Abstract
Chlamydia trachomatis (Ct) infection causes significant morbidity. In vitro studies demonstrate that Ct growth inhibition occurs by interferon-gamma (IFN-γ)–mediated depletion of intracellular tryptophan, and some Ct strains utilize extracellular indole to restore tryptophan levels. Whether tryptophan levels are associated with Ct infection clearance in humans remains unknown. We evaluated tryptophan, indole, and IFN-γ levels in cervicovaginal lavages from women with either naturally cleared or persisting Ct infection. Women who cleared infection had significantly lower tryptophan levels and trended toward lower IFN-γ levels compared to women with persisting infection. Due to its volatility, indole was not measurable in either group.
Keywords: tryptophan, interferon-gamma, indole, clearance, chlamydia.
Chlamydia trachomatis (Ct) causes the most prevalent bacterial sexually transmitted infection worldwide [1]. Ct infection can cause chronic inflammation of the reproductive tract, which can lead to complications such as tubal factor infertility. Despite public health efforts, Ct infection prevalence continues to increase [1], and our limited understanding of how Ct infection is cleared in humans is a hindrance to vaccine development.
Animal models of chlamydia infection have demonstrated that interferon-gamma (IFN-γ) is essential for infection clearance [2]. Limited human studies support the role of IFN-γ in protective immunity by demonstrating an association between IFN-γ and protection against Ct reinfection [3, 4]. In vitro studies demonstrate that IFN-γ induces depletion of intracellular tryptophan [5], which arrests Ct growth (as Ct is a tryptophan auxotroph) and can lead to Ct cell death [6]. To circumvent clearance, Ct has developed survival mechanisms. In response to tryptophan depletion, Ct survives by differentiating into an enlarged, noninfectious form (a process termed “persistence”), which results in a marked decrease in cellular metabolism and cellular division [7]. In addition, urogenital Ct strains encode a functional tryptophan synthase, which can synthesize tryptophan from extracellular precursor substrates, restoring tryptophan levels and permitting Ct survival in the presence of IFN-γ–mediated immune pressure [8]. In vitro studies also demonstrate that indole is a precursor that can be used for tryptophan synthesis [8]. Although unconfirmed in vivo, this relationship may explain why bacterial vaginosis (BV), an infection which may include indole-producing bacteria, is associated with a 2-fold increased risk for incident Ct infection [9]. Whether tryptophan, indole, or IFN-γ levels in the genital tract are associated with Ct infection clearance in humans remains to be elucidated.
To study the relationship between natural clearance of Ct infection and levels of tryptophan, indole, and IFN-γ, we evaluated levels of these compounds in cervicovaginal lavages (CVLs) collected from women with evidence of natural clearance of Ct infection or persisting Ct infection. Natural clearance of Ct infection (ie, resolving infection before treatment) occurs in approximately 11%–44% of women in the interval between a positive Ct screening test and returning for treatment [10]. Although the mechanisms involved in natural clearance are poorly understood, one study reported that women who naturally cleared Ct infection were 4-fold less likely to be reinfected within 6 months, suggesting that adaptive immunity (perhaps via IFN-γ–mediated tryptophan depletion) may contribute to natural clearance and protective immunity [11]. The primary objective of our study was to determine whether tryptophan, indole, and IFN-γ concentrations were associated with natural clearance of Ct infection in humans. A secondary objective was to determine whether participant characteristics or concomitant genital infections (eg, BV) were associated with concentrations of these compounds.
METHODS
Study Population and Procedures
The study population consisted of females ≥16 years of age presenting to the Jefferson County Department of Health (JCDH) Sexually Transmitted Diseases (STD) Clinic in Birmingham, Alabama, for treatment of a recent positive Ct screening nucleic acid amplification test (NAAT; Aptima Combo 2 [AC2]; Hologic, Marlborough, Massachusetts). All patients provided written consent prior to study enrollment. Women who were pregnant, had a prior hysterectomy, were coinfected with human immunodeficiency virus (HIV), syphilis, or gonorrhea, or had recently received antibiotics with anti-Ct activity were excluded. Participants were interviewed regarding their demographics, symptoms, sexual history, and hormonal contraception use. A pelvic examination was performed to obtain a vaginal swab specimen for wet mount testing to diagnose trichomoniasis, BV (by Amsel criteria), and candidiasis and an endocervical swab specimen for Ct and Neisseria gonorrhoeae testing by AC2 per the manufacturer’s instructions. A 10-mL CVL was collected in nonmenstruating women by continuously lavaging the cervix, posterior fornix, and vaginal walls with sterile saline for 1 minute using a 3-mL transfer pipette and then was stored at –80°C. All study participants received directly observed therapy with azithromycin 1 g given orally. The study was approved by the University of Alabama at Birmingham (UAB) Institutional Review Board and JCDH.
Multiplex
IFN-γ was measured in undiluted CVL supernatants using a validated multiplex cytokine assay (V-PLEX, Meso Scale Diagnostics, Rockville, Maryland) according to the manufacturer’s instructions.
High-Performance Liquid Chromatography–Mass Spectrometry
CVL cellular debris was removed by centrifugation at 16100g for 10 minutes, followed by protein precipitation with 4 volumes of ice-cold methanol for 30 minutes, repeat centrifugation, rotor-evaporation of supernatants, and reconstitution of dried residues in 500 µL water containing 0.1% formic acid. Reconstituted supernatant (10 µL) was injected into a Jupiter 2.0 × 150 mm, 4 µm C18 column (Phenomenex, Torrance, California) and analytes were resolved using a 5%–95% linear gradient of acetonitrile in 0.1% formic acid at 400 µL/minute and 50°C on a Shimadzu Prominence high-performance liquid chromatography (HPLC) system (Shimadzu, Kyoto, Japan). Peaks corresponding to tryptophan and indole were then measured by multiple reaction monitoring (MRM) on a SCIEX 4000 Triple Quadrupole Mass Spectrometer (Concord, Ontario, Canada) in the positive ion mode. Nebulization current was set at 3 with a heater interface temperature of 500°C. Curtain and GS1/GS2 gasses were set at 20, 50, and 5 pounds per square inch (PSI), respectively. Tryptophan was monitored using the MRM transition of 205.1/188.1 with a collision energy, declustering potential, entrance potential, and exit potential of 15 eV, 60 V, 10 V, and 12 V, respectively. Indole was monitored using the MRM transition of 118/91 with a collision energy, declustering potential, entrance potential, and exit potential of 20 eV, 60 V, 10 V, and 12 V, respectively. Fifty-millisecond dwell times were used per transition. The limits of quantification for tryptophan and indole were 0.1 ng/mL and 1 ng/mL, respectively. Tryptophan and indole concentrations were derived by comparison to known metabolite standards.
Statistical Analysis
Analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, North Carolina). Natural clearance of Ct infection was defined by a negative Ct result (by AC2) at the enrollment visit, while persisting infection was defined as having a positive Ct result. Participants defined as natural clearance who were Ct seronegative by Ct elementary body-enzyme-linked immunosorbent assay [12] were excluded, as their initial positive AC2 result may reflect Ct exposure without infection. Otherwise, we tested all women classified as natural clearance and a 1:1 matched group of women with persisting infection (matched by age, race, and coinfections). The Wilcoxon signed-rank test was used to compare tryptophan, indole, and IFN-γ levels between women with naturally cleared vs persisting Ct infection (matched pair groups). Associations of participant characteristics and coinfections with cleared vs persisting Ct infection was evaluated with the Pearson χ2 test or multinomial logistic regression, as appropriate. Tryptophan, indole, and IFN-γ levels between Ct infection only vs Ct infection with a coinfection (independent of Ct clearance status for both) were evaluated with the Mann-Whitney U test. Additional univariate analyses of covariates (age, number of partners, hormonal contraception use, and prior Ct infection) associated with tryptophan, indole, and IFN-γ levels were performed using Mann-Whitney U test, Spearman rank correlation, and Kendall τ-b correlation, as appropriate. A significance level of P < .05 was used for all analyses. Only groups with n > 5 were analyzed.
RESULTS
Among 245 women enrolled February 2014–June 2016, 36 (15%) participants had natural clearance of Ct infection and a CVL available for testing; 36 with persisting infection and a CVL available were matched to them (Table 1). The median interval between screening and enrollment was 10 days (range, 3–24 days). The median age was 25 years (range, 16–46 years) and 94% were African American. Thirty (42%) women were coinfected with either BV, trichomoniasis, or vulvovaginal candidiasis. No significant differences in contraception use, prior history of Ct infection, or interval between screening and enrollment was identified between the 2 groups.
Table 1.
Characteristics of Study Participants (N = 36 Pairs)a
| Characteristic | Total (N = 72) |
Cleared Ct (n = 36) |
Persisting Ct (n = 36) |
P Value |
|---|---|---|---|---|
| Age, y, median (range)b | 25 (16–46) | 25 (16–46) | 25 (16–46) | … |
| Race, No. (%)b | … | |||
| African American | 68 (94) | 34 (94) | 34 (94) | |
| White | 2 (3) | 1 (3) | 1 (3) | |
| Other | 2 (3) | 1 (3) | 1 (3) | |
| Partners in prior 3 mo, median (range)c | 1 (1–4) | 1 (1–3) | 1 (1–4) | .1399d |
| Contraception, No. (%)e | 25 (35) | 9 (25) | 16 (46) | .0677f |
| Prior Ct, No. (%) | 40 (56) | 18 (50) | 22 (61) | .3428f |
| Coinfection, No. (%)b | … | |||
| None (Ct only) | 42 (58) | 21 (58) | 21 (58) | |
| Bacterial vaginosis | 15 (21) | 8 (22) | 7 (19) | |
| Trichomoniasis | 3 (4) | 1 (3) | 2 (6) | |
| Candida | 10 (14) | 5 (14) | 5 (14) | |
| Visit intervalg, d, median (range) | 10 (3–24) | 9 (4–21) | 11 (3–24) | .1438d |
Abbreviation: Ct, Chlamydia trachomatis.
aCleared Ct and persisting Ct pairs were matched on age, race, and coinfection.
b P value not reported as data were matched.
cData missing for 3 women.
dMultinomial logistic regression.
eData missing for 1 woman.
fPearson χ2 test.
gDuration (days) between screening and treatment visits.
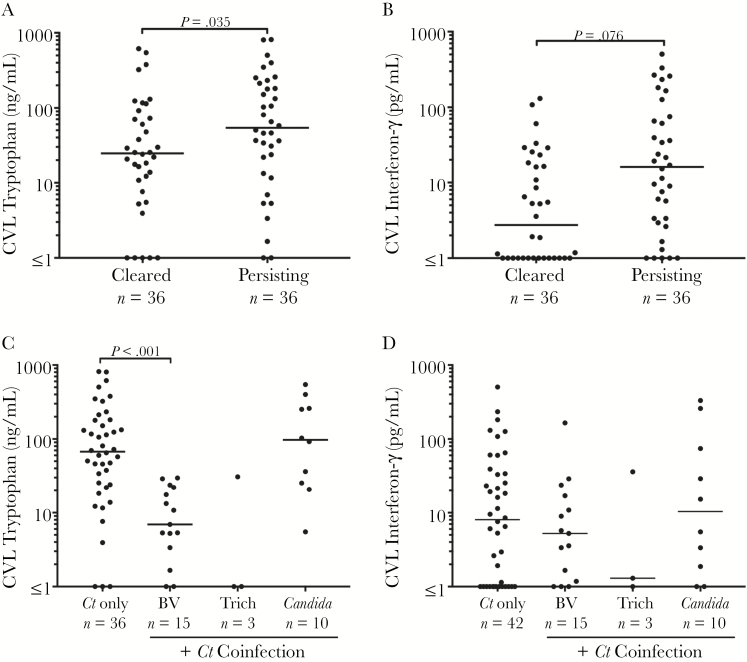
The median CVL tryptophan and IFN-γ levels were 36.4 ng/mL (range, 0–815.2 ng/mL) and 7.0 pg/mL (range, 0–504.2 pg/mL), respectively. Compared to women with persisting Ct infection, women with natural clearance of Ct infection had significantly lower CVL tryptophan levels (median, 25 vs 54 ng/mL; P = .035; Figure 1A) and a trend toward lower IFN-γ levels (median, 2.8 vs 16.1 pg/mL; P = .076; Figure 1B). Indole levels were below the limit of detection (<1 ng/mL) for all samples. As a control, we spiked CVL specimens with indole prior to extraction and did not detect indole by HPLC mass spectrometry (MS), suggesting that indole evaporated during the extraction process.
Figure 1.
Cervicovaginal lavage tryptophan and interferon-gamma (IFN-γ) concentrations in women with natural clearance of Chlamydia trachomatis (Ct) infection vs persisting Ct infection and also Ct monoinfection vs coinfections. Women who naturally cleared Ct infection had lower cervicovaginal lavage (CVL) tryptophan levels compared with women with persisting Ct infection (median, 25 vs 54 ng/mL; P = .035) (A) and a trend toward lower IFN-γ levels (median, 2.8 vs 16.1 pg/mL; P = .076) (B). Compared to Ct monoinfection (independent of Ct infection clearance status), significantly lower tryptophan levels were seen in women coinfected with bacterial vaginosis (BV) (median, 6.9 vs 67.4 ng/mL; P < .001) (C). IFN-γ levels did not differ by coinfection (D). Only coinfections with n > 5 were analyzed. The horizontal line denotes the median. The y-axis is shown as a logarithmic scale.
Stratifying tryptophan levels in Ct-infected women (independent of Ct infection clearance status) by coinfection showed that coinfection with BV was associated with an almost 10-fold lower median CVL tryptophan level compared with Ct monoinfection (median, 6.9 vs 67.4 ng/mL; P < .001; Figure 1C). No significant difference in tryptophan levels between trichomoniasis or vaginal candidiasis and Ct monoinfection was identified. IFN-γ levels did not significantly differ comparing Ct monoinfection and coinfections (Figure 1D).
We then evaluated the relationship between tryptophan or IFN-γ levels with age, race, hormonal contraception, or number of sex partners in the last 3 months, and no significant associations were found (data not shown). However, there was a trend in women with a history of prior Ct infection having a lower tryptophan level (median, 22.8 [range, 0–815] ng/mL vs 49.0 [range, 0.9–806] ng/mL; P = .0561; data not shown).
DISCUSSION
Natural clearance of Ct infection occurs slowly (approximately 50% after 1 year [13]), suggesting that Ct employs immune evasion mechanisms. Adaptive immunity likely contributes to this process, evidenced by the association between natural clearance of Ct infection and reduced Ct reinfection rates [11]. IFN-γ is crucial for effective Ct clearance in animal models [2], and likely humans [3, 4], by signaling tryptophan depletion, as shown in vitro [6]. To our knowledge, our study is the first to provide in vivo evidence that lower cervicovaginal tryptophan levels, and perhaps lower IFN-γ levels, are associated with Ct clearance. Together, our data suggest that persisting Ct infection is associated with elevated IFN-γ and tryptophan levels, the former likely reflecting the host immune response against Ct and the latter the organism’s effort to combat the IFN-γ–mediated depletion of tryptophan by de novo synthesis of tryptophan by a functional tryptophan synthase. Once Ct infection is cleared, host IFN-γ production declines and the heightened tryptophan synthesis is arrested. Our finding of a trend between a history of a prior Ct infection, a known correlate of protective immunity [14], and 2-fold lower tryptophan levels suggests that heightened IFN-γ production in protected individuals hastens Ct clearance, which would correlate with lower tryptophan levels.
An interesting finding in our study was the association between BV coinfection and lower CVL tryptophan levels. Given the well-established association of increased Ct incidence rates in women with BV [9], our finding suggests that BV may use tryptophan-independent mechanisms to promote effective Ct immune evasion (eg, BV biofilms, increasing vaginal pH).
An unexpected finding was that CVL indole was not detected. This is likely due to evaporation during the extraction process as spiked indole was not recovered. However, discovery-based relative quantitation HPLC-MS pilot studies we performed (data not shown) confirmed the presence of high levels of other indole-containing metabolites in CVL specimens (eg, indoleacetyl glutamine), which have been reported by others [15]. Whether these indole-containing metabolites may be a source of indole or whether indole is present in the cervicovaginal environment in women remains to be elucidated.
Our study has several limitations. First, the majority of subjects were African American, representative of our STD clinic population, and it is unknown if vaginal tryptophan or IFN-γ levels differ between races/ethnicities. Also, our study used Amsel criteria to diagnose BV, which is less specific than the Nugent score and could have overestimated the frequency of BV infections. Although we excluded women classified with naturally cleared Ct infection who were Ct seronegative (positive AC2 from Ct exposure, but not with established infection) to minimize misclassification of natural clearance, it is still possible misclassification may have occurred.
In summary, we demonstrated that natural clearance of Ct infection is associated with lower cervicovaginal tryptophan levels, and possibly lower levels of IFN-γ. Coinfection with BV, and possibly a prior Ct infection, was also associated with lower tryptophan levels. Further studies are needed to understand the interplay between coinfections and metabolites in the cervicovaginal environment.
Notes
Acknowledgments. We thank the UAB STD Program and the JCDH STD clinicians for their contributions.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was funded by a Development Research Project award (S. J. J., principal investigator [PI]) from the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH Sexually Transmitted Infection Cooperative Research Center (grant U19AI113212; E. Hook, PI) and by an NIH NIAID R01AI093692 award (W. M. G., PI). Funds for the SCIEX 4000 mass spectrometer used in these studies was obtained from the UAB Health Services Foundation General Endowment Fund Grant. Funds for the operation of the Targeted Metabolomics and Proteomics Laboratory come in part from the UAB O’Brien Acute Kidney Injury Center (award number P30 DK079337).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Newman L, Rowley J, Vander Hoorn S et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10:e0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rank RG, Whittum-Hudson JA. Protective immunity to chlamydial genital infection: evidence from animal studies. J Infect Dis 2010; 201(suppl 2): S168–77. [DOI] [PubMed] [Google Scholar]
- 3. Cohen CR, Koochesfahani KM, Meier AS et al. Immunoepidemiologic profile of Chlamydia trachomatis infection: importance of heat-shock protein 60 and interferon-gamma. J Infect Dis 2005; 192:591–9. [DOI] [PubMed] [Google Scholar]
- 4. Barral R, Desai R, Zheng X et al. Frequency of Chlamydia trachomatis-specific T cell interferon-γ and interleukin-17 responses in CD4-enriched peripheral blood mononuclear cells of sexually active adolescent females. J Reprod Immunol 2014; 103:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J 1991; 5:2516–22. [PubMed] [Google Scholar]
- 6. Beatty WL, Belanger TA, Desai AA, Morrison RP, Byrne GI. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun 1994; 62:3705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wyrick PB. Chlamydia trachomatis persistence in vitro: an overview. J Infect Dis 2010; 201(suppl 2):S88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fehlner-Gardiner C, Roshick C, Carlson JH et al. Molecular basis defining human Chlamydia trachomatis tissue tropism. A possible role for tryptophan synthase. J Biol Chem 2002; 277:26893–903. [DOI] [PubMed] [Google Scholar]
- 9. Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis 2003; 36:663–8. [DOI] [PubMed] [Google Scholar]
- 10. Geisler WM. Duration of untreated, uncomplicated Chlamydia trachomatis genital infection and factors associated with chlamydia resolution: a review of human studies. J Infect Dis 2010; 201(suppl 2):S104–13. [DOI] [PubMed] [Google Scholar]
- 11. Geisler WM, Lensing SY, Press CG, Hook EW 3rd. Spontaneous resolution of genital Chlamydia trachomatis infection in women and protection from reinfection. J Infect Dis 2013; 207:1850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bakshi R, Gupta K, Jordan SJ et al. Immunoglobulin-based investigation of spontaneous resolution of Chlamydia trachomatis infection. J Infect Dis 2017; doi:10.1093/infdis/jix194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molano M, Meijer CJ, Weiderpass E et al. The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow-up study. J Infect Dis 2005; 191:907–16. [DOI] [PubMed] [Google Scholar]
- 14. Katz BP, Batteiger BE, Jones RB. Effect of prior sexually transmitted disease on the isolation of Chlamydia trachomatis. Sex Transm Dis 1987; 14:160–4. [DOI] [PubMed] [Google Scholar]
- 15. Lewis ME, Belland RJ, AbdelRahman YM et al. Morphologic and molecular evaluation of Chlamydia trachomatis growth in human endocervix reveals distinct growth patterns. Front Cell Infect Microbiol 2014; 4:71. [DOI] [PMC free article] [PubMed] [Google Scholar]