sumare 2015 | Asian | BM 107.23 PFU | IR 0 | |||||
| ZIKU1/2015 | Human Rio de Janeiro | Asian | BM 106.56 PFU | IR 0 | |||||
| Cx. quinq. | Rio de Janeiro, SE Brazil | F>10 | ZIKVPE243 | Human; Recife | Asian | BM 106.36 PFU | IR 0 | ||
| ZIKVSPH2014 | Human Sumare 2015 | Asian | BM 107.23 PFU | IR 0 | |||||
| Cx. quinq. | Rio de Janeiro, SE Brazil | F1 | ZIKVPE243 | Human; Recife | Asian | BM 106.56 PFU | IR 0 | ||
| ZIKSPH2014 | Human Sumare2015 | Asian | BM 107.23 PFU | IR 0 | |||||
| Ae. aegypti | Rio de Janeiro, SE Brazil | F>10 | ZIKVPE243 | Human; Recife | BM 106.36 PFU | IR 68 DR 100 | |||
| ZIKVSPH2014 | Human Sumare 2015 | BM 107.23 PFU | IR 100 DR 100 | ||||||
| ZIKU1/2015 | Human Rio de Janeiro | BM 106.56 PFU 7 dpi | IR 75 DR 60 | ||||||
| Guedes et al., 2017 [67] | Ae. aegypti | Fernando de Noronha, PE Brazil | F1 - F2 | BRPE 243/2015 | Human, Brazil 2015 | Asian | BM 106 pfu 3,7,15 dpi day (15dpi shown) | IR 40 SR 0 | Cx. quinq. positive salivary glands; virus detected in salivary expectorates at rate similar to Ae. aegypti. |
| Cx. quinq. | Recife PE,Brazil | CqS Lab colony | IR 39 SR 28 | ||||||
| Ae. aegypti | Recife PE,Brazil | Rec Lab colony | IR 44 SR38 | ||||||
| Guo et al., 2016 [66] | Cx. quinq. | Hainan province southern China, 2014 | Lab colony | SZ01 | Human, Samoa, 2016. | Asian | BM, 105.48 PFU 2–18 dpi | 16d: IR 60 SR 0 (20% SG pos) 10 d: TR 89% mice pos |
Cx. quinq. transmitted to mice at 10 dpi, but no virus in saliva 16 dpi |
| Hall-Mendelin et al., 2016 [60] | Ae. aegypti | Queensland, Australia | F4 | MR 766 | Monkey, Uganda, 1947 | E African | BM, 106.7 TCID50 14 dpi | IR 57, DR 71, TR 27 |
Ae. aegypti competent. Three other Aedes species displayed none -moderate dissemination but no transmission. Three Culex species incompetent. |
| Ae. notoscriptus | Queensland, Australia | Field collected | IR 57, DR 0, TR 0 | ||||||
| Ae. procax | Queensland, Australia | Field collected | IR 33, DR 17, TR 0 | ||||||
| Ae. vigilax | Queensland, Australia | Field collected | IR 57, DR 27, TR 0 | ||||||
| Cx. annulirostris | Queensland, Australia | Field collected | IR 0, DR 0, TR 0 | ||||||
| Cx. quinq. | Queensland, Australia | Field collected | IR 7, DR 0, TR 0 | ||||||
| Cx. sitiens | Queensland, Australia | Field collected | IR 0, DR 0, TR 0 | ||||||
| Hart et al., 2017 [61] | Cx. quinq. | Gulf Coast, US | Lab colony | DAKAR41525 | 1985 Senegal | W African | BM 106 FFU 3–17 dp | IR 0, DR 0, TR 0 | Cx. quinq. and Ae. taeniorhynchus from the US Gulf Coast refractory to infection. In previous experiments, Ae. aegypti was competent for these virus strains. |
| FSS13025 | 2010 Cambodia | Asian | IR 0, DR 0, TR 0 | ||||||
| MEX1–7 | 2015 Mexico | Asian | IR 0, DR 0, TR 0 | ||||||
| MEX 1–44 | 2015 Mexico | Ásian | IR 0, DR 0, TR 0 | ||||||
| Cx. quinq. | Houston | F2 | FSS13025 | 2010 Cambodia | Asian | BM | IR 0, DR 0, TR 0 | ||
| MEX1–7 | 2015 Mexico | Asian | Murine | IR 0, DR 0, TR 0 | |||||
| PRV ABC59 | Human, Puerto Rico, 2015 | Asian | Murine | IR 0, DR 0, TR 0 | |||||
| Ae. taeniorhynchus | Gulf Coast, US | Lab colony | MEX 1–44 | 2015 Mexico | Ásian | BM | IR 0, DR 0, TR 0 | ||
| Heitmann et al. 2017 [64] | Ae. aegypti | Bayer company | Lab colony | FB-GWUH-2016 | Travel case (Mexico, Belize, & Guatemala) | Asian | BM 107 PFU/ml 14 & 21 dpi (14 dpi shown) |
18°C: IR 55, TR 0 27°C: IR49, TR22 |
Aedes spp. colonies competent only at 27°C at 14 dpi with similar transmission rates. Three Culex species, collected in Germany were not competent for ZIKV. |
| Ae. albopictus | Calabria, Italy 2016 | F7 | 18°C:IR 63, TR 0 27°C:IR 71, TR13 |
||||||
| Ae. albopictus | Freiburg, Germany 2016 | F7 | 18°C: IR 13, TR 0 27°C: IR 65, TR 13 |
||||||
| Cx. p. molestus | Heidelberg, Germany 2011 | Lab colony | 18°C: IR 29, TR 0 27°C: IR 24, TR 0 |
||||||
| Cx. p. pipiens | Hamburg, Germany | Field collected | 18°C: IR 47, TR 0 27°C: IR 8, TR 0 |
||||||
| Cx. torrentium | Hamburg, Germany | Field collected | 18°C: IR 31, TR 0 27°C: IR 11, TR0 |
||||||
| Huang et al., 2016 [55] | Cx. pipiens | Anderson, CA | F15 | PRV ABC59 | Human serum, Puerto Rico, 2015 | Asian | BM 10 6.52 TCID50 7 & 14 dpi | 7 dp: IR 0, DR 0 14 dpi IR 0, DR 0 | Cx. pipiens and Cx. quinq. incompetent |
| Cx. pipiens | Ewing, NJ | F7 | 7 dp: IR 0, DR 0 14 dpi IR 0, DR 0 | ||||||
| Cx. quinq. | Vero Beach, FL | F7 | 7 dp: IR 0, DR 0 14 dpi IR 0, DR 0 | ||||||
| Kenney et al., 2017 [56] | Cx quinq. | Florida 1988 | Lab colony | MR 766 | Monkey Uganda, 1947 | E African | BM: 106 PFU 14 dpi | IR 1, DR 0 | Minimal infection, but no dissemination. IT inoculation to bypass midgut: moderate infection but no transmission except by Ae.aegypti |
| IT: 106.7 PFU 7 dpi | IR 70, TR 0 | ||||||||
| PRV ABC59 | Human, Puerto Rico, 2015 | Asian | BM: 107.1 PFU 14 dpi | IR 0, T 0 | |||||
| IT: 106 PFU 7 dpi | IR 15, TR 0 | ||||||||
| R103451 | Human, Honduras 2016 | Asian | BM: 107.6 PFU 14 dpi | IR 0, TR 0 | |||||
| Cx pipiens | Chicago 2010 | Lab colony | MR 766 | Monkey, Uganda 1947 | E African | BM: 106 PFU 14 dpi | IR 5, DR 0 | ||
| PRV ABC59 | Human, Puerto Rico, 2015 | Asian | BM: 106 PFU 14 dpi | IR 10, DR 0 | |||||
| IT: 106 PFU 7 dpi | IR 61, TR 0 | ||||||||
| Ae aegypti | Poza Rica, Mexico | Lab colony | PRV ABC59 | Human, Puerto Rico, 2015 | Asian | IT: 106 PFU | IR 100, TR 67 | ||
| Ledermann et al., 2014 [12] | Ae. (Stegomyia) hensilli | Yap Island, Micronesia | F12- F15 | MR 766 | Monkey, Uganda, 1947 | E African | BM, 104.9 PFU 8 dpi | IR 86 DR 20 TR nd | Ae. hensilli exhibited high infection and moderate dissemination rates. |
| Li et al., 2012 [117] | Ae. aegypti | Singapore | Field | MR 766 | Monkey, Uganda, 1947 | E African | BM 107 TCID50, 1–14 dpi (14 dpi shown) | IR 100, DR 100, TF 100 | Ae aegypti highly competent. |
| Richard et al., 2016 [15] | Ae. aegypti | Tahiti 2014 | F16-F18 | PF13/251013–18 | Human, French Polynesia, 2013 | Asian | Blood meal, 107 TCID50 2–21 dpi (14 dpi shown) | IR 85, DR 85, TR 36 | Polynesian Ae. aegypti highly competent. Ae. polynesiensis showed moderate infection and dissemination but no transmission |
| Ae. polynesiensis | Tahiti 2014 | F16-F18 | IR 36, DR 18 TR 0 | ||||||
| Roundy et al. 2017 [52] | Ae. aegypti | Salvador, Brazil | F2 | DAK AR 41525 |
Senegal | W African | BM/murine 10 4-6 FFU/mL 4–14 dpi (results shown for 14 dpi 10 6 /mL) |
BM: IR 100 TR 100 | Transmission highest for Senegal strain. Blood meals from viremic mice were more infectious than artificial blood meals of comparable doses. |
| FSS 13025 | Cambodia | Asian | BM: IR 75 TR 0 Murine: IR 100 TR 40 | ||||||
| MEX1-7 | Mexico 2015 | Asian | BM: IR 75 TR 0 | ||||||
| Ae. aegypti | Dominican Republic, Caribbean | F6 | DAKAR 41525 | Senegal | W African | BM: IR 100 TR 100 | |||
| FSS 13025 | Cambodia | Asian | BM: IR 100 TR 18 | ||||||
| MEX1–7 | Mexico 2015 | Asian | BM: IR 90 TR 20 | ||||||
| Ae. aegypti | RioGrande Valley, Texas, US | F4 | DAKAR 41525 | Senegal | W African | BM: IR 100 TR 30 | |||
| FSS 13025 | Cambodia | Asian | BM: IR 40 TR 0 | ||||||
| MEX1–7 | Mexico 2015 | Asian | BM: IR 65 TR 0 | ||||||
| Weger- Lucarelli et al., 2016 [62] | Ae. aegypti | Poza Rica, MX | F11-13 | PRV ABC59 | Human, Puerto Rico, 2015 | Asian | BM, Fresh 106.3 PFU 14 dpi | IR 95, DR 92, TR 70 | ZIKV efficiently transmitted by Ae.aegypti from Mexico. Compared to frozen preparations, blood meals containing fresh virus resulted in higher infection and dissemination rates especially at early dpi. Fresh virus did not infect the three Culex species. |
| BM, Frozen 4 hr 107.2 PFU 14 dpi | IR 95, DR 80, TR 65 | ||||||||
| BM, Frozen 1 wk 107.2 PFU 14 dpi | IR 60, DR 40, TR 22 | ||||||||
| DAKAR 41525 | AE Africanus, Senegal, 1984 | W African | BM, Frozen 107.2 PFU 14 dpi | IR 75, DR 60, TR 55 | |||||
| PRV ABC59 | Human serum, Puerto Rico, 2015 | Asian | IR 60, DR 40, TR 25 | ||||||
| MR 766 | Monkey, Uganda, 1947 | E African | IR 58, DR 40, TR 37 | ||||||
| Cx. quinq. | Florida1988 | Lab colony | PRV ABC59 | Human serum, Puerto Rico, 2015 | Asian | BM, Fresh 106.7 PFU 14 dpi | IR 0 | ||
| Cx pipiens | Pennsylvania 2002 | Lab colony | IR 0 | ||||||
| Cx. tarsalis | California 1953 | Lab colony | IR 0 | ||||||
| Ae. aegypti | Poza Rica, MX | F11-13 | IR 96 | ||||||
| Wong et al., 2013 [118] | Ae. albopictus | Singapore | Field | MR 766 | Monkey, Uganda, 1947 | E African | BM, fresh 107.5 TCID50, 10 dpi | IR 83, DR 100, TR 100 | Ae. albopictus highly competent. |
Abbreviations: Ae., Aedes; An., Anopheles; BM, mosquitoes fed by artificial blood meal; Cx., Culex; Cx. quinq, Cx. quinquefasciatus; d or dpi, day post-infection; DR, dissemination rate, percentage of engorged females with virus in legs, head, and/or salivary glands; ID50, 50% infectious dose; IR, infection rate, percentage infected at 14 dpi unless otherwise stated; IT, intrathoracic inoculation; nd, not done; NIH, National Institute of Health; SR, salivary rate; TR, transmission rate, percentage of engorged females with virus in saliva at 14 dpi unless otherwise noted; murine: mosquitoes infected by feeding on an infected mouse; BM: mosquitoes fed on artificial blood meal; blood meal titers expressed as PFU (plaque forming unit), FFU (fluorescence focus units), TCID50 (50% tissue culture infectious dose), ID50 (50% infectious dose), per mL; temperature (°C): extrinsic incubation temperature.
aData in Table 1 is the best estimate of IR, DR, TR determined as proportion of all engorged females in the experiment, but because raw data was not available, slight differences from the actual results may be present.
Since multiple introductions of Ae. albopictus have been suggested in the United States [53], with both temperate and tropical origins, vector competence assays should be conducted with multiple populations, as it is known that there is intraspecific variation. Such variation may be behind the findings that Culex pipiens and its sibling species, Culex quinquefasciatus, are not competent ZIKV vectors in a total of at least 11 experimental laboratory studies [54–64] among others reviewed in [65]; but Cx. quinquefasciatus was demonstrated to transmit to mice when a population from Hainan province of southern China was infected with a 2016 isolate from Samoa [66], and the presence of ZIKV RNA and infectious virus in 3 of 80 pools of Cx. quinquefasciatus collected in field studies in Recife, Brazil, a hotspot for ZIKV [68]. In addition, laboratory studies demonstrated the presence of viral RNA in the saliva of perorally infected Culex [67, 68]. Other Brazilian scientists differ in their findings, concluding that Cx. quinquefasciatus has not played a role in the Rio de Janeiro outbreak; experimental studies with Cx. quinquefasciatus from areas with the highest incidence of microcephaly associated with ZIKV infections in the Northeast Region of Brazil demonstrated they are refractory to ZIKV [69]. One possible explanation for the discrepancy in results is mosquito population genetics, which are known to vary for Cx. quinquefasciatus populations [70], and virus genetics, which are known to affect vector competence. While this explanation seems unlikely, and Cx. pipiens/Cx. quinquefasciatus do not appear to play a significant role in ZIKV transmission, one cannot rule out that with some combinations of virus and vector strains, Cx. quinquefasciatus may possibly serve as secondary vectors.
VIRUS PERPETUATION
ZIKV is maintained primarily by transmission between humans and Aedes species mosquitoes. Some Aedes species capable of transmitting ZIKV and other viruses are likely to live year-round across certain tropical areas in the Americas, Africa, and Asia. However, in less suitable habitats, the virus may persist in the environment through alternative mechanisms. While there is no requirement for an enzootic amplification cycle, similar to DENV, CHIKV, and YFV once virus emerges from its sylvatic habitats, sylvatic transmission between nonhuman primates and forest-dwelling mosquitoes may serve to maintain the virus during periods of low urban transmission either due to climatic conditions or to high herd immunity in the human population. Antibody to ZIKV has been detected in other vertebrates besides nonhuman primates in Africa and Asia, but because of extensive cross-reactions among flaviviruses even in serum neutralization assays, these serologic assays are not confirmatory [71]. However, more studies are needed as experimental infections demonstrated susceptibility of diverse vertebrate species. The role of sylvatic virus in directly causing disease in humans is under investigation with DENV [72], and clearly occurs with YFV [73].
Another mechanism of viral maintenance is vertical transmission, which may serve to facilitate maintenance during low transmission seasons/years. Infection of male Ae. furcifer, a forest vector, has been reported [6]. ZIKV vertical transmission in Ae. aegypti mosquitoes was demonstrated in F1 adults following intrathoracic inoculation, yielding a minimum filial infection rate of 1:290 [74]. These investigators did not observe vertical transmission by Ae. albopictus similarly tested. But intrathoracic inoculation is not a natural means of infection. Following peroral infection of Ae. aegypti with 8.9–9.3 log10 plaque-forming units/mL ZIKV (Honduras), resulting in >93% disseminated infections, a filial infection rate (FIR) of 11.9 (range, 4.9–24.6) was found, equal to approximately 1:84, a rate higher than that generally observed for flaviviruses. Ae. albopictus, similarly tested, was determined to have an FIR of 11.8 (range, 1.7–134.8), thus also capable of vertical transmission [75]. Finally, alternate vectors must be considered as playing a role in maintenance of ZIKV. Little research has been done evaluating mosquito species other than Ae. aegypti and Ae. albopictus, with the exception of the studies mentioned above on Cx. quinquefasciatus.
ABIOTIC FACTORS
Mosquitoes are particularly susceptible to climate variability and climatic change as they are poikilothermic organisms; despite its domestic habits, Ae. aegypti is no exception. There have been numerous studies on association between temperature and development, current and future geographic distribution, and population dynamics of Ae. aegypti mostly in relation to DENV transmission [76, 77], but also looking specifically at impact on the vector itself [78, 79]. Temperature and precipitation affect both the immature stages of the mosquito, a holometabolus organism with immature stages confined to water-containing environments, and the adult stage. However, adults have a greater ability to survive in ostensibly inhospitable environments by moving to protected areas, (eg, cellars, sewers). That being said, Ae. aegypti larvae also have been found to breed very successfully in subterranean habitats, such as wells and service manholes in Australia [80]. Septic tanks in Puerto Rico have been demonstrated to be productive for Ae. aegypti larvae [81]. Such protected habitats allow Ae. aegypti to maintain DENV during the dry season. The identification of such habitats is especially important as Aedes control campaigns are directed at surface habitats primarily. New habitats more biologically accommodating to the vectors are also created by warming climates. For example, Ae. aegypti is now found at elevations up to 1420 m above sea level in Mexico, where elevations >1200 m above sea level had been prohibitive in the past [82]. Similarly, Lozano-Fuentes [83] commonly found Ae. aegypti at elevations as high as 1700 m, and occasionally from 1700 to 2130 m above sea level, in Mexico.
Other factors besides climate contribute equally, if not more, to increasing risk of infection with viruses such as ZIKV transmitted by Ae. aegypti. Such factors fall into social, economic, and epidemiologic groups. Passenger travel has increased significantly, allowing viremic individuals to travel with increasing speed to receptive environments containing susceptible mosquitoes. The estimated number of passengers flying internationally increased from 227 million in 1980 to 1.2 billion people in year 2016 [84, 85]. It is anticipated that by 2030, the number of people flying across international borders will exceed 1.8 billion per year. Because individuals with DENV, ZIKV, CHIKV, and YFV infections are infectious during the viremic phase for Ae. aegypti mosquitoes, these viruses may move even more frequently around the globe in the future. Furthermore, not only human travel, but also increased movement of goods around the world, creates an environment where eggs are transported inadvertently with cargo between regions. The vectors Ae. aegypti and Ae. albopictus are spreading geographically as a consequence of their invasiveness as container breeders and the ability of their eggs to withstand dry conditions. Anthropogenic changes increase breeding habitats and increase contact between humans and mosquitoes in increasingly dense urban population centers with substandard housing and lacking infrastructure to support the number of people living there.
PREVENTION AND CONTROL
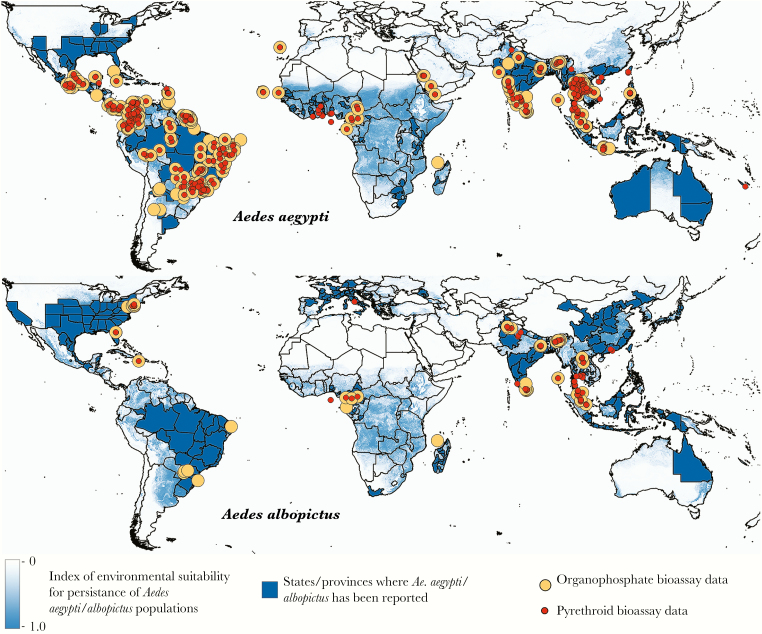
With the continued expansion of DENV and the recent introduction of ZIKV to the Americas, there has been a new impetus for vaccine development and antivirals against flaviviruses. Nonetheless, control of arboviral activity still rests largely with vector control at this time. This is problematic with the growing prevalence of insecticide resistance, particularly to commonly used organophosphates and pyrethroids, as well as carbamates and organochlorines and growing intolerance of the community to the use of such toxic agents, diminishing effectiveness of such intervention strategies. Moyes and colleagues [86] recently provided a comprehensive assessment of the geographical distribution of insecticide resistance in Ae. aegypti and Ae. albopictus, against the backdrop of environmental suitability for these species [87]. Figure 3 points out the locations of populations that have been bioassayed. This makes control of Ae. aegypti and Ae. albopictus and the diseases they cause a particularly grand challenge.
Figure 3.
Locations of bioassay data for the organophosphates and pyrethroids, 2006–2015. Locations of populations that have been bioassayed (susceptibility and dose response, adult and larval) are shown for both insecticide classes, overlaid on maps of environmental suitability for Aedes aegypti and Aedes albopictus. Adapted from Moyes et al [86] and Kraemer et al [87].
In the early 20th century, systematic elimination of Ae. aegypti breeding sites successfully led to vector suppression and consequent control of yellow fever [88]. In the 1950s, DDT (dichlorodiphenyltrichloroethane) was used to spray infested containers and solid surfaces to further control Ae. aegypti [89]. By the early 1960s, the species was declared absent from 22 countries. But when control efforts ceased, Ae. aegypti, which likely had not been completely eliminated by the earlier efforts, reemerged in force repopulating cities in Central and South America. Today, Ae. aegypti continues to pose a major public health threat.
The field of vector control is moving forward rapidly with promising new approaches. But the behavior and biology of Ae. aegypti and Ae. albopictus make these species particularly difficult to control over the long term. Specifically, their propensity to breed in artificial and natural containers in close proximity to people’s homes, where in addition the adults have access to protected resting sites, diminishes exposure to treatment. The number, diversity, and distribution of containers make it logistically difficult to treat all existing habitats to eliminate breeding. Furthermore, control of Ae. aegypti–transmitted pathogens is exacerbated by this species’ habit of feeding often and almost exclusively on human blood, thereby increasing the efficiency of transmission (R0) of each individual female [36].
As a consequence, a broad multifaceted approach to control is needed. With integrated pest management, a combination of methods is applied in concert, addressing prevention of transmission, reduction of the vector population, and the elimination of conditions that lead to mosquito infestations [90]. One aspect of this approach is adoption of personal protection measures by the community, education on Ae. aegypti and Ae. albopictus habits and on how to prevent mosquito bites, and source reduction. But such effort on its own has not been very successful in controlling disease. Additionally, ultra–low volume sprays and fogging with US Environmental Protection Agency–registered pesticides, generally conducted by mosquito control organizations or companies using aircraft or truck-mounted sprayers, have been used. But this method of control is fraught with problems not only because of indoor resting behavior of the vector, but also insecticide resistance, insecticide persistence in the environment, toxicity of the compounds for nontarget species, and public opinion. While peridomestic and indoor residual spraying may have been successful in the past, this approach is no longer acceptable to most people in an urban environment.
Biopesticides are being used as larvicides to circumvent the problem of resistance to and toxicity of chemical compounds. Examples include microbial control agents—for example, Bacillus thuringiensis (Berliner) [91] and Bacillus sphaericus Neide [92]—and insect growth regulators such as methoprene, pyriproxyfen, and diflubenzuron, which are chitin synthesis inhibitors [93, 94]. Pyriproxyfen not only inhibits the development of the adult mosquito, but in addition, when an adult mosquito comes in contact with this agent it can inadvertently transfer it to other containers [95]. It has been demonstrated in the field that low doses are effective in inhibiting Aedes and the residual activity can be maintained for 11–15 weeks [96]. But in the laboratory, resistance to some chitin synthesis inhibitors develops within a few generations [93]. Excellent thorough reviews on microbial control agents have been published and should be read for comprehensive information (eg, [97, 98], among many others). But it must be kept in mind that any introduced lethal agent, whether chemical or biological, affects the entire ecosystem because of the interdepence of all life.
Physical approaches to mosquito control include lethal ovitraps, insecticide-treated clothing, mosquito coils, and other such measures (see Ogoma and colleagues [99] for a review of spatial repellency testing methodologies). But both cultural and biological factors hinder integrated pest management methods, making development of novel methods a priority. To name a few such factors, people are hesitant to allow biological or chemical agents to be added to their drinking water; many breeding sites are cryptic and cannot be easily located; and breeding habitats may be ephemeral, making biological control ineffective.
Exciting novel approaches under development and in trial circumvent chemicals and biologicals and can be incorporated into an integrated vector control program. These approaches attack the adult stage of the mosquito and use the manipulated male mosquito as the delivery vehicle. They include sterile insect technique (SIT) [100], a modification of SIT where genetically engineered males carrying dominant lethal genes (release of insects carrying dominant lethal gene [RIDL]) are released into the wild [101], and release of Wolbachia-infected mosquitoes. Each of these approaches will be discussed briefly below.
Sterile Insect Technique
In this autocidal approach to mosquito control, large numbers of irradiated or chemosterilized males are released into the environment where they compete with wild-type males to ultimately suppress the F1 population of that species. Because SIT control is based on mating behavior, this approach is species-specific. But in field tests, the sterilized males lack competitiveness; furthermore, mated females may move into the area and lay eggs, further diminishing effectiveness. Repeated releases of the sterilized males is necessary as the procedure is not self-sustaining, but it is only native species that are being introduced, making the technique environmentally friendly. This technique has been extremely successful in eradicating the screw-worm fly (Cochliomyia hominivorax) from North and Central America [102].
Release of Insects Carrying Dominant Lethal Gene
Suppression is basically a modification of the SIT where, in place of irradiation or chemosterilization, a dominant lethal transgene is inserted during the embryonic stage. Its expression is artificially repressed during rearing in the laboratory but functional following release of the engineered males in the wild; any wild female mating with a RIDL male will produce offspring, all of which carry 1 copy of the RIDL system. All the female progeny will therefore die from the female-specific lethality. The male progeny, on the other hand, will survive, and because they carry 1 copy of the RIDL system, half of the next generation’s female progeny will die, and so on [101].
Oxitec (Oxitec Limited, Oxford, United Kingdom) has developed its own version of RIDL where the male mosquitoes are engineered to contain a self-limiting gene that causes their offspring to die, but allowing Oxitec insects to live and reproduce normally when they are fed a diet containing tetracycline (in the rearing facility). The self-limiting gene, tTAV (tetracycline repressible transactivator variant), is a gene variant that has been optimized to only work in insect cells. In the wild, offspring that contain the self-limiting gene make a nontoxic protein that ties up the cell’s machinery so its other genes are not expressed and the insect dies [103]. Field studies in the Cayman Islands led to a suppression of 80% of the natural population [104] and, in Brazil, a suppression of 78%–95% of the natural population [105].
Wolbachia
Wolbachia is an endosymbiotic bacteria that is naturally found in many arthropod species, with the exception of Ae. aegypti [106]. Infection of Ae. aegypti with Wolbachia has been shown to inhibit DENV and CHIKV replication [107] as well as ZIKV [108]. Contributing further to the effectiveness of Wolbachia is the phenomenon of cytoplasmic incompatibility [109], allowing it to spread efficiently in caged populations [110] and in the environment, as demonstrated in Australia with the release of Ae. aegypti infected with wMel Wolbachia. The frequency of these Wolbachia-infected Ae. aegypti remained at >90% for 3 years [111] demonstrating that Wolbachia is a highly efficient gene drive mechanism.
A pressing question is what level of population suppression of Ae. aegypti is necessary for elimination of virus transmission to humans, the target host. Since Ae. aegypti may take multiple interrupted blood meals in each gonotrophic cycle, mosquito populations with few infected females may still be effective in maintenance of the virus transmission cycle. Other issues that need to be addressed include lack of acceptance by the community, logistics of release, and certainty that nontarget insects are not harmed.
CONCLUSIONS
Aedes aegypti and Ae. albopictus are widely distributed globally and 2 of the most important vectors of human pathogens involved in the transmission of medically important arboviruses such as DENV, ZIKV, CHIKV, and YFV. This review has discussed some of the factors that have contributed to the ecological success of these species, including rapid development, desiccation-resistant eggs, resistance to the principal insecticide classes currently available on the market, preference for the urban environment and consequently proximity to humans, globalization (ie, increase of trade and travel), lack of effective surveillance, and lack of efficient control. Furthermore, Ae. albopictus demonstrates ecological plasticity and a strong competitive ability [112]. Relationships between climate and vector ecology and the social, economic, and epidemiological factors involved in virus transmission remain unclear. Models have not accounted for local microclimate effects, leading to difficulty interpreting results. But socioeconomic factors, including adequate healthcare and sanitation, may affect the current geographic distribution and human incidence of many diseases more significantly than climate. Clearly, integrated management approaches to control Ae. aegypti and Ae. albopictus must be undertaken if there is any hope of controlling these 2 important mosquito vectors.
Notes
Acknowledgments. We thank Mary Franke for her helpful assistance with the manuscript and supplementary data, and Alex Ciota for his suggestions for the table.
Financial support. This work was supported by the New York State Department of Health.
Supplement sponsorship. This work is part of a supplement sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH).
Potential conflicts of interest. Both authors: No reported conflicts of interest. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gaunt MW, Sall AA, de Lamballerie X, Falconar AK, Dzhivanian TI, Gould EA. Phylogenetic relationships of flaviviruses correlate with their epidemiology, disease association and biogeography. J Gen Virol 2001; 82:1867–76. [DOI] [PubMed] [Google Scholar]
- 2. Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–20. [DOI] [PubMed] [Google Scholar]
- 3. Haddow AJ, Williams MC, Woodall JP, Simpson DI, Goma LK. Twelve isolations of Zika virus from Aedes (Stegomyia) africanus (Theobald) taken in and above a Uganda forest. Bull World Health Organ 1964; 31:57–69. [PMC free article] [PubMed] [Google Scholar]
- 4. McCrae AW, Kirya BG. Yellow fever and Zika virus epizootics and enzootics in Uganda. Trans R Soc Trop Med Hyg 1982; 76:552–62. [DOI] [PubMed] [Google Scholar]
- 5. Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979; 83:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diallo D, Sall AA, Diagne CT et al. . Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One 2014; 9:e109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diagne CT, Diallo D, Faye O et al. . Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infect Dis 2015; 15:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boorman JP, Porterfield JS. A simple technique for infection of mosquitoes with viruses; transmission of Zika virus. Trans R Soc Trop Med Hyg 1956; 50:238–42. [DOI] [PubMed] [Google Scholar]
- 9. Cornet M, Robin Y. Transmission experimentale comparee du virus Zika chez Aedes aegypti. Ent med et Parasitology 1979; 17:47–53. [Google Scholar]
- 10. Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg 1969; 18:411–5. [DOI] [PubMed] [Google Scholar]
- 11. Duffy MR, Chen TH, Hancock WT et al. . Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–43. [DOI] [PubMed] [Google Scholar]
- 12. Ledermann JP, Guillaumot L, Yug L et al. . Aedes hensilli as a potential vector of chikungunya and Zika viruses. PLoS Negl Trop Dis 2014; 8:e3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paupy C, Kassa Kassa F, Caron M, Nkoghé D, Leroy EM. A chikungunya outbreak associated with the vector Aedes albopictus in remote villages of Gabon. Vector Borne Zoonotic Dis 2012; 12:167–9. [DOI] [PubMed] [Google Scholar]
- 14. Cao-Lormeau VM, Roche C, Teissier A et al. . Zika virus, French Polynesia, South Pacific, 2013. Emerg Infect Dis 2014; 20:1085–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richard V, Paoaafaite T, Cao-Lormeau VM. Vector competence of French Polynesian Aedes aegypti and Aedes polynesiensis for Zika virus. PLoS Negl Trop Dis 2016; 10:e0005024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lanciotti RS, Kosoy OL, Laven JJ et al. . Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caron M, Paupy C, Grard G et al. . Recent introduction and rapid dissemination of chikungunya virus and dengue virus serotype 2 associated with human and mosquito coinfections in Gabon, Central Africa. Clin Infect Dis 2012; 55:e45–53. [DOI] [PubMed] [Google Scholar]
- 18. Powell JR, Tabachnick WJ. History of domestication and spread of Aedes aegypti—a review. Mem Inst Oswaldo Cruz 2013; 108(suppl 1):11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB Jr. Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science 1987; 236:1114–6. [DOI] [PubMed] [Google Scholar]
- 20. Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis 2007; 7:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Enserink M. Entomology. A mosquito goes global. Science 2008; 320:864–6. [DOI] [PubMed] [Google Scholar]
- 22. Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol 2004; 18:215–27. [DOI] [PubMed] [Google Scholar]
- 23. Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog 2007; 3:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pan American Health Organization/World Health Organization. Zika epidemiological update, 21 April 2016. Washington, DC: PAHO/WHO, 2016. [Google Scholar]
- 25. Schuffenecker I, Iteman I, Michault A et al. . Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med 2006; 3:e263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sota T, Mogi M. Interspecific variation in desiccation survival time of Aedes (Stegomyia) mosquito eggs is correlated with habitat and egg size. Oecologia 1992; 90:353–8. [DOI] [PubMed] [Google Scholar]
- 27. Juliano SA, O’Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia 2002; 130:458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Faull KJ, Webb C, Williams CR. Desiccation survival time for eggs of a widespread and invasive Australian mosquito species, Aedes (Finlaya) notoscriptus (Skuse). J Vector Ecol 2016; 41:55–62. [DOI] [PubMed] [Google Scholar]
- 29. Hanson SM, Craig GB Jr. Relationship between cold hardiness and supercooling point in Aedes albopictus eggs. J Am Mosq Control Assoc 1995; 11:35–8. [PubMed] [Google Scholar]
- 30. Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition?Ecology 1998; 79:255–68. [Google Scholar]
- 31. Braks MAH, Honório NA, Lounibos LP, Lourenço-De-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Ann Entomol Soc Am 2014; 9:130–9. [Google Scholar]
- 32. Suwanchaichinda C, Brattsten LB. Induction of microsomal cytochrome P450s by tire-leachate compounds, habitat components of Aedes albopictus mosquito larvae. Arch Insect Biochem Physiol 2002; 49:71–9. [DOI] [PubMed] [Google Scholar]
- 33. Bargielowski IE, Lounibos LP. Satyrization and satyrization-resistance in competitive displacements of invasive mosquito species. Insect Sci 2016; 23:162–74. [DOI] [PubMed] [Google Scholar]
- 34. Tripet F, Lounibos LP, Robbins D, Moran J, Nishimura N, Blosser EM. Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors. Am J Trop Med Hyg 2011; 85:265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bargielowski IE, Lounibos LP, Shin D et al. . Widespread evidence for interspecific mating between Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in nature. Infect Genet Evol 2015; 36:456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ritchie SA. Dengue vector bionomics: why Aedes aegypti is such a good vector. In: Gubler DJO, Ooi EE, Vasudevan S, Farrar J, eds. Dengue and dengue haemorrhagic fever. Canberra, Australia: CSIRO Press, 2014:455–80. [Google Scholar]
- 37. Ponlawat A, Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol 2005; 42:844–9. [DOI] [PubMed] [Google Scholar]
- 38. Faraji A, Egizi A, Fonseca DM et al. . Comparative host feeding patterns of the Asian tiger mosquito, Aedes albopictus, in urban and suburban northeastern USA and implications for disease transmission. PLoS Negl Trop Dis 2014; 8:e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Richards SL, Ponnusamy L, Unnasch TR, Hassan HK, Apperson CS. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of central North Carolina. J Med Entomol 2006; 43:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Valerio L, Marini F, Bongiorno G et al. . Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in urban and rural contexts within Rome province, Italy. Vector Borne Zoonotic Dis 2010; 10:291–4. [DOI] [PubMed] [Google Scholar]
- 41. Miller BR, Monath TP, Tabachnick WJ, Ezike VI. Epidemic yellow fever caused by an incompetent mosquito vector. Trop Med Parasitol 1989; 40:396–9. [PubMed] [Google Scholar]
- 42. Hahn MB, Eisen RJ, Eisen L et al. . Reported distribution of Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus in the United States, 1995–2016 (Diptera: Culicidae). J Med Entomol 2016; 53:1169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tsetsarkin KA, McGee CE, Volk SM, Vanlandingham DL, Weaver SC, Higgs S. Epistatic roles of E2 glycoprotein mutations in adaption of chikungunya virus to Aedes albopictus and Ae. aegypti mosquitoes. PLoS One 2009; 4:e6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rezza G, Nicoletti L, Angelini R et al. . CHIKV Study Group Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 2007; 370:1840–6. [DOI] [PubMed] [Google Scholar]
- 45. Grandadam M, Caro V, Plumet S et al. . Chikungunya virus, southeastern France. Emerg Infect Dis 2011; 17:910–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lo Presti A, Ciccozzi M, Cella E et al. . Origin, evolution, and phylogeography of recent epidemic CHIKV strains. Infect Genet Evol 2012; 12:392–8. [DOI] [PubMed] [Google Scholar]
- 47. European Centre for Disease Prevention and Control. Aedes albopictus. http://ecdc.europa.eu/en/healthtopics/vectors/mosquitoes/Pages/aedes-albopictus.aspx. Accessed 15 May 2017. [Google Scholar]
- 48. Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect 2009; 11:1177–85. [DOI] [PubMed] [Google Scholar]
- 49. Juliano SA, LounibosLP. Invasions by mosquitoes: the roles of behaviour across the life cycle. In: Weis JS, Sol D, eds. Biological invasions and animal behaviour. Cambridge, UK: Cambridge University Press, 2016:245–65. [Google Scholar]
- 50. Hufbauer RA, Facon B, Ravigné V et al. . Anthropogenically induced adaptation to invade (AIAI): contemporary adaptation to human-altered habitats within the native range can promote invasions. Evol Appl 2012; 5:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. De Majo MS, Montini P, Fischer S. Egg hatching and survival of immature stages of Aedes aegypti (Diptera: Culicidae) under natural temperature conditions during the cold season in Buenos Aires, Argentina. J Med Entomol 2017; 54:106–13. [DOI] [PubMed] [Google Scholar]
- 52. Roundy CM, Azar SR, Rossi SL et al. . Variation in Aedes aegypti mosquito competence for Zika virus transmission. Emerg Infect Dis 2017; 23:625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marcombe S, Farajollahi A, Healy SP, Clark GG, Fonseca DM. Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. PLoS One 2014; 9:e101992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aliota MT, Peinado SA, Osorio JE, Bartholomay LC. Culex pipiens and Aedes triseriatus mosquito susceptibility to Zika virus. Emerg Infect Dis 2016; 22:1857–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang YJ, Ayers VB, Lyons AC et al. . Culex species mosquitoes and Zika virus. Vector Borne Zoonotic Dis 2016; 16:673–6. [DOI] [PubMed] [Google Scholar]
- 56. Kenney JL, Romo H, Duggal NK et al. . Transmission incompetence of Culex quinquefasciatus and Culex pipiens pipiens from North America for Zika virus. Am J Trop Med Hyg 2017; 96:1235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Amraoui F, Atyame-Nten C, Vega-Rua A, Lourenco-de-Oliveira R, Vazeille M, Failloux AB. Culex mosquitoes are experimentally unable to transmit Zika virus. Euro Surveill 2016; 21. doi:10.2807/1560-7917.ES.2016.21.35.30333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boccolini D, Toma L, Di Luca M et al. . Experimental investigation of the susceptibility of Italian Culex pipiens mosquitoes to Zika virus infection. Euro Surveill 2016; 21. doi:10.2807/1560-7917.ES.2016.21.35.30328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fernandes RS, Campos SS, Ferreira-de-Brito A et al. . Culex quinquefasciatus from Rio de Janeiro is not competent to transmit the local Zika virus. PLoS Negl Trop Dis 2016; 10:e0004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hall-Mendelin S, Pyke AT, Moore PR et al. . Assessment of local mosquito species incriminates Aedes aegypti as the potential vector of Zika virus in Australia. PLoS Negl Trop Dis 2016; 10:e0004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hart CE, Roundy CM, Azar SR et al. . Zika virus vector competency of mosquitoes, Gulf Coast, United States. Emerg Infect Dis 2017; 23:559–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Weger-Lucarelli J, Rückert C, Chotiwan N et al. . Vector competence of American mosquitoes for three strains of Zika virus. PLoS Negl Trop Dis 2016; 10:e0005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dodson BL, Rasgon JL. Vector competence of Anopheles and Culex mosquitoes for Zika virus. Peer J 2017; 5:e3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Heitmann A, Jansen S, Luhken R et al. . Experimental transmission of Zika virus by mosquitoes from Central Europe. Euro Surveill 2017; 22. doi:10.2807/1560-7917.ES.2017.22.2.30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lourenco-de-Oliveira R, Failloux AB. Lessons learned on Zika virus vectors. PLoS Negl Trop Dis 2017; 11:e0005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guo XX, Li CX, Deng YQ et al. . Culex pipiens quinquefasciatus: a potential vector to transmit Zika virus. Emerg Microbes Infect 2016; 5:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Guedes DR, Paiva MH, Donato MM et al. . Zika virus replication in the mosquito Culex quinquefasciatus in Brazil . Emerg Microbes Infect 2017; 6:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zika virus detected in Culex mosquito: Brazilian researchers. http://outbreaknewstoday.com/zika-virus-detected-in-culex-mosquito-brazilian-researchers-36669/. Accessed 25 July 2017. [Google Scholar]
- 69. Fernandes RS, Campos SS, Ribeiro PS, Raphael LM, Bonaldo MC, Lourenço-de-Oliveira R. Culex quinquefasciatus from areas with the highest incidence of microcephaly associated with Zika virus infections in the Northeast Region of Brazil are refractory to the virus. Mem Inst Oswaldo Cruz 2017; 112:577–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fonseca DM, Smith JL, Wilkerson RC, Fleischer RC. Pathways of expansion and multiple introductions illustrated by large genetic differentiation among worldwide populations of the southern house mosquito. Am J Trop Med Hyg 2006; 74:284–9. [PubMed] [Google Scholar]
- 71. Bueno MG, Martinez N, Abdalla L, Duarte Dos Santos CN, Chame M. Animals in the Zika virus life cycle: what to expect from megadiverse Latin American countries. PLoS Negl Trop Dis 2016; 10:e0005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vasilakis N, Cardosa J, Diallo M et al. . Sylvatic dengue viruses share the pathogenic potential of urban/endemic dengue viruses. Letter to the editor. J Virol 2010; 3726–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dyer O. Yellow fever stalks Brazil in Zika’s wake. BMJ 2017; 356:j707. [DOI] [PubMed] [Google Scholar]
- 74. Thangamani S, Huang J, Hart CE, Guzman H, Tesh RB. Vertical transmission of Zika virus in Aedes aegypti mosquitoes. Am J Trop Med Hyg 2016; 95:1169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ciota AT, Bialosuknia SM, Ehrbar DJ, Kramer LD. Vertical transmission of Zika virus by Aedes aegypti and Ae. albopictus mosquitoes. Emerg Infect Dis 2017; 23:880–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nicholls N. El niño-southern oscillation and vector-borne disease. Lancet 1993; 342:1284–5. [DOI] [PubMed] [Google Scholar]
- 77. Hales S, Weinstein P, Woodward A. Dengue fever epidemics in the South Pacific: driven by El Niño Southern oscillation?Lancet 1996; 348:1664–5. [DOI] [PubMed] [Google Scholar]
- 78. Hopp MJ, Foley JA. Global-scale relationships between climate and the dengue fever vector, Aedes aegypti. Climate Change 2001; 48:441–63. [Google Scholar]
- 79. Eisen L, Monaghan AJ, Lozano-Fuentes S, Steinhoff DF, Hayden MH, Bieringer PE. The impact of temperature on the bionomics of Aedes (Stegomyia) aegypti, with special reference to the cool geographic range margins. J Med Entomol 2014; 51:496–516. [DOI] [PubMed] [Google Scholar]
- 80. Russell BM, McBride WJ, Mullner H, Kay BH. Epidemiological significanceof subterranean Aedes aegypti (Diptera: Culicidae) breeding sites to dengue virus infection in Charters Towers, 1993. J Med Entomol 2002; 39:143–5. [DOI] [PubMed] [Google Scholar]
- 81. Burke R, Barrera R, Lewis M, Kluchinsky T, Claborn D. Septic tanks as larval habitats for the mosquitoes Aedes aegypti and Culex quinquefasciatus in Playa-Playita, Puerto Rico. Med Vet Entomol 2010; 24:117–23. [DOI] [PubMed] [Google Scholar]
- 82. Equihua M, Ibáñez-Bernal S, Benítez G, Estrada-Contreras I, Sandoval-Ruiz CA, Mendoza-Palmero FS. Establishment of Aedes aegypti (L.) in mountainous regions in Mexico: increasing number of population at risk of mosquito-borne disease and future climate conditions. Acta Trop 2017; 166:316–27. [DOI] [PubMed] [Google Scholar]
- 83. Lozano-Fuentes S, Hayden MH, Welsh-Rodriguez C et al. . The dengue virus mosquito vector Aedes aegypti at high elevation in Mexico. Am J Trop Med Hyg 2012; 87:902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. SUNY Levin Institute. Increased global travel. http://www.globalization101.org/increased-global-travel. Accessed 15 May 2017. [Google Scholar]
- 85. United Nations World Tourism Organization. UNWTO annual report 2016. http://www.e-unwto.org/doi/book/10.18111/9789284418725. Accessed 25 July 2017. [Google Scholar]
- 86. Moyes CL, Vontas J, Martins AJ et al. . Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis 2017; 11:e0005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kraemer MU, Sinka ME, Duda KA et al. . The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 2015; 4:e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Soper FL. The elimination of urban yellow fever in the Americas through the eradication of Aedes aegypti. Am J Public Health Nations Health 1963; 53:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Severo OP. Eradication of the Aedes aegypti mosquito from the Americas (1955). Yellow fever: a symposium in commemertion of Carlos Juan Finlay, 1955. Paper 6. 1955. [Google Scholar]
- 90. Rose RI. Pesticides and public health: integrated methods of mosquito management. Emerg Infect Dis 2001; 7:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mulla MS. Activity, field efficacy, and use of Bacillus thuringiensis israelensis against mosquitoes. In: Huguette de Barjac DJS, ed. Bacterial control of mosquitoes & black flies. Heidelberg: Springer Netherlands, 1990:134–60. [Google Scholar]
- 92. Mulla MS. Role of B.t.i. and Bacillus sphaericus in mosquito control programs. In: Sampson RA, Vlak JM, Peters D, eds. Fundamental and applied aspects of invertebrate pathology. Wageningen, the Netherlands: Foundation of the Fourth International Colloquium of Invertebrate Pathology, 1986:494–6. [Google Scholar]
- 93. Belinato TA, Valle D. The impact of selection with diflubenzuron, a chitin synthesis inhibitor, on the fitness of two Brazilian Aedes aegypti field populations. PLoS One 2015; 10:e0130719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Faraji A, Unlu I. The eye of the tiger, the thrill of the fight: effective larval and adult control measures against the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae), in North America. J Med Entomol 2016; 53:1029–47. [DOI] [PubMed] [Google Scholar]
- 95. Vythilingam I, Sam JI, Chan YF, Khaw LT, Sulaiman WY. New paradigms for virus detection, surveillance and control of Zika virus vectors in the settings of Southeast Asia. Front Microbiol 2016; 7:1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Vythilingam I, Luz BM, Hanni R, Beng TS, Huat TC. Laboratory and field evaluation of the insect growth regulator pyriproxyfen (Sumilarv 0.5G) against dengue vectors. J Am Mosq Control Assoc 2005; 21:296–300. [DOI] [PubMed] [Google Scholar]
- 97. Mulla MS. Field evaluation and efficacy of bacterial agents and their formulations against mosquito larvae. In: Laird M, Miles JW, eds. Integrated mosquito control methodologies. San Diego, CA: Academic Press, 1985:227–50. [Google Scholar]
- 98. Lacey LA, Undeen AH. Microbial control of black flies and mosquitoes. Annu Rev Entomol 1986; 31:265–96. [DOI] [PubMed] [Google Scholar]
- 99. Ogoma SB, Moore SJ, Maia MF. A systematic review of mosquito coils and passive emanators: defining recommendations for spatial repellency testing methodologies. Parasit Vectors 2012; 5:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Klassen W. Area-wide integrated pest management and the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS, eds. Sterile insect technique principles and practice in area-wide integrated pest management. Dordrecht, the Netherlands: Springer, 2005:39–68. [Google Scholar]
- 101. Thomas DD, Donnelly CA, Wood RJ, Alphey LS. Insect population control using a dominant, repressible, lethal genetic system. Science 2000; 287:2474–6. [DOI] [PubMed] [Google Scholar]
- 102. Wyss JH. Screwworm eradication in the Americas. Ann N Y Acad Sci 2000; 916:186–93. [DOI] [PubMed] [Google Scholar]
- 103. Oxitec. Oxitec: the science. http://www.oxitec.com/our-solution/technology/the-science/. Accessed 6 July 2017. [Google Scholar]
- 104. Harris AF, McKemey AR, Nimmo D et al. . Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat Biotechnol 2012; 30:828–30. [DOI] [PubMed] [Google Scholar]
- 105. Carvalho DO, McKemey AR, Garziera L et al. . Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl Trop Dis 2015; 9:e0003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 2008; 6:741–51. [DOI] [PubMed] [Google Scholar]
- 107. Moreira LA, Iturbe-Ormaetxe I, Jeffery JA et al. . A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell 2009; 139:1268–78. [DOI] [PubMed] [Google Scholar]
- 108. Tan CH, Wong PJ, Li MI, Yang H, Ng LC, O’Neill SL. wMel limits Zika and chikungunya virus infection in a Singapore Wolbachia-introgressed Ae. aegypti strain, wMel-Sg. PLoS Negl Trop Dis 2017; 11:e0005496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Caragata EP, Dutra HL, Moreira LA. Exploiting intimate relationships: controlling mosquito-transmitted disease with Wolbachia. Trends Parasitol 2016; 32:207–18. [DOI] [PubMed] [Google Scholar]
- 110. Walker T, Johnson PH, Moreira LA et al. . The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011; 476: 450–3. [DOI] [PubMed] [Google Scholar]
- 111. Hoffmann AA, Iturbe-Ormaetxe I, Callahan AG et al. . Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis 2014; 8:e3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Carvalho FD, Moreira LA. Why is Aedes aegypti Linnaeus so successful as a species?Neotrop Entomol 2017; 46:243–55. [DOI] [PubMed] [Google Scholar]
- 113. Chouin-Carneiro T, Vega-Rua A, Vazeille M et al. . Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl Trop Dis 2016; 10:e0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ciota AT, Bialosuknia SM, Zink SD et al. . Effects of Zika virus strain and Aedes mosquito species on vector competence. Emerg Infect Dis 2017; 23:1110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Di Luca M, Severini F, Toma L et al. . Experimental studies of susceptibility of Italian Aedes albopictus to Zika virus. Euro Surveill 2016; 21. doi:10.2807/1560-7917.ES.2016.21.18.30223. [DOI] [PubMed] [Google Scholar]
- 116. Dutra HL, Rocha MN, Dias FB, Mansur SB, Caragata EP, Moreira LA. Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe 2016; 19:771–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Li MI, Wong PS, Ng LC, Tan CH. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl Trop Dis 2012; 6:e1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wong PS, Li MZ, Chong CS, Ng LC, Tan CH. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis 2013; 7:e2348. [DOI] [PMC free article] [PubMed] [Google Scholar]