Historically, Bifidobacterium species were reported as abundant in the breastfed infant gut. However, recent studies in resource-rich countries show an increased abundance of taxa regarded as signatures of dysbiosis.
KEYWORDS: Bifidobacterium, biochemistry, infant microbiome, microbiome
ABSTRACT
Historically, Bifidobacterium species were reported as abundant in the breastfed infant gut. However, recent studies in resource-rich countries show an increased abundance of taxa regarded as signatures of dysbiosis. It is unclear whether these differences are the product of genetics, geographic factors, or interventions such as formula feeding, antibiotics, and caesarean section. Fecal pH is strongly associated with Bifidobacterium abundance; thus, pH could be an indicator of its historical abundance. A review of 14 clinical studies published between 1926 and 2017, representing more than 312 healthy breastfed infants, demonstrated a change in fecal pH from 5.0 to 6.5 (adjusted r2 = 0.61). This trend of increasing infant fecal pH over the past century is consistent with current reported discrepancies in Bifidobacterium species abundance in the gut microbiome in resource-rich countries compared to that in historical reports. Our analysis showed that increased fecal pH and abundance of members of the families Enterobacteriaceae, Clostridiaceae, Peptostreptococcaceae, and Veillonellaceae are associated, indicating that loss of highly specialized Bifidobacterium species may result in dysbiosis, the implications of which are not yet fully elucidated. Critical assessment of interventions that restore this ecosystem, measured by key parameters such as ecosystem productivity, gut function, and long-term health, are necessary to understand the magnitude of this change in human biology over the past century.
IMPLICATIONS
There is clear evidence that the infant gut microbiome has important long-term health implications, but changing the gut microbiome is challenging. We recently observed changes in fecal pH resulting from Bifidobacterium infantis EVC001 colonization owing to this bacterium’s selective and acidic fermentation of human milk oligosaccharides (HMOs), which was associated with a reduction in taxa that are signatures of dysbiosis. Although remodeling of the gut microbiome in breastfed infants fed B. infantis EVC001 improved gut function and ecosystem productivity, questions remain about whether differences in Bifidobacterium abundance and species between resource-rich and resource-poor countries are due to host genetics, geography, medical interventions, and/or demographics. Here, we show evidence for an increase in infant fecal pH over the past century, corresponding to an observed reduction of Bifidobacterium, the keystone infant gut symbiont. This may have implications for epidemic human immunological dysfunctions as perturbations in microbiota composition can lead to chronic inflammation and immune-mediated diseases.
EARLY DESCRIPTIONS OF THE INFANT MICROBIOME
In 1913, Logan described the breastfed infant gut microbiome as being an “almost pure culture” of a Gram-positive, acidiphilic “Bacillus bifidus” (Bifidobacterium) (1). This early microscopic characterization of diet-dependent infant microbiomes is in stark contrast to modern reports from resource-rich countries of unstable and highly diverse microbiomes (2). Recent comparisons of the infant gut microbiome from genetically similar but demographically diverse backgrounds indicated that Bifidobacterium was more abundant among infants from resource-poor locations (3), consistent with infants in sub-Saharan Africa and South Asia (4, 5). These differences are also notable at the species level, in that the Bifidobacterium in the feces of infants in Gambia and Bangladesh were shown to be predominantly Bifidobacterium longum subsp. infantis (B. infantis), whereas the Bifidobacterium species in stool samples from infants in the United States and Europe consisted predominantly of B. breve and B. longum subsp. longum (B. longum) (2, 6, 7). Substantial differences in Bifidobacterium composition and abundance among populations have led to questions as to whether medical interventions (e.g., caesarean section, antibiotic use) and formula feeding, or geographic and genetic differences alone, results in these differences (2, 6).
FECAL pH IN BREASTFED INFANTS IS DRIVEN BY BIFIDOBACTERIUM ABUNDANCE
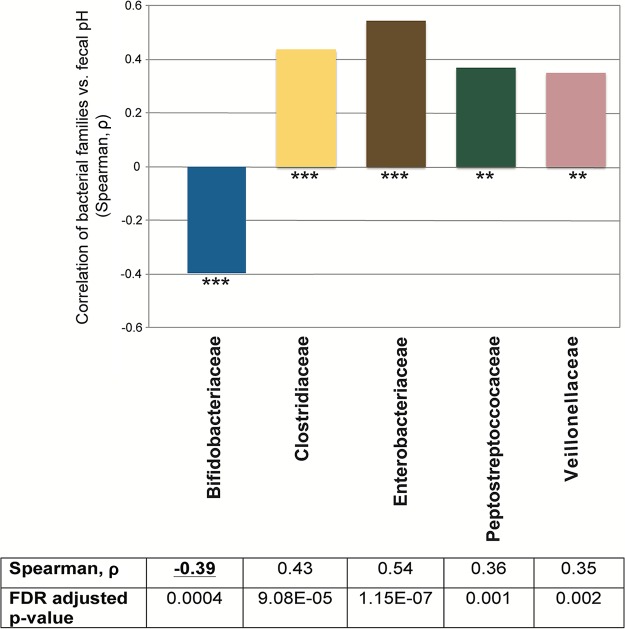
Recently, we found that breastfed infants fed B. infantis EVC001 developed a stable population of this strain and experienced substantial changes in intestinal biochemistry. Notably, fermentation of HMOs resulted in the increased production of lactate and acetate, which was markedly lower in infants who lacked populations of Bifidobacterium or were colonized by other Bifidobacterium species. This was concurrent with significantly higher fecal excretion of HMOs than that of infants fed B. infantis (7). Using data published by Frese et al. (7), we compared fecal pH measurements with bacterial taxa by using a Spearman correlation. Importantly, only one family was significantly associated with reduced fecal pH, i.e., Bifidobacteriaceae (P < 0.0004), indicating that while other bacteria can consume HMOs (e.g., Bacteroidaceae), only members of the family Bifidobacteriaceae convert them to acidic end products with a meaningful effect on fecal pH (Fig. 1). This corroborates previous findings linking infant fecal pH to Bifidobacteriaceae abundance (7, 8). This is a critical connection because although other bacteria (e.g., Lactobacillus, Clostridiaceae, Lachnospiraceae, and Ruminococcaceae) may produce organic acids during fermentation (e.g., lactate, acetate, butyrate, propionate), they were not significantly associated with the acidic fecal pH in breastfed infants. Infants colonized by B. infantis EVC001 had negligible levels of HMOs in their feces and an average fecal pH of 5.15, whereas infants lacking B. infantis had 10-fold higher levels of HMOs in their feces and a fecal pH of 5.97 (7). Further, quantitative PCR confirmed the association of low fecal pH with an increased abundance of Bifidobacterium, in agreement with another study (8).
FIG 1 .
Correlation of bacterial families identified via 16S rRNA marker gene sequencing with fecal pH. Corresponding P values were considered statistically significant when they were ≤0.05 with false-discovery rate (FDR) correction. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
INFANT FECAL pH CHANGES OVER GENERATIONS
Early 1900s reports suggest a rapid reduction in the fecal pH of breastfed infants during the first week after birth (9). Gyorgy and others identified a “bifidus factor,” whose abundance contributed to this reduction in fecal pH and an increase in Bifidobacterium in infant feces (10). This “bifidus factor” (now collectively described as HMOs), is selectively consumed by infant-associated Bifidobacterium; therefore, pH may be a reliable proxy of the breastfed infant gut microbiome. Infant fecal pH reported over the past century is independent of microbiological methodologies (e.g., microscopic examination versus 16S rRNA gene sequencing); thus, we speculated that historical reports of fecal pH could be used as an indirect measure of Bifidobacterium abundance.
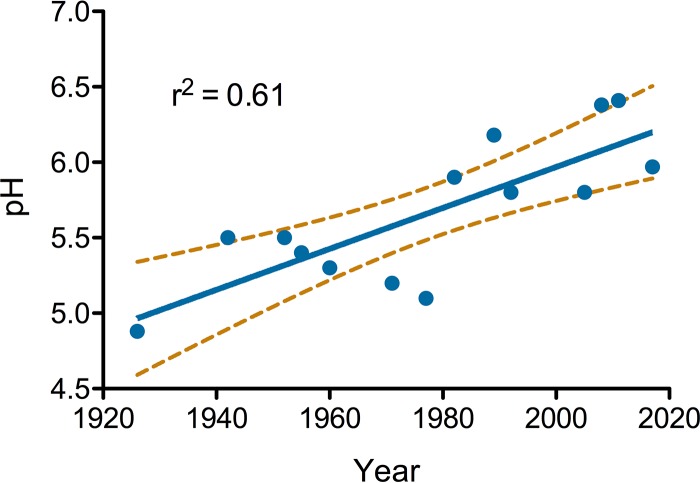
Fourteen peer-reviewed studies published between 1926 and 2017 and reporting 312 measurements from healthy, breastfed infants were found and included. A least-squares linear regression model revealed a strong positive trend with a high association between the publication year and fecal pH (slope = 0.014, adjusted r2 = 0.61; Fig. 2). These data suggest that the mean fecal pH of breastfed infants has increased from about 5.0 in 1926 to 6.5 in recent years (Table 1). Given our previous finding linking fecal pH to Bifidobacterium abundance (7) and reported differences in Bifidobacterium abundance across populations today (2, 3, 6, 7), this longitudinal change is consistent with a generational loss of Bifidobacterium in developed countries, most notably among infants born after 1980.
FIG 2 .
Fecal pH reported in studies along with the average, standard deviation, and numbers of samples measured (where reported) plotted by year of study publication. A linear trend (solid line) and 95% confidence interval (dashed lines) are plotted.
TABLE 1.
Studies examining the fecal pH of healthy, breastfed infants
| Study author(s) (country) | Yr | Fecal pH | SD | Sample size | Reference |
|---|---|---|---|---|---|
| Eitel (Germany)a | 1917 | 4.6–5.6 | NRb | NR | 19 |
| Freudenberg and Heller (Germany)a |
1921 | 4.8–5.6 | NR | NR | 20 |
| Tisdall (Canada)a | 1924 | 4.7–5.1 | NR | NR | 21 |
| Norton (United States) | 1926 | 4.88 | 0.22 | 19 | 9 |
| Uldall (Denmark) | 1942 | 5.5 | 0.56 | 17 | 22 |
| Barbero (United States) | 1952 | 5.5 | NR | 7 | 23 |
| Pratt (United States) | 1955 | 5.4 | NR | 71 | 24 |
| Nagai (Japan) | 1960 | 5.3 | 0.25 | 9 | 25 |
| Bullen (United Kingdom) | 1971 | 5.2 | 0.43 | 10 | 26 |
| Bullen (United Kingdom) | 1977 | 5.1 | NR | 13 | 27 |
| Simhon (United Kingdom) | 1982 | 5.9 | NR | 17 | 28 |
| Balmer (United Kingdom) | 1989 | 6.18 | 0.67 | 38 | 29 |
| Ogawa (Argentina) | 1992 | 5.8 | 0.6 | 7 | 30 |
| Knol (Germany) | 2005 | 5.8 | NR | 21 | 31 |
| Mohan (Germany) | 2008 | 6.38 | 0.1 | 32 | 32 |
| Holscher (United States) | 2011 | 6.41 | 0.11 | 33 | 33 |
| Matsuki (Japan)a | 2016 | 5.9 | 0.6 | 15 | 8 |
| Frese (United States) | 2017 | 5.97 | 0.57 | 18 | 7 |
Report excluded for insufficient data.
NR, not reported.
FACTORS LEADING TO THIS CHANGE IN INFANT FECAL pH
The absence of Bifidobacterium as a keystone symbiont in infants may explain the increase in fecal pH and can be linked to unintended historical and generational consequences of certain interventions that have otherwise significantly improved infant and maternal health. First, a rapid increase in the use of human milk replacers (e.g., evaporated milk and infant formula), which lack the bacterial selectivity of human milk, beginning in the 1920s may have resulted in the inability to foster high levels of specialized infant-associated Bifidobacterium in the infant gut among nonbreastfed infants. This may also explain why B. infantis, which is highly specialized for the consumption of HMOs, is now exceptionally rare among infants in the United States and Europe, whereas B. longum and B. breve, which can access mucin glycans and plant carbohydrates (11), remain relatively abundant. Second, increased caesarean section delivery since the 1980s further limits the natural fecal-oral transfer of Bifidobacterium from mother to infant associated with vaginal delivery (12). Third, antibiotic use has become increasingly common during labor and many infant-associated species of bifidobacteria are sensitive to antibiotics (13). For example, the use of antibiotics to prevent the transmission of group B Streptococcus during delivery and the use of caesarean section as the mode of delivery are both critically important interventions in public health but can alter the acquisition of gut microbes by the infant that begins at birth (13, 14). Together, these barriers may have played a role in the loss of Bifidobacterium over time and across generations, which is reflected in a higher fecal pH.
ARE THERE HEALTH IMPLICATIONS TO THIS CHANGE?
There is clear evidence that the infant gut microbiome has important long-term health implications, and perturbations of the microbiome composition may lead to chronic inflammation (15) and immune-mediated diseases (3, 16–18). These data highlight an increase in infant intestinal dysbiosis (16). Thus, the loss of Bifidobacterium and the profound change in the gut environment, as measured by fecal pH, present a compelling explanation for the increased incidence of allergic and autoimmune diseases observed in resource-rich nations. Longitudinal analyses studies comparing the incidence of autoimmune disorders with restored Bifidobacterium populations in the infant gut microbiome are essential to establish the role of Bifidobacterium in early immune development in the infant gut.
ACKNOWLEDGMENTS
We thank the mothers and their infants for participating in the clinical trial from which these samples were collected. We also thank Cora Morgan for her assistance with technical writing and editing of the manuscript.
This work was funded by Evolve BioSystems, and we are committed to making our data, materials, and analysis methods open and available upon request, where permitted.
REFERENCES
- 1.Logan WR. 1913. The intestinal flora of infants and young children. J Pathol 18:527–551. doi: 10.1002/path.1700180154. [DOI] [Google Scholar]
- 2.Tannock GW, Lee PS, Wong KH, Lawley B. 2016. Why don’t all infants have bifidobacteria in their stool? Front Microbiol 7:834. doi: 10.3389/fmicb.2016.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur TD, Hämäläinen A-M, Peet A, Tillmann V, Uibo R, Mokurov S, Dorshakova N, Ilonen J, Virtanen SM, Szabo SJ, Porter JA, Lähdesmäki H, Huttenhower C, Gevers D, Cullen TW, Knip M, DIABIMMUNE Study Group, Xavier RJ. 2016. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 165:842–853. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zivkovic AM, German JB, Lebrilla CB, Mills DA. 2011. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci U S A 108:4653–4658. doi: 10.1073/pnas.1000083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huda MN, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, Qadri F, Underwood MA, Mills DA, Stephensen CB. 2014. Stool microbiota and vaccine responses of infants. Pediatrics 134:e362–e372. doi: 10.1542/peds.2013-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis ZT, Totten SM, Smilowitz JT, Popovic M, Parker E, Lemay DG, Van Tassell ML, Miller MJ, Jin YS, German JB, Lebrilla CB, Mills DA. 2015. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 3:13. doi: 10.1186/s40168-015-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frese SA, Hutton AA, Contreras LN, Shaw CA, Palumbo MC, Casaburi G, Xu G, Davis JCC, Lebrilla CB, Henrick BM, Freeman SL, Barile D, German JB, Mills DA, Smilowitz JT, Underwood MA, Krajmalnik-Brown R. 2017. Persistence of supplemented Bifidobacterium longum subsp. infantis EVC001 in breastfed infants. mSphere 2:e00501-17. doi: 10.1128/mSphere.00501-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuki T, Yahagi K, Mori H, Matsumoto H, Hara T, Tajima S, Ogawa E, Kodama H, Yamamoto K, Yamada T, Matsumoto S, Kurokawa K. 2016. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat Commun 7:11939. doi: 10.1038/ncomms11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norton RC, Shohl AT. 1926. The hydrogen ion concentration of the stools of the new-born infants. Am J Dis Child 32:183–191. [Google Scholar]
- 10.Gyorgy P, Norris RF, Rose CS. 1954. A variant of Lactobacillus bifidus requiring a special growth factor. Arch Biochem Biophys 48:193–201. doi: 10.1016/0003-9861(54)90323-9. [DOI] [PubMed] [Google Scholar]
- 11.Pokusaeva K, Fitzgerald GF, van Sinderen D. 2011. Carbohydrate metabolism in bifidobacteria. Genes Nutr 6:285–306. doi: 10.1007/s12263-010-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Betrán AP, Ye J, Moller AB, Zhang J, Gülmezoglu AM, Torloni MR. 2016. The increasing trend in caesarean section rates: global, regional and national estimates: 1990–2014. PLoS One 11:e0148343-12. doi: 10.1371/journal.pone.0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duranti S, Lugli GA, Mancabelli L, Turroni F, Milani C, Mangifesta M, Ferrario C, Anzalone R, Viappiani A, van Sinderen D, Ventura M. 2017. Prevalence of antibiotic resistance genes among human gut-derived bifidobacteria. Appl Environ Microbiol 83:e02894-16. doi: 10.1128/AEM.02894-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stearns JC, Simioni J, Gunn E, McDonald H, Holloway AC, Thabane L, Mousseau A, Schertzer JD, Ratcliffe EM, Rossi L, Surette MG, Morrison KM, Hutton EK. 2017. Intrapartum antibiotics for GBS prophylaxis alter colonization patterns in the early infant gut microbiome of low risk infants. Sci Rep 7:16527. doi: 10.1038/s41598-017-16606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sommer F, Bäckhed F. 2013. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 16.Knip M, Honkanen J. 2017. Modulation of type 1 diabetes risk by the intestinal microbiome. Curr Diab Rep 17:105. doi: 10.1007/s11892-017-0933-9. [DOI] [PubMed] [Google Scholar]
- 17.Knip M, Siljander H. 2016. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol 12:154–167. doi: 10.1038/nrendo.2015.218. [DOI] [PubMed] [Google Scholar]
- 18.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, Subbarao P, Mandhane P, Becker A, McNagny KM, Sears MR, Kollmann T, CHILD Study, Mohn WW, Turvey SE, Finlay BB. 2015. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 7:307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 19.Eitel H. 1917. Die wahre Reaktion der Stühle gesunder Säuglinge bei verschiedener Ernährung. Z Kinderheilkd 16:13–62. doi: 10.1007/BF02222668. [DOI] [Google Scholar]
- 20.Freudenberg E, Heller O. 1921. Über Darmgärung II. Über den Einfluss von Eiweiss und Kalk auf die Gärung. Jahresber Kinderheilkd 95:314. [Google Scholar]
- 21.Tisdall FF. 1924. Studies of the acidity (hydrogen ion concentration) of infants’ stools. Arch Pediatr Adolesc Med 27:312–331. doi: 10.1001/archpedi.1924.01920100017003. [DOI] [Google Scholar]
- 22.Uldall C. 1942. Comparative studies on feces of healthy breast, bottle and spoon-fed infants. Acta Paediatr 29:339–366. doi: 10.1111/j.1651-2227.1942.tb16393.x. [DOI] [Google Scholar]
- 23.Barbero GJ, Runge G, Fischer D, Crawford MN, Torres FE, Gyorgy P. 1952. Investigations on the bacterial flora, pH, and sugar content in the intestinal tract of infants. J Pediatr 40:152–163. doi: 10.1016/S0022-3476(52)80176-3. [DOI] [PubMed] [Google Scholar]
- 24.Pratt AG, Read WT. 1955. Influence of type of feeding on pH of stool, pH of skin, and incidence of perianal dermatitis in the newborn infant. J Pediatr 46:539–543. doi: 10.1016/S0022-3476(55)80259-4. [DOI] [PubMed] [Google Scholar]
- 25.Nagai T. 1960. Clinical and experimental studies of ethyl-N-acetyl-d-glucosamine as bifidus factor. Pediatr Int 3:83–102. doi: 10.1111/j.1442-200X.1960.tb01617.x. [DOI] [Google Scholar]
- 26.Bullen CL, Willis AT. 1971. Resistance of the breast-fed infant to gastroenteritis. Br Med J iii:338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bullen CL, Tearle PV, Stewart MG. 1977. The effect of “humanised” milks and supplemented breast feeding on the faecal flora of infants. J Med Microbiol 10:403–413. doi: 10.1099/00222615-10-4-403. [DOI] [PubMed] [Google Scholar]
- 28.Simhon A, Douglas JR, Drasar BS, Soothill JF. 1982. Effect of feeding on infants’ faecal flora. Arch Dis Child 57:54–58. [PMC free article] [PubMed] [Google Scholar]
- 29.Balmer SE, Wharton BA. 1989. Diet and faecal flora in the newborn: breast milk and infant formula. Arch Dis Child 64:1672–1677. doi: 10.1136/adc.64.12.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa K, Ben RA, Pons S, de Paolo MI, Bustos Fernández L. 1992. Volatile fatty acids, lactic acid, and pH in the stools of breast-fed and bottle-fed infants. J Pediatr Gastroenterol Nutr 15:248–252. doi: 10.1097/00005176-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Knol J, Scholtens P, Kafka C, Steenbakkers J, Gro S, Helm K, Klarczyk M, Schöpfer H, Böckler HM, Wells J. 2005. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: more like breast-fed infants. J Pediatr Gastroenterol Nutr 40:36–42. doi: 10.1097/00005176-200501000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Mohan R, Koebnick C, Schildt J, Mueller M, Radke M, Blaut M. 2008. Effects of Bifidobacterium lactis Bb12 supplementation on body weight, fecal pH, acetate, lactate, calprotectin, and IgA in preterm infants. Pediatr Res 64:418–422. doi: 10.1203/PDR.0b013e318181b7fa. [DOI] [PubMed] [Google Scholar]
- 33.Holscher HD, Faust KL, Czerkies LA, Litov R, Ziegler EE, Lessin H, Hatch T, Sun S, Tappenden KA. 2012. Effects of prebiotic-containing infant formula on gastrointestinal tolerance and fecal microbiota in a randomized controlled trial. JPEN J Parenter Enteral Nutr 36:95S–105S. doi: 10.1177/0148607111430087. [DOI] [PubMed] [Google Scholar]