Abstract
Pathogenic mycobacteria trigger formation of organized granulomas. As granulomas mature, they induce angiogenesis and vascular permeability. Here, in a striking parallel to tumor pro-angiogenic signaling, we identify angiopoietin-2 (ANG-2) induction as an important component of vascular dysfunction during mycobacterial infection. Mycobacterial infection in humans and zebrafish results in robust induction of ANG-2 expression from macrophages and stromal cells. Using a small-molecule inhibitor closely related to one currently in clinical trials, we link ANG-2/TIE2 signaling to vascular permeability during mycobacterial infection. Targeting granuloma-induced vascular permeability via vascular endothelial-protein tyrosine phosphatase inhibition limits mycobacterial growth, suggesting a new strategy for host-directed therapies against tuberculosis.
Keywords: Mycobacterium tuberculosis, granuloma, vascular permeability, angiopoietin, TIE2, VE-PTP, zebrafish
Granulomas lie at the epicenter of tuberculosis biology. Pathology studies have identified key structural and immunological changes that occur during disease [1]. As granulomas mature, stromal tissue is remodeled, with recruitment of blood vessels reminiscent of ectopic and dysfunctional vasculature observed in tumors [2–4].
Vascular dysfunction in mycobacterial infection is characterized by two major components. There is ectopic growth of vessels around granulomas, resulting in increased vascular density. This growth is driven by the vascular endothelial growth factor (VEGF)–VEGF receptor (VEGFR) signaling axis and serves to alleviate granuloma hypoxia [3, 4]. Second, increased permeability is noted in the vasculature around granulomas. VEGFR inhibition also reduces infection-induced vascular permeability [3], but the role of vascular permeability in mycobacterial pathogenesis has not been examined in isolation.
Angiopoietin-1 (ANG-1) and ANG-2 are key regulators of endothelial cells via their common receptor, TIE2 [5]. Under physiological conditions, ANG-1 binds and activates TIE2 to promote vessel stability and quiescence, thereby limiting angiogenesis and vascular permeability. In contrast, ANG-2 binds TIE2 with similar affinity but acts as a context-dependent antagonist. ANG-2 expression is induced under pathological conditions and antagonizes the stabilizing effects of ANG-1/TIE2 signaling to facilitate angiogenesis and vascular remodeling. In cancer and inflammatory diseases, increased expression of ANG-2 in combination with an increased level of VEGF leads to enhanced tumor vascularity but with aberrant vessels that fail to mature, owing to persistent destabilizing effects of ANG-2 and VEGF. Stabilizing effects of ANG-1/TIE2 are further inhibited by vascular endothelial-protein tyrosine phosphatase (VE-PTP), which dephosphorylates TIE2, thereby blunting downstream signaling [6–8].
Here we identify robust ANG-2 expression in and around human tuberculous granulomas and improved host outcomes in the zebrafish–Mycobacterium marinum model via downstream modulation of this pathway. Using a member of a newly developed class of small-molecule inhibitors of VE-PTP, we show that stabilization of TIE2 signaling alleviates infection-induced vascular permeability independently of neovascularization. The rescue of vascular integrity limits mycobacterial growth, suggesting a novel target for a host stroma-directed therapy.
METHODS
Ethics Statement
Analysis of human granuloma samples was approved by the Duke Medicine Institutional Review Board. Zebrafish experiments were approved by the Duke University Institutional Animal Care and Use Committee.
Human Granulomas
We retrospectively ascertained patients who presented to the Duke University Health System during 2005−2014 and had surgical pathology specimens reported to contain granulomas. By use of DEDUCE, a Web-based tool that permits access to electronic clinical data, cases were ascertained by searching for keywords “granuloma” or “granulomatous” in combination with International Classification of Disease codes 010–018 (diseases due to tuberculosis) or 137 (late effects of tuberculosis). Record review confirmed microbiological confirmation of each case.
Detection of ANG-2
Five-micrometer sections were deparaffinized and rehydrated. Antigen retrieval was performed in a pressure cooker in 10 mM Tris/1 mM ethylenediaminetetraacetic acid (pH 9.0) for 10 minutes. Sections were blocked in phosphate-buffered saline/2.5% goat serum for 1 hour, followed by overnight incubation with either a 1:100 dilution of rabbit anti-ANG-2 (GTX28452; GeneTex) or a 1:100 dilution of rabbit immunoglobulin G isotype control antibody (10500C; ThermoFisher) and with a 1:50 dilution of mouse anti-CD68 (clone KP-1; Dako). Primary antibodies were detected by incubating with goat anti-rabbit Alexa 568– and goat anti-mouse Alexa 647–conjugated secondary antibodies, washed, and mounted in DAPI Fluoromount-G.
Zebrafish Infection Assays
Two days after fertilization, larvae were anesthetized in 160 µg/mL Tricaine (Sigma Aldrich) and injected via either the caudal vein or trunk 48 hours after fertilization with approximately 200 M. marinum organisms [3]. Infected larvae were recovered into water supplemented with 1-phenyl-2-thiourea (Sigma-Aldrich; final concentration, 45 µg/mL). After 6 days, larvae were anesthetized with tricaine prior to fixation, imaging, or analysis by a microangiography assay. For adults, zebrafish were anesthetized in 120 µg/mL Tricaine (Sigma Aldrich) and intraperitoneally injected with approximately 400 M. marinum organisms [3]. Infected adults were maintained at 28°C. After 14 days, adults were euthanized by tricaine overdose for determination of the number of colony-forming units (CFU).
Drug Treatment
AKB-9785 (Aerpio Therapeutics) was dissolved in dimethyl sulfoxide and administered to larvae by soaking immediately after infection at a concentration of 13 µM or to adults after the first week of infection at a concentration of 50 µM. Drug was refreshed every 2 days.
Microangiography
Six days after infection, larvae were anesthetized in 160 µg/mL tricaine and injected via caudal vein with 10 nL of dextran-Texas Red 70 000 MW (Life Technologies; 1 mg/mL concentration). Injected larvae were rinsed and immediately mounted in methylcellulose for fluorescence microscopy 10 minutes after dextran injection. Vascular leakage was calculated as a ratio of intersomitic dextran–Texas Red signal to aortic dextran–Texas Red signal.
Quantification of Bacterial Burden
Larval infection burden was measured as the number of pixels above background in ImageJ, using thresholding of single-channel images and the Analyze Particles function. Infected adult zebrafish were pretreated with 25 µg/mL hygromycin for 2 hours before harvesting, to reduce microbiota load. Zebrafish were euthanized by tricaine overdose and homogenized by a bead mill, using two 10-second bursts. Homogenate was plated on Middlebrook 7H10 (262 710; Difco) supplemented with OADC, hygromycin (H0654; Sigma-Aldrich, 50 µg/L), and amphotericin B (SV3007801; Thermo Scientific, 10 mg/L).
Statistical Analyses
Data are presented as mean ± standard deviation. Experiments were analyzed with unpaired Student t tests, or Welch's t test where appropriate.
RESULTS
ANG-2 Is Expressed in and Around Human Mycobacterial Granulomas
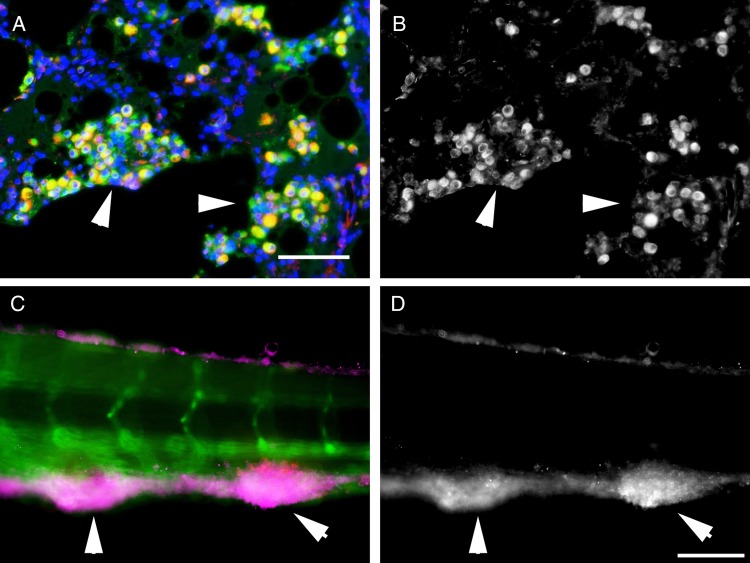
Robust induction of VEGFA in granuloma macrophages has been previously observed [3, 4]. We used dual immunofluorescence to investigate the expression of a parallel pro-angiogenic molecule ANG-2 by CD68-positive macrophages in tissue from patients infected with Mycobacterium tuberculosis. We observed strong expression of ANG-2 by granuloma-associated macrophages in lung tissue, as well as staining in cells negative for the CD68 macrophage marker, consistent with both a macrophage and stromal source in mycobacterial granulomas (Figure 1A and 1B and Supplementary Figures 1 and 2). Presumptive endothelial expression of ANG-2 was observed at a lower level than in macrophages, and uninvolved regions of the lung were largely negative for ANG-2.
Figure 1.
Angiopoietin-2 (ANG-2) is expressed around mycobacterial granulomas. A, Detection of ANG-2 expression, pseudo-colored red, in CD68-positive macrophages, pseudo-colored green, by fluorescent immunodetection in a section of human lung from a patient infected with Mycobacterium tuberculosis. The image is representative of 3 examined patient samples infected with M. tuberculosis. B, Detection of ANG-2 expression from the field of view in panel A. C, Detection of zebrafish Ang2 (magenta) by antibody staining around Mycobacterium marinum–tomato granulomas in a Tg(kdrl:egfp) embryo where the blood vessels are marked with enhanced green fluorescent protein expression. D, Detection of zebrafish Ang2 expression from the field of view in panel C. White arrowheads indicate the largest mycobacterial granulomas in each field of view. Scale bars represent 100 µm.
Ang2 Is Expressed Within M. marinum Granulomas in Zebrafish
The zebrafish–M. marinum infection model is a validated and tractable natural host-mycobacterial infection system that we have previously used to model infection-induced angiogenesis, with central elements of granulomas conserved with humans, including angiogenesis and vascular leakage [3, 4]. To examine whether mycobacterial granulomas recapitulate the ANG-2 expression observed in human granulomas, we performed antibody staining in larval granulomas. We identified consistent Ang2 induction within mycobacterial granulomas (Figure 1C and 1D and Supplementary Figure 3).
Inhibition of VE-PTP Reduces Infection-Induced Vascular Permeability
TIE2 is a critical regulator of vascular stability. ANG-2 acts directly as a TIE2 antagonist to limit vascular stability. An important downstream regulator of TIE2 signaling is VE-PTP, an endothelial cell-specific receptor-type protein tyrosine phosphatase that physically associates with, dephosphorylates, and inhibits TIE2 [9]. An extensive structure-based effort to target this phosphatase resulted in the development of a panel of highly selective pharmacological inhibitors of VE-PTP that bind the enzyme's active site with a subnanomolar Ki; treatment of endothelial cells with these compounds results in potent activation of TIE2 signaling [6]. Inactivation of VE-PTP with these compounds can activate TIE2 even in the presence of high levels of ANG-2 expression [6, 8]. The lead clinical compound in this class, AKB-9778, is currently in clinical trials for diabetic macular edema, a disease characterized by increased vascular permeability, where it has been well tolerated without safety concerns [10, 11], suggesting that these compounds could rapidly be translated to treatment of infectious diseases.
Zebrafish have conserved orthologs of ANG-2, TIE2, and VE-PTP; importantly, the active site and the stabilizing residues in zebrafish VE-PTP are conserved in humans [6, 12]. Owing to ongoing clinical trials with AKB-9778, we used AKB-9785, an inhibitor of VE-PTP that is highly structurally similar to AKB-9778, to investigate whether activation of TIE2 in the presence of an elevated ANG-2 level could alter vascular permeability and/or angiogenesis in the M. marinum model.
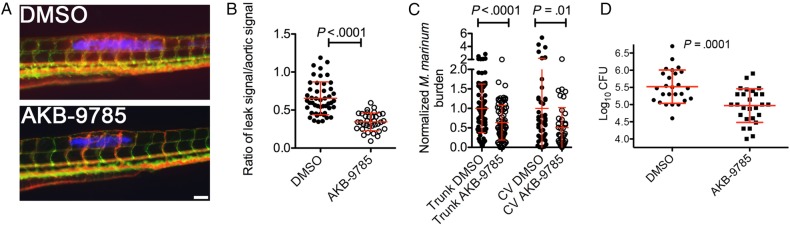
To assess infection-induced vasculopathies, we injected M. marinum into the trunk of transgenic Tg(kdrl:egfp)s843 zebrafish, where the blood vessels form stereotypical networks and are marked by green fluorescent protein expression, allowing intravital imaging of infection-induced angiogenesis and vascular permeability by detecting extravascular fluorescence following microangiography with high-molecular-weight dextran–Texas Red (Figure 2A and Supplementary Figure 4A) [3].
Figure 2.
Activation of TIE2 signaling reduces mycobacterial burden. A, Representative images of ectopic vascularization and vascular leak in Tg(kdrl:egfp) embryos infected with Mycobacterium marinum–cerulean and injected with dextran–Texas red. Scale bar represents 100 µm. B, Quantification of vascular leak in M. marinum–infected embryos treated with AKB-9785. C, Quantification of bacterial burden by fluorometry in M. marinum–infected embryos treated with AKB-9785. D, Quantification of the bacterial burden by a colony-forming units (CFU) recovery assay 2 weeks after M. marinum infection in adult zebrafish treated with AKB-9785 from 1 week after infection. Error bars represent standard deviations. Statistical testing was performed by unpaired Student t tests, with 3 independent biological replicates for each panel. Abbreviation: DMSO, dimethyl sulfoxide.
A previous report demonstrated reduced tumor-induced angiogenesis in zebrafish following VE-PTP inhibition with a relatively high dose of 150 µM AKB-9778 [7]. However, treatment of infected zebrafish with a cell-culture-equivalent dose of 13 µM AKB-9785 did not affect infection-induced angiogenesis, as similar levels of ectopic vasculature were observed in treated and control groups 6 days after infection (Supplementary Figure 4B).
However, analysis of the leakage of high-molecular-weight dextran into the somitic tissue around sites of infection revealed that treatment with AKB-9785 markedly decreased infection-induced vascular leakiness even around highly neovascularized granulomas (Figure 2B).
Inhibition of VE-PTP Limits Mycobacterial Growth
We next quantified the mycobacterial burden in infected zebrafish larvae to determine whether reduction in vascular permeability inhibited mycobacterial growth comparably to the total inhibition of angiogenesis by VEGFR inhibition [3]. We found that AKB-9785 treatment reduced the mycobacterial burden in trunk-injected zebrafish embryos by a similar magnitude to that previously reported for VEGFR inhibition (Figure 2C).
We reasoned that, since AKB-9785 treatment appeared to act independently of angiogenesis in our model, we would see a similar effect on mycobacterial infections established in areas with dense vascular beds. Therefore, we infected zebrafish embryos by caudal vein injection with M. marinum, which stereotypically results in granulomas forming adjacent to existing vascular beds. We found that AKB-9785 treatment reduced mycobacterial burden in this setting, confirming that the effects on mycobacterial growth were independent of an antiangiogenic effect (Figure 2C).
We investigated the effectiveness of AKB-9785 treatment in adult zebrafish infected with M. marinum [3]. We treated infected adults beginning 1 week after infection and found that AKB-9785 treatment reduced the mycobacterial burden by approximately 0.5 log10 CFU over 2 weeks of treatment (Figure 2D). Finally, to exclude the possibility that AKB-9785 is acting as an antibiotic against M. marinum, we showed that the drug had no effect during in vitro growth (Supplementary Figure 5).
DISCUSSION
Infection-mediated angiogenesis driven by the VEGF-VEGFR signaling axis has been previously identified as a tractable target for host-directed therapy against tuberculosis [3, 4]. However, long-term administration of VEGF inhibitors has produced unacceptable rates of arterial thromboembolic events [13]. This study complements the approach of broad inhibition of granuloma vascularization with a narrower approach of normalizing the permeability of the granuloma vasculature that may deliver a better safety profile than long-term administration of VEGFR-blocking agents. Notably, the related VE-PTP inhibitor, AKB-9778, has been well tolerated in clinical trials for diabetic macular edema thus far [10]. In a recent phase IIa study of patients with diabetic macular edema, it resulted in significant improvements when combined with anti-VEGF therapy but not as a monotherapy [11]. Nonetheless, our results support the possibility that targeting vascular permeability through TIE2 activation may provide an additional therapeutic target in tuberculosis.
The physiologically high density of lung vasculature is an important consideration in host-directed tuberculosis treatments. We previously observed decreased vascular leakiness in VEGFR inhibitor–treated zebrafish larvae, but this did not translate into better infection control when granulomas formed near dense vascular beds [3]. Here we found that specific targeting of vascular permeability with AKB-9785 helped to control infection even around sites with high-density vasculature. Taken together, these data suggest that TIE2 activation triggered by VE-PTP inhibition increases the integrity of both existing and ectopic vasculature to reduce mycobacterial growth.
Studies using a rodent Mycoplasma pulmonis model have demonstrated an important role for ANG-2 in destabilizing vasculature during Mycoplasma infection [14]. However, in the Mycoplasma infection model, the permeability changes may be mediated by changes in the recruitment of pericytes [14], a cell type only recently defined in zebrafish [15], whereas the effect of VE-PTP inhibition on vascular leak appears to be primarily through adherens junctions [6]. However, it is possible that both mechanisms could be involved or that they could be interrelated.
Overall, this study provides evidence of a pathological role for the ANG-2/TIE2/VE-PTP signaling axis in mycobacterial infection. Our findings suggest that VE-PTP inhibition and corresponding TIE2 stabilization may serve as novel host-directed therapies for mycobacterial infections.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Supplementary Material
Notes
Acknowledgments. We thank Kevin Peters and members of the Tobin laboratory for helpful discussions; Aerpio Therapeutics for AKB-9785; and John Madden and Wayne Terrell for assistance with human specimens.
Financial support. This work was supported by an Australian National Health and Medical Research Council (C. J. Martin Early Career Fellowship 1053407 and project grant 1099912 to S. H. O.); the American Cancer Society (postdoctoral fellowship PF-13-223-01-MPC to M. R. C.); Duke University (National Institutes of Health [NIH] AIDS training grant 5T32AI007392 to M. G. J.); the National Heart Lung and Blood Institute (grant R01 HL124444 to C. D. K. and J. H.); the Duke University Center for AIDS Research, an NIH-funded program (5P30 AI064518, small-project grant to D. M. T. and S. H. O.); the Searle Scholars Program (award to D. M. T.); the Vallee Foundation (Young Investigator Award to D. M. T.); and the NIH (Director′s New Innovator Award 1DP2-OD008614 to D. M. T.).
Potential conflicts of interest. C. D. K. reports receiving personal fees from Aerpio Therapeutics outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol 2012; 12:581–91. [DOI] [PubMed] [Google Scholar]
- 2. Tsai MC, Chakravarty S, Zhu G et al. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol 2006; 8:218–32. [DOI] [PubMed] [Google Scholar]
- 3. Oehlers SH, Cronan MR, Scott NR et al. Interception of host angiogenic signalling limits mycobacterial growth. Nature 2015; 517:612–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Datta M, Via LE, Kamoun WS et al. Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Proc Natl Acad Sci U S A 2015; 112:1827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer 2010; 10:575–85. [DOI] [PubMed] [Google Scholar]
- 6. Shen J, Frye M, Lee BL et al. Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. J Clin Invest 2014; 124:4564–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goel S, Gupta N, Walcott BP et al. Effects of vascular-endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. J Natl Cancer Inst Monogr 2013; 105:1188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gurnik S, Devraj K, Macas J et al. Angiopoietin-2-induced blood-brain barrier compromise and increased stroke size are rescued by VE-PTP-dependent restoration of Tie2 signaling. Acta Neuropathol 2016; 131:753–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fachinger G, Deutsch U, Risau W. Functional interaction of vascular endothelial-protein-tyrosine phosphatase with the angiopoietin receptor Tie-2. Oncogene 1999; 18:5948–53. [DOI] [PubMed] [Google Scholar]
- 10. Campochiaro PA, Sophie R, Tolentino M et al. Treatment of diabetic macular edema with an inhibitor of vascular endothelial-protein tyrosine phosphatase that activates Tie2. Ophthalmology 2015; 122:545–54. [DOI] [PubMed] [Google Scholar]
- 11. Campochiaro PA, Khanani A, Singer M et al. Enhanced benefit in diabetic macular edema from AKB-9778 Tie2 activation combined with vascular endothelial growth factor suppression. Ophthalmology 2016; 123:1722–30. [DOI] [PubMed] [Google Scholar]
- 12. Evdokimov AG, Pokross M, Walter R et al. Engineering the catalytic domain of human protein tyrosine phosphatase beta for structure-based drug discovery. Acta Crystallogr D Biol Crystallogr 2006; 62:1435–45. [DOI] [PubMed] [Google Scholar]
- 13. Qi WX, Shen Z, Tang LN, Yao Y. Risk of arterial thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: an up-to-date meta-analysis. Crit Rev Oncol Hemat 2014; 92:71–82. [DOI] [PubMed] [Google Scholar]
- 14. Le CT, Laidlaw G, Morehouse CA et al. Synergistic actions of blocking angiopoietin-2 and tumor necrosis factor-alpha in suppressing remodeling of blood vessels and lymphatics in airway inflammation. Am J Pathol 2015; 185:2949–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ando K, Fukuhara S, Izumi N et al. Clarification of mural cell coverage of vascular endothelial cells by live imaging of zebrafish. Development 2016; 143:1328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.