High levels of human papillomavirus vaccine–elicited neutralizing serum antibodies elicited to the first and second doses strongly correlated with reduced memory B-cell responses to the third dose, including antibody responses.
Keywords: vaccine, memory B cell, plasmablasts, HPV, quadrivalent
Abstract
Current guidance recommends that adolescents receive a 2-dose human papillomavirus (HPV) vaccine, whereas young adults and immunocompromised persons receive 3 doses. We examined secondary responses of vaccine-elicited memory B cells (Bmem) in naive women receiving 3 doses of the quadrivalent HPV vaccine to understand the quality of B-cell memory generated by this highly effective vaccine. Unexpectedly, we observed a lower Bmem response rate and magnitude of Bmem responses to the third dose than to a booster dose administered at month 24. Moreover, high titers of antigen-specific serum antibody at vaccination inversely correlated with Bmem responses. As the purpose of additional doses/boosters is to stimulate Bmem to rapidly boost antibody levels, these results indicate the timing of the third dose is suboptimal and lend support to a 2-dose HPV vaccine for young adults. Our findings also indicate more broadly that multidose vaccine schedules should be rationally determined on the basis of Bmem responses.
Three widely approved human papillomavirus (HPV) vaccines provide protection against the types of HPV that most commonly cause cancer or genital warts [1, 2]. The bivalent, quadrivalent, and nonavalent HPV vaccines differ in the number of HPV types they provide immunity against, but all elicit high titers of potent, type-specific antibodies (Abs) that prevent HPV infection. These Abs are directed against the major capsid protein, L1, from the different vaccine types, which each self-assemble to form virus-like particles that both are the basis of the vaccine and structurally and antigenically resemble native virions. Unlike other common subunit vaccines [3, 4], the HPV vaccines elicit highly durable Ab responses and thus presumably long-lived plasma cells [5]. Anamnestic Ab responses have been observed following a booster dose administered 60 months postvaccination in young women who received the quadrivalent HPV (4HPV) vaccine series, demonstrating that the HPV vaccines generate B-cell memory (Bmem) [6]. To learn more about the Bmem that HPV vaccines impart, we previously developed methods to identify HPV-specific Bmem in the blood of persons who received the 4HPV vaccine, using a combination of immunophenotyping, flow cytometry, and fluorescent pseudoviruses (psVs) comprised of the major and minor capsid proteins from 1 of the 4 vaccine types (HPV-16) to label antigen receptors on the surface of Bmem [7]. After singly sorting HPV-16+ Bmem and cloning Abs from them, we found these cells exhibited characteristics of germinal center participation [8] in that they encoded Abs that were somatically mutated, class-switched, and potently neutralized HPV-16 [7]. However, we were not able to evaluate Bmem responses to HPV vaccination using those samples. Therefore, we designed this study to examine Bmem responses in young women receiving the 4HPV vaccine series.
METHODS
Study Design
To study the Bmem responses elicited by 4HPV vaccination, 6 healthy women, aged 18–26 years, were enrolled in an exploratory, unblinded pilot study following written informed consent. Institutional review boards at the Fred Hutchinson Cancer Research Center and the University of Washington approved the study. Inclusion criteria included being self-reportedly sexually naive and never vaccinated against HPV, as well as testing negative for serum Abs against HPV-16 L1 in a previously described screen for HPV seropositivity [9]. Serum was designated seronegative if the amount of anti–HPV-16 L1 Abs was equal to or less than the mean value (plus or minus the standard deviation) obtained for a panel of sera from virgins.
Women received the licensed 4HPV vaccine (Gardasil) according to the prescribing information, that is, at month 0, month 2 (±1 month), and month 6 (±1 month). In addition, to examine vaccine-elicited Bmem responses, subjects received a 4HPV booster dose after having finished the 3-dose 4HPV vaccine series. This booster was scheduled during the memory phase at month 24 (ie, after peak Ab responses have contracted to a stable plateau [6]). We also examined Bmem responses to the third vaccine dose for comparison with other studies that have examined Bmem responses at month 7 [10, 11].
Blood samples (~60 mL) were collected at enrollment (month 0), month 6, month 6 + 1 week (±1 day), month 7 (±1 week), month 12 (±1 month), month 24 (±1 month), month 24 + 1 week (±1 day), and month 25 (±1 week). Plasma and peripheral blood mononuclear cells (PBMCs) were isolated from each sample. On days that included both vaccination and blood draws, blood draws immediately preceded vaccination.
Two women dropped out of the study due to moving from the area. Subject 15 dropped out following the month 6 + 1 week visit; whereas subject 16 dropped out following the month 12 visit.
Sample Preparation
Blood samples were collected and processed as before [9]. In brief, blood draws for serum (screening blood samples only) were collected into BD Vacutainer serum tubes, whereas blood draws for plasma and PBMCs were collected into BD Vacutainer acid citrate dextrose tubes. PBMCs were isolated using density centrifugation with Ficoll Paque Premium (GE Healthcare) according to the manufacturer’s instructions and frozen in ice-cold freezing media (10% dimethyl sulfoxide, 40% RPMI, and 50% heat-inactivated fetal bovine serum [FBS]). All samples were delivered for processing within 2 hours of collection.
Flow Cytometry
PBMC samples were thawed in prewarmed, heat-inactivated FBS and either directly stained for flow cytometry analysis/sorting in the case of plasmablasts, or enriched for B cells prior to staining using the Human B Cell Isolation Kit II (Miltenyi Biotec) in the case of Bmem. One million PBMCs were used for the kinetic analysis of plasmablast responses. To label HPV-16–specific B-cell receptors, Alexa Fluor 488 (AF488)–labeled HPV-16 psVs were generated as described [7].
Thawed samples were first stained in phosphate-buffered saline (PBS) with a Live/Dead Violet viability dye (Life Technologies) for 30 minutes. Samples were then washed and stained in 2% FBS-PBS for 30 minutes using the following Abs from BD Biosciences: anti-CD3 V500 (clone UCHT1; RRID:AB_10612021), anti–immunoglobulin D PE (clone IA6-2; RRID:AB_396114), anti-CD20 PerCP-Cy5.5 (clone 2H7; RRID:AB_1727451), anti-CD27 PE-Cy7 (clone M-T271; RRID:AB_1727456), anti-CD38 APC (clone HIT2; RRID:AB_398599), and anti-CD19 APC-Cy7 (SJ25-C1; RRID:AB_396873). AF488-labeled HPV-16 psVs were included in the cell surface stain for Bmem. All staining was conducted using pretitrated amounts of reagents and with samples on ice and protected from light. Samples were analyzed with a FACSAria II cell sorter (BD Biosciences) and AF488–HPV-16+ Bmem or plasmablasts were sorted as single cells directly into polymerase chain reaction (PCR) plates containing lysis buffer as described previously [9].
Ab Cloning, Sequence Analysis, Expression, and Purification
Full-length heavy and light chain Ab variable regions were amplified from the RNA of single HPV-16+ Bmem or plasmablasts and cloned into Ab expression vectors. These vectors were used for recombinant expression of paired heavy and light Ab chains as full-length Abs in 293F cells (RRID: CVCL_6642). The resulting human monoclonal Abs were then purified from supernatants. All steps were conducted as previously described, including Ab sequence analysis using V-QUEST [7, 12], with the following modifications:
Kappa, lambda, and heavy chain PCRs were conducted in parallel. For analytical gels, up to 10 μL of the PCR reaction was analyzed. Ten picograms of amplification PCR product was used for vector cloning PCR reaction.
PCR purification was conducted using total volume of PCR reaction (~34 μL) and 30 μL Agencourt AMPure XP beads (Beckman Coulter) per well in a 96-well format. After mixing and a 5-minute incubation, magnetic plate was applied for 2 minutes. Cleared solution was aspirated and beads washed twice in 200 μL 70% ethanol on magnet. Beads were then left on magnet to dry for <5 minutes. Samples were then removed from magnet and resuspended in 20 μL molecular biology–grade water for 1 minute. Samples were then returned to plate magnet and eluent collected by pipetting.
For human monoclonal Ab purification, 5-mL plastic columns were used with 1 mL protein G resin (Thermo Scientific Pierce). Supernatant was applied to column 3 times in total. Following purification, Ab-containing fractions were dialyzed in 20K MWCO Slide-A-Lyzer G2 Dialysis Cassettes (Thermo Scientific Pierce).
HPV-16 Neutralization Assay and L1 Binding Assay
Both assays were conducted as before [9] for analyzing the titer of neutralizing or binding anti–HPV-16 Abs in plasma. Human monoclonal Abs cloned from AF488–HPV-16+ Bmem or plasmablasts were also evaluated for HPV-16 neutralization potency. The HPV neutralization assay utilizes non–fluorescently labeled psVs and 293TT cells (RRID: CVCL_1D85) [13]. Neutralization assay dilution series started at a final plasma dilution of 1/100 in PBS and a monoclonal Ab concentration of 50 μg/mL. Neutralization assay controls included psVs in media and media only. Each plasma sample was evaluated in monoplicate at least twice. Monoclonal Abs were evaluated in monoplicate once, and if 50% neutralization was not achieved, deemed as nonneutralizing. However, if the monoclonal Ab was neutralizing in the initial screen, a second experiment was repeated at least once in triplicate. The average signal obtained from the media controls was subtracted from the signals of the psV control and samples, and percentage neutralization was calculated using the following formula: (Abs 405 nmpsV only − Abs 405 nmAb/plasma + psV) / (Abs 405 nmpsV only) × 100. The theoretical concentration or dilution at which a given sample exhibited 50% neutralization (IC50) was determined by nonlinear regression analysis of the neutralization curve using the log (inhibitor) vs response formula (GraphPad Prism).
Statistical Analyses
Pearson correlation coefficient (R) was used to identify correlations between HPV-16+ Bmem or plasmablast responses and preexisting Ab levels, or between monoclonal Ab somatic hypermutation and neutralization potency. A 2-tailed P value of <.05 indicates statistical significance.
RESULTS
Naive Adult Women Received a 3-Dose HPV Vaccine and a Booster Dose at Month 24
Women, aged 18–26 years (n = 6), who were self-reportedly sexually naive and seronegative for HPV-16, were enrolled following informed consent to receive the 4HPV vaccine as prescribed at study months 0, 2, and 6. Four subjects completed the study. These subjects received an additional 4HPV booster dose at month 24 to evaluate secondary Bmem responses, because peak vaccine-elicited Ab titers reach a stable plateau by this time [6]. Blood samples were collected at enrollment (month 0), prior to vaccination at month 6, month 6 + 1 week, month 7, month 12, prior to boosting at month 24, month 24 + 1 week, and month 25 (Supplementary Figure 1).
Bmem Responses to the Third Dose and Booster Dose Inversely Correlate With Preexisting Ab Titers
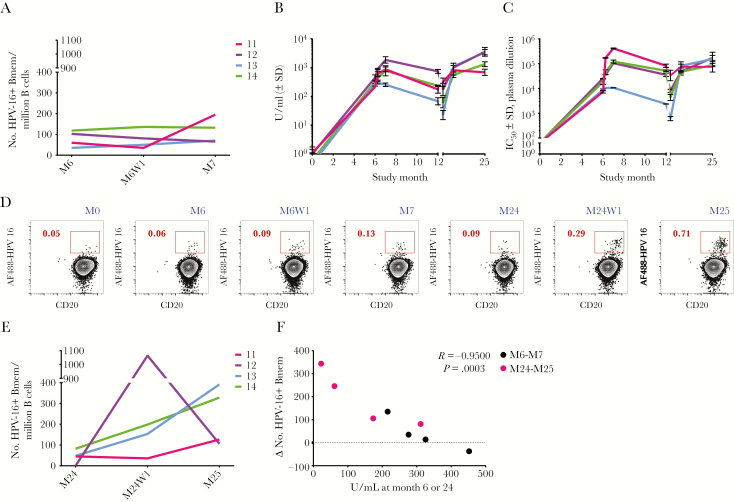
Changes in HPV-16+ Bmem numbers were evaluated by flow cytometry following the third dose at month 6. Only a small, positive Bmem response was observed at month 6 + 1 week or month 7 in 3 of the 4 subjects (Figure 1A). These low-magnitude Bmem responses were initially surprising, given that these subjects have HPV-16+ Bmem at month 6, and Bmem reexposed to antigen characteristically become reactivated and proliferate; yet, this was not observed. Moreover, in an earlier study, we had observed greater HPV-16+ Bmem responses to a single 4HPV dose in unvaccinated, previously HPV-infected subjects who possessed low levels of naturally elicited anti–HPV-16 Abs [9]. One notable difference between the previously infected cohort and this study’s cohort was that anti–HPV-16 binding Ab titers were at least 1 order of magnitude higher at the time the third dose was received in this study (Figure 1B) than at the time of vaccination in the previously infected cohort [9]. Ab titers in both studies were measured at the same time, using the same HPV-16 L1 binding assay, and expressed relative to an international serum standard. Thus, we hypothesized that the high Ab titers elicited in response to the first and second 4HPV doses in this study were neutralizing the vaccine antigen in the third dose and preventing reactivation of Bmem and thus secondary Bmem responses (Supplementary Figure 2). If Bmem responses were being indirectly curbed due to the presence of high vaccine-specific Ab titers, then Bmem responses would be predicted to be greater following a booster dose at month 24 when Ab titers have waned.
Figure 1.
The magnitude of the memory B-cell response inversely correlates with preexisting antibody (Ab) titers. A, Absolute numbers of human papillomavirus type 16 (HPV-16)–positive memory B cells (Bmem) in blood prior to administration of the third quadrivalent HPV vaccine (4HPV) dose at study month 6 (M6) and at 1 week and 1 month postvaccination (M6W1 and M7). Absolute numbers were calculated by multiplying the frequencies of the gated populations together with the absolute number of enriched B cells used in the experiment and normalizing to 1 million B cells. B, Binding anti–HPV-16 immunoglobulin G (IgG) levels in plasma over time are expressed in U/mL relative to an international serum standard. n ≥ 5 experiments. C, Neutralizing anti–HPV-16 Ab titers are estimated by the plasma dilution at which half-maximal viral inhibition occurred in vitro. n ≥ 2 experiments. Month 24 is represented by a tick to the right of month 12. D, Dot plots showing a representative HPV-16+ Bmem response from subject 13 to the third dose at M6 and a booster 4HPV dose at M24. Numbers associated with the red inset indicate the percentage of HPV-16+ Bmem. CD20 is a B-cell marker. E, Absolute numbers of HPV-16+ Bmem in blood on the day the booster dose was administered at month 24 (M24) and at 1 week and 1 month postvaccination (M24W1 and M25). F, The changes (mostly increases) in numbers of HPV-16+ Bmem from M6 to M7 in response to the third dose (black dots) or from M24 to M25 in response to the booster dose (pink dots) were inversely correlated with preexisting binding anti–HPV-16 IgG titers at the time of vaccination. Pearson correlation. n = 4 human subjects. Abbreviations: Bmem, memory B cell; HPV-16, human papillomavirus; IC50, plasma dilution at which pseudovirus infection was inhibited by 50%; SD, standard deviation.
To test this hypothesis, neutralizing Ab titers in blood were also evaluated across different study time points using a psV neutralization assay. Both binding and neutralizing anti–HPV-16 Ab titers were then compared between month 6 and month 24 when the third dose and booster dose were received, respectively. In keeping with the literature, Ab responses at month 24 were generally lower than those at month 6, with the exception of subject 11 (Figure 1B and 1C, and Supplementary Table 1). Furthermore, the HPV-16+ Bmem response rate was 100% following the booster dose, and the magnitude of Bmem responses was greater at month 24 + 1 week and month 25 than that following the third dose at month 6 + 1 week and month 7 (Figure 1D and 1E; gating for HPV-16+ Bmem in Figure 1D shown in Supplementary Figure 3). Again, the only exception was subject 11, whose HPV-16+ Bmem numbers were higher at month 7 (195 HPV-16+ Bmem) than at month 25 (126 HPV-16+ Bmem).
When the titers of anti–HPV-16 Abs in blood at the time of vaccination (either month 6 or month 24) were plotted against the changes in the number of HPV-16+ Bmem (from month 6 to month 7 or month 24 to month 25), a significant inverse correlation was found (Figure 1F). It should also be noted that the fold increase in anti–HPV-16–specific Ab responses following vaccination were greater following the booster dose at month 25 (subject 11 = 2-fold increase, subject 12 = 21-fold, subject 13 = 154-fold, subject 14 = 22-fold; Figure 1B and Supplementary Table 1) than following the third dose at month 7 (subject 11 = 4-fold; subject 12 = 4-fold; subject 13 = unchanged; subject 14 = 2-fold), with the exception of subject 11. Neutralizing anti–HPV-16 Ab titers followed the same trend (Figure 1C and Supplementary Table 1).
More Plasmablast Responses Observed After Vaccine-Elicited Ab Levels Wane
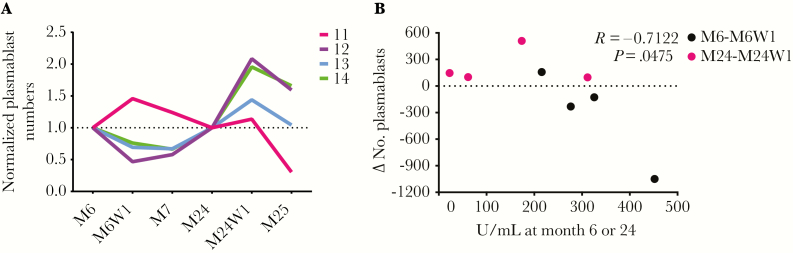
Upon reactivation, Bmem not only proliferate, but also differentiate into Ab-secreting plasmablasts that can be transiently detected in the blood at 1 week postvaccination [14, 15]. Therefore, subject samples were also analyzed for plasmablast numbers following the third dose at month 6 + 1 week and following the booster dose at month 24 + 1 week. Plasmablasts were identified and quantified on the basis of their surface receptor phenotype [15] (example gating shown in Supplementary Figure 4). To facilitate visualization of plasmablast responses to the vaccine, plasmablast numbers at month 6 + 1 week and month 7 were normalized to month 6, whereas plasmablast numbers at month 24 + 1 week and month 25 were normalized to month 24 (Figure 2A). The only subject exhibiting a peak plasmablast response to the third dose was subject 11 (25% plasmablast response rate), who also exhibited the largest magnitude HPV-16+ Bmem response to the third dose. In contrast, all 4 of the subjects elicited a peak plasmablast response to the booster dose, which was received when anti–HPV-16 Ab titers were generally lower. When anti–HPV-16 Ab titers at the time of vaccination were plotted against the change in plasmablast numbers following the third dose or booster dose, a statistically significant inverse correlation was once again found (Figure 2B).
Figure 2.
The magnitude of the plasmablast response also inversely correlates with preexisting antibody (Ab) titers. A, Absolute numbers of plasmablasts in blood at 1 week and 1 month postvaccination (M6W1 and M7) were normalized to numbers at M6, when subjects received their third quadrivalent human papillomavirus (4HPV) vaccine dose. Likewise, numbers of plasmablasts at M24W1 and M25 were normalized to numbers at M24, when subjects received a fourth booster 4HPV dose. B, Changes in the numbers of plasmablasts from M6 to M6W1 in response to the third dose at M6 (black dots) or from M24 to M24W1 in response to the booster dose at M24 (pink dots) inversely correlated with the preexisting binding anti–HPV-16 immunoglobulin G (IgG) titers at the time of vaccination. Pearson correlation. n = 4 human subjects.
No Evidence of Affinity Maturation Following Third Dose
To confirm the specificity of the isolated B cells and understand whether HPV-16–specific Bmem were becoming more somatically mutated and affinity matured over time or after subsequent doses—suggesting continued evolution or reentry into germinal centers—Abs were cloned from singly sorted HPV-16+ Bmem and characterized. To assess specificity, the resulting Bmem-derived monoclonal Abs were tested for their ability to neutralize HPV-16 in a psV neutralization assay. Monoclonal Ab neutralization potency ranged from 0.2 pM to 68.3 nM (Table 1 and Supplementary Table 2). The frequency of Bmem expressing neutralizing Abs varied from 3 of 11 (~27%) at month 6 to 2 of 2 (100%) at month 6 + 1 week. Interestingly, the frequency of Bmem expressing neutralizing Abs appeared to track with the neutralizing Ab titer in blood.
Table 1.
The frequency of memory B cells (Bmem) expressing neutralizing antibodies (Abs) tracks with the neutralizing Ab titers in blood
| M6 | M7 | M24 | M25 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | mAb | mAb IC50 | Plasma IC50 | Subject | mAb | mAb IC50 | Plasma IC50 | Subject | mAb | mAb IC50 | Plasma IC50 | Subject | mAb | mAb IC50 | Plasma IC50 |
| 11 | HPV-16.77 | >333 nM | 7350 | 11 | HPV-16.55 | 1.6 pM | 414000 | 11 | HPV-16.95 | >333 nM | 33500 | 11 | HPV-16.128 | >333 nM | 74100 |
| HPV-16.78 | >333 nM | HPV-16.56 | 6.2 nM | HPV-16.96 | 5.3 pM | HPV-16.129 | 30.2 pM | ||||||||
| HPV-16.79 | >333 nM | HPV-16.57 | 0.2 pM | HPV-16.97 | 1.0 pM | HPV-16.130 | 6.8 pM | ||||||||
| HPV-16.80 | 39.1 pM | HPV-16.58 | 14.2 pM | HPV-16.98 | >333 nM | 12 | HPV-16.131 | 15.9 pM | 172000 | ||||||
| HPV-16.81 | >333 nM | HPV-16.59 | 48.4 nM | HPV-16.99 | 8.5 pM | HPV-16.132 | 21.2 pM | ||||||||
| 13 | HPV-16.83 | >333 nM | 8750 | HPV-16.60 | 0.4 pM | HPV-16.166 | 30.7 pM | HPV-16.133 | >333 nM | ||||||
| HPV-16.84 | 6.2 pM | HPV-16.61 | 0.2 pM | 12 | HPV-16.100 | 36 pM | 8070 | HPV-16.134 | >333 nM | ||||||
| HPV-16.85 | >333 nM | HPV-16.62 | >333 nM | 13 | HPV-16.101 | >333 nM | 611 | HPV-16.135 | 3.0 pM | ||||||
| HPV-16.86 | >333 nM | HPV-16.63 | 3.4 pM | 14 | HPV-16.102 | >333 nM | 4310 | HPV-16.136 | 2.2 pM | ||||||
| HPV-16.87 | >333 nM | HPV-16.64 | 5.1 pM | 13 | HPV-16.137 | 7.0 pM | 155000 | ||||||||
| 14 | HPV-16.82 | 8.1 pM | 18500 | HPV-16.65 | >333 nM | HPV-16.138 | 23.4 pM | ||||||||
| HPV-16.66 | 38.1 pM | HPV-16.139 | 75.6 pM | ||||||||||||
| HPV-16.67 | >333 nM | HPV-16.140 | >333 nM | ||||||||||||
| 12 | HPV-16.68 | 6.3 nM | 105000 | HPV-16.141 | 1.1 pM | ||||||||||
| HPV-16.69 | 1.7 pM | HPV-16.142 | 68.3 nM | ||||||||||||
| HPV-16.70 | 3.3 pM | HPV-16.143 | 0.7 pM | ||||||||||||
| HPV-16.71 | 6.7 pM | HPV-16.144 | 0.9 pM | ||||||||||||
| HPV-16.72 | 3.9 pM | HPV-16.145 | 6.8 pM | ||||||||||||
| HPV-16.74 | 0.7 pM | HPV-16.146 | 3.8 pM | ||||||||||||
| 13 | HPV-16.90 | 1.2 pM | 10700 | HPV-16.147 | 0.9 pM | ||||||||||
| 14 | HPV-16.73 | 1.8 pM | 122000 | HPV-16.148 | 16.9 pM | ||||||||||
| HPV-16.75 | >333 nM | HPV-16.149 | 0.1 nM | ||||||||||||
| HPV-16.150 | 5.0 pM | ||||||||||||||
| HPV-16.151 | 6.0 pM | ||||||||||||||
| HPV-16.152 | 11.9 pM | ||||||||||||||
| 14 | HPV-16.153 | >333 nM | 87900 | ||||||||||||
| HPV-16.154 | 0.8 pM | ||||||||||||||
| HPV-16.155 | 11.6 pM | ||||||||||||||
| HPV-16.156 | 6.7 nM | ||||||||||||||
| HPV-16.157 | 24.3 pM | ||||||||||||||
| HPV-16.158 | 1.9 pM | ||||||||||||||
| HPV16.159 | 1.0 pM | ||||||||||||||
| HPV16.160 | 67.0 pM | ||||||||||||||
| HPV16.161 | 8.1 pM | ||||||||||||||
| HPV16.162 | 2.8 pM | ||||||||||||||
| HPV16.163 | 2.9 pM | ||||||||||||||
| HPV-16.164 | 0.4 pM | ||||||||||||||
At each indicated time point, monoclonal antibodies (mAbs) cloned from human papillomavirus type 16 (HPV-16)–specific Bmem are shown alongside their neutralization potency, or the concentration at which they achieved half-maximal virus inhibition. No mAbs were successfully cloned from the few HPV-16+ Bmem isolated from subject 12 at month (M) 6. The levels of neutralizing anti–HPV-16 Abs in blood, or plasma dilution at which half-maximal virus inhibition (IC50) occurred, are also shown for comparison. The frequency of neutralizing mAbs cloned from HPV-16+ Bmem at each time point was ~31% (M6), ~82% (M7), ~56% (M24), and ~86% (M25). Neutralization potencies for mAbs cloned from Bmem at M6W1 and M24W1 are shown in Supplementary Table 1.
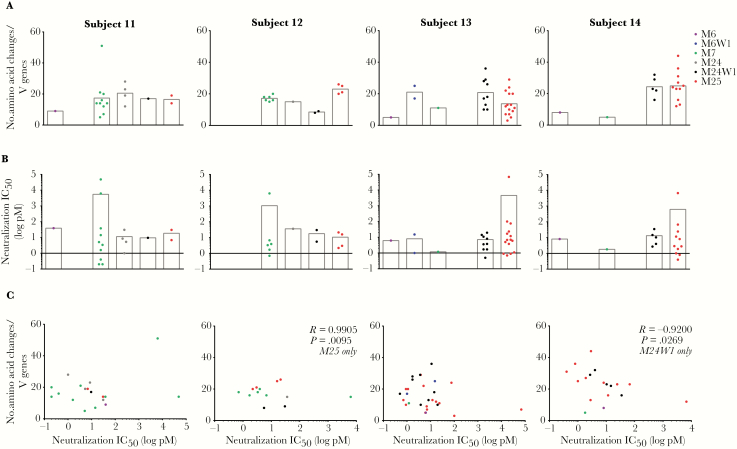
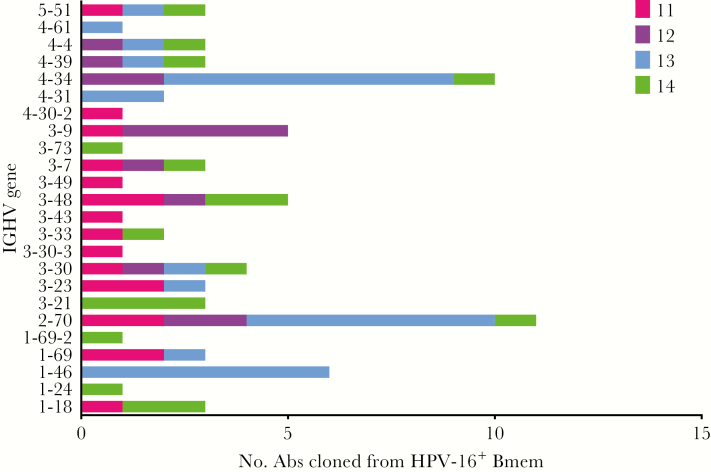
Heavy and light chain variable region sequences from Bmem-derived monoclonal Abs exhibiting HPV-16–specific neutralization activity were analyzed for their predicted germline variable gene assignments and level of somatic hypermutation (ie, numbers of nucleotide mutations or amino acid changes from germline). There was no evidence that Abs cloned from HPV-16–specific Bmem at later time points consistently exhibited more somatic hypermutation (Figure 3A), greater neutralization potency (Figure 3B), or a correlation between somatic hypermutation and neutralization potency (ie, affinity maturation; Figure 3C). However, there are too few numbers of HPV-16–specific Bmem at certain time points to draw firm conclusions. There was also no strong evidence for selection of HPV-16–specific Bmem with particular heavy chain germline variable genes. For although the most common heavy chain variable genes utilized by Bmem-derived neutralizing Abs were IGHV2-70 and IGHV4-34 overall, the most commonly utilized heavy chain variable genes differed on a per-subject basis (Figure 4).
Figure 3.
No evidence of human papillomavirus type 16 (HPV-16)–specific memory B cell (Bmem) affinity maturation beyond third quadrivalent HPV (4HPV) vaccine dose. Antibodies (Abs) cloned from HPV-16+ Bmem isolated at M6 (when the third dose was received), M6W1, M7, M24 (when the booster dose was received), M24W1, and M25 were analyzed for somatic hypermutation and neutralization potency. Only neutralizing monoclonal Abs are shown here. A, The level of somatic hypermutation is represented by the number of amino acid changes from germline in the Bmem’s variable genes (heavy and light Ab chains combined). B, The concentration (log pM) at which each Bmem-derived Ab inhibited 50% of HPV-16 pseudovirus (psV) infection in a neutralization assay, or neutralization IC50, is also shown. An IC50 below zero represents more potent activity. C, The level of somatic hypermutation for each Bmem-derived Ab was plotted against its neutralization IC50. Each dot represents an Ab and is colored by time point. For all Bmem -derived Abs characterized, only 2 statistically significant correlations were observed within a subject for a given time point, and they showed opposing correlations: A positive correlation between somatic hypermutation and neutralization potency was observed for Bmem-derived Abs in subject 12 at M25, indicating that with increasing somatic hypermutation, these Abs exhibited weaker neutralization activity. This positive correlation signifies a lack of affinity maturation. In contrast, a negative correlation was observed between somatic hypermutation and neutralization potency in subject 14 at M24W1, indicating that with increasing somatic hypermutation, Bmem-derived Abs exhibited more potent neutralization activity, signifying affinity maturation. Pearson correlation coefficient (R); P < .05 indicates statistical significance.
Figure 4.
Heavy chain variable gene usage of human papillomavirus type 16 (HPV-16)–specific memory B cells (Bmem). The predicted germline heavy chain variable genes (IGHV) of neutralizing monoclonal antibodies (Abs) cloned from HPV-16+ Bmem are shown on the y-axis. The number of Bmem utilizing a given IGHV for each subject is shown on the x-axis. For example, in the case of IGHV5-51, 1 HPV-16+ Bmem each from subjects 11, 13, and 14 were predicted to have utilized this heavy chain variable gene.
DISCUSSION
The HPV vaccines are among the most effective subunit vaccines developed to date. To better understand the quality of B-cell memory that the 4HPV vaccine generates, we sought to directly evaluate the secondary responses induced by HPV vaccine–elicited Bmem, as such responses are the hallmark of immunological memory. We designed the current study to examine Bmem and plasmablast responses in young women following the third dose of the 4HPV vaccine series, as well as following a single booster 4HPV vaccine dose at month 24.
Unexpectedly, we found that both the magnitude of the Bmem responses and Bmem response rate were lower following the third vaccine dose than following the booster dose. Similarly, we observed a lower plasmablast response rate and consistently found lower peak binding and neutralizing Ab titers following the third dose than following the booster dose. The primary exception was subject 11, whose HPV-16+ Bmem numbers, plasmablast numbers, and peak Ab titers were modestly higher following the third dose than following the booster dose. We hypothesized that high preexisting Ab titers at the time of vaccination prevented the expansion of Bmem in response to vaccination, as well as their differentiation into plasmablasts and the boosting of Ab titers. In support of this hypothesis, we found that the magnitudes of both the HPV-16+ Bmem and plasmablast responses inversely correlated with the binding Ab titers present in blood at the time of vaccination. Interestingly, subject 11 possessed lower binding Ab titers in blood at month 6 than at month 24, perhaps explaining why she exhibited greater secondary responses following the third dose than following a booster dose. Thus, despite being an exception to the trend we observed with other subjects, subject 11’s responses fit this inverse correlation.
One might argue that the third dose improves the quality of Bmem or serum Abs elicited, for example, by improving their antigen affinity. However, we found no evidence of increasing somatic hypermutation, neutralization potency, or affinity maturation following the third dose among the HPV-16–specific Bmem-derived monoclonal Abs. These results are consistent with the limited avidity maturation observed for serum Abs after the third dose in 4HPV vaccinees [16].
Taken together, these results show that secondary vaccine responses (Bmem, plasmablast, and Ab responses) are limited in the presence of high levels of vaccine-specific Abs induced by the first and second 4HPV doses. This finding suggests that a third HPV vaccine dose is suboptimal as scheduled and that a 2-dose series, similar to that recommended for adolescents, might be equally effective for young adults. There are clinical efficacy data for the bivalent HPV vaccine in young adults that support such a shift in strategy, albeit deriving from low numbers and as a post hoc analysis [17]. Studies with influenza have also found that high preexisting Ab levels attenuate secondary B-cell responses [18–21]. However, studying HPV vaccine responses in naive subjects provides a more straightforward system for evaluating the role of preexisting Ab titers in regulating Bmem responses than studying influenza vaccine responses in subjects with complex immunological histories of previous influenza infections and/or vaccinations, which could be confounded by subject-dependent levels of cross-reactive Abs. Ultimately, this study suggests that the timing of multidose vaccine schedules should be rationally determined by analysis of Bmem responses, specifically plasmablast response rates, as the purpose of additional vaccine doses is to activate Bmem to differentiate into plasmablasts that boost Ab levels.
Our finding that the frequency of Bmem expressing HPV-16 neutralizing Abs tracks with HPV-16 neutralizing Ab titers in blood also suggests that spikes in Ab responses immediately following vaccine boosters derive from Bmem undergoing expansion and differentiation into transiently detected Ab-secreting plasmablasts [22]. However, we acknowledge that steady-state serum Ab levels are maintained by long-lived plasma cells in the bone marrow, which are thought to be largely unrelated to circulating plasmablasts [23–25].
The limitations of this study include (1) the inclusion of a relatively small number of subjects; (2) the analysis of only HPV-16–specific Bmem and Ab responses (though plasmablast responses are representative of all vaccine types); and (3) the low number of HPV-16+ Bmem isolated at certain time points. It should be noted that this was a pilot study, and the correlation between serum Ab titers and both Bmem and plasmablast responses warrants validation with a larger cohort. Moreover, similar studies involving the clonal analysis of humoral vaccine responses have been conducted with small cohort sizes (n = 4) [18, 26].
In addition to inducing durable Ab responses and somatically mutated, class-switched Bmem encoding potently neutralizing Abs, the results from this study demonstrate that the HPV vaccine also generates Bmem capable of generating robust secondary responses when peak vaccine-elicited Ab levels have waned. Thus, the HPV vaccines provide a prototype for the quality of B-cell memory and immunity sought for more, if not all, subunit vaccines.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institutes of Health (grant numbers R01 AI038382 to D. A. G.; K24 AI071113 to A. W.; and STD/AIDS Research Training Fellowship T32 AI07140 to E. M. S.) and a Walker Immunotherapy Fellowship (to E. M. S).
Potential conflicts of interest. D. A. G. was a member of Merck's Global Advisory Board for HPV and receives funding from Merck unrelated to this study. A. W. serves on a Merck data and safety monitoring board for a clinical trial not related to HPV. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 30th International Papillomavirus Conference, Lisbon, Portugal, 17–21 September 2015; A Nature Conference: Immune Profiling in Health and Disease, Seattle, Washington, 9–11 September 2015; and 29th International Papillomavirus Conference, Seattle, Washington, 21–25 August 2014.
References
- 1. de Sanjose S, Quint WG, Alemany L et al. ; Retrospective International Survey and HPV Time Trends Study Group Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–56. [DOI] [PubMed] [Google Scholar]
- 2. Garland SM, Steben M, Sings HL et al. . Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis 2009; 199:805–14. [DOI] [PubMed] [Google Scholar]
- 3. Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med 2007; 357:1903–15. [DOI] [PubMed] [Google Scholar]
- 4. Mendy M, Peterson I, Hossin S et al. . Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian villages: no need for a booster dose. PLoS One 2013; 8:e58029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity 1998; 8:363–72. [DOI] [PubMed] [Google Scholar]
- 6. Olsson SE, Villa LL, Costa RL et al. . Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine 2007; 25:4931–9. [DOI] [PubMed] [Google Scholar]
- 7. Scherer EM, Smith RA, Simonich CA, Niyonzima N, Carter JJ, Galloway DA. Characteristics of memory B cells elicited by a highly efficacious HPV vaccine in subjects with no pre-existing immunity. PLoS Pathog 2014; 10:e1004461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol 2012; 30:429–57. [DOI] [PubMed] [Google Scholar]
- 9. Scherer EM, Smith RA, Gallego DF et al. . A single human papillomavirus vaccine dose improves B cell memory in previously infected subjects. EBioMedicine 2016; 10:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Einstein MH, Baron M, Levin MJ et al. ; HPV-010 Study Group Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin 2009; 5:705–19. [DOI] [PubMed] [Google Scholar]
- 11. Smolen KK, Gelinas L, Franzen L et al. . Age of recipient and number of doses differentially impact human B and T cell immune memory responses to HPV vaccination. Vaccine 2012; 30:3572–9. [DOI] [PubMed] [Google Scholar]
- 12. Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res 2008; 36:W503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med 2005; 119:445–62. [DOI] [PubMed] [Google Scholar]
- 14. Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol 2015; 15:149–59. [DOI] [PubMed] [Google Scholar]
- 15. Wrammert J, Smith K, Miller J et al. . Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 2008; 453:667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrin DM, Coates EE, Costner PJ et al. . Comparison of adaptive and innate immune responses induced by licensed vaccines for human papillomavirus. Hum Vaccin Immunother 2014; 10:3446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kreimer AR, Struyf F, Del Rosario-Raymundo MR et al. ; Costa Rica Vaccine Trial Study Group Authors; PATRICIA Study Group Authors; HPV PATRICIA Principal Investigators/Co-Principal Investigator Collaborators; GSK Vaccines Clinical Study Support Group Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol 2015; 16:775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee J, Boutz DR, Chromikova V et al. . Molecular-level analysis of the serum antibody repertoire in young adults before and after seasonal influenza vaccination. Nat Med 2016; 22:1456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andrews SF, Kaur K, Pauli NT, Huang M, Huang Y, Wilson PC. High preexisting serological antibody levels correlate with diversification of the influenza vaccine response. J Virol 2015; 89:3308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sasaki S, He XS, Holmes TH et al. . Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One 2008; 3:e2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Angeletti D, Gibbs JS, Angel M et al. . Defining B cell immunodominance to viruses. Nat Immunol 2017; 18:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lewis GK, DeVico AL, Gallo RC. Antibody persistence and T-cell balance: two key factors confronting HIV vaccine development. Proc Natl Acad Sci U S A 2014; 111:15614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Halliley JL, Tipton CM, Liesveld J et al. . Long-lived plasma cells are contained within the CD19(-)CD38(hi)CD138(+) subset in human bone marrow. Immunity 2015; 43:132–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mei HE, Wirries I, Frölich D et al. . A unique population of IgG-expressing plasma cells lacking CD19 is enriched in human bone marrow. Blood 2015; 125:1739–48. [DOI] [PubMed] [Google Scholar]
- 25. Lavinder JJ, Wine Y, Giesecke C et al. . Identification and characterization of the constituent human serum antibodies elicited by vaccination. Proc Natl Acad Sci U S A 2014; 111:2259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andrews SF, Huang Y, Kaur K et al. . Immune history profoundly affects broadly protective B cell responses to influenza. Sci Transl Med 2015; 7:316ra192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.