The mechanisms of IBA transport and conversion are covered in this review, and specific roles for IBA-derived auxin in driving certain developmental events are discussed.
Keywords: Auxin, IBA, plant development, root development, shoot development
Abstract
The plant hormone auxin is a central regulator of plant growth and development. Because auxin plays critical roles in cell division and cell expansion, plants use a number of cellular mechanisms to regulate auxin levels and response. Among these mechanisms is regulated input from the auxin precursor indole-3-butyric acid (IBA) toward the pool of active auxin [indole-3-acetic acid (IAA)]. In this review, we cover the mechanisms of IBA transport and conversion, and discuss specific roles for IBA-derived auxin in driving certain developmental events. We further discuss multiple open questions remaining for the IBA field.
Introduction
The term auxin is derived from the Greek word ‘auxein’, which means ‘to grow’. Because auxin is a potent regulator of cell division, cell expansion, and cell differentiation (reviewed in Enders and Strader, 2015), it is involved in nearly every aspect of plant development. Therefore, regulation of auxin levels and response is critical for normal plant form and function. Plants use a number of cellular mechanisms to regulate auxin levels and response, including transport, de novo biosynthesis, and management of inputs from various auxin precursors and storage forms (reviewed in Korasick et al., 2013).
The predominant active auxin, indole-3-acetic acid (IAA), is transported long distances through plants via the combined action of distinct families of transporters (reviewed in Zazímalová et al., 2010). The AUX1/LAX family of transporters act as IAA uptake carriers, whereas members of the ABCB and PIN family of transporters facilitate IAA efflux. Together, these transporters facilitate long-distance, directional transport of IAA through the plant to regulate numerous aspects of plant development.
The main auxin biosynthesis pathway uses the TRYPTO PHAN AMINOTRANSFERASE OF ARABIDOPSIS1 (TAA1) and YUCCA family of enzymes (reviewed in Zhao, 2012). In this pathway, tryptophan is converted to indole-3-pyruvic acid (IPyA) through the activity of the TAA1 family of aminotransferase enzymes. The YUCCA family of flavin monooxygenase-like enzymes then converts IPyA to IAA. Conversion of IPyA to IAA is the rate-limiting step in this process; ovexpression of YUCCA family members results in elevated auxin levels. Further, tissue-specific expression of various YUCCA family members allows for de novo auxin biosynthesis to drive specific aspects of plant development (reviewed in Zhao, 2010).
In addition to biosynthesis of IAA via the IPyA pathway, the pool of active auxin can be modulated by inputs from additional storage forms and precursors, such as IAA conjugates and indole-3-butyric acid (IBA). These auxin inputs can drive distinct aspects of plant development (reviewed in Korasick et al., 2013). In this review, we focus on specific roles for IBA-derived auxin in plant development.
IBA conversion and transport mechanisms
For decades, IBA was described as a ‘synthetic auxin’ that elicited auxin-like effects such as root initiation, stem bending, and leaf epinasty (Zimmerman and Wilcoxon, 1935). Indeed, IBA is the active ingredient in plant propagation media, such as Rootone®, used to induce adventitious rooting in stem cuttings. Later studies have demonstrated that IBA is an endogenous compound in a variety of examined plant species (reviewed in Korasick et al., 2013).
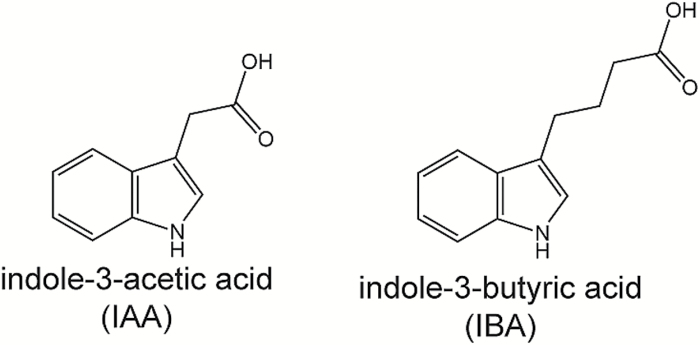
The side chain in the 3 position on the indole ring of IBA has four carbons, as opposed to the two-carbon side chain of IAA (Fig. 1); this lengthened side chain results in a molecule that is probably unable to adopt a conformation for binding into the TIR1–Aux/IAA co-receptor pocket (Uzunova et al., 2016). Indeed, surface plasmon resonance analysis suggests that IBA has no measured binding activity (Uzunova et al., 2016), consistent with the genetic evidence that IBA activity is through its conversion to IAA (reviewed in Strader and Bartel, 2011).
Fig. 1.
IAA and IBA. The side chain in the 3 position on the indole ring of IBA has four carbons, as opposed to the two-carbon side chain of IAA.
IBA is likely to be converted to IAA in a process similar to fatty acid β-oxidation. Many plants convert IBA into IAA (reviewed in Epstein and Ludwig-Müller, 1993), including Arabidopsis (Strader et al., 2010), hazelnut (Kreiser et al., 2016), and elm (Kreiser et al., 2016). In Arabidopsis, this process is peroxisome dependent (Strader et al., 2010), and multiple mutants defective in peroxisome biogenesis and peroxisomal enzymes have been identified for IBA resistance while retaining sensitivity to the active auxin IAA (reviewed in Hu et al., 2012). The PEROXISOMAL TRANSPORTER1/COMATOSE/ABCD1 (PXA1/CTS/ABCD1) transporter is likely to move IBA into the peroxisome for metabolism into active auxin (reviewed in Strader and Bartel, 2011; Michniewicz et al., 2014). Whereas some peroxisomal enzymes, such as the PED1 3-ketoacyl-CoA thiolase, are likely to act in both fatty acid (Hayashi et al., 1998) and IBA β-oxidation (Zolman et al., 2000), other peroxisome enzymes appear to be specific to IBA β-oxidation. Specifically, the predicted short-chain dehydrogenase/reductase INDOLE-3-BUTYRIC ACID RESPONSE1 (IBR1) (Zolman et al., 2008), the acyl-CoA dehydrogenase/oxidase-like IBR3 (Zolman et al., 2007), the predicted enoyl-CoA hydratase IBR10 (Zolman et al., 2008), and the predicted enoyl-CoA hydratase ENOYL-COA HYDRATASE2 (ECH2) (Strader et al., 2011) are enzymes that may act solely in the conversion of the auxin precursor IBA to active IAA.
Similar to mechanisms regulating IAA levels (reviewed in Zazímalová et al., 2010; Korasick et al., 2013), mechanisms to regulate IBA levels include formation of IBA conjugates and IBA transport (Fig. 2). IBA exists in both amide- and ester-linked amide forms (reviewed in Woodward and Bartel, 2005; Bajguz and Piotrowska, 2009; Ludwig-Müller, 2011). Whereas some members of the Arabidopsis GH3 amino acid synthetase family, including GH3.4, GH3.5, GH3.6, and GH3.17, display adenylation activity with both IBA and IAA (Staswick et al., 2005), enzymes specifically conjugating IBA to amino acids have not yet been reported. The hydrolases Triticium aestivum TaIAR3 (Campanella et al., 2004), Brassica rapa BrIAR3 (Savić et al., 2009), and B. rapa BrILL2 (Savić et al., 2009) display higher affinity for IBA–amino acid conjugates than for IAA–amino acid conjugates, consistent with the possibility that IBA may be stored in amino acid conjugate form. Additionally, IBA is likely to be stored as conjugates to sugar. The enzymes UGT74E2 (Tognetti et al., 2010) and UGT75D1 (Zhang et al., 2016) promote the formation of IBA–glucose; overexpression of either UGT74E2 (Tognetti et al., 2010) or UGT75D1 (Zhang et al., 2016) results in elevated IBA–glucose levels. For example, determination that GH3.1, GH3.2, GH3.5, and GH3.17 conjugated amino acids to IAA led to examination of the respective knockout lines to determine the importance of IAA–amino acid conjugates in regulating IAA homeostasis in planta (Staswick et al., 2005). Similarly, identification of ILR1, IAR3, ILL1, and ILL2 as IAA hydrolases was instrumental in understanding how IAA conjugates are cleaved and contribute to the free IAA pool in planta (LeClere et al., 2002). Identification of the IBA-specific synthetases and hydrolases will similarly inform our understanding of how plants regulate their pools of free IBA.
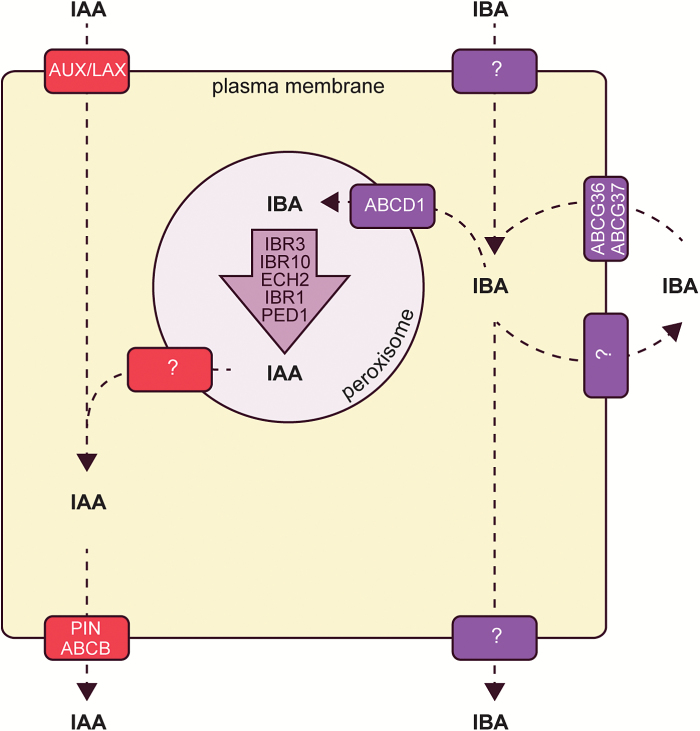
Fig. 2.
Cellular model of IAA and IBA transport. IAA and IBA use distinct transporters for movement into and out of cells and into and out of the peroxisome.
Auxin distribution throughout the body of the plant is mediated by cellular auxin transport to achieve the long-distance movement of IAA. Similarly, IBA and/or IBA conjugates are thought to move long distances through the plant (reviewed in Strader and Bartel, 2011; Michniewicz et al., 2014). Tracking of radiolabel in plants treated with [3H]IBA allowed for the acropetal and basipetal movement of signal in Cleopatra mandarin midrib sections (Epstein and Sagee, 1992), and in various Arabidopsis tissues (Ludwig-Müller et al., 1995b; Rashotte et al., 2003). However, these radiotracer studies are complicated by IBA metabolism to IAA and to IBA conjugates because the identity of the moving molecule tracked is unknown; it is only certain that it is derived from the radiolabeled precursor. For example, later studies determined that most of the radioactive transported material was not the original [3H]IBA, but rather [3H]IAA derived from [3H]IBA in an Arabidopsis columella cell transport assay (Růžička et al., 2010). Likewise, in a study using heavy IBA feeding followed by analysis by MS, [13C1]IAA, ester–[13C1]IBA conjugates, or amide–[13C1]IBA conjugates derived from [13C1]IBA were predominantly transported through Arabidopsis hypocotyls, rather than the supplied [13C1]IBA itself (Liu et al., 2012a). These studies are consistent with IBA conjugates being the major form of transported IBA.
Although IBA may be predominantly transported in the form of conjugates, uptake of the IBA molecule itself is a saturable process (Ludwig-Müller et al., 1995b; Rashotte et al., 2003), which suggests that IBA uptake into plant cells is carrier mediated. Further, examined transporters of IAA, including AUX1, PIN2, PIN7, ABCB1, and ABCB19, do not appear to facilitate the transport of IBA (reviewed in Michniewicz et al., 2014), suggesting that other carriers act in the transport of IBA.
Several IBA transporters have been identified, although there are likely to be additional carriers that have yet to be identified (reviewed in Strader and Bartel, 2011; Michniewicz et al., 2014). IBA efflux is promoted by ATP-BINDING CASSETTE G36/PLEIOTROPIC DRUG RESISTANCE 8/PENETRATION 3 (ABCG36/PDR8/PEN3) (Strader and Bartel, 2009), ABCG37/PDR9/PIS1 (Strader et al., 2008; Růžička et al., 2010), and possibly by additional members of the PDR subclade of the ABCG family (Michniewicz et al., 2014). Mutants defective in ABCG29 (Michniewicz et al., 2014), ABCG33 (Michniewicz et al., 2014), ABCG36 (Strader and Bartel, 2009), or ABCG37 (Strader et al., 2008; Růžička et al., 2010) display increased sensitivity to the auxin precursor IBA and retain wild-type sensitivity to the active auxin IAA. Consistent with the IBA hypersensitivity displayed, root tips excised from mutants defective in either ABCG36 (Strader and Bartel, 2009) or ABCG37 (Strader et al., 2008; Růžička et al., 2010) hyperaccumulate [3H]IBA, but not [3H]IAA. The IBA hypersensitivity combined with the hyperaccumulation of [3H]IBA in these mutants is consistent with roles for ABCG36 and ABCG37 in effluxing IBA from the root.
Although ABCG36 and ABCG37 appear to transport the auxin precursor IBA, but not active IAA, they probably transport additional substrates, as is common for members of the PLEIOTROPIC DRUG RESISTANCE family of transporters. In particular, ABCG37 is likely to transport the synthetic auxin 2,4-dichlorophenoxy acetic acid (2,4-D) (Ito and Gray, 2006; Strader et al., 2008; Růžička et al., 2010), the synthetic auxin precursor 2,4-dichlorophenoxy butyric acid (2,4-DB) (Strader et al., 2008; Růžička et al., 2010), 1-N-naphthylphthalamic acid (NPA) (Ito and Gray, 2006), the auxin breakdown product oxIAA-Hex (Peer et al., 2013), and non-auxinic phenolic coumarin compounds (Fourcroy et al., 2014). Further, ABCG36 probably transports the synthetic auxin precursor 2,4-DB (Strader and Bartel, 2009), oxIAA-Hex (Peer et al., 2013), cadmium and cadmium conjugates (Kim et al., 2007), coumarin (Fourcroy et al., 2014), and a precursor to 4-O-β-d-glucosyl-indol-3-yl formamide (Lu et al., 2015). Clearly, these transporters have roles outside of their regulation of cellular IBA levels.
Analysis of mutants with altered IBA to IAA conversion, altered management of storage forms, and altered transport have revealed roles for IBA-derived auxin in multiple specific developmental processes.
IBA-derived auxin drives aspects of root development
IBA-derived auxin has strong roles in various aspects of root development, including regulation of root apical meristem size, root hair elongation, lateral root development, and formation of adventitious roots. Mutations disrupting IBA metabolism and chemicals that affect IBA metabolism result in multiple root phenotypes, revealing specific roles for IBA-derived auxin in these processes.
The root apical meristem is a collection of undifferentiated cells at the root tip region that display indeterminate growth. The balanced cell division and differentiation in this tissue gives rise to new root tissue, while maintaining a small group of cells that undergo occasional cell division, called the quiescent center. Maintaining proper auxin levels and establishment of an auxin gradient in these tissues is essential to establish root patterning and meristem formation (reviewed in Iyer-Pascuzzi and Benfey, 2009). The ech2 ibr10 double mutant, defective in β-oxidation enzymes required for IBA to IAA conversion, displays decreased DR5-GUS (β-glucuronidase) activity in root tips. Further the ech2 ibr1 ibr3 ibr10 quadruple mutant, defective in multiple IBA conversion enzymes, has reduced free IAA levels in the root tip and displays a reduced meristem size (Strader et al., 2011), consistent with IBA conversion to IAA acting as a major input into the auxin pool in this tissue.
Root hairs are long tubular outgrowths protruding from the epidermal cell layer of roots that aid in nutrient and water acquisition by increasing the root surface area. Auxin affects the positioning of the root hair outgrowth site and promotes root hair elongation (reviewed in Honkanen and Dolan, 2016). Multiple lines of evidence suggest that IBA-derived auxin promotes root hair expansion. First, mutants defective in the ABCG36 or ABCG37 transporters, which act to efflux IBA out of the root, display longer root hairs (Strader and Bartel, 2009; Růžička et al., 2010). Further, blocking IBA to IAA conversion suppresses the long root hair phenotype observed in abcg36 mutants (Strader et al., 2010), suggesting that elevated IBA-derived IAA levels in the abcg36 mutant cause the elongated root hair phenotype. In addition, mutants defective in IBA conversion enzymes display root hairs that can be rescued with exogenous auxin (Strader et al., 2010, 2011), consistent with the possibility that blocking IBA conversion can result in decreased auxin levels in root epidermal cells.
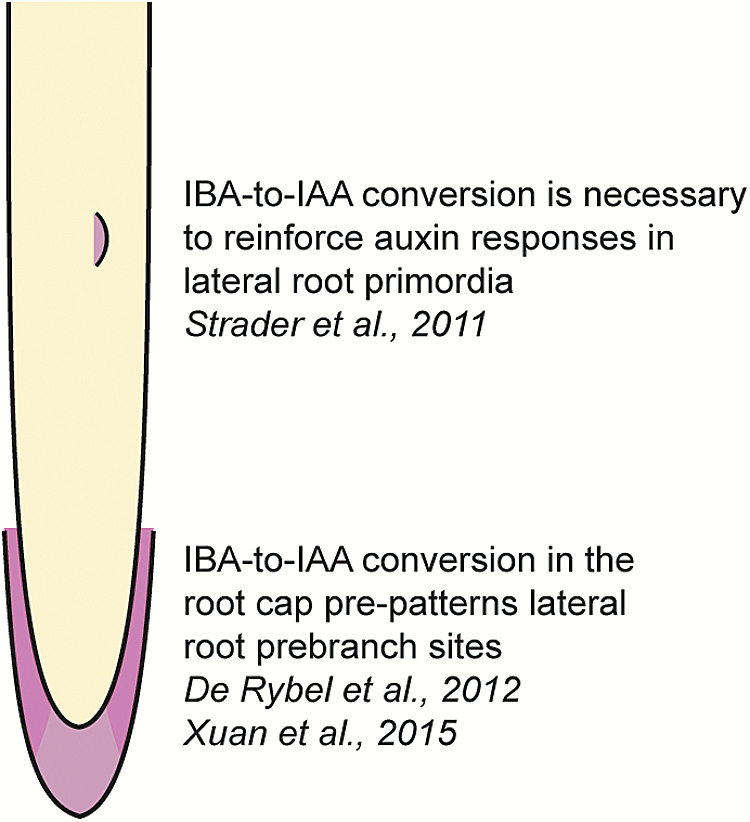
Lateral roots are post-embryonic organs originating from the primary root. The number and positioning of lateral roots is critical to establish the ideal root system for adaptation to local environments (reviewed in Rellán-Álvarez et al., 2016). Auxin drives both lateral root initiation and lateral root emergence (reviewed in Laskowski and Ten Tusscher, 2017). Mutants defective in IBA conversion enzymes display greatly decreased production of lateral roots (Strader et al., 2011). Further, treatment of seedlings with the compound naxillin results in increased lateral root production with limited effects on primary root elongation (De Rybel et al., 2012). Naxillin activity requires an intact IBA to IAA conversion pathway (De Rybel et al., 2012), consistent with the possibility that naxillin promotes IBA conversion to active IAA. Furthermore, IBA to IAA conversion occurs in the lateral root cap and contributes to the priming of lateral root pre-branch sites by setting up the amplitude and frequency of auxin oscillations through the root (De Rybel et al., 2012; Xuan et al., 2015). These oscillations are necessary to establish the lateral root pre-branch sites (Xuan et al., 2015), pointing to an important role for IBA-derived auxin in driving lateral root development (Fig. 3). In addition to roles for IBA-derived auxin originating in the lateral root cap, IBA to IAA conversion in the lateral root primordia themselves is also likely to contribute to lateral root development. In particular, mutants defective in IBA conversion display decreased DR5-GUS staining in lateral root primordia, even after the addition of active auxins (Strader et al., 2011), suggesting that IBA to IAA conversion acts to reinforce auxin responses in these tissues.
Fig. 3.
IBA-derived auxin drives lateral root development. In addition to roles for IBA-derived auxin originating in the lateral root cap (De Rybel et al., 2012; Xuan et al., 2015), IBA to IAA conversion in the lateral root primordia themselves also likely contributes to lateral root development (Strader et al., 2011). (This figure is available in colour at JXB online.)
Adventitious roots are similar to lateral roots in many regards, but are defined by their origination from aerial tissues, such as stems or leaves. Adventitious root formation is often a part of adaptive responses to stress, and shares both common and distinct regulatory mechanisms to lateral root formation (reviewed in Bellini et al., 2014). Zimmerman and Wilcoxin first reported that IBA could stimulate adventitious rooting in cuttings of several species in 1935 (reviewed in Preece, 2003). Throughout the 1930s, IBA arose as the compound of choice for horticulturalists to induce adventitious roots on stem cuttings for plant propagation and is the active ingredient in many modern rooting compounds, such as Rootone® or Hormodin®. In Arabidopsis, IBA promotion of adventitious rooting requires its conversion to IAA; the ech2 ibr10 mutant, defective in IBA to IAA conversion enzymes, fails to produce adventitious roots in response to IBA (Veloccia et al., 2016). Indeed, cuttings from elm cultivars displaying higher levels of IBA to IAA conversion also display relatively high rates of adventitious rooting in response to rooting compounds (Kreiser et al., 2016), suggesting that IBA conversion may be critical for plant propagation in certain species.
IBA-derived auxin drives aspects of shoot development
In addition to its varied roles in root development, IBA-derived auxin plays distinct roles in shoot development, with particular roles in cotyledon expansion and apical hook formation.
Altering IBA homeostasis or IBA conversion to IAA has striking effects on cotyledon expansion. For example, mutants defective in the ABCG36 transporter display larger cotyledons than the wild type (Strader and Bartel, 2009), consistent with its role in IBA efflux and suggesting that auxin levels are elevated in the cotyledons of this mutant. Combining the abcg36 mutation with mutations in IBA conversion enzymes suppresses this large cotyledon phenotype (Strader et al., 2010), suggesting that IBA-derived IAA, rather than IBA itself, drives the increased cotyledon expansion observed in the abcg36 mutant. Further, a strong genetic block in IBA to IAA conversion results in dramatically reduced cotyledon expansion, concomitant with decreased cotyledon epidermal cell size (Strader et al., 2011). Likewise, overexpression of the IBA glycosylating enzyme UGT75D1 results in decreased cotyledon size (Zhang et al., 2016), consistent with decreased contributions to the auxin pool in these overexpression lines. IBA-derived auxin also appears to play a role in compensated cell enlargement (CCE), a phenomenon that allows for increased cell expansion to occur when cell numbers are limited in order to achieve a ‘normal’ organ size in plants. Mutants defective in ECH2, an enzyme required for IBA to IAA conversion, are defective in CCE in cotyledons (Katano et al., 2016), suggesting that IBA-derived auxin is important for driving cotyledon cell expansion not only during normal development but also under conditions where cell numbers are limiting.
Auxin is a critical driver of pavement cell lobing (reviewed in Pan et al., 2015). Auxin-driven intercalary growth results in lobes and indentations among neighboring cotyledon and leaf epidermal cells. The ech2 ibr1 ibr3 ibr10 mutant, defective in IBA to IAA conversion, displays a strong defect in pavement cell lobing, with decreased lobe indentation and decreased pavement cell size, in addition to decreased cotyledon size (Strader et al., 2011), consistent with IBA-derived auxin contributing to the lobing process. Additionally, overexpressing the IBA glycosylating enzyme UGT75D1 results in small cotyledon pavement cells (Zhang et al., 2016) that appear to display decreased lobing. Further research will be required to understand how IBA to IAA conversion is regulated to affect pavement cell lobing.
Contributions to the auxin pool by IBA in shoot tissues are not limited to the cotyledons. In addition, IBA-derived auxin affects hypocotyls (Strader et al., 2011), rosettes (Tognetti et al., 2010), and shoot branching (Tognetti et al., 2010). Dark-grown dicotyledonous seedlings display an apical hook and closed cotyledons as a mechanism to avoid damaging the shoot apical meristem during soil emergence (Goeschl et al., 1966; Guzmán and Ecker, 1990). Mutant seedlings defective in IBA to IAA conversion enzymes display decreased apical hook curvature and also fail to maintain an apical hook for the same length of time as the wild type (Strader et al., 2011). Further, DR5-GUS activity on the inner side of the apical hook is less in IBA conversion mutants than in the wild type (Strader et al., 2011), suggesting that auxin responses in the region are lessened in the absence of auxin pool contributions by IBA. IBA-derived auxin appears important for apical hook formation and maintenance. In addition to playing roles in apical hook formation in dark-grown hypocotyls, IBA-derived auxin plays roles in high temperature responses in light-grown hypocotyls. Growth of seedlings at high temperature results in elevated auxin levels and increased hypocotyl elongation (Gray et al., 1998). Under high temperature conditions, IBA conversion mutants display decreased hypocotyl elongation compared with the wild type (Strader et al., 2011), suggesting that IBA to IAA conversion contributes to this process. Altering IBA homeostasis affects both shoot branching and rosette shape. In Arabidopsis, overexpression of UGT74E2, encoding an enzyme promoting IBA glycosylation, results in a compact rosette shape comprised of leaves with short petioles and dark-green leaves (Tognetti et al., 2010). Further, these UGT74E2 overexpression lines display decreased stature and increased numbers of apical branches (Tognetti et al., 2010), suggesting roles for IBA homeostasis in regulating aspects of shoot architecture. The strong effects of IBA-derived auxin on multiple aspects of plant growth and development suggest that IBA is an important contributor to the auxin pool.
Considering developmental roles of IBA in both aerial and root tissue over the plant life cycle, a unifying theme is that IBA acts as an auxin reserve within the plant. Conversion of this auxin reserve pool is crucial for a variety of important developmental events, as enumerated above, but these processes may not represent the totality of IBA-dependent development. Although we understand the importance of IBA to IAA conversion in many facets of development, other questions about roles for IBA-derived auxin are sure to refine further our understanding of this important auxin. These open questions in IBA biology are expanded upon in the next section.
Open questions
Why do plants need IBA-derived auxin in addition to other pathways?
Auxin regulates a wide spectrum of developmental processes. The local distribution of auxin, modified by its transport or its metabolism, is critical for control of these developmental processes. In Arabidopsis, altering IBA contributions to the auxin pool results in a distinct set of developmental and growth effects, as detailed above, suggesting specific roles for this pathway in modulating auxin levels during seedling development and under stress conditions. Further, genes encoding enzymes required for IBA to IAA conversion are conserved throughout all examined plants (Strader et al., 2011), suggesting that IBA contributions to the auxin pool may also be conserved. Thus, it seems likely that IBA conversion plays roles in plant growth and development that cannot be easily compensated for by other pathways; otherwise, we would expect this pathway to be lost, at least in some species.
Difficulties in detecting IBA
The auxin precursor IBA has been identified as an endogenous compound in numerous plant species, including various monocots and dicots (reviewed in Korasick et al., 2013). However, multiple labs have had difficulty identifying IBA (personal communication), including a report that questioned its presence when it was undetected by GC-MS in samples from Arabidopsis, Populus, and wheat (Novák et al., 2012). Further, IBA concentrations are often reported to be at lower levels than IAA concentrations (Sutter and Cohen, 1992; Ludwig-Müller et al., 1993, 1997; Liu et al., 2012b), and detection of IBA in maize kernels varies by variety examined (Epstein et al., 1989; Ludwig-Müller et al., 1993, 1997). However, IBA has been detected in Arabidopsis by MS (Ludwig-Müller et al., 1993; Strader et al., 2010; Liu et al., 2012b). Further, mutants defective in enzymes required for IBA to IAA conversion display developmental phenotypes consistent with an auxin deficiency and decreased levels of free IAA (Strader et al., 2011), in agreement with endogenous IBA contributing to the auxin pool. These differences in detection of IBA in different labs and in different samples may reflect biological differences in IBA accumulation under different growth conditions (e.g. media used, light conditions, temperature) or may reflect differences in the methods used to extract and quantify IBA levels, although this seems less likely because recovery of the internal heavy IBA standard seems to be adequate in all reports of IBA quantification, whether endogenous IBA was detected or not. If differing growth conditions are truly the underlying basis for variability in detected IBA, this could lead to potentially interesting insight into how IBA homeostasis is influenced by environmental conditions.
Missing IBA transporters
IBA and IAA appear to use independent transport systems (reviewed in Strader and Bartel, 2011; Michniewicz et al., 2014). Thus far, only two IBA carriers have been reported, ABCG36 (Strader and Bartel, 2009) and ABCG37 (Strader et al., 2008; Růžička et al., 2010). The arm2 mutant in rice displays decreased IBA uptake and response and unaltered IAA uptake or response (Chhun et al., 2005), suggesting that this mutant is defective in an IBA uptake carrier. Likewise, the rib1 mutant in Arabidopsis displays IBA resistance and response and unaltered IAA uptake or response (Poupart and Waddell, 2000; Poupart et al., 2005), suggesting that this mutant is also defective in an IBA uptake carrier. Because IBA uptake is a saturable process (Ludwig-Müller et al., 1995b; Rashotte et al., 2003), IBA uptake is likely to be a carrier-mediated process. Perhaps identification of the defective gene in arm2 or rib1 could provide insight into the molecular basis of IBA uptake. Identification of additional IBA carriers will be instrumental in understanding regulation of IBA homeostasis and contributions to the auxin pool.
Regulation of IBA-derived IAA in the auxin pool
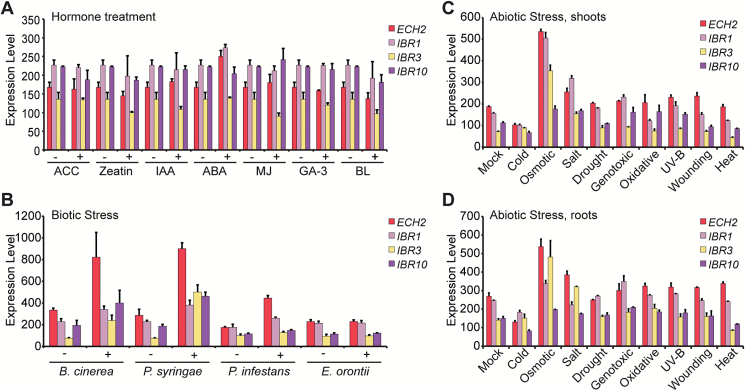
Because IBA-derived auxin plays critical roles in plant development, mechanims to regulate IBA contributions to the auxin pool are likely to exist. Mechanisms to regulate these contributions could include regulated transport, formation and release from conjugates, and transcriptional control of IBA conversion enzymes. Evidence already suggests that regulation of IBA contributions to the auxin pool is important for stress responses. For example, overexpression of UGT74E2 results in elevated IBA–glucose levels, increased tolerance to drought and salt stress, and increased shoot branching (Tognetti et al., 2010). Similarly, overexpression of UGT75D1 results in increased tolerance to osmotic stress (Zhang et al., 2016). Additionally, eFP Browser-annotated (Schmid et al., 2005; Winter et al., 2007) expression of genes encoding the IBA conversion enzymes ECH2, IBR1, IBR3, and IBR10, although seemingly unaffected by treatment with various hormones (Fig. 4A), is up-regulated by several biotic (Fig. 4B) and abiotic (Fig. 4C, D) stresses, consistent with the possibility that IBA to IAA conversion plays roles in stress response. Future research will be needed to verify whether IBA conversion genes are indeed regulated by these stresses and to elucidate those conditions in which IBA contributions to the auxin pool affect growth and stress responses.
Fig. 4.
Genes encoding IBA conversion enzymes are regulated by various stresses. Relative transcript levels of ECH2, IBR1, IBR3, and IBR10 obtained from the eFP Browser database (Schmid et al., 2005; Winter et al., 2007) in response to (A) hormone treatment, (B) biotic stress, (C) abiotic stress in shoots, or (D) abiotic stress in roots.
IBA activity outside of its conversion to IAA
In the earliest studies of auxinic compounds in rooting and propagation assays, IBA was reported to be more effective than IAA (reviewed in Preece, 2003), causing speculation that IBA itself can act as a signaling molecule (reviewed in Ludwig-Müller, 2000). In addition, IBA is more effective than IAA at inducing crown roots in maize (Martínez-de la Cruz et al., 2015). The lrt1 mutant in rice displays decreased lateral rooting and decreased gravitropism. Application of IAA rescues the lateral root phenotypes of lrt1, but not agravitropic growth, whereas IBA application rescues both the lateral root and gravitropism phenotypes (Chhun et al., 2003), consistent with the possibility that IBA plays some roles that IAA cannot. Additionally, some stress conditions caused increased accumulation of IBA, but not IAA (Ludwig-Müller et al., 1995c). Further, inoculation of maize roots with arbuscular mycorrhizal fungi results in elevated IBA, but not IAA levels (Ludwig-Müller et al., 1997). These conditions under which IBA levels are elevated, combined with the potency of IBA in rooting assays, provide some measure of support for roles in which IBA, rather than IBA-derived IAA, might act as a signaling molecule.
In contrast to these reports of roles for IBA as a signaling molecule, genetic data in Arabidopsis suggest that IBA has no discernible activity outside of its conversion to IAA (Zolman et al., 2000, 2007, 2008; Strader et al., 2010, 2011). Further, IBA is unlikely to adopt a conformation for binding into the TIR1–Aux/IAA co-receptor pocket and has no measured binding activity (Uzunova et al., 2016). Potential explanations for the effectiveness of IBA in promoting rooting include the stability of IBA against degradation (Nordström et al., 1991) and effects of nitric oxide produced during the IBA to IAA conversion process (Schlicht et al., 2013), which contribute to lateral root formation. Although data in Arabidopsis are consistent with IBA activity caused by IBA-derived IAA, it remains a formal possibility that IBA could act as a signaling molecule under specialized conditions or in other organisms.
IBA synthesis
We do not currently know the molecular mechanism of IBA synthesis. IBA synthesis from IAA has been demonstrated in microsomal membrane preparations from maize (Ludwig-Müller et al., 1995a) or Arabidopsis (Ludwig-Müller, 2007) seedlings when provided with acetyl-CoA and ATP. Identifying the enzymes required for IBA synthesis will be important in understanding IBA biosynthesis and its roles in the plant. Further, generating mutants defective in IBA synthesis will allow for experiments to understand roles for IBA-derived auxin, and perhaps IBA itself, in plant development.
Acknowledgements
We thank Hongwei Jing and Katie Schreiber for critical comments on the manuscript and helpful discussion. This research was supported by the National Science Foundation (IOS-1453750 to LCS), the NSF Science and Technology Center for Engineering Mechanobiology (CMMI-1548571), the United States Department of Agriculture–National Institute of Food and Agriculture Fellowship Program (2016-67011-25096 to EMF), and the National Institutes of Health (R01 GM112898 to LC.S).
Glossary
Abbreviations:
- ABCG36
ATP-BINDING CASSETTE G36
- ECH2
ENOYL-COA HYDRATASE2
- IAA
indole-3-actetic acid
- IBA
indole-3-butyric acid
- IBR1
IBA RESPONSE1
- IBR3
IBA RESPONSE3
- IBR10
IBA RESPONSE10
- IPyA
indole-3-pyruvic acid.
References
- Bajguz A, Piotrowska A. 2009. Conjugates of auxin and cytokinin. Phytochemistry 70, 957–969. [DOI] [PubMed] [Google Scholar]
- Bellini C, Pacurar DI, Perrone I. 2014. Adventitious roots and lateral roots: similarities and differences. Annual Review of Plant Biology 65, 639–666. [DOI] [PubMed] [Google Scholar]
- Campanella JJ, Olajide AF, Magnus V, Ludwig-Müller J. 2004. A novel auxin conjugate hydrolase from wheat with substrate specificity for longer side-chain auxin amide conjugates. Plant Physiology 135, 2230–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhun T, Taketa S, Ichii M, Tsurumi S. 2005. Involvement of ARM2 in the uptake of indole-3-butyric acid in rice (Oryza sativa L.) roots. Plant and Cell Physiology 46, 1161–1164. [DOI] [PubMed] [Google Scholar]
- Chhun T, Taketa S, Tsurumi S, Ichii M. 2003. The effects of auxin on lateral root initiation and root gravitropism in a lateral rootless mutant Lrt1 of rice (Oryza sativa L.). Plant Growth Regulation 39, 161–170. [Google Scholar]
- De Rybel B, Audenaert D, Xuan W et al. . 2012. A role for the root cap in root branching revealed by the non-auxin probe naxillin. Nature Chemical Biology 8, 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders TA, Strader LC. 2015. Auxin activity: past, present, and future. American Journal of Botany 102, 180–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E, Chen K-H, Cohen JD. 1989. Identification of indole-3-butyric acid as an endogenous constituent of maize kernels and leaves. Plant Growth Regulation 8, 215–223. [Google Scholar]
- Epstein E, Ludwig-Müller J. 1993. Indole-3-butyric acid in plants: occurrence, synthesis, metabolism, and transport. Physiologia Plantarum 88, 382–389. [Google Scholar]
- Epstein E, Sagee O. 1992. Effect of ethylene treatment on transport and metabolism of indole-3-butyric acid in citrus leaf midribs. Plant Growth Regulation 11. [Google Scholar]
- Fourcroy P, Sisó-Terraza P, Sudre D et al. . 2014. Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytologist 201, 155–167. [DOI] [PubMed] [Google Scholar]
- Goeschl JD, Rappaport L, Pratt HK. 1966. Ethylene as a factor regulating the growth of pea epicotyls subjected to physical stress. Plant Physiology 41, 877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Östin A, Sandberg G, Romano CP, Estelle M. 1998. High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proceedings of the National Academy of Sciences, USA 95, 7197–7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. 1990. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. The Plant Cell 2, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Toriyama K, Kondo M, Nishimura M. 1998. 2,4-Dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid beta-oxidation. The Plant Cell 10, 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkanen S, Dolan L. 2016. Growth regulation in tip-growing cells that develop on the epidermis. Current Opinion in Plant Biology 34, 77–83. [DOI] [PubMed] [Google Scholar]
- Hu J, Baker A, Bartel B, Linka N, Mullen RT, Reumann S, Zolman BK. 2012. Plant peroxisomes: biogenesis and function. The Plant Cell 24, 2279–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Gray WM. 2006. A gain-of-function mutation in the Arabidopsis pleiotropic drug resistance transporter PDR9 confers resistance to auxinic herbicides. Plant Physiology 142, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer-Pascuzzi AS, Benfey PN. 2009. Transcriptional networks in root cell fate specification. Biochimica et Biophysica Acta 1789, 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano M, Takahashi K, Hirano T, Kazama Y, Abe T, Tsukaya H, Ferjani A. 2016. Suppressor screen and phenotype analyses revealed an emerging role of the monofunctional peroxisomal enoyl-CoA hydratase 2 in compensated cell enlargement. Frontiers in Plant Science 7, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y. 2007. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. The Plant Journal 50, 207–218. [DOI] [PubMed] [Google Scholar]
- Korasick DA, Enders TA, Strader LC. 2013. Auxin biosynthesis and storage forms. Journal of Experimental Botany 64, 2541–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiser M, Giblin C, Murphy R, Fiesel P, Braun L, Johnson G, Wyse D, Cohen JD. 2016. Conversion of indole-3-butyric acid to indole-3-acetic acid in shoot tissue of hazelnut (Corylus) and elm (Ulmus). Journal of Plant Growth Regulation 35, 710. [Google Scholar]
- Laskowski M, Ten Tusscher KH. 2017. Periodic lateral root priming: what makes it tick?The Plant Cell 29, 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClere S, Tellez R, Rampey RA, Matsuda SP, Bartel B. 2002. Characterization of a family of IAA–amino acid conjugate hydrolases from Arabidopsis. Journal of Biological Chemistry 277, 20446–20452. [DOI] [PubMed] [Google Scholar]
- Liu X, Barkawi L, Gardner G, Cohen JD. 2012a Transport of indole-3-butyric acid and indole-3-acetic acid in Arabidopsis hypocotyls using stable isotope labeling. Plant Physiology 158, 1988–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hegeman AD, Gardner G, Cohen JD. 2012b Protocol: high-throughput and quantitative assays of auxin and auxin precursors from minute tissue samples. Plant Methods 8, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Dittgen J, Piślewska-Bednarek M et al. . 2015. Mutant allele-specific uncoupling of PENETRATION3 functions reveals engagement of the ATP-binding cassette transporter in distinct tryptophan metabolic pathways. Plant Physiology 168, 814–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Müller J. 2000. Indole-3-butyric acid in plant growth and development. Plant Growth Regulation 32, 219–230. [Google Scholar]
- Ludwig-Müller J. 2007. Indole-3-butyric acid synthesis in ecotypes and mutants of Arabidopsis thaliana under different growth conditions. Journal of Plant Physiology 164, 47–59. [DOI] [PubMed] [Google Scholar]
- Ludwig-Müller J. 2011. Auxin conjugates: their role for plant development and in the evolution of land plants. Journal of Experimental Botany 62, 1757–1773. [DOI] [PubMed] [Google Scholar]
- Ludwig-Müller J, Hilgenberg W, Epstein E. 1995a The in vitro biosynthesis of indole-3-butyric acid in maize. Phytochemistry 40, 61–68. [Google Scholar]
- Ludwig-Müller J, Kaldorf M, Sutter EG, Epstein E. 1997. Indole-3-butyric acid (IBA) is enhanced in young maize (Zea mays L.) roots colonized with the arbuscular mycorrhizal fungus Glomus intraradices. Plant Science 125, 153–162. [Google Scholar]
- Ludwig-Müller J, Raisig A, Hilgenberg W. 1995b. Uptake and transport of indole-3-butyric acid in Arabidopsis thaliana: comparison with other natural and synthetic auxins. Journal of Plant Physiology 147, 351–354. [Google Scholar]
- Ludwig-Müller J, Sass S, Sutter EG, Wodner M, Epstein E. 1993. Indole-3-butyric acid in Arabidopsis thaliana. I. Identification and quantification. Plant Growth Regulation 13, 179–187. [Google Scholar]
- Ludwig-Müller J, Schubert B, Pieper K. 1995c Regulation of IBA synthetase from maize (Zea mays L.) by drought stress and ABA. Journal of Expimental Botany 46, 423–432. [Google Scholar]
- Martínez-de la Cruz E, García-Ramírez E, Vázquez-Ramos JM, Reyes de la Cruz H, López-Bucio J. 2015. Auxins differentially regulate root system architecture and cell cycle protein levels in maize seedlings. Journal of Plant Physiology 176, 147–156. [DOI] [PubMed] [Google Scholar]
- Michniewicz M, Powers SK, Strader LC. 2014. IBA transport by PDR proteins. In: Geisler M, ed. Plant ABC transporters. Switzerland: Springer International Publishing, 313–331. [Google Scholar]
- Nordström AC, Jacobs FA, Eliasson L. 1991. Effect of exogenous indole-3-acetic acid and indole-3-butyric acid on internal levels of the respective auxins and their conjugation with aspartic acid during adventitious root formation in pea cuttings. Plant Physiology 96, 856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák O, Hényková E, Sairanen I, Kowalczyk M, Pospíšil T, Ljung K. 2012. Tissue-specific profiling of the Arabidopsis thaliana auxin metabolome. The Plant Journal 72, 523–536. [DOI] [PubMed] [Google Scholar]
- Pan X, Chen J, Yang Z. 2015. Auxin regulation of cell polarity in plants. Current Opinion in Plant Biology 28, 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Cheng Y, Murphy AS. 2013. Evidence of oxidative attenuation of auxin signalling. Journal of Experimental Botany 64, 2629–2639. [DOI] [PubMed] [Google Scholar]
- Poupart J, Rashotte AM, Muday GK, Waddell CS. 2005. The rib1 mutant of Arabidopsis has alterations in indole-3-butyric acid transport, hypocotyl elongation, and root architecture. Plant Physiology 139, 1460–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poupart J, Waddell CS. 2000. The rib1 mutant is resistant to indole-3-butyric acid, an endogenous auxin in Arabidopsis. Plant Physiology 124, 1739–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preece JD. 2003. A century of progress with vegetative plant propogation. HortScience 38, 1015–1025. [Google Scholar]
- Rashotte AM, Poupart J, Waddell CS, Muday GK. 2003. Transport of the two natural auxins, indole-3-butyric acid and indole-3-acetic acid, in Arabidopsis. Plant Physiology 133, 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellán-Álvarez R, Lobet G, Dinneny JR. 2016. Environmental control of root system biology. Annual Review of Plant Biology 67, 619–642. [DOI] [PubMed] [Google Scholar]
- Růžička K, Strader LC, Bailly A et al. . 2010. Arabidopsis PIS1 encodes the ABCG37 transporter of auxinic compounds including the auxin precursor indole-3-butyric acid. Proceedings of the National Academy of Sciences, USA 107, 10749–10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savić B, Tomić S, Magnus V, Gruden K, Barle K, Grenković R, Ludwig-Müller J, Salopek-Sondi B. 2009. Auxin amidohydrolases from Brassica rapa cleave the alanine conjugate of indolepropionic acid as a preferable substrate: a biochemical and modeling approach. Plant and Cell Physiology 50, 1587–1599. [DOI] [PubMed] [Google Scholar]
- Schlicht M, Ludwig-Müller J, Burbach C, Volkmann D, Baluska F. 2013. Indole-3-butyric acid induces lateral root formation via peroxisome-derived indole-3-acetic acid and nitric oxide. New Phytologist 200, 473–482. [DOI] [PubMed] [Google Scholar]
- Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Schölkopf B, Weigel D, Lohmann JU. 2005. A gene expression map of Arabidopsis thaliana development. Nature Genetics 37, 501–506. [DOI] [PubMed] [Google Scholar]
- Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W. 2005. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. The Plant Cell 17, 616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Bartel B. 2009. The Arabidopsis PLEIOTROPIC DRUG RESISTANCE8/ABCG36 ATP binding cassette transporter modulates sensitivity to the auxin precursor indole-3-butyric acid. The Plant Cell 21, 1992–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Bartel B. 2011. Transport and metabolism of the endogenous auxin precursor indole-3-butyric acid. Molecular Plant 4, 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Culler AH, Cohen JD, Bartel B. 2010. Conversion of endogenous indole-3-butyric acid to indole-3-acetic acid drives cell expansion in Arabidopsis seedlings. Plant Physiology 153, 1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Monroe-Augustus M, Rogers KC, Lin GL, Bartel B. 2008. Arabidopsis iba response5 suppressors separate responses to various hormones. Genetics 180, 2019–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Wheeler DL, Christensen SE, Berens JC, Cohen JD, Rampey RA, Bartel B. 2011. Multiple facets of Arabidopsis seedling development require indole-3-butyric acid-derived auxin. The Plant Cell 23, 984–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter EG, Cohen JD. 1992. Measurement of indolebutyric acid in plant tissues by isotope dilution gas chromatography–mass spectrometry analysis. Plant Physiology 99, 1719–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetti VB, Van Aken O, Morreel K et al. . 2010. Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. The Plant Cell 22, 2660–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova VV, Quareshy M, Del Genio CI, Napier RM. 2016. Tomographic docking suggests the mechanism of auxin receptor TIR1 selectivity. Open Biology 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloccia A, Fattorini L, Della Rovere F, Sofo A, D’Angeli S, Betti C, Falasca G, Altamura MM. 2016. Ethylene and auxin interaction in the control of adventitious rooting in Arabidopsis thaliana. Journal of Experimental Botany 67, 6445–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. 2007. An ‘Electronic Fluorescent Pictograph’ browser for exploring and analyzing large-scale biological data sets. PLoS One 2, e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. 2005. Auxin: regulation, action, and interaction. Annals of Botany 95, 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan W, Audenaert D, Parizot B et al. . 2015. Root cap-derived auxin pre-patterns the longitudinal axis of the Arabidopsis root. Current Biology 25, 1381–1388. [DOI] [PubMed] [Google Scholar]
- Zazímalová E, Murphy AS, Yang H, Hoyerová K, Hosek P. 2010. Auxin transporters—why so many?Cold Spring Harbor Perspectives in Biology 2, a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GZ, Jin SH, Jiang XY, Dong RR, Li P, Li YJ, Hou BK. 2016. Ectopic expression of UGT75D1, a glycosyltransferase preferring indole-3-butyric acid, modulates cotyledon development and stress tolerance in seed germination of Arabidopsis thaliana. Plant Molecular Biology 90, 77–93. [DOI] [PubMed] [Google Scholar]
- Zhao Y. 2010. Auxin biosynthesis and its role in plant development. Annual Review of Plant Biology 61, 49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. 2012. Auxin biosynthesis: a simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Molecular Plant 5, 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman PW, Wilcoxon F. 1935. Several chemical growth substances which cause initiation of roots and other responses in plants. Contributions from Boyce Thompson Institute 7, 209–229. [Google Scholar]
- Zolman BK, Martinez N, Millius A, Adham AR, Bartel B. 2008. Identification and characterization of Arabidopsis indole-3-butyric acid response mutants defective in novel peroxisomal enzymes. Genetics 180, 237–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman BK, Nyberg M, Bartel B. 2007. IBR3, a novel peroxisomal acyl-CoA dehydrogenase-like protein required for indole-3-butyric acid response. Plant Molecular Biology 64, 59–72. [DOI] [PubMed] [Google Scholar]
- Zolman BK, Yoder A, Bartel B. 2000. Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 156, 1323–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]